Abstract
Spontaneous rhythmic constrictions known as vasomotion are developed in several microvascular beds in vivo. Vasomotion in arterioles is considered to facilitate blood flow, while venular vasomotion would facilitate tissue metabolite drainage. Mechanisms underlying vasomotion periodically generate synchronous Ca2+ transients in vascular smooth muscle cells (VSMCs). In visceral organs, mural cells (pericytes and VSMCs) in arterioles, capillaries and venules exhibit synchronous spontaneous Ca2+ transients. Since sympathetic regulation is rather limited in the intra-organ microvessels, spontaneous activity of mural cells may play an essential role in maintaining tissue perfusion. Synchronous spontaneous Ca2+ transients in precapillary arterioles (PCAs)/capillaries appear to propagate to upstream arterioles to drive their vasomotion, while venules develop their own synchronous Ca2+ transients and associated vasomotion. Spontaneous Ca2+ transients of mural cells primarily arise from IP3 and/or ryanodine receptor-mediated Ca2+ release from sarcoendoplasmic reticulum (SR/ER) Ca2+ stores. The resultant opening of Ca2+-activated Cl- channels (CaCCs) causes a membrane depolarisation that triggers Ca2+ influx via T-type and/or L-type voltage-dependent Ca2+ channels (VDCCs). Mural cells are electrically coupled with each other via gap junctions, and thus allow the sequential spread of CaCC or VDCC-dependent depolarisations to develop the synchrony of Ca2+ transients within their network. Importantly, the synchrony of spontaneous Ca2+ transients also requires a certain range of the resting membrane potential that is maintained by the opening of Kv7 voltage-dependent K+ (Kv7) and inward rectifier K+ (Kir) channels. Thus, a depolarised membrane would evoke asynchronous, ‘premature’ spontaneous Ca2+ transients, while a hyperpolarised membrane prevents any spontaneous activity.
Keywords: smooth muscle, pericyte, vasomotion, microvasculature, intracellular calcium
Abbreviations.
CaCC: Ca2+-activated Cl– channel; CICR: Ca2+-induced Ca2+ release; IK channel: intermediate-conductance Ca2+-activated K+ channel; Kir channel: inward rectifier K+ channel; Kv7 channel: Kv7 voltage-dependent K+ channel; LVDCC: L-type voltage-dependent Ca2+ channel; NO: nitric oxide; NOS: nitric oxide synthase; PCA: precapillary arteriole; PCV: postcapillary venule; SK channel: small-conductance Ca2+-activated K+ channel; SMC: smooth muscle cell; SR/ER: sarcoendoplasmic reticulum; STD: spontaneous transient depolarisation; TVDCC: T-type voltage-dependent Ca2+ channel; VDCC: voltage-dependent Ca2+ channel; VSMC: vascular smooth muscle cell.
Spontaneous Vasomotion of Microvessels
Arterioles and venules in several vascular beds in vivo exhibit periodic spontaneous constrictions known as spontaneous vasomotion (1). Arteriolar vasomotion is considered to facilitate arteriolar blood flow into capillaries (2,3,4), while venular vasomotion facilitates venular drainage (5). Since spontaneous vasomotion is also generated in vitro (6), it is likely that the spontaneous activity originates from microvasculature themselves rather than driven by systemic humoral or neuronal factors.
The mural cells, i.e. vascular smooth muscle cells (VSMCs) or pericytes, in the microvasculature of visceral organs exhibit synchronous spontaneous Ca2+ transients with or without vasoconstrictions (7, 8). Therefore, visualisations of the spontaneous changes in intracellular Ca2+ dynamics of mural cells are fundamental to explore the origin of spontaneous vasomotion in microvascular networks. This short review summarises recent advances in understanding the mechanisms underlying spontaneous Ca2+ transients in mural cells, particularly focusing on their synchrony.
Morphological Properties of Microvascular Mural Cells
In capillaries, pericytes with a morphology distinct from spindle-shaped VSMCs have been recognised since the 19th century using various staining methods including silver impregnation (9). Transmission electron microscopy revealed that the basement membrane is not observed between the pericyte and endothelial cell. Thus, pericytes and the endothelium make frequent membranous contacts in capillaries and postcapillary venules (PCVs) (10). Scanning electron microscopy using enzymatically-digested specimens demonstrates that capillary pericytes have an oval cell body with primary processes extending in the longitudinal directions (9, 11, 12). The mural cells of precapillary arterioles (PCAs) have an oval or round cell body with several circumferentially-oriented processes, while PCV mural cells have an oval or round cell body and several processes extending in various directions (9, 11, 12).
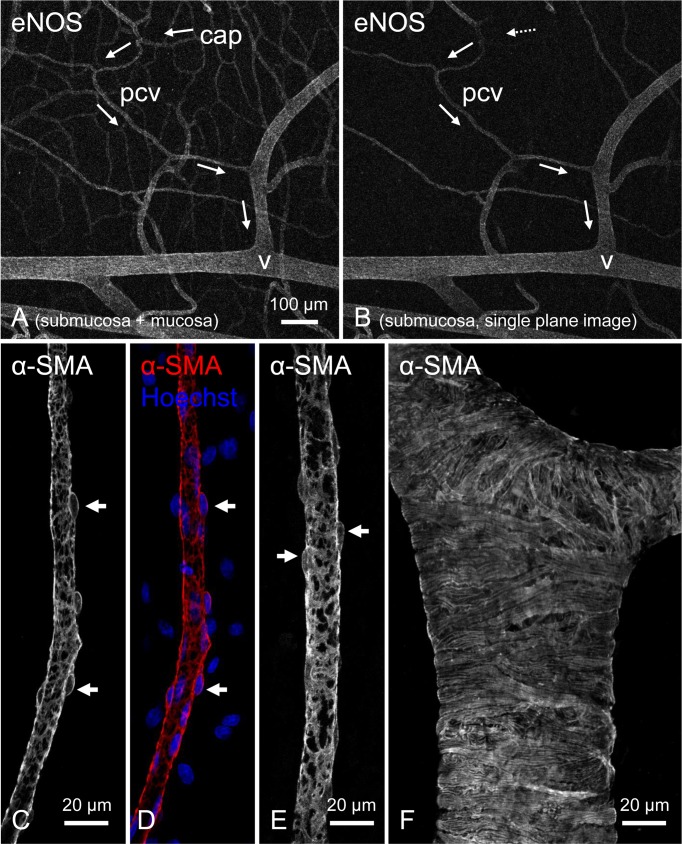
In thin whole mount preparations, immunohistochemistry using specific markers for mural cells such as α-smooth muscle actin (α-SMA) or NG2 chondroitin sulphate proteoglycan (NG2) revealed the arrangement of mural cells in different segments of microvessels (13,14,15,16,17,18). The entire network of microvessels or just the microvascular segment in a single plane can also be visualised by immunohistochemistry using endothelial markers such as endothelial nitric oxide synthase (eNOS, Fig. 1A, B) (16), von Willebrand factor (vWF) (16) or CD31 (19).
Fig. 1.
Immunohistochemical demonstration of postcapillary venules (PCVs) using confocal laser scanning microscope.
Immunoreactivity for endothelial nitric oxide synthase (eNOS) reveals a microvascular network in a submucosal/mucosal preparation of rat stomach (A). Arrows indicate the direction of venular drainage pathway originating from the mucosal capillary network (cap) that connects to a submucosal PCV (pcv) and is finally collected into a larger venule (v). An extracted single plane image of the same area shows the submucosal PCV and connecting larger venule but not the mucosal capillary network (B). Immunohistochemistry for α-smooth muscle actin (α-SMA) reveals the stellate morphology of mural cells (pericytes or vascular smooth muscle cells) with a round cell body (arrows) in a PCV of rat submucosal specimen (C–E). Hoechst 33342 was used for nuclei staining. In a larger venule, vascular smooth muscle cells are circumferentially arranged (F). All micrographs are reproduced from (16) with permission.
Mural cells express immunoreactivity for α-SMA in most microvascular segments with the exception of capillary pericytes in some tissues (13, 15). Consistent with the expression of α-SMA, α-SMA-positive mural cells in arterioles or PCAs of the mouse bladder suburothelium are contractile, while capillary pericytes do not contract during their synchronous spontaneous Ca2+ transients (19). In contrast, capillary pericytes in the central nervous system and heart appear to be contractile (20,21,22) corresponding with their expression of α-SMA immunoreactivity (22, 23).
Morphological characteristics of mural cells in PCVs or venules can also be visualised by immunohistochemistry for α-SMA. At a higher magnification, the stellate-shaped mural cells in PCVs of rat gastric submucosa (Fig. 1C–E) are clearly distinct from the circumferentially-oriented, tightly-packed smooth muscle cells (SMCs) in venules of the same vascular network (Fig. 1F) (16). The heterogeneity of mural cell morphology in different vascular segments of gastric microvasculature is very similar to that in the mouse and rat bladder (15) and also corresponds to scanning electron microscopy observations in the rat mammary gland (11), suggesting that morphological features of mural cells are preserved in different tissues.
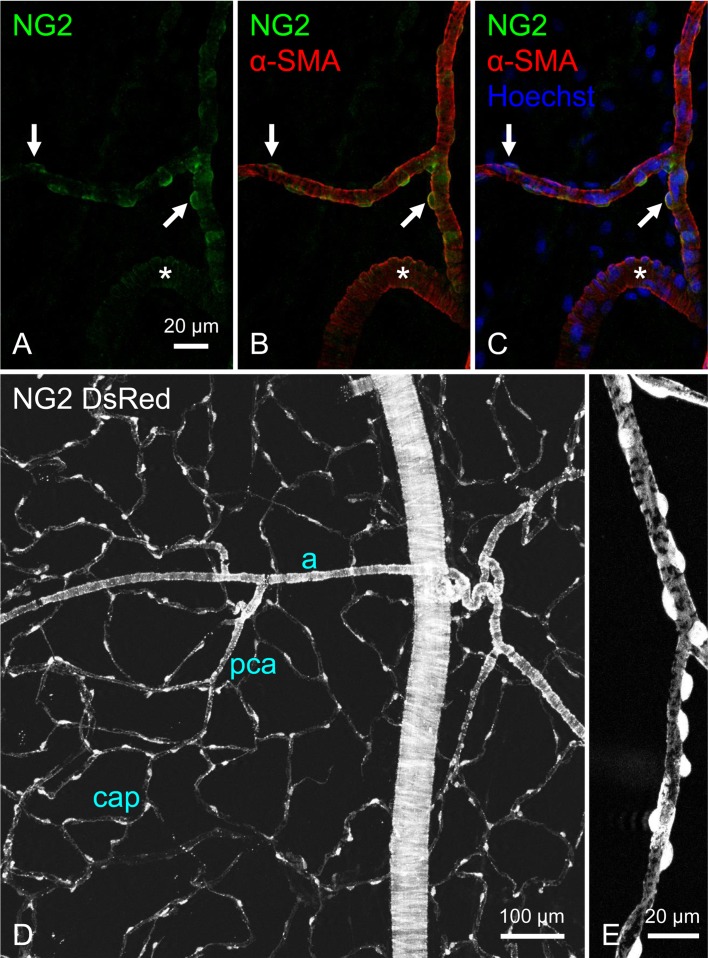
The mural cells of arterioles and capillaries but not venules are immunoreactive for NG2 chondroitin sulphate proteoglycan (NG2) in hollow visceral organs (Fig. 2A–C) (15, 17, 22, 24). Similar expression patterns of NG2 have also been reported in the mesentery (14), subcutaneous tissue (14), skeletal muscles (14) and retina (23). Tg(Cspg4-DsRed.T1)1Akik/J mice (NG2 DsRed mice) have the advantage of allowing the visualisation of NG2-expressing cells and their morphology (25) in different segments of the microvascular (Fig. 2D, E) (19). In combination with green fluorescent Ca2+ indicators, intracellular Ca2+ dynamics of the mural cells can be examined in a microvascular segment-specific manner (19). More recently, NG2cre:GCaMP3 mice have allowed intracellular Ca2+ imaging of the microvascular mural cells in the somatosensory cortex in vivo where rhythmic spontaneous Ca2+ transients are generated in the mural cells (26). Interestingly, arterioles and PCAs but not capillaries show detectable spontaneous changes in vessel diameter (26).
Fig. 2.
Visualisation of NG2 chondroitin sulphate proteoglycan (NG2)-positive mural cells in precapillary arterioles (PCAs).
Double immunostaining for NG2 (green) and α-smooth muscle actin (α-SMA, red) combined with nuclear staining (blue) reveals the round cell bodies of mural cells (arrows) in the PCAs of rat rectal submucosa (A–C). Smooth muscle cells in the connecting larger arteriole (asterisks) are also faintly immunopositive for NG2. A bladder mucosal specimen of NG2 DsRed mouse demonstrates the NG2-expressing suburotherial microvasculature consisting of a branching arteriolar tree (a), PCAs (pca) and a capillary meshwork (cap) (D). Mural cells in the PCA shows an oval or round cell body and circumferentially-oriented processes (E). Micrographs in A–C are reproduced from (17), and those in D and E are from (19) with permission.
Basis of Spontaneous Ca2+ Transients in Mural Cells
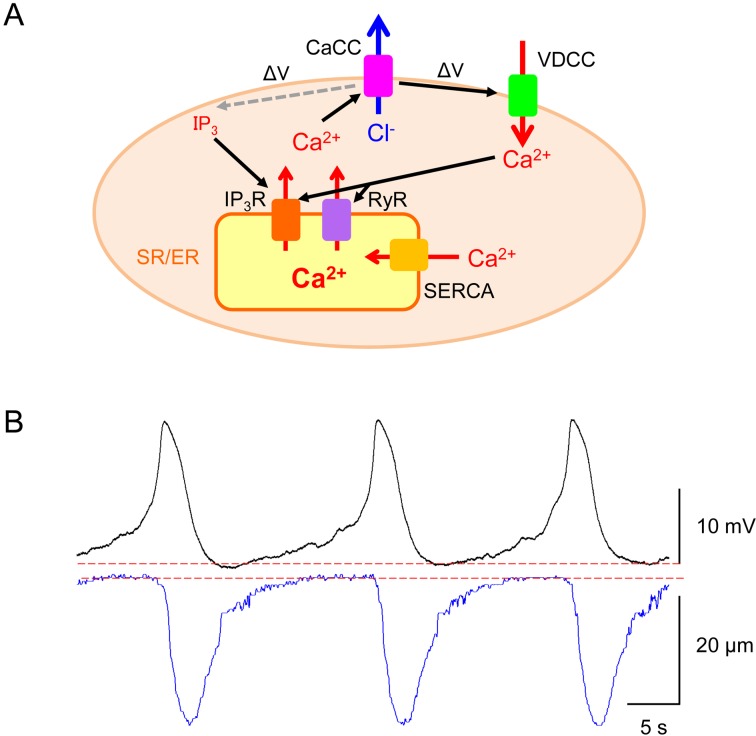
Spontaneous Ca2+ transients in mural cells and associated vasomotion are primarily arise from the spontaneous release of Ca2+ from the sarcoendoplasmic reticulum (SR/ER) (1, 6,7,8) as shown in Fig. 3A. Inhibition of SR/ER Ca2+-ATPase with cyclopiazonic acid or thapsigargin abolishes spontaneous Ca2+ transients and/or vasomotion in arterioles (27), PCAs (28), venules (29) or PCVs (16). Spontaneous Ca2+ or contractile activity is also prevented upon the inhibition of IP3 and/or ryanodine receptors. In addition, spontaneous Ca2+ cycling is terminated by the blockade of store-operated Ca2+ entry but not the sodium calcium exchanger 3 (24).
Fig. 3.
Proposed mechanisms underlying synchronous spontaneous Ca2+ transients in mural cells.
A: Spontaneous Ca2+ release from sarco-endoplasmic reticulum (SR/ER) via IP3 receptors (IP3R) and/or ryanodine receptors (RyR) triggers the opening of Ca2+-activated Cl- channels (CaCCs) to depolarise the membrane (ΔV) (cf. reference 41). The CaCC-dependent depolarisation further activates voltage-dependent Ca2+ channels (VDCCs). Ca2+ influx through VDCCs stimulates Ca2+-induced Ca2+ release (CICR) via RyR and/or IP3R, and CaCC-dependent membrane depolarisation (ΔV) would increase IP3 production to facilitate IP3-induced Ca2+ release. The sequestration of cytosolic Ca2+ is mediated by sarco-endoplasmic reticulum Ca2+-ATPase (SERCA). B: In a rat bladder suburothelial venule, individual spontaneous action potentials of venular smooth muscle cells (upper trace) precede each vasoconstriction as shown by a reduction in venular diameter (lower trace). Traces in B are reproduced from (38) with permission.
Mural cells generate spontaneous transient depolarisations (STDs) that can sum to develop larger ‘pacemaker’ depolarisations to drive spontaneous vasomotion of the microvessels (Fig. 3B). Ca2+ released from SR/ER triggers the opening of Ca2+-activated Cl- channel (CaCC) allowing Cl- efflux to depolarise the membrane. The CaCC-dependent depolarisations further activate voltage-dependent Ca2+ channels (VDCCs), resulting in Ca2+ influx that triggers Ca2+-induced Ca2+ release (CICR) from the SR/ER via ryanodine receptors (Fig. 3A). In addition, the CaCC-dependent depolarisations may facilitate IP3 production that in turn triggers SR/ER Ca2+ release resulting in the further amplification of CaCC-dependent depolarisations (Fig. 3A) (1, 8). Thus, there seems to be a reciprocal facilitation between SR/ER Ca2+ cycling and plasmalemmal ion channels (1, 8). STDs arising from the activation of ion channels also play a critical role in maintaining the synchrony of Ca2+ transients amongst mural cells, in turn, vasomotion (as will be discussed later).
Roles of Gap Junctions in the Synchrony of Spontaneous Ca2+ Transients
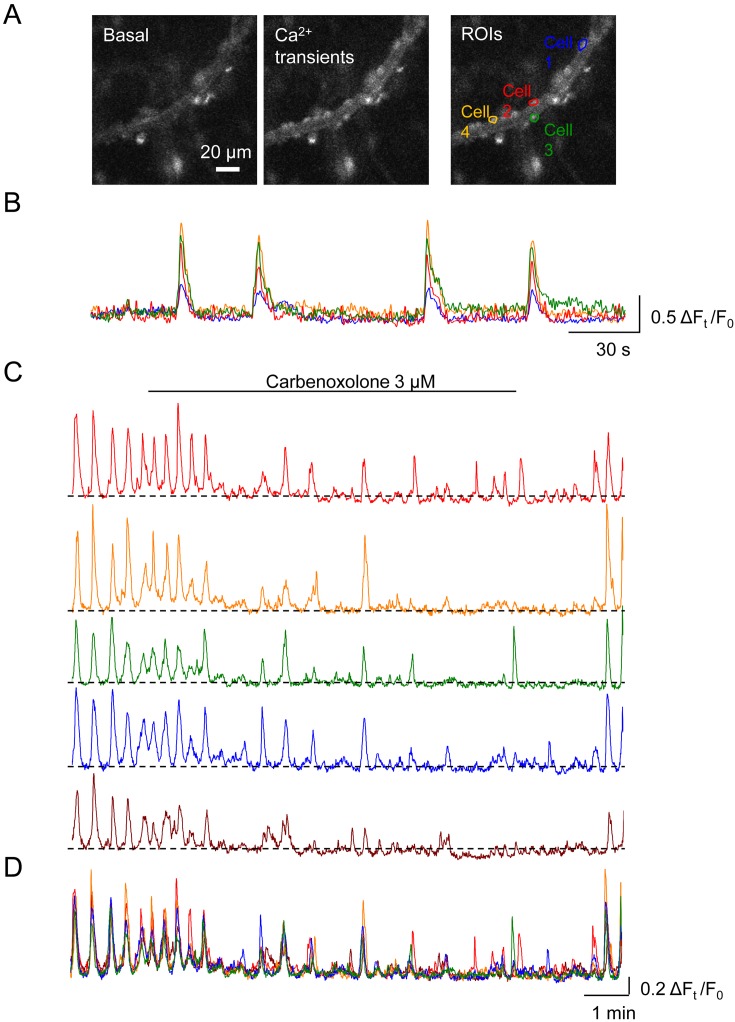
In the submucosal PCAs of rat rectum, mural cells develop synchronous spontaneous Ca2+ transients (Fig. 4A, B) (17). Similarly, mural cells in gastric PCVs exhibit synchronous spontaneous Ca2+ transients (Fig. 4C) (16) and corresponding spontaneous vasomotion. Carbenoxolone (3 μM), a gap junction blocker, reversibly disrupts the synchrony of Ca2+ transients leaving asynchronous Ca2+ transients in the individual mural cells (Fig. 4C, D) and also attenuates spontaneous vasomotion. In the mouse bladder suburothelium, stellate-shaped PCV pericytes also develop synchronous spontaneous Ca2+ transients and associated vasomotion (19). Carbenoxolone (10 μM) abolishes the spontaneous Ca2+ transients in the mural cells of PCVs or disrupts their synchrony, while preventing Ca2+ transients in venules (19). Thus, the synchrony of spontaneous Ca2+ transients among PCV mural cells depends on intercellular coupling via gap junctions, and such coupling is required for generating spontaneous vasomotion.
Fig. 4.
Roles of gap junction in maintaining the synchrony of spontaneous Ca2+ transients.
In a submucosal precapillary arteriole (PCA) of rat rectum loaded with Cal-520, mural cells with a round shaped cell body exhibit spontaneous Ca2+ transients (A). Four mural cells are randomly selected as regions of interests (ROIs). Corresponding traces demonstrate the synchrony of spontaneous Ca2+ transients in the four cells (B). In a postcapillary venule (PCV) of rat gastric submucosa loaded with Cal-520, spontaneous Ca2+ transients in five mural cells are synchronous (C). Carbenoxolone (3 μM), a gap junction blocker, disrupts the synchrony of spontaneous Ca2+ transients amongst the five cells with a reduction in their amplitude. The gradual disruption of the synchrony is evident in the merged traces corresponding to C (D). Images and/or traces are reproduced from (17) (A, B) and (16) (C, D) with permission.
Gap junction-mediated intercellular communication between mural cells has been well demonstrated by simultaneous patch clamp recordings of two mural cells in isolated PCAs of the rat kidney (30). Because of spread of the angiotensin II-induced depolarisation in a PCA mural cell to an adjacent endothelial cell, gap junction-mediated communication between mural cell and endothelial cells are indicated (30).
Origin of Spreading Synchronous Spontaneous Ca2+ Transients in the Microvasculature
In the bladder suburothelium of NG2-DsRed mice, ‘non-contractile’ capillary pericytes exhibit synchronous spontaneous Ca2+ transients that propagate to PCAs resulting in diameter changes (19). Carbenoxolone (10 μM) disrupts the synchrony of spontaneous Ca2+ transients in capillary pericytes, while preventing spontaneous Ca2+ transients in mural cells of connecting PCAs, indicating that capillary pericytes function as pacemaker cells to drive the upstream PCAs (8, 19). In the guinea-pig stomach, synchronous spontaneous Ca2+ transients in PCA mural cells spread to SMCs of arterioles to evoke spontaneous vasomotion (28).
Thus, in arteriole-capillary networks, synchronous spontaneous Ca2+ transients in mural cells appear to be predominantly generated in PCAs or capillaries and can retrogradely propagate to arterioles to evoke spontaneous vasomotion. The spreading nature of synchronous spontaneous Ca2+ transients may explain the fact that spontaneous vasomotion is preferentially observed in small arteriolar branches less than 20 μm in diameter in vitro (18, 19, 27) and in vivo (2, 26, 31,32,33,34).
Spontaneous Depolarisations as a Means of the Synchrony of Spontaneous Ca2+ Transients
In vitro studies have demonstrated rhythmically generated pacemaker potentials arising from summated STDs in VSMCs of the rat irideal or basilar arterioles (27, 35) or human pial arteries (36). Pacemaker potentials are associated with Ca2+ transients and corresponding spontaneous vasoconstrictions. Rhythmic pacemaker depolarisations in venular SMCs of the cat gastric submucosa are also associated with spontaneous constrictions (37).
In the lamina propria preparation of rat bladder, pacemaker potentials of venular SMCs precede each spontaneous vasoconstriction (Fig. 3B) (38). The resting membrane potential of spontaneously-active venular SMCs in the rat and mouse bladder suburothelium is about −43 mV and −45 mV, respectively (19, 38). These values are close to the activation threshold of L-type voltage-dependent Ca2+ channels (LVDCCs) (39). Indeed, blockade of LVDCCs suppressed slow waves and disrupted their synchrony amongst venular SMCs leaving asynchronous STDs, indicating that STDs sum to trigger the opening of LVDCCs to generate slow waves and associated vasomotion (19). The spontaneous vasomotion is associated with synchronous spontaneous Ca2+ transients in circumferentially-oriented SMCs or stellate pericytes in bladder venules (24), supporting the notion that synchronous Ca2+ influx through LVDCCs in these cells is required for the generation of spontaneous vasomotion.
Roles of Voltage-dependent Ca2+ Channels in the Synchrony of Spontaneous Ca2+ Transients
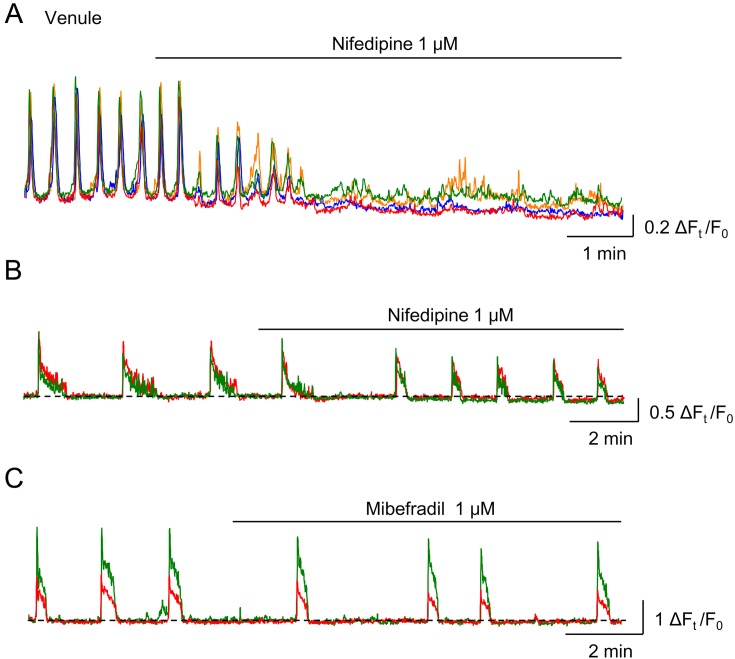
Inhibitors of LVDCCs, nifedipine or nicardipine, disrupt the synchrony of spontaneous Ca2+ transients in the mural cells of venules (Fig. 5A) and inhibit spontaneous venular vasomotion (16, 24, 29, 38, 40). Thus, the intercellular coupling amongst venular mural cells appears to be mediated by the spread of LVDCC-dependent depolarisations, presumably via gap junctions. Nifedipine also disrupts the synchrony of spontaneous Ca2+ transients in the SMCs of basilar arterioles and abolishes their vasomotion (35).
Fig. 5.
Roles of voltage-dependent Ca2+ channels (VDCCs) in maintaining the synchrony of spontaneous Ca2+ transients.
In a submucosal venule of rat rectum, nifedipine (1 μM), an L-type VDCC blocker, disrupts the synchrony of spontaneous Ca2+ transients among four regions of interest (A). In a submucosal precapillary arteriole (PCA) of rat rectum, two mural cells exhibit synchronous spontaneous Ca2+ transients, and nifedipine does not affect the synchrony of spontaneous Ca2+ transients, while their duration is reduced by nifedipine (B). Mibefradil (1 μM), a T-type VDCC blocker, also has no effect on the synchrony of spontaneous Ca2+ transients of two PCA mural cells in the rat rectum (C). Mibefradil decreases their frequency. Traces are reproduced from (17) with permission.
In contrast to venules or arterioles, the synchrony of spontaneous Ca2+ transients in PCA mural cells of the guinea-pig gastric myenteric layer (28), rat rectal submucosa (17) and mouse bladder suburothelium (19) are not disrupted by the blockade of LVDCCs (Fig. 5B). In contrast, the blockade of T-type voltage-dependent Ca2+ channels (TVDCCs) by ML218 or mibefradil disrupts the synchrony of spontaneous Ca2+ transients among mural cells in the PCAs of guinea-pig stomach (28). However, blockade of TVDCCs decreases their frequency in PCAs of rat rectum without disrupting their synchrony (Fig. 5C) (17). In the rat rectal PCA mural cells, TVDCCs appears to be involved in regulating the frequency of spontaneous Ca2+ transients, while LVDCCs contributes to the duration of spontaneous Ca2+ transients (Fig. 5B, C) (17).
Roles of Ca2+-activated Cl- Channels in Generating Synchronous Spontaneous Ca2+ Transients
The synchrony of spontaneous Ca2+ transients among mural cells in the rat rectal PCAs is not affected by blockers of LVDCCs and TVDCCs but disrupted by lowering extracellular Cl- from 134.4 mM to 12.4 mM or in the presence of a Ca2+-activated Cl- channel (CaCC) blocker (17). Lowering extracellular Cl- or CaCC blocker also disrupts the synchrony of spontaneous Ca2+ transients among mural cells and/or inhibits spontaneous vasomotion in several vascular beds (16, 35, 38). Thus, spontaneous Ca2+ release from SR/ER opens CaCCs to cause membrane depolarisations that appear to play a fundamental role in intercellular signal spread. The inhibitors of Na+-K+-Cl- co-transporter bumetanide and furosemide decrease the amplitude of spontaneous Ca2+ transients and resultant constrictions without disrupting their synchrony (16), suggesting that Cl- accumulation in mural cells is partly dependent on these co-transporters and that other Cl- accumulation mechanisms such as anion exchangers may also be operating (41).
CaCC-dependent depolarisations are also fundamental in generating slow waves in interstitial cells of Cajal (ICC), gastrointestinal pacemaker cells, to electrically drive SMCs (42, 43) or rhythmic spontaneous constrictions of lymphatic vessels (44,45,46). Consistent with this, immunoreactivity for TMEM16A (also known as ANO1), a CaCC, is detected in ICC (47) or lymphatic SMCs (46). In contrast, TMEM16A immunoreactivity is not detected in mural cells of the microvascular (24, 29), suggesting that the mural cells may have other types of CaCCs such as TMEM16B (48).
Roles of K+ channels in maintaining the synchrony of spontaneous Ca2+ transients
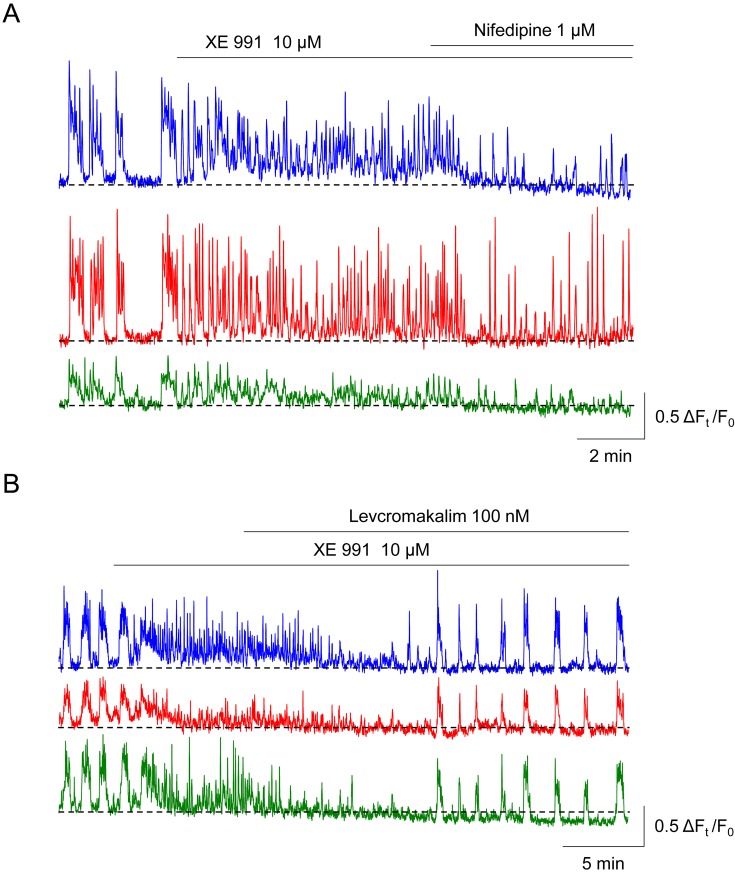
In the PCA mural cells of the rat rectum, the Kv7 voltage-dependent K+ (Kv7) channel blocker XE 991 (10 μM, Fig. 6A) or an increase in [K+]o from 5.9 mM to 29.7 mM converts synchronous Ca2+ transients into asynchronous, high-frequency Ca2+ transients (49). Thus, Kv7 channels in PCA mural cells of the rectum appear to be constitutively open to maintain their relatively hyperpolarised membrane potential to prevent ‘premature’ asynchronous Ca2+ transients in individual cells. Since subsequent application of nifedipine decreases their frequency but fails to recover their intercellular synchrony (Fig. 6A), the activation of LVDCCs appears to be involved in the generation of asynchronous, high-frequency Ca2+ transients, but not a critical cause of the disruption of intercellular synchrony. Levcromakalim, a KATP channel opener that is known to hyperpolarise mural cells (19), restores the synchrony of spontaneous Ca2+ transients (Fig. 6B), suggesting that repolarisations of the mural cell membrane potential are required to restore the synchrony.
Fig. 6.
Roles of Kv7 voltage-dependent K+ (Kv7) channels in maintaining the synchrony of spontaneous Ca2+ transients.
In three mural cells of rat rectal precapillary arteriole (PCA), XE 991 (10 μM), a blocker of Kv7 channels, converts synchronous spontaneous Ca2+ transients into asynchronous, high-frequency Ca2+ transients and increases the basal Ca2+ level (A). Subsequent nifedipine decreases their frequency but does not restore the synchrony. In an XE991-treated rectal PCA where asynchronous spontaneous Ca2+ transients are generated, levcromakalim, an ATP-sensitive K+ channel opener that is known to hyperpolarise mural cells, restores their intercellular synchrony (B). Traces are reproduced from (49) with permission.
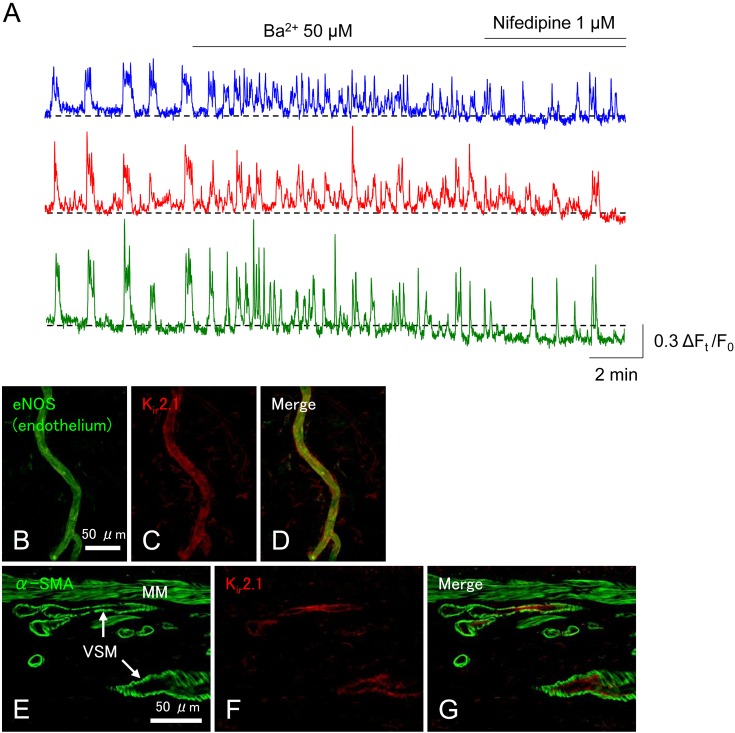
Ba2+ (50 μM), a known blocker of inward rectifier K+ (Kir) channels also changes the synchronous spontaneous Ca2+ transients into asynchronous, high-frequency Ca2+ transients (Fig. 7A) (49). Thus, some Kir channels in the endothelial cells and/or mural cells of rectum PCAs appear to be open under resting condition to prevent asynchronous, high-frequency Ca2+ transients. A small increase in [K+]o from 5.9 mM to 10.7 mM, which is known to activate Kir channels (50), inhibits the spontaneous Ca2+ transients, presumably by opening more Kir channels, this hyperpolarising the membrane of mural cells (50). Consistent with the functional Kir channel (Kir2.1) expression in PCAs, immunoreactivity for Kir2.1 is detected in the endothelial cells but not mural cells in the submucosal PCAs in the rat rectum (Fig. 7B–G) (49) as is the case of endothelial cells of brain capillaries (51). These data indicate that Kir channel-dependent hyperpolarisations generated in the endothelium can be transmitted to mural cells via myoendothelial gap junctions. Nevertheless, other subtypes of Ba2+-sensitive Kir channel subunits may also be expressed in the mural cells or the endothelium. Functional expression of Kir channels has been demonstrated in PCA mural cells of the rat kidney (vasa recta) and retina (52, 53).
Fig. 7.
Roles of endothelial inward rectifier K+ (Kir) channels in maintaining the synchrony of spontaneous Ca2+ transients.
Ba2+ (50 μM), a blocker of Kir channels, converts synchronous spontaneous Ca2+ transients into asynchronous, high-frequency Ca2+ transients in the rat rectal precapillary arteriole (PCA) (A). Subsequent nifedipine does not recover the synchrony. Immunohistochemistry reveals that eNOS-positive endothelium of PCA in the rat rectum expresses Kir2.1 (B–D). In a cross section of rat rectum, Kir2.1 immunoreactivity is not colocalised with α-smooth muscle actin (α-SMA)-immunoreactive vascular smooth muscle cells (VSM) of submucosal blood vessels (E–G). MM indicates the muscularis mucosae. Traces and micrographs are reproduced from (49) with permission.
The blockade of both small (SK)- and intermediate (IK)-conductance Ca2+-activated K+ channels has no effect on the synchronous spontaneous Ca2+ transients in the PCA mural cells of rat rectum (49). Consistently, endothelial cells of mouse brain capillaries do not express SK and IK channels (51), suggesting that capillaries or connecting PCAs do not have functional SK/IK channels. This is in contrast to SMCs of rat mesenteric arteries, in which endothelial SK and IK channel play a role in maintaining the intercellular synchrony of phenylephrine-evoked rhythmic Ca2+ transients and resultant vasomotion (54, 55). Large conductance Ca2+-activated K+ channels are not involved in the synchronisation of spontaneous Ca2+ transients in the rat basilar artery (35) and rectal PCA (49), while these channels contribute to suppress the basal Ca2+ level in the basilar artery. Thus, Ca2+-activated K+ channels only play a marginal role in maintaining the synchrony of spontaneous Ca2+ transients in the PCA mural cells.
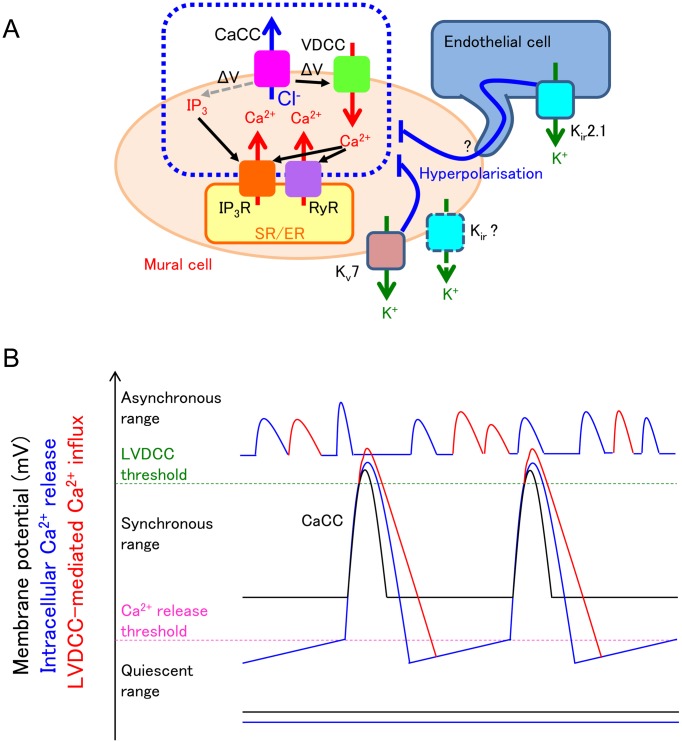
A hyperpolarised membrane potential suppresses VDCC-mediated Ca2+ influx and resultant CICR as well as IP3 receptor-mediated Ca2+ release from the SR/ER (Fig. 8A). In the conditions where ‘premature’ Ca2+ transients are prevented, SR/ER Ca2+ stores are allowed to be fully refilled so that regenerative Ca2+ release is periodically generated. Regenerative Ca2+ releases trigger CaCC-dependent depolarisations that are sufficiently large in their amplitude to speared to distant cells. Since levcromakalim alone suppresses or abolishes spontaneous Ca2+ transients (49), setting the resting membrane potential within a certain range appears to be critical for generating synchronous Ca2+ transients (Fig. 8B).
Fig. 8.
Fundamental roles of the membrane potential in maintaining the synchrony of spontaneous Ca2+ transients.
A: Kv7 voltage-dependent K+ (Kv7) channels and inward rectifier K+ (Kir) channels are open under resting condition to hyperpolarise the mural cells. This hyperpolarisation inhibits voltage-dependent IP3 production and subsequent IP3 receptor (IP3R)-mediated Ca2+ release and also decreases voltage-dependent Ca2+ channel (VDCC)-mediated Ca2+ influx and subsequent Ca2+-induced Ca2+ release via ryanodine receptors (RyR) and/or IP3R. A resultant decrease in the frequency of spontaneous Ca2+ release from the sarcoendoplasmic reticulum (SR/ER) ensures the enough Ca2+ refilling in the SR/ER; thus, each spontaneous Ca2+ release from SR/ER is large enough to induce Ca2+-activated Cl- channel (CaCC)-mediated depolarisation that leads to recruitment/activation of more store Ca2+ release events and their synchronisation within the network of mural cells. B: When the resting membrane potential of mural cells are within the ‘synchronous range’, cyclical spontaneous Ca2+ release from SR/ER (blue line) opens CaCCs to induce cyclical spontaneous depolarisation (black line). The depolarisation causes L-type voltage-dependent Ca2+ channel (LVDCC)-mediated Ca2+ influx (red line). These spontaneous activities spread to neighbouring mural cells via gap junctions. When the resting membrane potential of mural cells are within the ‘quiescent range’, voltage-dependent IP3 production is suppressed. Thus, cyclical spontaneous Ca2+ release from SR/ER is now not generated (blue flat line), and membrane potential change is not detected (black flat line). When the resting membrane potential is higher than the threshold of LVDCC, i.e., within the ‘asynchronous range’, both cyclical spontaneous Ca2+ release from SR/ER (blue line) and LVDCC-mediated Ca2+ influx (red line) cause high frequency spontaneous Ca2+ transients that are generated independently among mural cells.
Roles of Endothelium in Maintaining the Synchrony of Ca2+ Transients
Nitric oxide (NO) released from the endothelium appears to play a critical role in maintaining the synchrony of spontaneous activity of mural cells in the rat gastric PCVs. Tadalafil, an inhibitor of phosphodiesterase type 5 (PDE5), disrupts the synchrony of Ca2+ transients in the mural cells and inhibits associated spontaneous vasomotion of gastric PCVs (16). Since tadalafil failed to inhibit vasomotion in PCVs that had been pre-treated with nitric oxide synthase (NOS) inhibitor, constitutive PDE5 activity in the mural cells counteracts NO/cGMP signalling to maintain the synchronous Ca2+ transients.
In arterioles, the endothelial layer functions as a low-resistance pathway that allows the conduction of electrical signals from adjacent SMCs via myoendothelial gap junctions to distant SMCs (56). Such electrical coupling has also been demonstrated between mural cell and endothelial cells in PCAs or capillaries (30, 51). Thus, it is envisaged that the synchrony of spontaneous Ca2+ transients in mural cells in the PCA or capillary depends on the low-resistance endothelial layer rather than electrical coupling between mural cells. Indeed, in brain capillaries where the bipolar pericytes are sparsely distributed, the tips of the elongated processes of two adjacent pericytes come within a very close proximity, but do not contact or overlap, indicating a lack of a direct electrical coupling between pericytes (57).
Putative Physiological roles of Microvascular Synchronous Activity
Increases in the intraluminal pressure of the dog gastric corpus (58) and colon (59) induce wall distention resulting in the reduction of mucosal and/or submucosal blood supply (i.e., ischaemia) associated with a diminished oxygen consumption in the mucosa (59). Spontaneous vasomotion in submucosal arterioles and venules is expected to facilitate the mucosal circulation and thus may well preserve oxygen/nutrients supply to the mucosa even during organ wall distensions.
Synchronous Ca2+ transients in the mural cells of capillaries or PCAs propagate to upstream arteriolar branches to evoke vasomotion in the mouse bladder (19) and guinea-pig stomach (28). Since capillaries are the site of substance exchange between blood and tissue where metabolic demand and environmental changes (e.g., oxygen, nutrients and pH) can be finely sensed, it is reasonable that synchronous spontaneous Ca2+ transients originate in capillary pericytes and spread to upstream PCAs or arterioles to drive spontaneous vasomotion (7, 49). This will meet the tissue demands of oxygen/nutrients and reset the tissue pH appropriately.
Sympathetic nerve projections become sparser along PCAs and PCVs in the mouse bladder suburothelium (19) and virtually absent in capillaries of the mouse bladder suburothelium and myenteric plexus of mouse stomach (19, 60). Thus, these microvascular segments appear not to be under tight sympathetic control, suggesting that their spontaneous activity plays a critical role in maintaining their perfusion. This is in contrast to PCAs/capillaries in the central nervous system, where the blood flow appears to be precisely regulated by neural activity (20, 26) as well as by their own spontaneous activity (26).
The disruption of spontaneous activity of microvascular mural cells may underlies disease states, and thus could a therapeutic target. Diabetic rat models show an impairment of contractility of mural cells in the retinal PCA/capillary network (61). Gap junctions-mediated intercellular coupling of mural cells is also disrupted in the PCA/capillary of diabetic rat retina (61). Therefore, further investigations into changes in spontaneous arteriolar vasomotion (4) in the diabetic human eye are clinically relevant. Ischaemia in the rat heart or brain causes the sustained contraction and eventual rigor mortis of mural cells in capillaries and/or PCAs, suggesting their critical roles in developing no-flow phenomenon after reperfusion of upstream arteries (21, 22). In visceral organs, the causal relationship between circularity dysfunction of the bladder and lower urinary tract symptoms, particularly overactive bladder, is well documented. Since previous studies focused on bladder ischaemia subsequent to the occlusion of bladder feeding arteries (62), microvasculature within the bladder wall that regulates the transmural distribution of blood supply should be a fruitful research target. More specifically, functions and dysfunctions of the suburothelial microvascular network that play a key role in determining the mucosal blood flow in the bladder storage phase should be explored.
Conclusions
Besides the previously established mechanisms underlying spontaneous Ca2+ transients in mural cells, namely the reciprocal interaction between SR/ER Ca2+ cycling and plasmalemmal ion channels, the significance of the resting membrane potential in maintaining the synchrony of spontaneous Ca2+ transients has become increasingly evident. While the spread of regenerative depolarisations triggered by Ca2+ transients is critical for the intercellular coupling, K+ channels also play a fundamental role in maintaining the resting membrane potential within an appropriate range. Future studies investigating changes in the resting membrane potential in disease states and means of its restoration will be of great interest.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to thank Dr Richard Lang (Monash University) for critical reading of the manuscript. The authors gratefully acknowledge that this study was supported by grants-in-aid from The Japan Society for Promotion of the Science (JSPS) to R.M. (No. 26860521, 16K19361, 19K08426) and H.H. (No. 21659377, 23659763, 17K11187) and a grant-in-aid of the 24th General Assembly of the Japanese Association of Medical Sciences to R.M.
References
- 1.Aalkjaer C, Nilsson H. Vasomotion: cellular background for the oscillator and for the synchronization of smooth muscle cells. Br J Pharmacol. 2005; 144(5): 605–16. doi: 10.1038/sj.bjp.0706084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984; 246(4 Pt 2): H508–17. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003; 3(2): 79–89 51. doi: 10.1124/mi.3.2.79 [DOI] [PubMed] [Google Scholar]
- 4.Bek T, Jeppesen P, Kanters JK. Spontaneous high frequency diameter oscillations of larger retinal arterioles are reduced in type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. 2013; 54(1): 636–40. doi: 10.1167/iovs.12-11182 [DOI] [PubMed] [Google Scholar]
- 5.Dongaonkar RM, Quick CM, Vo JC, Meisner JK, Laine GA, Davis MJ, Stewart RH. Blood flow augmentation by intrinsic venular contraction in vivo. Am J Physiol Regul Integr Comp Physiol. 2012; 302(12): R1436–42. doi: 10.1152/ajpregu.00635.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005; 566(Pt 3): 645–56. doi: 10.1113/jphysiol.2005.086405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashitani H, Lang RJ. Spontaneous activity in the microvasculature of visceral organs: role of pericytes and voltage-dependent Ca(2+) channels. J Physiol. 2016; 594(3): 555–65. doi: 10.1113/JP271438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashitani H, Mitsui R. Role of pericytes in the initiation and propagation of spontaneous activity in the microvasculature. Adv Exp Med Biol. 2019; 1124: 329–56. doi: 10.1007/978-981-13-5895-1_14 [DOI] [PubMed] [Google Scholar]
- 9.Sims DE. The pericyte--a review. Tissue Cell. 1986; 18(2): 153–74. doi: 10.1016/0040-8166(86)90026-1 [DOI] [PubMed] [Google Scholar]
- 10.Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968; 25(5): 452–500. doi: 10.1016/S0022-5320(68)80098-X [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara T, Uehara Y. The cytoarchitecture of the wall and the innervation pattern of the microvessels in the rat mammary gland: a scanning electron microscopic observation. Am J Anat. 1984; 170(1): 39–54. doi: 10.1002/aja.1001700104 [DOI] [PubMed] [Google Scholar]
- 12.Fawcett DW, Raviola E. Bloom and Fawcett, a textbook of histology. 12th ed. New York: Chapman & Hall; 1994. 964p. [Google Scholar]
- 13.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991; 113(1): 147–54. doi: 10.1083/jcb.113.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005; 12(2): 151–60. doi: 10.1080/10739680590904955 [DOI] [PubMed] [Google Scholar]
- 15.Mitsui R, Hashitani H. Immunohistochemical characteristics of suburothelial microvasculature in the mouse bladder. Histochem Cell Biol. 2013; 140(2): 189–200. doi: 10.1007/s00418-012-1074-5 [DOI] [PubMed] [Google Scholar]
- 16.Mitsui R, Hashitani H. Mechanisms underlying spontaneous constrictions of postcapillary venules in the rat stomach. Pflugers Arch. 2016; 468(2): 279–91. doi: 10.1007/s00424-015-1752-y [DOI] [PubMed] [Google Scholar]
- 17.Mitsui R, Hashitani H. Properties of synchronous spontaneous Ca2+ transients in the mural cells of rat rectal arterioles. Pflugers Arch. 2017; 469(9): 1189–202. doi: 10.1007/s00424-017-1978-y [DOI] [PubMed] [Google Scholar]
- 18.Fukuta H, Mitsui R, Takano H, Hashitani H. Contractile properties of periosteal arterioles in the guinea-pig tibia. Pflugers Arch. 2017; 469(9): 1203–13. doi: 10.1007/s00424-017-1980-4 [DOI] [PubMed] [Google Scholar]
- 19.Hashitani H, Mitsui R, Miwa-Nishimura K, Lam M. Role of capillary pericytes in the integration of spontaneous Ca2+ transients in the suburothelial microvasculature in situ of the mouse bladder. J Physiol. 2018; 596(16): 3531–52. doi: 10.1113/JP275845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006; 443(7112): 700–4. doi: 10.1038/nature05193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014; 508(7494): 55–60. doi: 10.1038/nature13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife. 2017; 6: e29280. doi: 10.7554/eLife.29280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, Schallek J, Kılıç K, Can A, Di Polo A, Dalkara T. Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. eLife. 2018; 7: e34861. doi: 10.7554/eLife.34861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashitani H, Mitsui R, Shimizu Y, Higashi R, Nakamura K. Functional and morphological properties of pericytes in suburothelial venules of the mouse bladder. Br J Pharmacol. 2012; 167(8): 1723–36. doi: 10.1111/j.1476-5381.2012.02125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008; 135(1): 145–57. doi: 10.1242/dev.004895 [DOI] [PubMed] [Google Scholar]
- 26.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015; 87(1): 95–110. doi: 10.1016/j.neuron.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill CE, Eade J, Sandow SL. Mechanisms underlying spontaneous rhythmical contractions in irideal arterioles of the rat. J Physiol. 1999; 521(Pt 2): 507–16. doi: 10.1111/j.1469-7793.1999.00507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashitani H, Mitsui R, Masaki S, Van Helden DF. Pacemaker role of pericytes in generating synchronized spontaneous Ca2+ transients in the myenteric microvasculature of the guinea-pig gastric antrum. Cell Calcium. 2015; 58(5): 442–56. doi: 10.1016/j.ceca.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 29.Mitsui R, Hashitani H. Functional properties of submucosal venules in the rat stomach. Pflugers Arch. 2015; 467(6): 1327–42. doi: 10.1007/s00424-014-1576-1 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Payne K, Pallone TL. Descending vasa recta endothelial membrane potential response requires pericyte communication. PLoS One. 2016; 11(5): e0154948. doi: 10.1371/journal.pone.0154948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slaaf DW, Tangelder GJ, Teirlinck HC, Reneman RS. Arteriolar vasomotion and arterial pressure reduction in rabbit tenuissimus muscle. Microvasc Res. 1987; 33(1): 71–80. doi: 10.1016/0026-2862(87)90008-2 [DOI] [PubMed] [Google Scholar]
- 32.Meyer JU, Lindbom L, Intaglietta M. Coordinated diameter oscillations at arteriolar bifurcations in skeletal muscle. Am J Physiol. 1987; 253(3 Pt 2): H568–73. [DOI] [PubMed] [Google Scholar]
- 33.Hundley WG, Renaldo GJ, Levasseur JE, Kontos HA. Vasomotion in cerebral microcirculation of awake rabbits. Am J Physiol. 1988; 254(1 Pt 2): H67–71. [DOI] [PubMed] [Google Scholar]
- 34.Bouskela E. Vasomotion frequency and amplitude related to intraluminal pressure and temperature in the wing of the intact, unanesthetized bat. Microvasc Res. 1989; 37(3): 339–51. doi: 10.1016/0026-2862(89)90051-4 [DOI] [PubMed] [Google Scholar]
- 35.Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002; 545(2): 615–27. doi: 10.1113/jphysiol.2002.027904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gokina NI, Bevan RD, Walters CL, Bevan JA. Electrical activity underlying rhythmic contraction in human pial arteries. Circ Res. 1996; 78(1): 148–53. doi: 10.1161/01.RES.78.1.148 [DOI] [PubMed] [Google Scholar]
- 37.Morgan KG. Comparison of membrane electrical activity of cat gastric submucosal arterioles and venules. J Physiol. 1983; 345: 135–47. doi: 10.1113/jphysiol.1983.sp014970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashitani H, Takano H, Fujita K, Mitsui R, Suzuki H. Functional properties of suburothelial microvessels in the rat bladder. J Urol. 2011; 185(6): 2382–91. doi: 10.1016/j.juro.2011.02.046 [DOI] [PubMed] [Google Scholar]
- 39.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004; 92(5): 2633–41. doi: 10.1152/jn.00486.2004 [DOI] [PubMed] [Google Scholar]
- 40.Mitsui R, Miyamoto S, Takano H, Hashitani H. Properties of submucosal venules in the rat distal colon. Br J Pharmacol. 2013; 170(5): 968–77. doi: 10.1111/bph.12347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bulley S, Jaggar JH. Cl− channels in smooth muscle cells. Pflugers Arch. 2014; 466(5): 861–72. doi: 10.1007/s00424-013-1357-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003; 553(Pt 3): 803–18. doi: 10.1113/jphysiol.2003.051334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kito Y, Mitsui R, Ward SM, Sanders KM. Characterization of slow waves generated by myenteric interstitial cells of Cajal of the rabbit small intestine. Am J Physiol Gastrointest Liver Physiol. 2015; 308(5): G378–88. doi: 10.1152/ajpgi.00308.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol. 1993; 471: 465–79. doi: 10.1113/jphysiol.1993.sp019910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von der Weid PY, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008; 295(5): H1989–2000. doi: 10.1152/ajpheart.00007.2008 [DOI] [PubMed] [Google Scholar]
- 46.Mohanakumar S, Majgaard J, Telinius N, Katballe N, Pahle E, Hjortdal V, Boedtkjer D. Spontaneous and α-adrenoceptor-induced contractility in human collecting lymphatic vessels require chloride. Am J Physiol Heart Circ Physiol. 2018; 315(2): H389–401. doi: 10.1152/ajpheart.00551.2017 [DOI] [PubMed] [Google Scholar]
- 47.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009; 296(6): G1370–81. doi: 10.1152/ajpgi.00074.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011; 286(3): 2365–74. doi: 10.1074/jbc.M110.175109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsui R, Hashitani H. Role of K+ channels in maintaining the synchrony of spontaneous Ca2+ transients in the mural cells of rat rectal submucosal arterioles. Pflugers Arch. 2019; 471(7): 1025–40. doi: 10.1007/s00424-019-02274-3 [DOI] [PubMed] [Google Scholar]
- 50.Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015; 22(3): 183–96. doi: 10.1111/micc.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017; 20(5): 717–26. doi: 10.1038/nn.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao C, Goo JH, Lee-Kwon W, Pallone TL. Vasa recta pericytes express a strong inward rectifier K+ conductance. Am J Physiol Regul Integr Comp Physiol. 2006; 290(6): R1601–7. doi: 10.1152/ajpregu.00877.2005 [DOI] [PubMed] [Google Scholar]
- 53.Matsushita K, Puro DG. Topographical heterogeneity of K(IR) currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol. 2006; 573(Pt 2): 483–95. doi: 10.1113/jphysiol.2006.107102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okazaki K, Seki S, Kanaya N, Hattori J, Tohse N, Namiki A. Role of endothelium-derived hyperpolarizing factor in phenylephrine-induced oscillatory vasomotion in rat small mesenteric artery. Anesthesiology. 2003; 98(5): 1164–71. doi: 10.1097/00000542-200305000-00019 [DOI] [PubMed] [Google Scholar]
- 55.Mauban JR, Wier WG. Essential role of EDHF in the initiation and maintenance of adrenergic vasomotion in rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2004; 287(2): H608–16. doi: 10.1152/ajpheart.01084.2003 [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol. 2001; 535(Pt 1): 181–95. doi: 10.1111/j.1469-7793.2001.00181.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berthiaume AA, Grant RI, McDowell KP, Underly RG, Hartmann DA, Levy M, Bhat NR, Shih AY. Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell Rep. 2018; 22(1): 8–16. doi: 10.1016/j.celrep.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edlich RF, Borner JW, Kuphal J, Wangensteen OH. Gastric blood flow. I. Its distribution during gastric distention. Am J Surg. 1970; 120(1): 35–7. doi: 10.1016/S0002-9610(70)80139-8 [DOI] [PubMed] [Google Scholar]
- 59.Boley SJ, Agrawal GP, Warren AR, Veith FJ, Levowitz BS, Treiber W, Dougherty J, Schwartz SS, Gliedman ML. Pathophysiologic effects of bowel distention on intestinal blood flow. Am J Surg. 1969; 117(2): 228–34. doi: 10.1016/0002-9610(69)90308-0 [DOI] [PubMed] [Google Scholar]
- 60.Fu YY, Peng SJ, Lin HY, Pasricha PJ, Tang SC. 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol. 2013; 304(1): G1–11. doi: 10.1152/ajpgi.00209.2012 [DOI] [PubMed] [Google Scholar]
- 61.Ivanova E, Kovacs-Oller T, Sagdullaev BT. Vascular pericyte impairment and connexin43 gap junction deficit contribute to vasomotor decline in diabetic retinopathy. J Neurosci. 2017; 37(32): 7580–94. doi: 10.1523/JNEUROSCI.0187-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersson KE, Nomiya M, Sawada N, Yamaguchi O. Pharmacological treatment of chronic pelvic ischemia. Ther Adv Urol. 2014; 6(3): 105–14. doi: 10.1177/1756287214526768 [DOI] [PMC free article] [PubMed] [Google Scholar]