Abstract
Purpose
To describe the clinical and swept source OCT angiographic features of a patient with acute syphilitic posterior placoid chorioretinitis (ASPPC).
Observations
A 67-year-old man presented with acute loss of vision in the left eye. On exam, we noted a yellowish placoid lesion in the macula. Optical coherence tomography (OCT) imaging showed RPE nodularity and disruption of the inner segment-outer segment region in the left eye. Fluorescein angiography showed early hyperfluorescent and late staining within the placoid lesions. Wide field swept source OCT angiography (SS-OCTA) showed macular choriocapillaris perfusion flow deficits. Laboratory tests revealed positive 1:128 rapid plasma reagin titer and fluorescent treponemal antibody absorption (FTA-ABS) tests. OCT imaging revealed complete restoration of the IS-OS boundary layer with near complete resolution of the RPE granularity after adequate penicillin therapy. SS-OCTA showed resolution of choriocapillaris flow deficit in the left eye. Improvement in BCVA correlated with improvement in choriocapillaris perfusion.
Conclusions and importance
This is the first case that describes long-term SS-OCTA findings in ASPPC. SS-OCTA is a fast, safe, and easily repeatable imaging modality that offers valuable insights in our understanding of the pathophysiology and the response to treatment of ASPPC.
Keywords: Acute syphilitic posterior placoid chorioretinitis, Swept source OCT angiography, Multimodal imaging
1. Introduction
Acute syphilitic posterior placoid chorioretinitis (ASPPC) is a rare manifestation of ocular syphilis characterize by a rounded, yellowish, placoid lesion in the macula or the posterior pole.1 Syphilitic ocular involvement is more frequently observed in the secondary and tertiary stages of the disease.2 With the recent worldwide rise in the incidence of syphilis, clinicians should have a high index of suspicion when evaluating patients with ocular inflammation even in the absence of other syphilitic manifestations.3 In this case report, we present the evolution over one year of multimodal imaging including macular choriocapillaris perfusion on swept source OCT angiography (SS-OCTA) in a patient treated for ASPPC.
2. Case report
A 67-year-old man presented to Bascom Palmer Eye Institute complaining of acute vision loss in the left eye of 9 days duration. He gave a medical history of an epidural steroid injection 1 month prior to his presentation. He had a history of genital herpes and was on valacyclovir prophylactic therapy. He denied any viral prodrome or recent illness. He was diagnosed with central serous chorioretinopathy by an outside physician. His best corrected visual acuity (BCVA) was 20/20 in his right eye and 20/125 in the left eye. Intraocular pressure was normal. His anterior segment exam was unremarkable. Funduscopic examination and color fundus imaging showed a normal right eye and a yellow placoid lesion involving the macula in the left eye (Fig. 1). Fundus autofluorescence revealed foci of punctate hyper-autofluorescence in the area of the placoid lesion in the left eye (Fig. 1). Swept source OCT (SS-OCT; PLEX Elite 9000; Carl Zeiss Meditec, Inc, Dublin, CA) imaging showed a normal right eye, while in the left eye, hyperreflective foci were identified along the anterior surface of the retinal pigment epithelium (RPE) along with disruption of the inner segment-outer segment (IS-OS) junction (Fig. 2H) and thickening of the macular retina (Fig. 2 B). An en face structural slab with boundaries that included the inner segment-outer segment (IS-OS) region showed scattered hyper-reflective spots (Fig. 2 D) that corresponded to the hyper-reflective foci seen on the B-scan. En face SS-OCT angiography (SS-OCTA) imaging using a choroidal slab adjacent to the RPE in the region of the choriocapillaris showed decreased flow associated with the placoid lesion in the left eye (Fig. 3). An inspection of the corresponding structural image shows that the decreased detection of flow cannot be explained by the presence of uneven illumination or a transmission defect. SS-OCTA imaging was normal in the right eye.
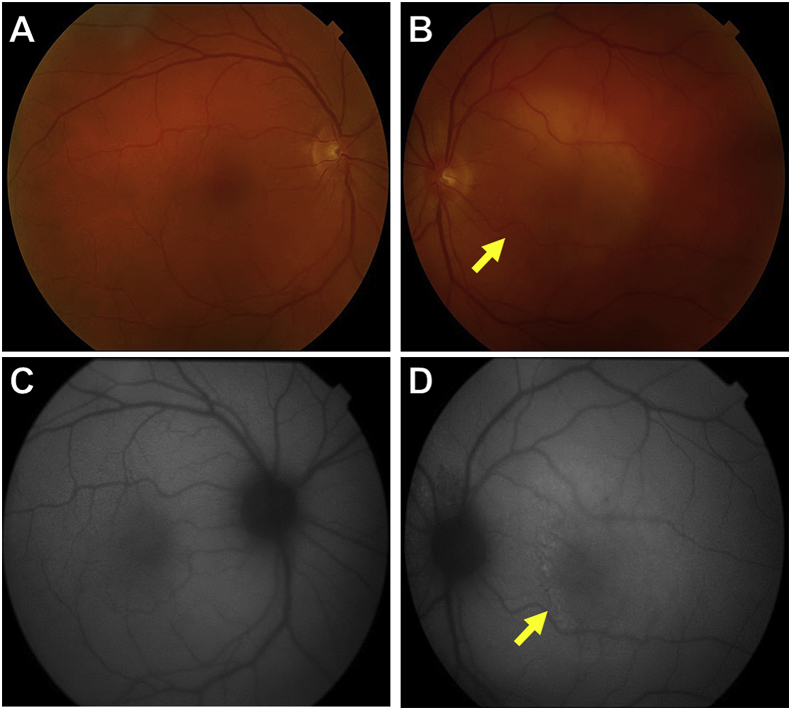
Fig. 1.
Color photographs and autofluorescence of both eyes at presentation.
A and C: Color photograph of the right eye with the corresponding autofluorescence show normal findings. B and D: Color photograph of the left eye reveals a yellowish placoid lesion involving the macula (yellow arrow in B), and the autofluorescence of the left eye demonstrates punctate hyper-autofluorescence corresponding to the placoid lesion in the macula (yellow arrow in D). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
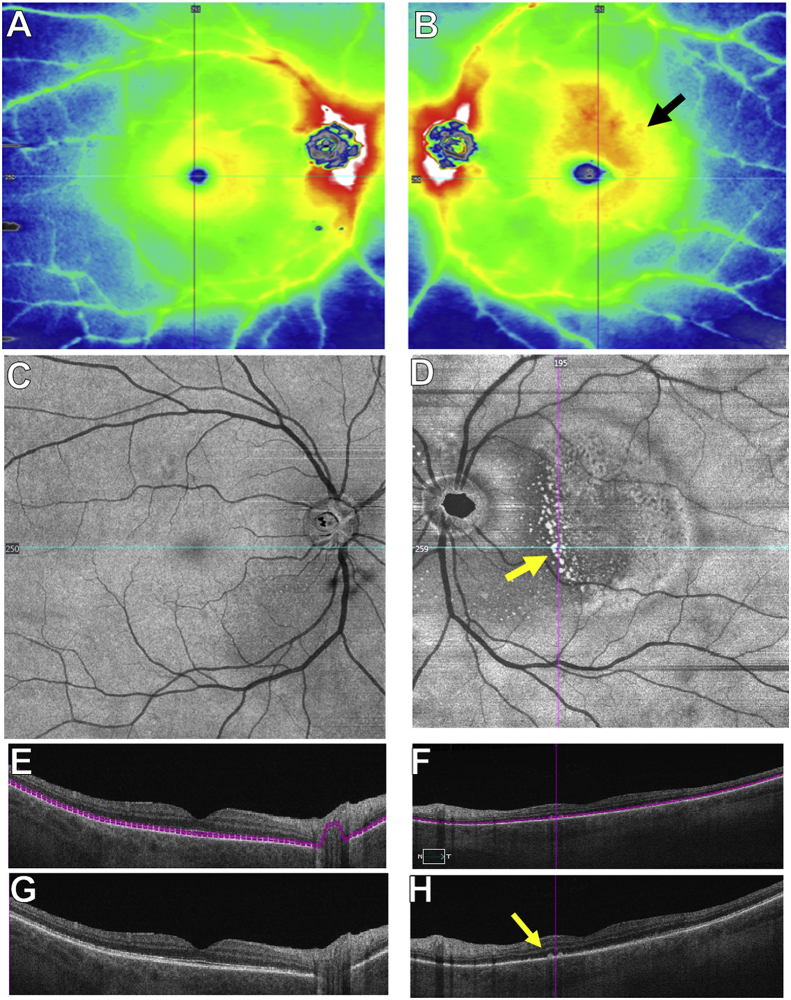
Fig. 2.
SS-OCTA 12 × 12 mm images at presentation in both eyes.
A and B: The SS-OCTA 12 × 12 mm thickness map is normal in the right eye, and the left eye shows thickening in the macula (black arrow in B). C and D: SS-OCTA 12 × 12 mm en face structural slab derived from the inner segment-outer segment (IS-OS) region (see segmentation lines in E-F) show normal findings in the right eye and bright spots within the macula (yellow arrow in D) that correspond to RPE deposits seen on B-scan (yellow arrow in H). E and G: Horizontal B-scans of the right eye with or without segmentation lines showing normal anatomy. F and H: Horizontal B-scans of the left eye with or without segmentation lines show nodular deposits along the RPE (yellow arrow in H) and IS-OS disruption. The blue lines through the fovea in C and D correspond to the horizontal B-scans shown in E-H. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
SSOCTA 12 × 12 mm en face choriocapillaris images at presentation in both eyes.
A and B: En face choriocapillaris flow and structural images are normal in the right eye. C and D: SSOCTA 12 × 12 mm en face choriocapillaris flow image of the left eye shows decreased flow within the macula (yellow arrow in C) with the corresponding en face structural images showing a homogeneous signal with no decreased signal suggestive of a shadowing artifact (D). E and F: Horizontal B-scans of the right eye with or without segmentation lines and color-coded flow showing normal flow and structure. Red color represents flow in the retina and the green color represents flow in the choroid. G and H: Horizontal B-scans of the left eye with or without segmentation lines and color-coded flow showing decreased choriocapillaris flow. The blue lines through the fovea in A-D correspond to the horizontal B-scans shown in E-H. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
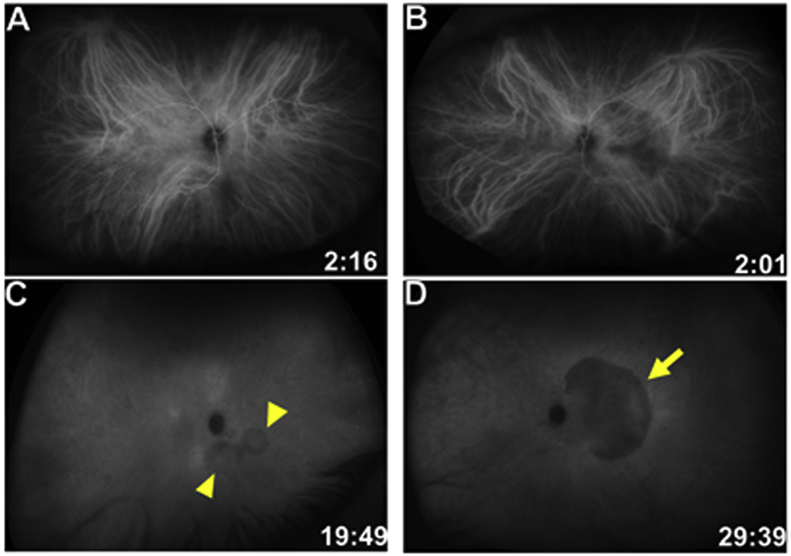
Five days later, the patient was seen by a uveitis specialist. His BCVA decreased to 20/200 in the left eye. On slit lamp examination, trace anterior vitritis was seen in the right eye and 1+ anterior vitritis was observed in the left eye. Fundus exam of the left eye revealed expansion of the yellow placoid lesion beyond the superior and inferior arcades with evidence of stippled RPE hyperpigmentation. The right eye continued to appear normal. Widefield autofluorescence imaging showed patches of hyper-autofluorescence inferior and inferonasal to the optic nerve in the right eye and stippled hyper-autofluorescence in the left eye corresponding to the placoid lesion (Fig. 4). Fluorescein angiography (FA) showed early faint hyperfluorescence and late staining without leakage in both eyes (Fig. 4), and indocyanine green angiography (ICGA) showed early and late hypofluorescence in the corresponding areas (Fig. 5). Upon further questioning, the patient disclosed a positive HIV history. Laboratory tests revealed positive 1:128 rapid plasma reagin titer and fluorescent treponemal antibody absorption (FTA-ABS) tests. Cerebrospinal fluid (CSF) Venereal Disease Research Laboratory (VDRL) test was also reactive, and findings were consistent with the diagnosis of neurosyphilis. He denied any ulcers or mucocutaneous lesions or other systemic symptoms. Brain MRI imaging was unremarkable. The patient was started on intravenous penicillin G treatment.
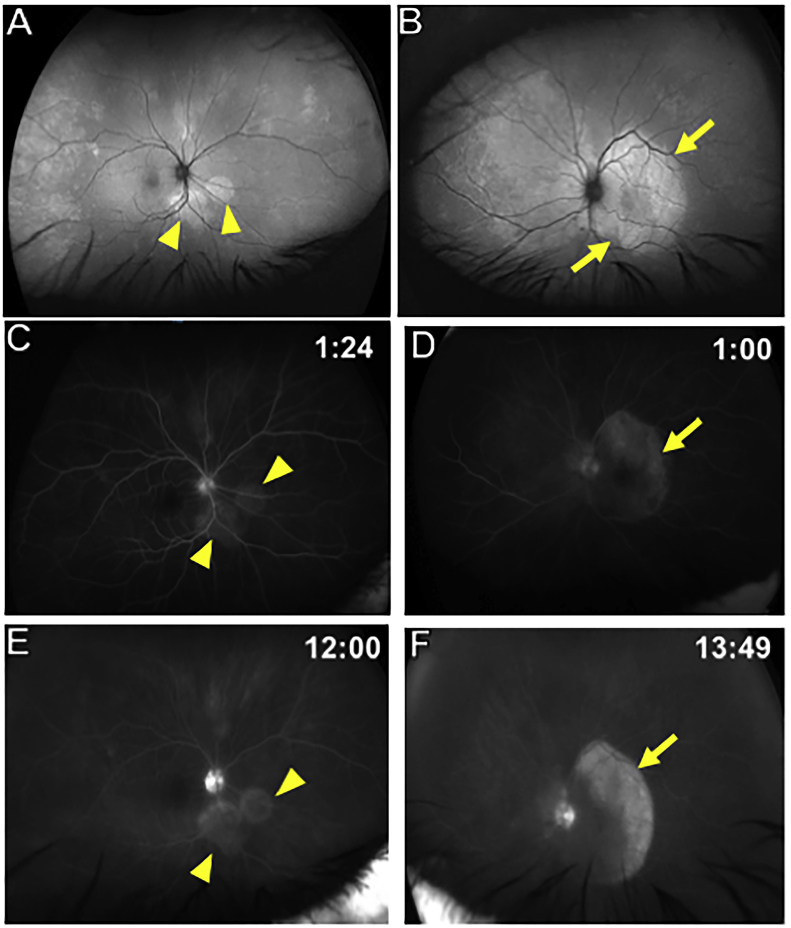
Fig. 4.
Wide-field Autofluorescence and fluorescein angiography findings of both eyes 5 days after presentation.
A: Wide-field autofluorescence of the right eye shows new peripapillary punctate hyper-autofluorescent lesions (yellow arrowheads) that were not initially present. B: Wide-field autofluorescence of the left eye shows macular hyper-autofluorescence with the lesion reaching the superior and inferior arcades (yellow arrows) and extends nasal to the disc, which corresponds to an area of decreased fluorescence on ICG as seen in Fig. 5D. C and E: Fluorescein angiography of the right eye shows early hyperfluorescence (yellow arrowheads in C) with late staining of the peripapillary lesions (yellow arrowheads in E). D and F: Fluorescein angiography of the left eye shows early hyperfluorescence (yellow arrow in D) with late staining of the macular lesion (yellow arrow in F). . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Indocyanine green (ICG) angiography of both eyes 5 days after presentation.
A and C: ICG angiography of the right eye reveals hypofluorescence in early and late frames of the peripapillary lesions (yellow arrowheads in C). B and D: ICG angiography of the left eye shows early and late hypofluorescence of the macular lesion (yellow arrow in D). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
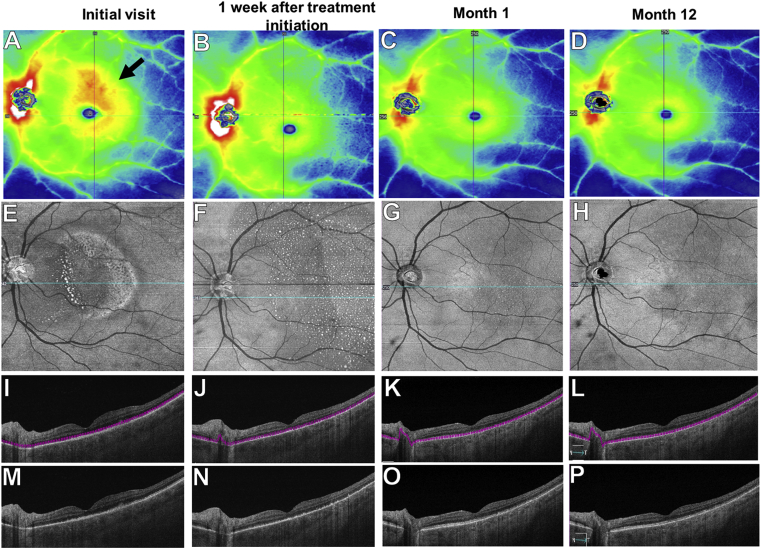
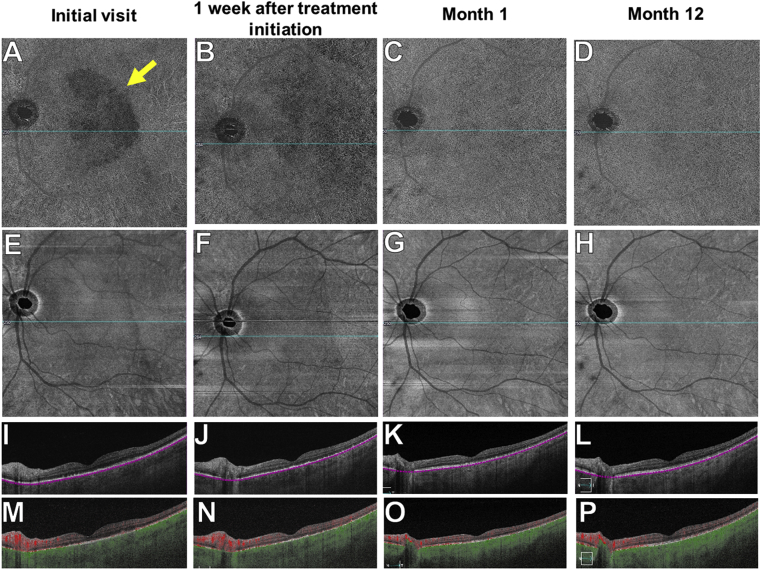
One month later, his BCVA improved to 20/20 in the left eye. There was resolution of intraocular inflammation. The placoid lesion resolved on fundus exam. There was partial restoration of the IS-OS junction in the left eye with decreased RPE granularity (Fig. 6). SS-OCTA imaging showed gradual resolution of the choriocapillaris flow deficits in the left eye (Fig. 7). At 12 months follow up, BCVA remained 20/20 in both eyes. SS-OCT imaging revealed complete restoration of the IS-OS boundary layer with near complete resolution of the RPE granularity (Fig. 6). SS-OCTA imaging revealed restoration of choriocapillaris perfusion in both eyes (Fig. 7). The SS-OCT thickness map showed resolution of the macular thickening in the left eye noted at presentation (Fig. 6).
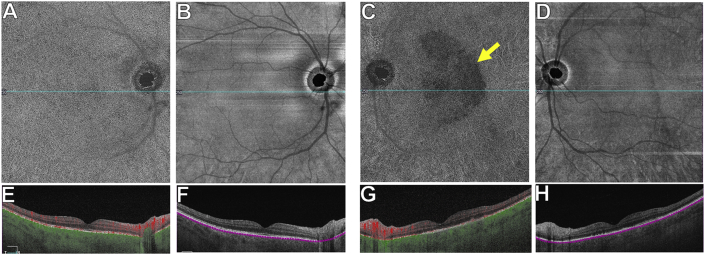
Fig. 6.
SS-OCTA 12 × 12 mm structural images of the left eye over 12 months.
A-D: Thickness maps show macular thickening at presentation (black arrow in A), and resolution of the thickening at month-1 (C) after penicillin therapy. E-H: En face structural slabs from the inner segment-outer segment (IS-OS) region show near complete resolution by month-12 of the bright spots corresponding to the RPE deposits (H). I-P: Horizontal B-scans with or without segmentation lines through the fovea show gradual improvement of the RPE deposits and resolution of the IS-OS disruption by month-12 (P). The blue lines through the fovea in E-H correspond to the horizontal B-scans shown in I-P. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 7.
SS-OCTA 12 × 12 mm en face choriocapillaris images of the left eye over 12 months.
A-D: En face choriocapillaris flow images show flow deficits at presentation (yellow arrow in A) that resolve by month-1 after penicillin therapy (C). E-H: En face choriocapillaris structural images show uniform reflectivity with no evidence of shadow artifacts. I-P: Horizontal B-scans with or without segmentation lines and color-coded flow show resolution of the choriocapillaris flow abnormalities after penicillin treatment by month-1 (O) and month-12 (P). The blue lines through the fovea in A-H correspond to the horizontal B-scans shown in I-P. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
The term ASPPC was coined by Gass et al. to characterize single or several placoid yellowish lesions at the level of the retinal pigment epithelium and outer retina in the macula and juxtapapillary regions.1 Similarly to prior studies, we noted localized hyper-autofluorescence in the area of the placoid lesion that corresponded to the granular deposits on the RPE.4 The hyper-autofluorescence noted in the lesions of both eyes may be a consequence of photoreceptor outer segment abnormalities that correspond to an increase in the detection of RPE autofluorescence.5 It may also be the result of RPE dysfunction leading to incomplete phagocytosis of outer segments with accumulation of photoreceptor outer segment material between the photoreceptors and RPE.4 Widefield autofluorescence also revealed focal patchy areas of increased autofluorescence that otherwise appeared normal on fundus exam. This finding was also noted by Tsui et al.6 Our patient also had OCT findings previously reported with syphilis, including irregular nodular thickening of the RPE, external limiting membrane disruption, and segmental loss of the IS-OS region reflectivity.7, 8, 9 While subretinal fluid has been reported as an early transient feature in patients with ASPPC who had imaging performed within the first few days of onset of symptoms,8 we did not detect subretinal fluid in our patient, perhaps due to his delayed presentation to our office. After initiating antibiotic treatment, we observed gradual improvement of OCT findings as seen in other reports.7,9 Some authors report complete resolution of OCT abnormalities, which we have not yet observed in our patient.10,11 En face images segmented at the level of IS-OS region demonstrated hyperreflective lesions that correlated to the RPE nodular deposits seen on OCT B-scans, and these lesions gradually improved with therapy resulting in total restoration of the IS-OS region and near complete resolution of the RPE granularity. While our patient was HIV positive and tested positive for both VDRL and FTA-ABS, it is noteworthy that the clinical features of ASPPC in both HIV-positive and HIV-negative patients are similar.7
Using SS-OCTA imaging and a widefield 12 × 12 mm field of view scans with slabs that encompassed the anterior choroidal vasculature, which included the choriocapillaris, we were able to document decreased perfusion that we interpreted as diminished choriocapillaris flow that corresponded to the placoid lesion. Decreased choriocapillaris perfusion was reported previously in a smaller scan area.6 This reduced anterior choroidal flow appears to be real and not the result of a shadow artifact from the overlying vitreous or retina. With appropriate treatment, areas of decreased choriocapillaris flow appeared to improve. Improvement in BCVA to 20/20 correlated with improvement in choriocapillaris perfusion despite subtle persistent outer retinal abnormalities. As a result, we speculate that detection of choriocapillaris perfusion abnormalities may help predict final visual acuity outcomes in ASPPC. Other authors have speculated on the impact of decreased choriocapillaris perfusion on visual acuity in various chorioretinal diseases such as macular degeneration, and these choriocapillaris abnormalities likely affect vision indirectly by causing RPE dysfunction.12,13 Visual prognosis for ASPPC patients is usually good after adequate therapy, and our observed improvement in vision correlated with the improvement in choriocapillaris perfusion.
The pathophysiology of ASPPC is not clearly understood. Gass et al. postulated that an inflammatory reaction was present at the level of the choriocapillaris–RPE–photoreceptor complex.1 Later studies using ICGA, FAF, and SD-OCT have been in accordance with this theory.9,11 Our present case demonstrates transient disruptions of choriocapillaris perfusion and outer retinal architecture on SS-OCTA imaging that support the theory regarding the inflammatory involvement of the inner choroid and outer retina in ASPPC. Additional studies are needed to demonstrate the role of the choriocapillaris in predicting visual acuity outcomes following treatment of ASPPC as well as the role of SS-OCTA imaging in differentiating various placoid diseases and their response to treatment.
4. Conclusion
This is the first case report to describe long-term SS-OCTA imaging in ASPPC. SS-OCTA is a fast, safe, and easily repeatable imaging modality that offers valuable insights in our understanding of the pathophysiology and the response to treatment of this condition by providing both structural and flow information of the retina and choroid over a 40-degree 12 × 12 mm field of view at baseline and at frequent follow-up visits.
Patient consent
Written consent to publish case details was obtained from the patient.
Funding
Research supported by grants from Carl Zeiss Meditec, Inc. (Dublin, CA), the Salah Foundation, an unrestricted grant from the Research to Prevent Blindness, Inc. (New York, NY), and the National Eye Institute Center Core Grant (P30EY014801) to the Department of Ophthalmology, University of Miami Miller School of Medicine. The funding organizations had no role in the design or conduct of the present research.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
Philip Rosenfeld and Giovanni Gregori received research support from Carl Zeiss Meditec, Inc. and the University of Miami co-owns a patent that is licensed to Carl Zeiss Meditec, Inc.
Philip Rosenfeld also receives grant support from Boehringer-Ingelheim and Stealth BioTherapeutics. He is also a consultant for Apellis, Boehringer-Ingelheim, Carl Zeiss Meditec, Chengdu Kanghong Biotech, Ocunexus Therapeutics, Ocudyne, and Unity Biotechnology. Philip Rosenfeld has equity interest in Apellis, Verana Health, and Ocudyne.
Janet Davis receives grant support from EyePharma, Nightstar/Biogen. She is a consultant for Kodiak Sciences.
The following author has nothing to disclose: AB.
Acknowledgements
None.
References
- 1.Gass J.D., Braunstein R.A., Chenoweth R.G. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288–1297. doi: 10.1016/s0161-6420(90)32418-1. [DOI] [PubMed] [Google Scholar]
- 2.Aldave A.J., King J.A., Cunningham E.T., Jr. Ocular syphilis. Curr Opin Ophthalmol. 2001;12(6):433–441. doi: 10.1097/00055735-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kojima N., Klausner J.D. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5(1):24–38. doi: 10.1007/s40471-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto Y., Spaide R.F. Autofluorescence imaging of acute syphilitic posterior placoid chorioretinitis. Retin Cases Brief Rep. 2007;1(3):123–127. doi: 10.1097/01.iae.0000242759.80833.39. [DOI] [PubMed] [Google Scholar]
- 5.Freund K.B., Mrejen S., Jung J., Yannuzzi L.A., Boon C.J. Increased fundus autofluorescence related to outer retinal disruption. JAMA Ophthalmol. 2013;131(12):1645–1649. doi: 10.1001/jamaophthalmol.2013.5030. [DOI] [PubMed] [Google Scholar]
- 6.Tsui E., Gal-Or O., Ghadiali Q., Freund K.B. Multimodal imaging adds new insights into acute syphilitic posterior placoid chorioretinitis. Retin Cases Brief Rep. 2018;12(Suppl 1):S3–S8. doi: 10.1097/ICB.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 7.Eandi C.M., Neri P., Adelman R.A., Yannuzzi L.A., Cunningham E.T., Jr. International Syphilis Study G. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina. 2012;32(9):1915–1941. doi: 10.1097/IAE.0b013e31825f3851. [DOI] [PubMed] [Google Scholar]
- 8.Pichi F., Ciardella A.P., Cunningham E.T., Jr. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. 2014;34(2):373–384. doi: 10.1097/IAE.0b013e3182993f11. [DOI] [PubMed] [Google Scholar]
- 9.Burkholder B.M., Leung T.G., Ostheimer T.A., Butler N.J., Thorne J.E., Dunn J.P. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4(1):2. doi: 10.1186/1869-5760-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sridhar J., Shahlaee A., Sharma P. En face: disappearing outer retinal dots in treated syphilitic chorioretinitis. Ophthalmology. 2016;123(3):557. doi: 10.1016/j.ophtha.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Lima L.H., de Andrade G.C., Vianello S., Zett C., Farah M.E., Belfort R., Jr. Multimodal imaging analyses of hyperreflective dot-like lesions in acute syphilitic posterior placoid chorioretinopathy. J Ophthalmic Inflamm Infect. 2017;7(1):1. doi: 10.1186/s12348-016-0119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesper P.L., Soetikno B.T., Fawzi A.A. Choriocapillaris nonperfusion is associated with poor visual acuity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2017;174:42–55. doi: 10.1016/j.ajo.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrelli E., Mastropasqua R., Senatore A. Impact of choriocapillaris flow on multifocal electroretinography in intermediate age-related macular degeneration eyes. Invest Ophthalmol Vis Sci. 2018;59(4):AMD25–AMD30. doi: 10.1167/iovs.18-23943. [DOI] [PubMed] [Google Scholar]