Abstract
The objective of the present retrospective comparative cohort study was to compare the impact of wearing glasses versus an orthokeratology (Ortho-K) lens on peripheral optical properties and myopia progression in a population of South Korean children. Participants included children with myopia, between 8 and 12 years of age (n = 22 eyes), and divided into two groups: those who used glasses (Group I, n = 9) and those who used an Ortho-K lens (Group II, n = 13). Myopia progression over one year was quantified by changes in the central axial length of the eye. Keratometry and corneal aberrations on both the anterior and posterior surfaces of the eye were obtained using a Scheimpflug camera. A custom-developed Shack-Hartmann aberrometer was also used to measure peripheral aberrations across the horizontal visual field, up to 30°, and along the nasal-temporal meridian in 10-degree steps. Central axial elongation was larger in Group I (0.59 ± 0.21 mm) than in Group II (0.34 ± 0.18 mm) (P = .01). Relative peripheral spherical refractions at 10 and 20° nasally and at 10° temporally (P = 0.04, 0.049, and 0.042, respectively) relative to the fovea were positively correlated with central axial elongation in Group II. Group II exhibited an increase in peripheral ocular high order aberrations, such as horizontal coma and asymmetric trefoil. The use of Ortho-K lenses was found to slow the rate of central axis elongation in children with myopia. This effect might be related to an increase in both peripheral spherical refraction and peripheral ocular higher order aberrations with Ortho-K lens use.
Keywords: Physiology, Ophthalmology, Eye-ear-nose-throat, Clinical research, Myopia, Aberrometry, Child
Physiology; Ophthalmology; Eye-ear-nose-throat; Clinical research; Myopia; Aberrometry; Child.
1. Introduction
Despite substantial recent improvement in our understanding of myopia due to studies in both humans and animals, the fundamental mechanisms underlying myopia's etiology remain unclear. Some hypotheses include the retinal defocus theory, the accommodation lag theory, and the mechanical theory, all of which have been tested [1, 2, 3, 4, 5, 6]. Specifically, the currently prevailing retinal defocus theory states that peripheral hyperopic blur stimulates an increase in central axial length, while the accommodation lag theory posits that retinal blur or hyperopic defocus caused by an increase in accommodation lag leads to the central axial elongation that results in myopia [1, 2]. The mechanical theory posits that the central axial elongation or increased accommodation lag often found in myopia are due to an increase in ciliary choroidal tension at the anterior aspect of the eyeball [7]. Other hypotheses, such as the association between light spectrum exposure and dopamine release [8], as well as those which involve environmental factors, such as time spent pursing outdoor activities [9, 10, 11], have also been tested to explain the pathophysiology of myopia development.
Among these hypotheses, the peripheral retinal defocus theory, described above, has been tested using both experimental and clinical approaches since Smith et al.‘s original report that peripheral hyperopic visual signals affected the elongation of the central axis in infant monkeys [12]. Several clinical studies of the hyperopic defocus theory have also demonstrated the significance of peripheral defocus to myopia [1, 13, 14, 15, 16, 17, 18, 19]. Among the clinical interventions used to control myopia progression, the use of orthokeratology (Ortho-K) lenses has been shown to successfully slow the progression of myopia in some children with myopia [1, 14, 15, 16, 17, 18, 19, 20, 21]. Changes in peripheral refraction are induced by corneal shape changes induced by wearing an Ortho-K lens [22]. More recently, soft multifocal contact lenses have also been introduced for the management of myopia. The application of these lenses is based on the hypothesis that a decrease in relative peripheral hyperopic refractive error might slow the progression of myopia [23, 24, 25, 26, 27]. Walline et al. (the BLINK study group) [27] used a double-masked, randomized clinical trial design to evaluate the effect of and mechanism underlying soft multifocal contact lenses control of myopia's progression. In summary, peripheral refractive error has been the primary hypothesis explored with regard to the role of defocusing in myopia, with some finding that relative peripheral hyperopia is not predictive of myopia but rather plays only a small role in its progression [5, 28, 29, 30, 31].
The Shin-Nippon NVision K5001 open field autorefractor is currently considered to be the most useful commercial device for the measurement of peripheral refraction [32]. Unlike autorefractors, such ocular wavefront sensors measure not only conventional refractive errors, but also higher order aberrations. Peripheral aberrations, including both astigmatism and higher order aberrations, are increased compared to those measured at the fovea [33, 34] and the ability to quantify these aberrations in the peripheral visual field with natural foveal fixation is critical to developing a more complete understanding of the peripheral optical quality of the eye [34, 35].
Although most clinical studies have demonstrated the effectiveness of using an Ortho-K lens to slow the progression of myopia, little has been done to understand how the reshaping of the cornea affects peripheral optics and their potential relationship with myopia's progression. A previous study of lower order aberrations revealed that Ortho-K-lens-corrected myopia within 10 degrees of the central visual field led to a relative myopic change in the periphery [36]. Additionally, Oshika et al. recently reported a negative correlation between on-axis corneal and ocular high order aberrations (HOAs) with central axial elongation [6, 37]. Zhong et al. [14] also used on-axis measurements to assess the effect of summed corneal power changes along the diameter of a measuring circle in myopic children before and after Ortho-K lens use.
Given this background, the goal of the present study was to investigate the relationship between the peripheral optical properties of the eye and its effect on myopia progression. To do this, we used a customized aberrometer to objectively measure refractive error and HOAs across the horizontal visual field in children with myopia who used an Ortho-K lens or glasses for approximately one year.
2. Material and methods
2.1. Subjects
We used a retrospective comparative cohort study design and assessed new Ortho-K lens wearers and new glasses wearers who used their respective aids for at least 1 year. Follow-ups were conducted between November 2014 and December 2016 at Seoul St. Mary's Hospital, Seoul, South Korea. Patients using Ortho-K lenses or glasses were grouped according their treatment type. Patients (and their parents) made all decisions about their treatment independently; no specific suggestions were made and all subjects were informed of the benefits and possible disadvantages of use of both Ortho-K lenses and glasses. All participants and their parents provided prior informed consent to allow for their clinical data to be used for research purposes. The present study was conducted in accordance with the ethical standards of the Declaration of Helsinki and with the approval of Seoul St. Mary's Hospital's (Seoul, Republic of Korea) Institutional Review Board (IRB #KC15TISI0093).
At their baseline visit, subjects provided demographic information and underwent thorough ophthalmologic examinations including a slit-lamp examination and assessments of visual acuity, cycloplegic manifest refraction, peripheral spherical refraction in the cycloplegic condition, corneal topography, tear break-up time, and intraocular pressure, as well as corneal endothelial cell counts and the Schirmer test. Patients with complete clinical data and who underwent those examinations were included in the present study. Ophthalmologic records inclusive of peripheral refractive error were collected 2 weeks after Ortho-K lens use began or at the time glasses were first prescribed, as per the participant's group assignment. All participants had a visual acuity with distance correction of 0.1 logMAR (20/25) or better.
The following inclusion criteria were applied to both Ortho-K lens and glasses wearers: age between 8 and 12 years old; no prior history of wearing glasses or contact lenses to correct myopia; spherical equivalent on cycloplegic manifest refraction assessment between −0.75 and −4.00 D in both eyes; less than a −1.50 D astigmatism in both eyes; less than 1.50 D of anisometropia; normal ocular alignment; horizontal corneal diameter greater than 11.0 mm; no history of inflammatory ocular disease or surgery; and a willingness to provide signed written consent. Children were divided into two groups based the correction method used for their myopia—glasses (Group I) or Ortho-K lenses (Group II).
Children within any of the following exclusion criteria were excluded from the present study: Schirmer test results of less than 10 mm or tear break-up time shorter than 10 s; any signs of keratoconus or corneal degeneration; abnormal findings during slit-lamp microscopy; ocular allergies; contraindications for wearing contact lenses; and inflammation, erosions, ulcers after Ortho-K lens wear (among Ortho-K lens wearers only). Follow-up appointments were conducted every four months to check for side effects and for power modification of the glasses or Ortho-K lenses. Subjects who started wearing Ortho-K lenses were evaluated every four months after successful fitting of the lenses. Finally, we retrospectively created a final cohort of 22 patients (n = 9 and 13 in groups I and II, respectively) who were able return for a follow-up evaluation 1 year after their first visit.
2.2. Biometric measurements
Cycloplegic manifest refraction was assessed with the administration of 1 % cyclopentolate and 0.5 % phenylephrine eye drops. Both eye drops were applied to each eye three times in 10-min intervals starting 30 min prior to cycloplegic manifest refraction measurements. Axial length was the main outcome of biometric measurements using a partial coherence interferometer (IOL master®, Carl Zeiss, Jena, Germany) and used to assess the progression of myopia. Corneal topography was evaluated with a rotating Scheimpflug camera (Pentacam®, Oculus, Lynwood, WA) to assess keratometry, corneal wavefront values, and to create a corneal power map. Cycloplegic manifest refraction and axial length were checked at the time of enrollment and again approximately 12 months later. A corneal power map, obtained via the Scheimpflug camera, was created every 4 months and used to confirm the shape of corneal surface during follow-ups.
2.3. Prescription for glasses and Ortho-K lenses
Subjects in Group I (glasses) received a prescription for glasses powered per their cycloplegic manifest refraction. At screening, subjects in Group II were informed of the precautions that should be taken when using the Ortho-K lens as well as potential adverse reactions. Ortho-K lenses (Lucid Korea, Bonghwa, South Korea) were prescribed to subjects in Group II. The base curve and power Ortho-K lenses were determined using each patient's individual corneal topography and cycloplegic manifest refraction, respectively. Fitting for Ortho-K lenses was confirmed using corneal topography and slit-lamp examination. A clinically acceptable Ortho-K lens fit was defined by both an uncorrected distant visual acuity of 0.1 logMAR (20/25) or better and by the presence of a bull's eye pattern on the corneal topography. A contact lens expert (Dr. K.S. Na) verified that there was adequate pressure on the cornea and that a space was maintained between the back of the Ortho-K lens and the anterior of the cornea. Moderate to severe Efron grading test scores [38] and other pathological findings in the kerato-conjunctival area were considered indicators of an adverse reaction to use of the Ortho-K lens. When a poor lens fitting was detected during the follow-up period, the Ortho-K lens was changed to achieve the appropriate base curve and power. All subjects were instructed to wear Ortho-K lenses for an average of seven hours during sleep and to refrain from use of any contact lens during the daytime.
2.4. Measurements of peripheral spherical refractive error
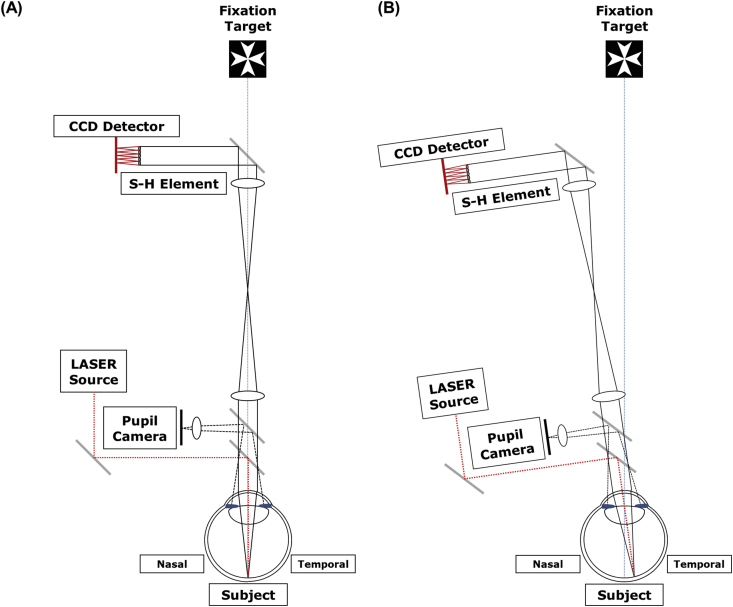
Ocular wavefront aberrations across the horizontal visual field were measured using a custom-developed Shack-Hartmann aberrometer (Figure 1) [39] at the time of glasses prescription (group 1) and after 2 weeks of wearing an Ortho-K lens (group 2). This aberrometer uses an infrared laser (λ = 830 nm) and the entire device is rotated around the eye's pupil center (Figure 1B) as in measuring ocular aberrations rather than having patients fixate eccentrically. Ocular wavefront aberrations were measured across a 30-degree visual field along the nasal-temporal meridian. The central 6 mm of the pupil was assessed for any aberrations.
Figure 1.
Schematics of the wavefront analyzer used for measuring peripheral wavefront aberrations in the retina. Measurements were performed from the fixation target axis (A) to an off-axis target (B). The laser source and components were positioned vertically in the customized aberrometer so as to not interfere with the subject's view of the fixation target.
After cycloplegia was achieved, subjects, without corrected refractive error, were positioned on a chin rest with a forehead rest to minimize head movements. Each subject was asked to fixate on a luminous Maltese spot target while their left eye was occluded with a black eye patch. The pupil was precisely aligned with a pupil camera. Individual aberration coefficients were calculated with custom software and averaged across three measurements for each subject.
Lower order aberration coefficients across a 6.0-mm diameter were converted to spherical refraction (S), asymmetric astigmatism (), and symmetric astigmatism () in diopters according to the following equations, as used previously [40]:
| (1) |
| (2) |
| (3) |
where , , and are defocus, asymmetric astigmatism, and symmetric astigmatism terms for Zernike polynomials corresponding to a 6-mm pupil diameter, respectively, and is the pupil radius used for the calculation of aberration. The defocus term was rescaled to compensate for longitudinal chromatic aberrations induced by the difference between two wavelengths: 830-nm for wavefront sensing, and 555-nm (waveform at which the human eye's spectral sensitivity peaks). Relative peripheral spherical refractive error was calculated by subtracting the foveal spherical refraction from those at each off-axis point.
2.5. Statistical analyses
All subjects' right eyes were included in the present analysis. Statistical analyses were performed using SAS software (version 9.3, SAS Institute, Cary, NC, USA). Continuous variables are expressed as means and standard deviation, while categorical variables are expressed as frequencies and percentage. Measurement outcomes were compared between the two groups using Wilcoxon signed-rank tests for continuous variables and Fisher's exact tests for categorical variables. Spearman correlation analyses were used to assess for correlations between axial elongation and clinical outcome measures, including peripheral spherical refractive error. A P-value < .05 was considered to indicate statistical significance.
3. Results
Of the 22 eyes assessed in the present study (from 22 subjects) 9 were categorized into Group 1 while 13 were categorized into group II. There was no difference in age between the two groups. Likewise, pre-treatment spherical equivalent values did not differ between the two groups. Differences in sex, corneal spherical aberrations (SA), corneal coma-like aberration, keratometry, or axial length were statistically insignificant (Table 1).
Table 1.
Patients demographics.
| Glasses (Group I, n = 9) | Ortho-K Lens (Group II, n = 13) | P-value | |
|---|---|---|---|
| Duration of wear (months) | 12 | 12 | N/A |
| Age | 8.8 ± 1.0 | 9.4 ± 1.6 | 0.30∗ |
| Sex (Male) | 4 (44.4 %) | 7 (53.8 %) | 0.67† |
| Spherical Equivalent (D) | −2.72 ± 0.87 | −3.30 ± 0.51 | 0.07∗ |
| Corneal Spherical Aberration (μm) | 0.11 ± 0.11 | 0.14 ± 0.17 | 0.36∗ |
| Corneal Coma-like Aberration (μm) | 0.12 ± 0.15 | 0.10 ± 0.12 | 0.42∗ |
| Keratometry (D) | 43.5 ± 1.5 | 42.8 ± 1.6 | 0.71∗ |
| Axial Length (mm) | 24.6 ± 0.6 | 25.0 ± 0.5 | 0.11∗ |
Spherical equivalent was calculated based on the cycloplegic manifest refraction.
Wilcoxon signed-rank test.
Fisher's exact test.
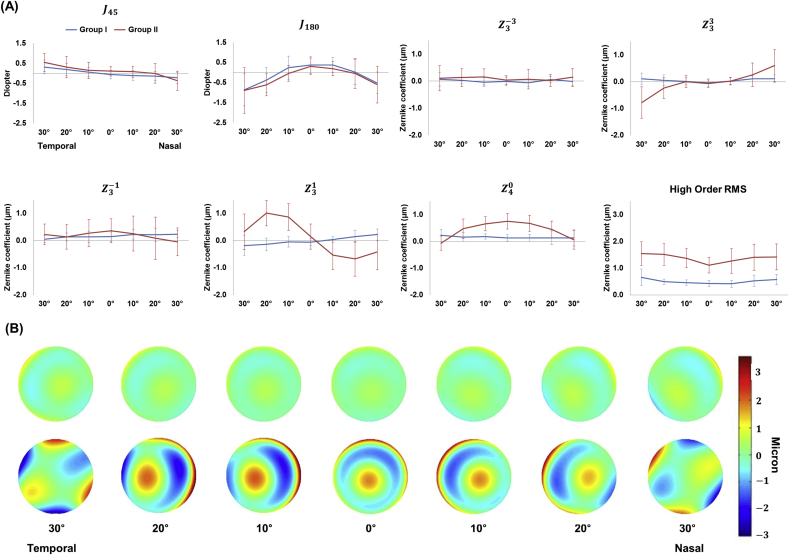
Figure 2 displays ocular aberrations along the horizontal meridian in both groups. High order RMS values along the horizontal meridian were larger in Group II than in Group I (Figure 2A). The coefficient was also larger in Group II than in Group I at multiple points along the horizontal meridian except for those 30° from the central axis in both the nasal and temporal directions. HOAs such as , , and exhibited a symmetric wavefront graph pattern along the horizontal meridian in both groups. On the other hand, aberration terms such as and exhibited an asymmetric pattern in both groups. in Group II exhibited a more asymmetric pattern than in Group I. and in Group II exhibited more variation along the horizontal meridian than in Group I. When the corneal wavefront map was reconstructed based on corneal HOAs and without low order aberrations in both groups, corneal region flattening shifted to the periphery along the horizontal axis (Figure 2B). Coma was dominant at 10 and 20° towards both the nasal and temporal sides in Group II while trefoil was dominant at 30° towards both the nasal and temporal sides in Group II.
Figure 2.
High order aberrations based on Zernike polynomials from peripheral wavefront measurement (A), such as asymmetric astigmatism (J_(45)), symmetric astigmatism (J_(180)), symmetric trefoil (Z_3ˆ(-3)), asymmetric trefoil (Z_3ˆ3), vertical coma (Z_3ˆ(-1)), horizontal coma (Z_3ˆ1), spherical aberration (Z_4ˆ0), and high order root mean square (RMS). High order aberrations were mounted and reconstructed on aberration maps (B) along the horizontal meridian in both Group I (upper row) and Group II (lower row).
After a 1-year follow-up period, axial length elongation from the corneal endothelium to the retina for subjects in Group I was 0.591 ± 0.214, while it was only 0.330 ± 0.182 mm in Group II (P < .05) (Table 2). Changes in cycloplegic manifest refraction during this year were significantly more myopic in Group I than in Group II (P < .001). In contrast, there was no difference in the curvature of the corneal posterior surface (P = .11), while the anterior surface of the cornea was flatter in Group II than in Group I (P < .001). Group II exhibited decreased corneal thickness, higher SA, and coma-like aberrations. The occurrence of these changes differed significantly different between the two groups (P < .05).
Table 2.
Outcome measurements after 1 year of wearing glasses (Group I) and orthokeratology (Ortho-K) lenses (Group II).
| Glasses (Group I, n = 9) | Ortho-K Lens (Group II, n = 13) | P-value | |
|---|---|---|---|
| Central Axial Length Elongation (mm) | |||
| From Corneal Epithelium to Retina | 0.591 ± 0.214 | 0.330 ± 0.182 | 0.010∗ |
| From Corneal Endothelium to Retina | 0.590 ± 0.214 | 0.341 ± 0.183 | 0.014∗ |
| Cycloplegic Manifest Refraction (D) | |||
| Spherical Equivalent | −3.33 ± 1.12 | −0.47 ± 0.27 | <0.001∗ |
| Difference (Initial to 12 month) | −0.61 ± 0.68 | 2.89 ± 0.40 | <0.001∗ |
| Corneal Measurements | |||
| Keratometry (Km, D) | |||
| Anterior surface | 43.56 ± 1.18 | 40.70 ± 1.56 | <0.001∗ |
| Posterior surface | −6.30 ± 0.12 | −6.15 ± 0.25 | 0.11∗ |
| Change in Corneal Thickness (within 1 year, pupil center, μm) | 6.25 ± 9.98 | −14.25 ± 6.73 | 0.017∗ |
| Corneal Spherical Aberration (μm) | 0.21 ± 0.22 | 0.85 ± 0.29 | <0.001∗ |
| Corneal Coma-like Aberration (μm) | 0.15 ± 0.06 | 0.64 ± 0.40 | 0.002∗ |
Spherical equivalent was calculated based on cycloplegic manifest refraction.
Wilcoxon signed-rank test.
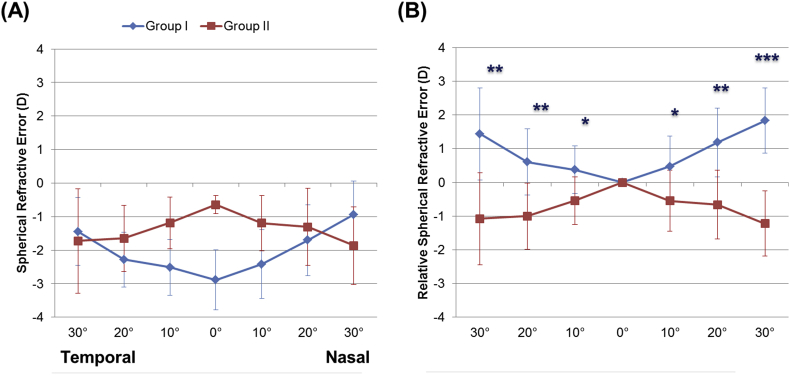
Figure 3A presents the spherical refractive error across the visual field in both groups. The relative peripheral spherical refractive error was 0.37 ± 0.71, 0.61 ± 0.98, and 1.44 ± 1.36 D at 10, 20, and 30° from the temporal side of the fovea in Group I, respectively (Figure 3B). For the nasal side of the fovea, these values were 0.47 ± 0.91, 1.18 ± 1.02, and 1.94 ± 1.11 D, also respectively. In contrast to Group I, Group II exhibited peripheral myopia for all off-axis measurements from the fovea (P < .05).
Figure 3.
Peripheral spherical refractions in Group I and II. (A). Children with glasses (Group I) and orthokeratology lenses (Group II) were measured at the time at which they were prescribed (Group I) and 2 weeks after stabilization using an orthokeratology lens (Group II). Peripheral spherical refractions were calculated from ocular aberrations in the right eyes of subjects across a 6.0-mm diameter scan site. After applying an offset, which was equal to the amount of spherical refractive error on the central axis, differences between peripheral and central spherical refractive error was defined as the relative spherical refractive error in Groups I and II (B). ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.001. Error bars indicate 95% confidence intervals for mean values.
In Group II, there was a moderately positive correlation between central axial length elongation and peripheral relative spherical refractive error at 10 and 20° relative to the nose and at 10° temporally from the fovea (Table 3). No correlation was found in terms of corneal SA, coma-like aberration, or high order root mean square (RMS) compared to the elongation in the axial dimension in either groups.
Table 3.
Correlation between peripheral relative spherical refractive error and central axial length elongation.
| Glasses (Group I, n = 9) |
Ortho-K Lens (Group II, n = 13) |
||||
|---|---|---|---|---|---|
| Correlation Coefficient, R | P-value∗ | Correlation Coefficient, R | P-value∗ | ||
| Nasal | 30 Degrees | .050 | .898 | .250 | .409 |
| 20 Degrees | .550 | .125 | .556 | .049 | |
| 10 Degrees | .367 | .332 | .583 | .036 | |
| Temporal | 10 Degrees | .467 | .205 | .569 | .042 |
| 20 Degrees | .517 | .154 | .113 | .714 | |
| 30 Degrees | .483 | .187 | .396 | .180 | |
Central axial length was measured from the corneal endothelial layer to the retina.
Spearman Correlation.
4. Discussions
The present retrospective study demonstrated the effects of reshaping the anterior surface of the cornea with an Ortho-K lens on myopia progression. After one year of use, central axis elongation in children who wore an Ortho-K lens was found to be smaller than that which occurred in those who wore glasses. A significant increase was found in spherical aberration and other HOAs in the cornea with Ortho-K lens use (versus use of glasses), a changed which may affect eye growth.
Aberration profiles, including HOAs, provide more complete information about peripheral optical quality changes after wearing an Ortho-K lens than do conventional refractive error measurements (obtained with an autorefractor). Peripheral wavefront measurements can be obtained when the head position is rotated or when the eye fixates eccentrically. Although Radhakrishnan et al. [41] did not find a difference in peripheral refraction between these two methods, rotating the head and realigning the system prior to obtaining each measurement is time consuming and requires user experience. To overcome this methodological limitation, we instead used a customized aberrometer for assessment of children with natural foveal fixation, as was previously described by Jaeken et al. [42].
Several studies of Ortho-K lens users have suggested that a myopic shift in peripheral refractive error might play an important role in the deceleration of central axial elongation with myopia [1, 14, 15, 16, 17, 18, 19]. Previous reports found that central axial elongations in children from China and Hong Kong who wore an Ortho-K lens for two years were 0.37 ± 0.27 mm and 0.40 ± 0.25 mm, respectively [14, 15]. These numbers are smaller than those found in the present study, possibly due to differences in age distribution and the degree of myopia already present when subjects were enrolled. In the present study, we found a weak but statistically significant correlation between relative peripheral refractive error and axial eye elongation at 10° in both the temporal and nasal directions and at 20° in the nasal direction. Several clinical trials have reported that Ortho-K lenses lead to deceleration of central axis elongation with myopia, although the pathophysiology underlying these findings has not yet been described [1, 14, 15, 16, 17, 18, 19]. Another confounding factor in studies like the present one and those mentioned here is whether peripheral myopia was present in the cases assessed. It is not clear whether peripheral myopia is a cause or a result of myopia, as has been pointed out by Atchison et al. [5, 28] However, the peripheral refractive changes and progression of myopia found here are likely not fully explained by the prevailing retinal defocus theory. Hence, additional potential mechanisms that might underlie the effects of Ortho-K lenses on the progression of myopia remain critical to examine.
Ortho-K lenses render the central and peripheral cornea flatter and steeper, respectively [21, 43]. In normal eyes, an increase in refractive power and HOAs occurs in eccentric corneas [34, 35]. This is due to the fact that a nodal point inside of the human eye causes light to propagate to the peripheral retina and pass obliquely through different areas of the cornea and lens. Moreover, abnormal curvature changes in the central and peripheral cornea induced by the use of Ortho-K lenses may amplify changes in the peripheral cornea. Increased incidence of aberrations in the peripheral cornea, for example, is the most common unique manifestation of wearing Ortho-K lenses and might contribute to the slowing of myopia's progression [44].
To evaluate the effects of corneal reshaping on peripheral refractive error, we will expand on two representative cases (see Figure 1A, Supplemental Content), one each from Groups I and II. The relative corneal sagittal power was larger in all peripheral axes for the Group II case than for the Group I case and it was a main contributor to the peripheral ocular high order RMS difference between the two groups (see Figure 1B, Supplemental Content).
HOAs in the eye typically produce multifocality, extending the depth of focus (DoF) [45]. An extended DoF, within which variation in retinal image quality is relatively small or is not obvious, may play a role in rendering the visual system less sensitive to differentiating myopic or hyperopic defocus blurring. In a previous peripheral wavefront study, the constant and unique direction of astigmatisms and comas was reported according to the meridian of each in the visual field [46]. The direction of blur caused by these aberrations in the periphery of the retina may be one factor that stimulates the eye's elongation.
If peripheral retina aberrations can result in eye elongation, any potentially effective method for slowing the progression of myopia should also neutralize or symmetrize intrinsically asymmetric peripheral optical blur. This can be achieved clinically with the use of bifocal contact lenses, which are designed to induce additional aberrations [47]. While present study did not find any statistically significant correlation between HOAs (e.g. coma-like aberration or SA) and central axial elongation, as has been reported by others [48], a negative correlation between these factors was previously reported by Oshika et al. [6, 37].
The present study also has some limitations which warrant discussion. For instance, it used a retrospective study design, which encompassed a relatively short period of time and a small number of subjects, all of whom had already been diagnosed with myopia. Future research should include more long-term outcomes and a prospective study design in which full-field peripheral refraction is used to evaluate the effects of peripheral image quality on central axial elongation in children wearing glasses or Ortho-K lenses.
5. Conclusions
In summary, the use of Ortho-K lenses increases HOAs considerably across the horizontal visual field as well as decreases the progression of myopia comparing to wearing glasses. We suggest that the mechanism underlying the Ortho-K lens's therapeutic effects on myopia, as well as those of bifocal and multifocal lenses, is related to their peripheral optical properties, including increased HOAs, which result in extending the DoF with the increased focus allowed by these lenses. Further studies are necessary to improve our understanding of how changes in the corneal surface impact ocular optics and thus retinal image quality in eccentric visual fields.
Declarations
Author contribution statement
Y. Yoo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
K. Na and G. Yoon: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
D. Kim: Analyzed and interpreted the data; Wrote the paper.
Y. Byun, M. Park and C. Joo: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Q. Ji, I. Chung, H. Kim and W. Whang: Analyzed and interpreted the data.
Funding statement
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Sejong City, South Korea [2016R1A6A1A03010528, 2017R1C1B1012060, 2019R1F1A1061421] and by Research to Prevent Blindness (RPB), Rochester, NY.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We wish to thank Chloe Degre, who critically reviewed this manuscript and provided assistance with grammar editing.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sankaridurg P. Contact lenses to slow progression of myopia. Clin. Exp. Optom. 2017;100(5):432–437. doi: 10.1111/cxo.12584. [DOI] [PubMed] [Google Scholar]
- 2.Wallman J., Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Wallman J., Turkel J., Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201(4362):1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 4.Charman W.N. Near vision, lags of accommodation and myopia. Ophthalmic Physiol. Optic. 1999;19(2):126–133. doi: 10.1046/j.1475-1313.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Atchison D.A., Rosén R. The possible role of peripheral refraction in development of myopia. Optom. Vis. Sci. 2016;93(9):1042–1044. doi: 10.1097/OPX.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 6.Hiraoka T., Kotsuka J., Kakita T., Okamoto F., Oshika T. Relationship between higher-order wavefront aberrations and natural progression of myopia in schoolchildren. Sci. Rep. 2017;7(1):7876. doi: 10.1038/s41598-017-08177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berntsen D.A., Mutti D.O., Zadnik K. Study of theories about myopia progression (STAMP) design and baseline data. Optom. Vis. Sci. 2010;87(11):823–832. doi: 10.1097/OPX.0b013e3181f6f776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashby R.S., Schaeffel F. The effect of bright light on lens compensation in chicks. Invest. Ophthalmol. Vis. Sci. 2010;51(10):5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 9.Morgan I.G., He M., Rose K.A. Epidemic of Pathological Myopia: what can laboratory studies and epidemiology tell us? Retina. 2017;37(5):989–997. doi: 10.1097/IAE.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 10.Wu P.C., Tsai C.L., Wu H.L., Yang Y.H., Kuo H.K. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120(5):1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 11.He M., Xiang F., Zeng Y. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. J. Am. Med. Assoc. 2015;314(11):1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 12.Smith E.L., 3rd, Hung L.F., Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vis. Res. 2009;49(19):2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogerheide J., Rempt F., Hoogenboom W.P. Acquired myopia in young pilots. Ophthalmologica. 1971;163(4):209–215. doi: 10.1159/000306646. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Y., Chen Z., Xue F., Miao H., Zhou X. Central and peripheral corneal power change in myopic orthokeratology and its relationship with 2-year axial length change. Invest. Ophthalmol. Vis. Sci. 2015;56(8):4514–4519. doi: 10.1167/iovs.14-13935. [DOI] [PubMed] [Google Scholar]
- 15.Cho P., Cheung S.W. Protective role of orthokeratology in reducing risk of rapid axial elongation: a reanalysis of data from the ROMIO and TO-SEE studies. Invest. Ophthalmol. Vis. Sci. 2017;58(3):1411–1416. doi: 10.1167/iovs.16-20594. [DOI] [PubMed] [Google Scholar]
- 16.Huang J., Wen D., Wang Q. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4):697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Leo S.W. Scientific bureau of world society of paediatric Ophthalmology and strabismus (WSPOS). Current approaches to myopia control. Curr. Opin. Ophthalmol. 2017;28(3):267–275. doi: 10.1097/ICU.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 18.González-Méijome J.M., Peixoto-de-Matos S.C., Faria-Ribeiro M. Strategies to regulate myopia progression with contact lenses: a Review. Eye Contact Lens. 2016;42(1):24–34. doi: 10.1097/ICL.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 19.Pauné J., Morales H., Armengol J. Myopia control with a novel peripheral gradient soft lens and orthokeratology: a 2-year clinical trial. BioMed Res. Int. 2015;2015:507572. doi: 10.1155/2015/507572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santodomingo-Rubido J., Villa-Collar C., Gilmartin B., Gutiérrez-Ortega R., Sugimoto K. Long-term efficacy of orthokeratology contact lens wear in controlling the progression of childhood myopia. Curr. Eye Res. 2017;42(5):713–720. doi: 10.1080/02713683.2016.1221979. [DOI] [PubMed] [Google Scholar]
- 21.Morgan I.G., French A.N., Ashby R.S. The epidemics of myopia: aetiology and prevention. Prog. Retin. Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kang P., Swarbrick H. Time course of the effects of orthokeratology on peripheral refraction and corneal topography. Ophthalmic Physiol. Optic. 2013;33(3):277–282. doi: 10.1111/opo.12027. [DOI] [PubMed] [Google Scholar]
- 23.Robboy M.W., Hilmantel G., Tarver M.E., Eydelman M.B. Assessment of clinical trials for devices intended to control myopia progression in children. Eye Contact Lens. 2018;44(4):212–219. doi: 10.1097/ICL.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 24.Fedtke C., Ehrmann K., Thomas V., Bakaraju R.C. Peripheral refraction and aberration profiles with multifocal lenses. Optom. Vis. Sci. 2017;94(9):876–885. doi: 10.1097/OPX.0000000000001112. [DOI] [PubMed] [Google Scholar]
- 25.Li S.M., Kang M.T., Wu S.S. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: a meta-analysis. Ophthalmic Physiol. Optic. 2017;37(1):51–59. doi: 10.1111/opo.12332. [DOI] [PubMed] [Google Scholar]
- 26.Sankaridurg P., Holden B., Smith E., 3rd Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest. Ophthalmol. Vis. Sci. 2011;52(13):9362–9367. doi: 10.1167/iovs.11-7260. [DOI] [PubMed] [Google Scholar]
- 27.Walline J.J., Gaume Giannoni A., Sinnott L.T., BLINK Study Group A randomized trial of soft multifocal contact lenses for myopia control: baseline data and methods. Optom. Vis. Sci. 2017;94(9):856–866. doi: 10.1097/OPX.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atchison D.A., Li S.M., Li H. Relative peripheral hyperopia does not predict development and progression of myopia in children. Invest. Ophthalmol. Vis. Sci. 2015;56(10):6162–6170. doi: 10.1167/iovs.15-17200. [DOI] [PubMed] [Google Scholar]
- 29.Lee T.T., Cho P. Relative peripheral refraction in children: twelve-month changes in eyes with different ametropias. Ophthalmic Physiol. Optic. 2013;33(3):283–293. doi: 10.1111/opo.12057. [DOI] [PubMed] [Google Scholar]
- 30.Sng C.C., Lin X.Y., Gazzard G. Change in peripheral refraction over time in Singapore Chinese children. Invest. Ophthalmol. Vis. Sci. 2011;52(11):7880–7887. doi: 10.1167/iovs.11-7290. [DOI] [PubMed] [Google Scholar]
- 31.Mutti D.O., Sinnott L.T., Mitchell G.L., CLEERE Study Group Relative peripheral refractive error and the risk of onset and progression of myopia in children. Invest. Ophthalmol. Vis. Sci. 2011;52(1):199–205. doi: 10.1167/iovs.09-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedtke C., Ehrmann K., Holden B.A. A Review of peripheral refraction techniques. Optom. Vis. Sci. 2009;86(5):429–446. doi: 10.1097/OPX.0b013e31819fa727. [DOI] [PubMed] [Google Scholar]
- 33.Lundström L., Gustafsson J., Unsbo P. Vision evaluation of eccentric refractive correction. Optom. Vis. Sci. 2007;84(11):1046–1052. doi: 10.1097/OPX.0b013e318159aa7a. [DOI] [PubMed] [Google Scholar]
- 34.Lundström L., Gustafsson J., Svensson I., Unsbo P. Assessment of objective and subjective eccentric refraction. Optom. Vis. Sci. 2005;82(4):298–306. doi: 10.1097/01.opx.0000159366.61943.62. [DOI] [PubMed] [Google Scholar]
- 35.Atchison D.A. Comparison of peripheral refractions determined by different instruments. Optom. Vis. Sci. 2003;80(9):655–660. doi: 10.1097/00006324-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Charman W.N., Mountford J., Atchison D.A., Markwell E.L. Peripheral refraction in orthokeratology patients. Optom. Vis. Sci. 2006;83(9):641–648. doi: 10.1097/01.opx.0000232840.66716.af. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka T., Kakita T., Okamoto F., Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122(1):93–100. doi: 10.1016/j.ophtha.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 38.Efron N., Pritchard N., Brandon K. A survey of the use of grading scales for contact lens complications in optometric practice. Clin. Exp. Optom. 2011;94(2):193–199. doi: 10.1111/j.1444-0938.2010.00549.x. [DOI] [PubMed] [Google Scholar]
- 39.Jeong T.M., Menon M., Yoon G. Measurement of wave-front aberration in soft contact lenses by use of a Shack-Hartmann wave-front sensor. Appl. Optic. 2005;44(21):4523–4527. doi: 10.1364/ao.44.004523. [DOI] [PubMed] [Google Scholar]
- 40.Thibos L.N., Wheeler W., Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom. Vis. Sci. 1997;74(6):367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 41.RadhakrishnanH CharmanWN. Peripheral refraction measurement: does it matter if one turns the eye or the head? Ophthalmic Physiol. Optic. 2008;28(1):73–82. doi: 10.1111/j.1475-1313.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 42.Jaeken B., Artal P. Optical quality of emmetropic and myopic eyes in the periphery measured with high-angular resolution. Invest. Ophthalmol. Vis. Sci. 2012;53(7):3405–3413. doi: 10.1167/iovs.11-8993. [DOI] [PubMed] [Google Scholar]
- 43.Santodomingo-Rubido J., Villa-Collar C., Gilmartin B., Gutiérrez-Ortega R. Short-term and long-term changes in corneal power are not correlated with axial elongation of the eye induced by orthokeratology in children. Eye Contact Lens. 2018;44(4):260–267. doi: 10.1097/ICL.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q., Li M., Yuan Y. Interaction between corneal and internal ocular aberrations induced by orthokeratology and its influential factors. BioMed Res. Int. 2017;2017:3703854. doi: 10.1155/2017/3703854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benard Y., Lopez-Gil N., Legras R. Optimizing the subjective depth-of-focus with combinations of fourth- and sixth-order spherical aberration. Vis. Res. 2011;51(23-24):2471–2477. doi: 10.1016/j.visres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Mathur A., Atchison D.A., Charman W.N. Myopia and peripheral ocular aberrations. J. Vis. 2009;9(10):1–12. doi: 10.1167/9.10.15. [DOI] [PubMed] [Google Scholar]
- 47.Ji Q., Yoo Y.S., Alam H., Yoon G. Through-focus optical characteristics of monofocal and bifocal soft contact lenses across the peripheral visual field. Ophthalmic Physiol. Optic. 2018;38(3):326–336. doi: 10.1111/opo.12452. [DOI] [PubMed] [Google Scholar]
- 48.Santodomingo-Rubido J., Villa-Collar C., Gilmartin B., Gutiérrez-Ortega R., Suzaki A. Short- and long-term changes in corneal aberrations and axial length induced by orthokeratology in children are not correlated. Eye Contact Lens. 2017;43(6):358–363. doi: 10.1097/ICL.0000000000000290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.