Highlights
-
•
We performed vertex-wise analyses comparing grey matter in youth with and without perinatally-acquired HIV (PHIV).
-
•
PHIV youth had reduced cortical thickness, surface area, and gyrification compared to control youth.
-
•
PHIV youth did not exhibit the same pattern of inverse grey matter-age relationships that were observed in control youth.
Keywords: Perinatally-acquired HIV, Neurodevelopment, Grey matter, Brain
Abstract
Youth with perinatally-acquired HIV (PHIV) experience specific and global cognitive deficits at increased rates compared to typically-developing HIV-uninfected youth. In youth with PHIV, HIV infects the brain early in development. Neuroimaging studies have demonstrated altered grey matter morphometry in youth with PHIV compared to typically-developing youth. This study examined cortical thickness, surface area, and gyrification of grey matter in youth (age 11–20 years old) with PHIV (n = 40) from the Pediatric HIV/AIDS Cohort Study (PHACS) compared to typically-developing presumed HIV uninfected and unexposed youth (n = 80) from the Pediatric Imaging, Neurocognition and Genetics Study (PING) using structural magnetic resonance imaging. This study also examined the relationship between grey matter morphometry and age. Youth with PHIV had reduced cortical thickness, surface area, and gyrification compared to typically-developing youth. In addition, an inverse relationship between age and grey matter volume was found in typically-developing youth, but was not observed in youth with PHIV. Longitudinal studies are necessary to understand the neurodevelopmental trajectory of youth with PHIV.
1. Introduction
Perinatal infection with HIV (PHIV) affects the brain early in life. Selective or global cognitive deficits have been observed in infancy, childhood and/or adolescence, despite well-controlled disease, and have implications for mental health, well-being, and successful transitioning to adulthood (Harris et al., 2018; Malee et al., 2016; Nichols et al., 2016; Smith and Wilkins, 2015). Previous neuroimaging studies of youth with PHIV have demonstrated differences in grey matter cortical and subcortical volume, white matter integrity, and global network connectivity in youth with PHIV compared to typically-developing youth (Blokhuis et al., 2016; Cohen et al., 2016; Dean et al., 2020; Herting et al., 2015; Hoare et al., 2018; Lewis-de los Angeles et al., 2016, Lewis-de los Angeles et al., 2017; Li et al., 2018; Sarma et al., 2014; Uban et al., 2015). Youth with PHIV may be particularly vulnerable to structural brain damage given HIV infection during early, critical periods of brain development and prolonged exposure to the virus and chronic inflammation intermittently throughout development (Lewis-de los Angeles et al., 2016).
Normative patterns of structural brain development throughout the lifespan, particularly cortical grey matter, have previously been characterized by inverted-U curves. Past structural magnetic resonance imaging (MRI) have shown that cortical grey matter volumes increase and peak before puberty and then have shown a decrease in grey matter volume as synapses prune across adolescence into adulthood. Previous studies have differed on when this peak occurs (i.e., childhood vs. adolescence), with most findings showing widespread cortical thinning with increasing age during adolescence (Tamnes et al., 2017). Moreover, these normative patterns of development are heterogeneous by anatomic regions, grey matter metrics, individual subject characteristics, and different trajectories among disorders of neurodevelopment (Astley et al., 2009; Giedd and Rapoport, 2010; Walhovd et al., 2016). To date, few neuroimaging studies have focused on how HIV alters brain structure at different ages (Van den Hof et al., 2019). In adult males with HIV, brain structure trajectories exhibited more atrophy in the absence of, compared to those with continued, cART medication (Sanford et al., 2018). However, how trajectories differ during key developmental time periods with HIV infection and with or without medication requires future investigation.
Various cortical grey matter components, such as surface area, cortical thickness, and gyrification, can be differentially affected in neurodevelopment of youth with PHIV. These grey matter metrics overlap in their changes over time, but also offer unique variance and are thought to relate to different biological factors (Storsve et al., 2014) with different rates of maturation (Raznahan et al., 2011). Previous studies have examined the relationships between these measures of cortical morphometry and PHIV in pre-defined regions-of-interest of the brain (Nwosu et al., 2018). This study adds vertex-level analysis of grey matter anatomical differences of the brain, not limited to predefined regions of interest. The current study examined the relationships between specific morphometric measures of grey matter and PHIV, as well as the relationship between age and grey matter volume on the cortical surface in a cross-sectional sample of youth with PHIV. We hypothesized that youth with PHIV would have reduced grey matter measures (surface area, cortical thickness, and local gyrification) both globally and regionally compared to typically-developing youth.
2. Methods
2.1. Study population
We evaluated a cohort of 40 youth (age 11–20 years) with PHIV enrolled at a single site in 2007–2009 (the Ann and Robert H. Lurie Children's Hospital of Chicago) of the Adolescent Master Protocol (AMP) study, conducted by the Pediatric HIV/AIDS Cohort Study (PHACS) network (https://phacsstudy.org/), who underwent multi-modal MRI acquisition. In general, the PHACS AMP study is a longitudinal cohort study aimed at investigating long-term effects of HIV infection and antiretroviral therapy among youth with PHIV. This particular cohort of 40 youth with PHIV has previously been described (Herting et al., 2015; Lewis-de los Angeles et al., 2016, Lewis-de los Angeles et al., 2017; Uban et al., 2015). Institutional review boards (IRB) at the participating site and the Harvard T. H. Chan School of Public Health approved the study. Parents, legal guardians, and youth with PHIV who were 18 years or older provided written informed consent for research participation; participating minor adolescents provided assent.
As a comparison group with harmonized MRI acquisition protocols, we selected a subset of 80 youth, presumed HIV-unexposed and uninfected, from the Pediatric, Imaging, Neurocognition, and Genetics (PING) study. The selection was made to 2:1 ratio of number of PING and PHACS participants, with group matching for age, race, ethnicity, caregiver education, and household income. For patients with missing data, multiple imputation was performed. Study population demographic, clinical, cognitive, and substance use data as well as imaging data were obtained through the PING portal after execution of data use requests and agreement to data use policies (Brown et al., 2012). We note that neuroimaging data in PING and PHACS were collected according to the same protocol.
2.2. Image acquisition and processing
All MRIs of the brains were collected on 3.0 Tesla Siemens Magnetom Tim Trio Scanner as described in previous studies (Herting et al., 2015; Lewis-de los Angeles et al., 2016, Lewis-de los Angeles et al., 2017; Uban et al., 2015). Structural MRIs were conducted using a T1-weighted, MP-RAGE sequence (sagittal, TR/TE/TI = 2,170/4.37/1,100 ms, FOV = 256 × 256 mm, flip angle = 7o, voxel resolution = 1 × 1 × 1.2 mm3, scan time = 8:08). No sedation was used for participants in either study. All selected subjects from the PING study and the PHACS study were scanned on 3T Siemens scanners using the same imaging protocol, except for 8 channel head coil used in the control youth and a 12 channel head coil in youth with PHIV. As in previous studies, PHACS structural MRI data were analyzed using the same processing pipeline as the PING participants (Herting et al., 2015; Lewis-de los Angeles et al., 2016, Lewis-de los Angeles et al., 2017; Uban et al., 2015). Specifically, scans were analyzed using FreeSurfer 5.3.0 (processed on CentOS 6 × 86_64) to generate cortical surfaces (see http://surfer.nmr.mgh.harvard.edu/). The automated process involved motion correction, intensity normalization, Talairach transformation, segmentation of grey and white matter volumes, tessellation of boundaries, topology correction, and surface deformation to generate cortical surfaces. Inflated surfaces registered to spherical atlases allowed for analyses of surface morphometry, including cortical thickness, surface area, and local gyrification index (Dale et al., 1999; Desikan et al., 2006; Fischl et al., 2002, 2004; Fischl and Dale, 2000; Schaer et al., 2012). Quality assurance procedures were carried out according to FreeSurfer recommended protocols. SurfStat was used to perform exploratory vertex-wise analyzes on the surface (see http://www.math.mcgill.ca/keith/surfstat/) (Chung et al., 2010; Worsley et al., 2009).
2.3. Statistical analyses
Demographic characteristics of PHIV and control youth were summarized and compared using Fisher's exact tests for binary measures, Chi-Square tests for categorical variables, and Wilcoxon rank sum test for continuous measures. Spatial distribution vertex-wise analyses were performed to identify vertices (points on the cortical surface of the brain) and clusters (statistically significant spatially-contiguous regions) that were associated with morphometric differences between PHIV youth and control youth (from the PING sample). Mean cortical volume at each vertex over the whole brain was compared in youth with PHIV and control youth. Multivariate (multiple) linear regression was used to obtain clusters of significant (p < 0.05) relationships between grey matter metrics on cortical surfaces (volume, surface area, cortical thickness, and local gyrification index) (Dale et al., 1999; Desikan et al., 2006; Schaer et al., 2012) and group (PHIV vs. control youth). Multiple comparisons were corrected with random field theory (RFT) (Chung et al., 2010); vertices that survived cluster-level correction using familywise error rate (FWER) <0.05 were identified as significant on the surface using SurfStat (Worsley et al., 2009). Models for each grey matter metric were used to test for group effect (PHIV vs. controls), adjusting for age at imaging (continuous) and sex. In this cross-sectional design, we evaluated the relationship of age with each grey matter metric (area, thickness, gyrification) by fitting a separate model within each of the groups (PHIV and control youth) for each metric as a function of age, adjusting for sex. For the analyses relating age to grey matter metric, two models were used. First, within-group analyses of grey matter metric (volume, cortical thickness, surface area, local gyrification) at each vertex was related to age, adjusting for sex. Second, as an exploratory analysis, scatter plots for both control youth and youth with PHIV were generated depicting age-grey matter volume relationships in regions identified significantly related to age in the control youth. Linear regression was performed. We fit a second model to explore a group by age interaction.
3. Results
3.1. Study population
Characteristics for the 40 youth with PHIV and 80 typically-developing youth are shown in Table 1; as a result of group matching, distributions with respect to age, race, ethnicity, caregiver education, and household income were very similar. However, the female participants tended to be younger within the PHIV group than in the control youth (mean age (years) = 15.9 vs 17.1 years, respectively, p = 0.07) while the males tended to be older within the PHIV group than in control youth (mean age (years) = 17.6 vs 15.7 years, respectively, p = 0.005).
Table 1.
Characteristics of study population by group
| PHIV (n = 40) | PING (n = 80) | p-value | |
|---|---|---|---|
| Female (n(%)) | 21 (53%) | 40 (50%) | 0.80 |
| Age (mean (SD)) | 16.7 (2.4) | 16.3 (2.8) | 0.66 |
| Race (n(%)) | 0.44 | ||
| Black | 29 (73%) | 52 (65%) | |
| White | 10 (25%) | 28 (35%) | |
| Hispanic ethnicity (n(%)) | 5 (13%) | 10 (13%) | 1.00 |
| Caretaker education (n(%))a | 0.35 | ||
| Less than high school | 7 (18%) | 8 (10%) | |
| High School or GED | 13 (33%) | 28 (35%) | |
| Some college or 2 year degree | 10 (25%) | 25 (31%) | |
| 4 year college | 3 (7.5%) | 12 (15%) | |
| Graduate School | 7 (18%) | 7 (8.8%) | |
| Annual household income (n(%))b | 0.52 | ||
| Less than 10000 | 2 (5%) | 5(6.3%) | |
| 10001–20000 | 10 (25%) | 11 (14%) | |
| 20001–30000 | 6 (15%) | 14 (18%) | |
| 30001–40000 | 6 (15%) | 14 (18%) | |
| 40001–50000 | 3 (7.5%) | 3 (3.8%) | |
| 50001–100000 | 11 (28%) | 21 (26%) | |
| greater than 100000 | 2 (5%) | 12 (15%) | |
| HIV disease-severity measures (median (Q1, Q3) or N (%) for PHIV | |||
| CD4% < 15% | 17 (43%) | ||
| Nadir CD4% | 16.5 (8.0,23.8) | ||
| Age (years) at nadir CD4% | 5.7 (2.2,10.9) | ||
| Nadir CD4 count | 236.0 (112.0, 392.5) | ||
| Age (years) at nadir CD4 count | 8.2 (3.8, 11.6) | ||
| Most recent* CD4 count | 627 (453.0, 846.0) | ||
| Most recent* CD4% | 35.9 (27.6,42.9) | ||
| Age (years) at most recent CD4%/CD4 count | 17.0 (14.9,18.2) | ||
| Log peak HIV RNA viral load (copies/mL) | 5.7 (5.2,5.9) | ||
| Age (years) at peak RNA | 2.5 (0.6,5.3) | ||
| Most recent* RNA count > 400 copies/mL | 6 (15) | ||
| Age (years) at most recent RNA | 17.0 (14.9, 18.2) | ||
| % of viral load > 1000 copies/mL in past 5 years | 6.5 (0.0, 29.0) | ||
| Age at start of cART | 3.5 (1.4 -6.3) | ||
| ARV regimen at the time of scan | |||
| cART | 37(92%) | ||
| Not on cART | 1 (3%) | ||
| Not on any antiretrovirals | 2 (5%) | ||
| CDC ‘C’ Classification | 9 (22%) | ||
GED = General Education Development test passed.
Cart = combination antiretrovial therapy (regimen including at least 3 drugs from at least 2 drug classes).
CDC = Centers for Disease Control.
Most recent = closest clinical data to neuroimaging scan.
One missing value of PING subjects imputed.
Eleven missing values of PING subjects imputed.
3.2. Comparison of grey matter metrics between PHIV youth and control youth
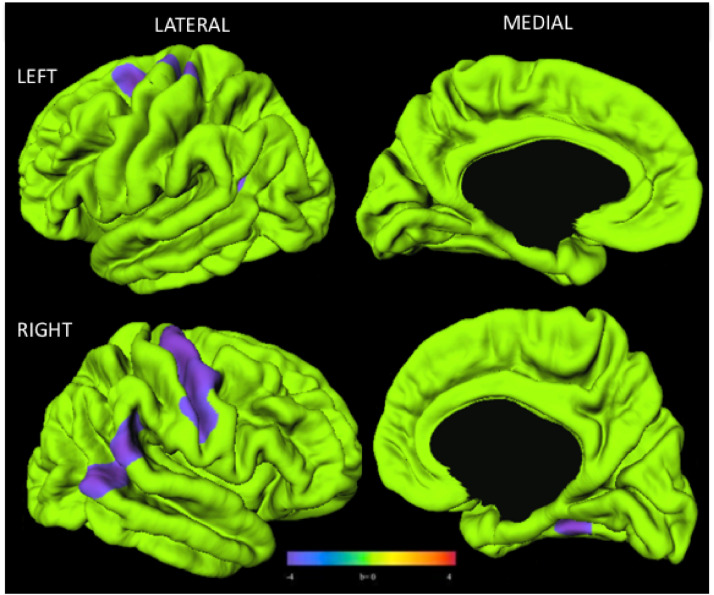
Mean cortical volume for each vertex across the whole brain was significantly different in youth with PHIV compared to control youth (youth with PHIV: 1.41, control youth 1.53, t(118) = −3.55, p < 001). Compared to control youth, youth with PHIV exhibited cortical thinning that appeared limited in terms of both degree and range in anatomical locations overall: thinning appeared largely concentrated on the right hemisphere. Between-group analyses revealed that youth with PHIV had smaller cortical thickness than control youth in regions of the right and left primary motor areas, right superior frontal, left inferior parietal cortices, left posterior temporal, and right temporal lobes (Fig. 1).
Fig. 1.
Youth with PHIV(n = 40) had smaller cortical thickness than control youth (n = 80). Surface clusters (purple) indicate significantly smaller cortical thickness in PHIV than control youth with adjustment for age and sex (RFT-FWER, p < 0.05). Cooler colors indicate youth with PHIV had smaller cortical thickness than controls. Warmer colors would be indicative of a thicker cortex in PHIV compared to controls, although no such findings were observed. Scale bar indicates range of standardized regression coefficients. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
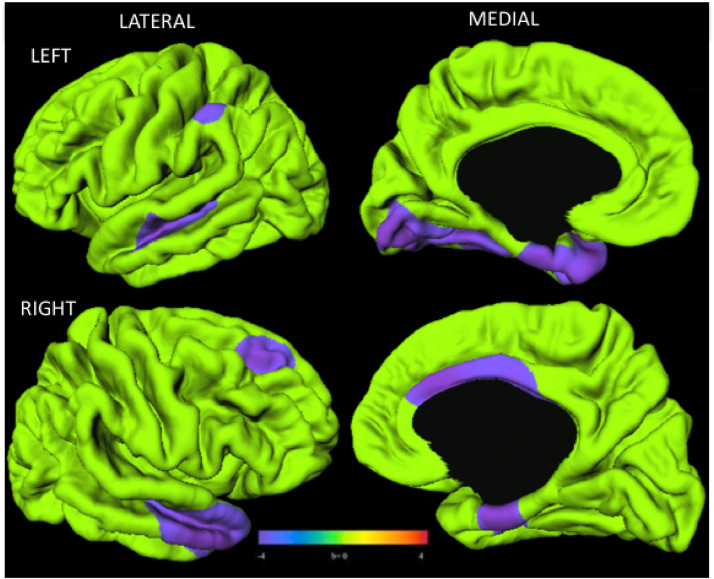
In youth with PHIV, reduced surface area was found bilaterally, with most reductions in the lateral superior and temporal lobe bilaterally, as well as left medial temporal lobe and right cingulate cortex (Fig. 2).
Fig. 2.
Youth with PHIV (n = 40) had smaller surface area than control youth (n = 80). Surface clusters (purple) indicate significantly smaller surface area in PHIV than control youth with adjustment for age and sex (RFT-FWER, p < 0.05). Cooler colors indicate youth with PHIV had smaller surface area than controls. Warmer colors would be indicative of a larger surface area in PHIV compared to controls, although no such findings were observed. Scale bar indicates range of standardized regression coefficients. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
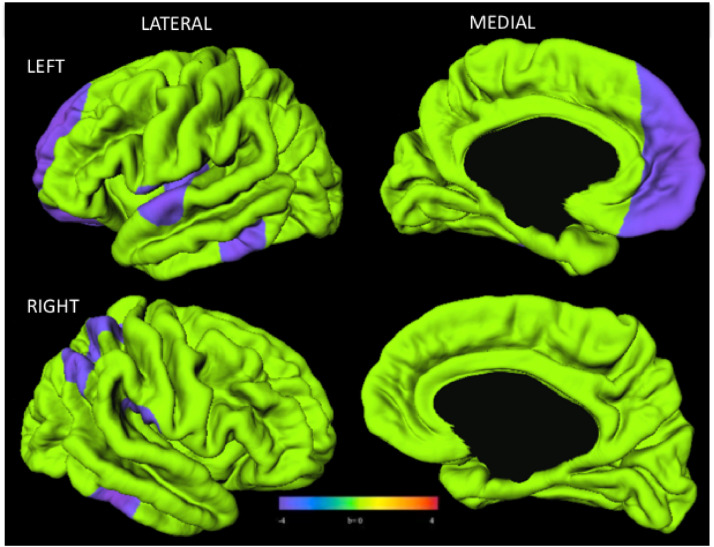
Youth with PHIV exhibited significantly reduced local gyrification index, primarily in the left hemisphere. Specifically, youth with PHIV had lower gyrification than control youth in regions of the left lateral and medial frontal lobes, the left and right temporal lobes, and the right lateral parietal cortex and temporal lobe (Fig. 3).
Fig. 3.
Youth with PHIV (n = 40) had lower gyrification index than control youth (n = 80). Surface clusters (purple) indicate significantly lower gyrification index in PHIV than control youth with adjustment for age and sex (RFT-FWER, p < 0.05). Cooler colors indicate youth with PHIV had lower gyrification index than control youth. Warmer colors would indicate youth with PHIV had larger gyrification index than controls, although no such findings were observed. Scale bar indicates range of standardized regression coefficients. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Relationship between grey matter metrics correlation and age: within-group analyses
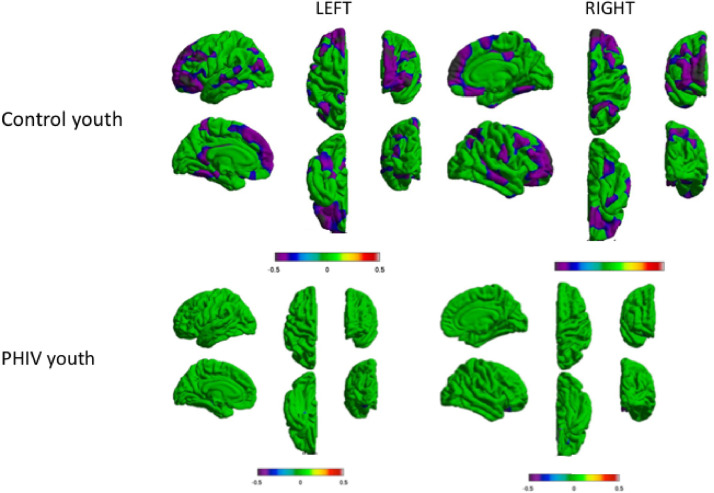
In the control youth, distributed symmetric clusters in the left and right hemispheres, largely in the frontal cortex, showed negative association between age and grey matter volume. There were also additional clusters of negative associations between age and grey matter volume in the medial and lateral temporal lobe, though these clusters were smaller than those in the frontal lobe. In PHIV youth, there were no statistically significant relationships between age and grey matter volume (Fig. 4). We did not observe any regions of the brain that had significant interaction between age and group, though the study may have been underpowered to detect such an effect. Similar results were found for cortical thickness, surface area, and local gyrification index (Supplemental Fig. 2). There were significant associations between age and grey matter metric (cortical thickness and gyrification) in distributed regions in bilateral frontal, parietal, and temporal lobe in the control youth, but not in youth with PHIV. For surface area, there were clusters in the left medial temporal lobe and right occipital lobe associated with age in the control youth, but not in youth with PHIV. Linear regression for age related to volume in regions identified significantly related to age in the control youth were performed. In youth with PHIV, there was no significant relationship (r = −0.1359, p = 4066.). In control youth, there was a significant relationship between age and volume (r = −0.5332, p < 001.)
Fig. 4.
Youth with PHIV (n = 40) did not show age-related volume reduction typically seen in control youth (n = 80). Clusters indicate significant relationship between age and volume within PHIV and control youth with adjustment for sex. Cooler colors indicate negative association between volume and age (RFT-FWER, p < 0.05). Warmer colors would indicate there is a positive association between volume and age, though no such relationships were observed. Scales indicates ranges of coefficient of association for the magnitude of the volume-age relationships.
4. Discussion
Using vertex-level analysis, we identified surface clusters of reduced grey matter thickness, surface area, and gyrification index in youth with PHIV compared to typically-developing youth in distributed regions throughout the cortex, including the frontal and parietal regions identified in previous studies of grey matter volume, but also in the temporal lobe of youth in the age range of 11–20 years old (Cohen et al., 2016; Lewis-de los Angeles et al., 2017). These findings occurred in the setting of mean cortical volume reduction over the whole brain in youth with PHIV compared to control youth. This confirms our hypothesis that multiple cortical grey matter morphometric measures (i.e., cortical thickness, surface area, and gyrification index) are vulnerable to HIV infection. This pattern is supported by earlier studies (Cohen et al., 2016), and the present study adds to earlier grey matter studies of this sample of youth with PHIV enrolled in the PHACS study (Lewis-de los Angeles et al., 2016, Lewis-de los Angeles et al., 2017) . These findings of smaller grey matter volumes, reduced cortical surface area and decreased gyrification index have also been identified in a younger cohort 9–11-year old children with PHIV (Hoare et al., 2018). Further, a recent study comparing volume, cortical thickness, and surface area between HIV- infected youth and HIV-exposed uninfected found subtle differences between the two groups, suggesting both in utero and postnatal effects of the virus (Dean et al., 2020). Interestingly, Dean et al., (2020) identify reductions in surface area in the right temporal lobe as well as cortical thickness in the left frontal lobe, but not in other regions when comparing HIV-infected and HIV-exposed uninfected youth. These regions may be particularly vulnerable to HIV, while other regions found to be altered in the present study but not in Dean et al., (2020) may relate more to timing of successful viral suppression, which differs between the studied populations.
A recently published systematic review highlighted that previous studies of structural magnetic resonance imaging have identified both similar and different grey matter regions affected across studies, which is also true in our findings (Van den Hof et al., 2019). It is unclear why both different and similar regions or lateralization of findings have been identified in these neuroimaging studies. One potential explanation could be differential microglia concentration. In particular, animal studies have shown that in general, microglia have different concentrations in different regions of the brain (Lawson et al., 1990; Tan et al., 2020). As microglia are a major reservoir of HIV in the central nervous system, one possibility is that this differential brain geographic concentration of microglia contributes to the observed grey matter differences observed in youth with HIV. Another potential reason that different regions have been identified from prior neuroimaging studies may be due to differences in comparison samples, for example comparing to typically-developing youth versus comparing to HIV-exposed uninfected, or comparing samples with slightly different age groups, geographic locations, or timing of viral suppression. Alternatively, other methodological differences such as in the analysis technique may be responsible for heterogenous findings.
Generally, reductions in surface area and gyrification in youth with PHIV were found in the frontal lobe, whereas the extent of frontal lobe differences in cortical thickness measures was much smaller. The pattern of changes in grey matter components identified in this study may reflect how PHIV most significantly affects neurodevelopment. Examining individual components such as cortical thickness (possibly reflecting changes in neuronal size, number of spines and synapses) and surface area (possibly reflecting cortical column generation) (Klein et al., 2014; Raznahan et al., 2011; Storsve et al., 2014), is helpful to more fully understand specific deficits and neurological insults of PHIV across the duration of brain development. In addition, gyrification reflects radial expansion of cortex and can be especially sensitive to early defects of cortical development (Klein et al., 2014; Schaer et al., 2012). The current understanding of gyrification is that cortical folds appear while in utero and gyrification increases through early childhood, stabilizes, then decreases in adolescence until early adulthood (Klein et al., 2014; Schaer et al., 2012). The pattern of reductions in gyrification and surface area, but not thickness, may be partially explained by the observation that by the second year of life, cortical thickness has achieved 97% of adult values, compared with a total surface area of only 69% of expected young adult size (Lyall et al., 2015), leading to continued surface area development throughout the first two decades of life. Thus, youth with PHIV may have more significant continued effects on cortical column generation and radial expansion of the cortex, and thus surface area and gyrification, respectively, in the frontal lobe beyond this period, rather than neuronal properties (cell size, arborization, spines) as reflected by lack of changes in cortical thickness.
Whether youth with PHIV have similar rates of decline or altered thinning in this age group compared to typically-developing youth is not yet known. Disorders of neurodevelopment, such as attention-deficit hyperactivity disorder, schizophrenia, and fetal alcohol syndrome have demonstrated distinct and varied trajectories of grey matter development (Astley et al., 2009; Giedd & Rapoport, 2010). It is possible that, similarly to these neurodevelopmental disorders, greater benefits may be observed with earlier induction of interventions for individuals with PHIV in terms of determining trajectory of brain development (Giedd and Rapoport, 2010; Castellanos et al., 2002). This cross-sectional study of the relationship of age with grey matter volume suggests that typically-developing youth demonstrate linear cortical volume reduction in this age range, thought to be a sign of effective maturation and pruning, whereas youth with PHIV may not undergo this maturation process in adolescence. This study may suggest that youth with PHIV have reduced grey matter cortical thickness and surface area to begin with, but do not undergo effective synaptic maturation. Other studies of blood markers of epigenetic aging suggest that youth with PHIV may have accelerated aging (Horvath et al., 2018); however, how this correlates with grey matter morphometry is not yet understood.
The interpretations of these analyses must be understood in the context of their limitations. Given the limited sample size of the HIV-infected group, it is likely that we did not have sufficient power to detect group interactions on the association with age. Moreover, the non-linear inverted U shape relationship between cortical gray matter and age shown by previous larger studies spanned wider age ranges (for one study, the age range was 3–20 years (Brown et al., 2012), for another study, 4–22 years (Giedd et al., 1999)). For these studies, grey matter appeared to peak in later childhood, 10–12 years. The smaller, older age range of our study (11–20 years) may have prevented us from testing the non-linear hypothesis. In our study, analyses examining relationships between age and grey matter metrics made a simplifying assumption of linearity in age, which appeared to be satisfied within the typically-developing youth group. However, given the limited size of the HIV-infected group it is unclear whether we had too little power to detect associations with age, or whether the relationship with age was potentially non-linear and thereby obscured overall trends with age. Possible non-linear relationships may have also impacted our tests for interaction between age and HIV status. Larger future studies should study adolescents across a wide range of ages to more fully understand the complex relationships with age in this population.
Further, given our smaller sample size, it is not yet clear if HIV affects males and females differently. It should also be noted that sex is one of the strongest predictors of brain size, especially in the adolescent age range (Giedd and Rapoport, 2010). Since males and females enter puberty at different ages, and synaptic pruning and the characteristic downward slope of the inverted-U grey matter occurs after the start of puberty (Giedd, 2004; Giedd and Rapoport, 2010), it is possible that males and females experience the interacting effects of HIV and brain volume changes differently.
Another limitation of our study is healthy volunteer bias. It is possible that the individuals who volunteer to participate in studies, including neuroimaging studies, may represent a healthier and wealthier sample of the general population (Ganguli et al., 2015; Oswald et al., 2013). Socioeconomic status has been shown to affect brain volumes in prior structural magnetic resonance imaging studies (Brito and Noble, 2014; Lawson et al., 2013; Noble et al., 2012, Noble et al., 2015). To minimize the effect of socioeconomic status, the present study matched the samples utilizing grouped ranges for two SES factors (annual household incomes and caregiver education). Despite these efforts, there were residual sociodemographic differences, with a higher frequency of participants with PHIV towards the lower end of the lowest income range compared to controls. Likewise, there were more control youth towards the high end of highest income range compared to youth with PHIV
Finally, this was a cross-sectional study of the relationship of age with cortical grey matter, providing a snapshot of possible longitudinal relationships. One study that examined subcortical abnormalities longitudinally observed shape differences in youth with PHIV at the initial evaluation, and the differences diminished over a year (Wade et al., 2019). This suggests that longitudinal differences may be minor when examining an endpoint; however, diminished changes at one year could also reflect an altered neurodevelopmental trajectory. Future studies should evaluate the correlations of these structural abnormalities with cognitive and behavioral outcomes. In order to further characterize the neurodevelopmental trajectories of youth with PHIV, larger longitudinal neuroimaging studies with multiple timepoints across the lifespan are warranted.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with co-funding by the National Institute on Drug Abuse (NIDA), the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). This work was also supported by NIDA (RC2 DA029475) through the Pediatrics Imaging-Genomics Data Resource (PING) study. C.P.L-d.l.A. was additionally supported by the Northwestern University Training Program in the Neuroscience of Human Cognition (NIH T32 NS047987), the Northwestern University Medical Scientist Training Program (NIH T32 GM008152), F30 (NIH HD090842-02), and the Dr. John N. Nicholson Fellowship. Additionally, K01AA026889 to K.A.U., and K01MH108761 to M.M.H.
CRediT authorship contribution statement
C. Paula Lewis-de los Angeles: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data curation, Visualization, Writing - original draft, Writing - review & editing. Paige L. Williams: Data curation, Resources, Supervision, Writing - review & editing. Lisanne M. Jenkins: Software, Visualization, Formal analysis, Writing - review & editing. Yanling Huo: Data curation, Writing - review & editing. Kathleen Malee: Writing - review & editing. Kathryn I. Alpert: Methodology, Software, Visualization. Kristina A. Uban: Data curation, Writing - review & editing. Megan M. Herting: Data curation, Writing - review & editing. John G. Csernansky: Writing - review & editing, Supervision. Sharon L. Nichols: Writing - review & editing. Russell B. Van Dyke: Funding acquisition, Resources, Writing - review & editing. Elizabeth R. Sowell: Conceptualization, Resources, Supervision, Funding acquisition, Writing - review & editing. Lei Wang: Conceptualization, Methodology, Software, Resources, Supervision, Writing - review & editing.
Acknowledgments
We thank the participants and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, the Office of AIDS Research, and the National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George R Seage III; Program Director: Liz Salomon) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP and AMP Up in 2018, in alphabetical order: Ann & Robert H. Lurie Children's Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Kathleen Malee, Yoonsun Pyun; Baylor College of Medicine: William Shearer, Mary Paul, Chivon McMullen-Jackson, Mandi Speer, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Mirza Baig, Alma Villegas; Children's Diagnostic & Treatment Center: Lisa Gaye-Robinson, Sandra Navarro, Patricia Garvie; Boston Children's Hospital: Sandra K. Burchett, Michelle E. Anderson, Adam R. Cassidy; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Ray Shaw, Raphaelle Auguste; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson, Karen Surowiec; St. Christopher's Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Taesha White, Mitzie Grant; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Megan Loughran, Veronica Figueroa, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Carrie Chambers, Emily Barr, Mary Glidden; University of Miami: Gwendolyn Scott, Grace Alvarez, Juan Caffroni, Anai Cuadra.
C.P.L.-d.l.A was also supported by award numbers 1F30HD090842 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, T32-NS047987 from the National Institute of Neurological Disorders and Stroke (Northwestern University Training Program in the Neuroscience of Human Cognition) and the Northwestern University Medical Scientist Training Program. This work was also supported by NIDA (RC2 DA029475) through the Pediatrics Imaging-Genomics Data Resource (PING) study.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Footnotes
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102246.
Appendix. Supplementary materials
References
- Astley S.J., Aylward E.H., Olson H.C., Kerns K., Brooks A., Coggins T.E., Davies J., Dorn S., Gendler B., Jirikowic T., Kraegel P., Maravilla K., Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhuis C., Kootstra N.A., Caan M.W.A., Pajkrt D. Neurodevelopmental delay in pediatric HIV/AIDS: current perspectives. Neurobehav. HIV Med. 2016;7(1):1–13. [Google Scholar]
- Brito N.H., Noble. K.G. Socioeconomic Status and Structural Brain Development. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.T., Kuperman J.M., Chung Y., Erhart M., McCabe C., Hagler D.J., Venkatraman V.K., Akshoomoff N., Amaral D.G., Bloss C.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kaufmann W.E., Kenet T., Kennedy D.N., Murray S.S., Sowell E.R., Jernigan T.L., Dale A.M. Neuroanatomical assessment of biological maturity. Curr. Biol. 2012;22(18):1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos X.F., Lee P.P., Sharp W., Jeffries N.O., Greenstein D.K., Clasen L.S., Blumenthal J.D., James R.S., Ebens C.L., Walter J.M., Zijdenbos A., Evans A.C., Giedd J.N., Rapoport J.L. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J. Am. Med. Assoc. 2002 doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chung M.K., Worsley K.J., Nacewicz B.M., Dalton K.M., Davidson R.J. General multivariate linear modeling of surface shapes using SurfStat. Neuroimage. 2010;53(2):491–505. doi: 10.1016/j.neuroimage.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Caan M.W.A., Mutsaerts H.J., Scherpbier H.J., Kuijpers T.W., Reiss P., Majoie C.B.L.M., Pajkrt D. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2016;86(1):19–27. doi: 10.1212/WNL.0000000000002209. [DOI] [PubMed] [Google Scholar]
- Dale A., Fischl B., Martin S. Cortical Surface-Based Analysis. Neuroimage. 1999;194:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dean O., Buda A., Adams H.R., Mwanza-Kabaghe S., Potchen M.J., Mbewe E.G., Kabundula P.P., Mohajeri Moghaddam S., Birbeck G.L., Bearden D.R. Brain magnetic resonance imaging findings associated with cognitive impairment in children and adolescents with human immunodeficiency virus in Zambia. Pediatr. Neurol. 2020;102:28–35. doi: 10.1016/j.pediatrneurol.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Paul Maguire R., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Kouwe A.V.D., Destrieux C., Halgren E., Ségonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004 doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Kouwe A.V.D., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Ganguli M., Lee C.-W., Hughes T., Snitz B.E>, Jakubcak J., Duara R., Chang C.-C.H. Who Wants a Free Brain Scan? Assessing and Correcting for Recruitment Biases in a Population-Based SMRI Pilot Study. Brain Imaging Behav. 2015;9(2):204–212. doi: 10.1007/s11682-014-9297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004;1021(1):77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L.L., Chernoff M.C., Nichols S.L., Williams P.L., Garvie P.A., Yildirim C., McCauley S.R., Woods S.P. Prospective memory in youth with perinatally-acquired HIV infection. Child Neuropsychology. 2018;24(7):938–958. doi: 10.1080/09297049.2017.1360854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Uban K.A., Williams P.L., Gautam P., Huo Y., Malee K., Yogev R., Csernansky J., Wang L., Nichols S., Dyke R.V., Sowell E.R. Default mode connectivity in youth with perinatally acquired HIV. Medicine (Baltimore). 2015;94(37):e1417. doi: 10.1097/MD.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hof M., Marleen ter Haar A., Matthan W., Caan A., Spijker R., van der Lee J.H., Pajkrt D. Brain structure of perinatally HIV-infected patients on long-term treatment. Neurology. 2019;9(5):433–442. doi: 10.1212/CPJ.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J., Fouche J.P., Phillips N., Joska J.A., Myer L., Zar H.J., Stein D.J. Structural brain changes in perinatally HIV-infected young adolescents in South Africa. AIDS. 2018;18:2707–2718. doi: 10.1097/QAD.0000000000002024. London, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Stein D.J., Phillips N., Heany S.J., Kobor M.S., Lin D.T.S., Myer L., Zar H.J., Levine A.J., Hoare J. Perinatally acquired HIV infection accelerates epigenetic aging in South African adolescents. AIDS. 2018;32(11):1465–1474. doi: 10.1097/QAD.0000000000001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D., Rotarska-Jagiela A., Genc E., Sritharan S., Mohr H., Roux F., Cheol E. H., Kaiser M., Singer W., Uhlhaas Peter J. Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0084914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson L.J., Perry V.H., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lewis-de los Angeles C.Paula, Paula C., Kathryn I., Paige A., Williams L., Kathleen M., Huo Y., Csernansky J.G., Yogev R., Russell B. V.D., Sowell E.R., Wang L., for the Pediatric HIV/AIDS Cohort Study (PHACS) Deformed subcortical structures are related to past HIV disease severity in youth with perinatally acquired HIV infection. J. Pediatric Infect. Dis. Soc. 2016;5(suppl 1):S6–14. doi: 10.1093/jpids/piw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-de los A., Paula C., Williams P.L., Huo Y., Wang S.D., Kristina U.A., Megan H.M., Kathleen M., Yogev R., Csernansky J.G., Sharon N., Van Dyke R.B., Sowell E.R., Wang L., Pediatric HIV/AIDS Cohort Study (PHACS) and the Pediatric Imaging, Neurocognition, and Genetics (PING) Study Lower total and regional grey matter brain volumes in youth with perinatally-acquired HIV infection: associations with HIV disease severity, substance use, and cognition. Brain Behav. Immun. 2017;62:100–109. doi: 10.1016/j.bbi.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Gao L., Wen Z., Zhang J., Wang P., Tu N., Lei H., Lin F., Gui X., Wu G. Structural covariance of gray matter volume in HIV vertically infected adolescents. Sci. Rep. 2018;8(1):1182. doi: 10.1038/s41598-018-19290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall A.E., Shi F., Geng X., Woolson S., Li G., Wang Li, Hamer R.M., Shen D., Gilmore J.H. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb. Cortex. 2015;25(8):2204–2212. doi: 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malee K.M., Smith R.A., Mellins C.A. Brain and cognitive development among U.S. youth with perinatally acquired human immunodeficiency Virus infection. J. Pediatric Infect. Dis. Soc. 2016;5(suppl 1):S1–S5. doi: 10.1093/jpids/piw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols S.L., Chernoff M.C., Malee K.M., Sirois P.A., Woods S.P., Williams P.L., Yildirim C., Delis D., Kammerer B. Executive functioning in children and adolescents with perinatal HIV infection and perinatal HIV exposure. J. Pediatric Infect. Dis. Soc. 2016;5:S15–S23. doi: 10.1093/jpids/piw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural Correlates of Socioeconomic Status in the Developing Human Brain. Developmental Science. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M. Family Income, Parental Education and Brain Structure in Children and Adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwosu, Emmanuel C., Robertson F.C., Holmes M.J., Cotton M.F., Dobbels E., Little F., Laughton B., van derKouwe A., Meintjes E.M. Altered brain morphometry in 7-year old HIV-infected children on early ART. Metab. Brain Dis. 2018;33(2):523–535. doi: 10.1007/s11011-017-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald L.M., Wand G.S., Zhu S., Selby V. Volunteerism and Self-Selection Bias in Human Positron Emission Tomography Neuroimaging Research. Brain Imaging Behav. 2013;7(2):163–176. doi: 10.1007/s11682-012-9210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R., Ances B.M., Meyerhoff D.J., Price R.W., Fuchs D., Zetterberg H., Spudich S., Louis Collins D. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin. Infect. Dis. 2018;67(11):1697–1704. doi: 10.1093/cid/ciy362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M.K., Nagarajan R., Keller M.A., Kumar R., Nielsen-Saines K., Michalik D.E., Deville J., Church Ja., Albert Thomas M. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M., Cuadra M.B., Schmansky N., Fischl B., Thiran J.-P., Eliez S. How to measure cortical folding from MR Images: a step-by-step tutorial to compute local gyrification index. J. Visual. Exp. 2012;59:1–8. doi: 10.3791/3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Wilkins M. Perinatally acquired HIV infection: long-term neuropsychological consequences and challenges ahead. Child Neuropsychol. 2015;21(2):234–268. doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- Storsve A.B., Fjell A.M., Tamnes C.K., Westlye L.T., Overbye K., Aasland H.W., Walhovd K.B. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J. Neurosci. 2014;34(25):8488–8498. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A..L., Meuwese R., Blakemore S.-J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017;37(12):3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.-L., Yuan Yi, Tian Li. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry. 2020;25(2):351–367. doi: 10.1038/s41380-019-0609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban K.A., Herting M.M., Williams P.L., Ajmera T., Gautam P., Huo Y., Malee K.M., Yogev R., Csernansky J.G., Wang L., Nichols S.L., Sowell E.R. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS. 2015;29(9):1035–1044. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B.S.C., Valcour V.G., Puthanakit T., Saremi A., Gutman B.A., Nir T.M., Watson C., Aurpibul L., Kosalaraksa P., Ounchanum P., Kerr S., Dumrongpisutikul N., Visrutaratna P., Srinakarin J., Pothisri M., Narr K.L., Thompson P.M., Ananworanich J., Paul R.H., Jahanshad N. Mapping abnormal subcortical neurodevelopment in a cohort of Thai children with HIV. NeuroImage. 2019;23 doi: 10.1016/j.nicl.2019.101810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Giedd J., Dale A.M., Brown T.T. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb. Cortex. 2016;1989(June) doi: 10.1093/cercor/bhv301. bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K.J., Taylor J.E., Carbonell F., Chung M.K., Duerden E., Bernhardt B., Lyttelton O., Boucher M., Evans A.C. SurfStat: a Matlab Toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. Neuroimage. 2009;47(July 2009):S102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.