Summary
We disclose the Cu-catalyzed enantioselective synthesis of 3-methyl-3-propargyl-indolines, which contain a quaternary stereogenic carbon center, via the decarboxylative [4 + 1] annulation of 4-methyl-4-propargyl-benzoxazinanones with variety of sulfur ylides. The reaction proceeds predominantly through a γ-attack at the Cu-allenylidene intermediates by sulfur ylides to provide the corresponding indolines in good yield and high enantioselectivity (up to 91% ee). In contrast, the reaction of 4-trifluoromethyl-4-propargyl-benzoxazinanones with sulfur ylides delivers 3-trifluoromethyl-2-functionalized indoles in good to high yield via an unexpected α-attack at the Cu-allenylidene intermediates. Control over the α/γ-attack at the Cu-allenylidene intermediates by the same interceptors was achieved for the first time by the use of trifluoromethyl substituents.
Subject Areas: Organic Chemistry, Organic Synthesis, Physical Organic Chemistry
Graphical Abstract

Highlights
-
•
Fluorine changes the catalytic decarboxylative annulation modes
-
•
All carbon quarternary stereocentered indolines, up to 91% ee
-
•
An unexpected α-attack at the Cu-allenylidene intermediate with CF3
-
•
3-CF3-substituted indoles with a 2-functional group
Organic Chemistry; Organic Synthesis; Physical Organic Chemistry
Introduction
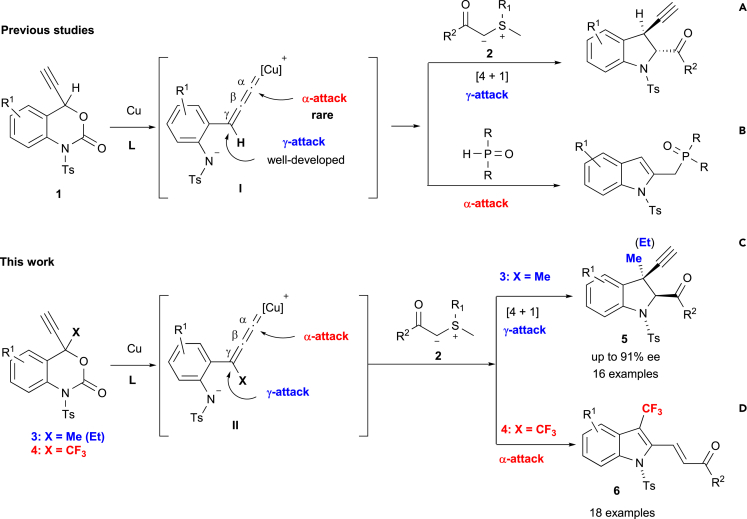
Transition-metal-catalyzed annulation reactions have been extensively investigated, especially in the context of constructing multiply functionalized nitrogen (N)-containing heterocycles (D'Souza and Muller, 2007, Gulevich et al., 2013, Nakamura and Yamamoto, 2004, Patil and Yamamoto, 2008, Qiao et al., 2019, Reen et al., 2019, Sole and Fernandez, 2018, Yamamoto, 2014). Indoles and indolines have received a significant amount of that attention, as these heterocycles represent privileged structural fragments in pharmaceuticals and natural products (Sundberg, 1996, Kochanowska-Karamyan and Hamann, 2010, Sharma et al., 2010, Zhang et al., 2011, Kaushik et al., 2013, Ishikura et al., 2015, Mo et al., 2015, Patil et al., 2016, Zeeli et al., 2018, Cacchi and Fabrizi, 2011, Li et al., 2014, Guo et al., 2015, Giorgio, 2017, Liang and Xia, 2017, Mancuso and Dalpozzo, 2018, Huang and Yin, 2019, Silva et al., 2019). Among the multitude of synthetic methods for the preparation of indoles and indolines, we were particularly interested in annulation reactions with 4-propargyl benzoxazinanones (1) (Wang et al., 2016, Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018c, Li et al., 2016, Li et al., 2017, Li et al., 2018, Song et al., 2017, Lu et al., 2017, Lu et al., 2018a, Lu et al., 2018b, Shao and You, 2017, Chen et al., 2018, Jiang et al., 2018, Zhang et al., 2018a, Zhang et al., 2019, Ji et al., 2018, Simlandy et al., 2019, Sun et al., 2019), which were first reported by Xiao, Lu, and co-workers in 2016 (Wang et al., 2016) and have since rapidly attracted attention as attractive reactants for the preparation of N-heterocycles via metal-catalyzed annulation reactions (Wang et al., 2016, Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018c, Li et al., 2016, Li et al., 2017, Li et al., 2018, Song et al., 2017, Lu et al., 2017, Lu et al., 2018a, Shao and You, 2017, Chen et al., 2018, Jiang et al., 2018, Zhang et al., 2018a, Zhang et al., 2019, Ji et al., 2018, Simlandy et al., 2019, Sun et al., 2019). Crucial for annulation reactions involving 1 is the decarboxylative generation of Cu-stabilized allenylidene zwitterionic intermediates (I), which can be trapped by suitable interceptors to construct various types of N-heterocycles. Accordingly, new types of annulation reactions can be easily developed by judiciously choosing the interceptors.
It should be noted that annulation reactions involving 1 may proceed via two different reaction modes as the Cu-allenylidenes of the type I contain two reactive electrophilic positions, i.e., α and γ relative to the Cu atom. For example, the decarboxylative [4 + 1] cycloaddition of 1 with sulfur ylides 2 provides enantio-enriched 3-propargyl indolines via the γ-addition (Wang et al., 2016, Wang et al., 2018a, Wang et al., 2018b, Li et al., 2016, Li et al., 2017, Li et al., 2018, Song et al., 2017, Lu et al., 2017, Shao and You, 2017, Chen et al., 2018, Jiang et al., 2018, Zhang et al., 2018a, Zhang et al., 2019, Ji et al., 2018, Simlandy et al., 2019, Sun et al., 2019) of I (Scheme 1A) (Wang et al., 2016). Such a α-addition at I has been reported for the use of phosphonates as interceptors, which exclusively provides 2-phosphorylmethyl indoles (Scheme 1B) (Wang et al., 2018c). Although the α/γ chemo-selectivity at I can be controlled by the interceptors (nucleophiles) as mentioned above, most of these induce γ-addition reactions (Wang et al., 2016, Wang et al., 2018a, Wang et al., 2018b, Li et al., 2016, Li et al., 2017, Li et al., 2018, Song et al., 2017, Lu et al., 2017, Shao and You, 2017, Chen et al., 2018, Jiang et al., 2018, Zhang et al., 2018a, Zhang et al., 2019, Ji et al., 2018, Simlandy et al., 2019, Sun et al., 2019), whereas the α-addition-mode is very rare (Wang et al., 2018c). In other words, controlling the α/γ chemoselectivity at Cu-allenylidene zwitterionic intermediates of the type I to induce the α-addition mode remains highly challenging.
Scheme 1.
Decarboxylative Annulations of 4-Substituted Benzoxazinanones via Cu-Allenylidene Intermediates
(A) and (B): Previous studies.
(C) and (D): Present work.
Herein, we disclose the first successful attempt to control the α/γ chemo-selectivity at Cu-allenylidene zwitterionic intermediates via a fluorine effect. Specifically, the Cu-catalyzed decarboxylative annulation of non-fluorinated 4-methyl (Me)-4-propargylic benzoxazinanones 3 with sulfur yields 2 furnished chiral non-racemic 3-Me-3-propargyl-indolines 5 in a γ-selective fashion in good to high yield with high enantioselectivity (up to 91% ee; Scheme 1C). As examples of the generation of all-carbon quaternary stereocenters at the propargylic position are rare (Tsuchida et al., 2016, Sanz-Marco et al., 2016, Shemet and Carreira, 2017, Wendlandt et al., 2018, Zhang et al., 2018a, Li et al., 2019, Xu and Hu, 2019), the obtained results might help to activate the corresponding area of research. On the other hand, the α-selective addition was predominantly observed for the Cu-catalyzed decarboxylative annulation of fluorinated variants such as 4-trifluoromethyl (CF3)-4-propargylic benzoxazinanones 4 with 2, which led to the formation of 3-CF3-2-functionalized indoles 6 in good to high yield with high E/Z-selectively via a rare α-attack at the Cu-allenylidene zwitterionic intermediates (Scheme 1D). Given that CF3-containing N-heterocycles have gained considerable attention in academic and industrial research on pharmaceutics and agrochemicals (Kawai and Shibata, 2014, Engl et al., 2015, Huang et al., 2015, Meyer, 2016, He et al., 2019), CF3-substituted indoles 6 that contain 2-functional groups should represent versatile building blocks for the preparation of drug candidates. To the best of our knowledge, this is the first example of controlling the α/γ chemoselectivity at Cu-allenylidene zwitterionic intermediates that does not depend on the interceptor.
Results and Discussion
Optimization
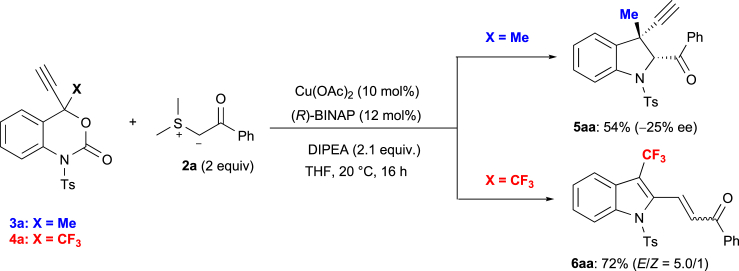
Recently, we reported the Pd-catalyzed decarboxylation of 4-trifluoromethyl benzoxazinanones (Punna et al., 2018, Punna et al., 2019, Das et al., 2018) with sulfur ylides 2 to provide 3-CF3-substituted indolines with high diastereoselectivity (Punna et al., 2018). Stimulated by the seminal work of Xiao, Lu, and co-workers (Scheme 1A) (Wang et al., 2016), we were interested in the enantioselective formation of previously unknown 3-propargyl indolines with an all-carbon quaternary stereogenic center such as 5 by the reaction of 4-tetrasubstituted propargyl benzoxazinanones (3, 4) with sulfur ylides 2 via a catalytic decarboxylative [4 + 1] cycloaddition. To our great surprise, the targeted 3-Me-3-propargyl-indoline 5aa was obtained in 54% yield with 25% ee when we treated 4-Me-4-propargyl benzoxazinanone 3a with benzoyl sulfur ylide 2a and i-Pr2NEt (DIPEA, 2.1 equiv.) in the presence of a catalytic amount of Cu(OAc)2 and (R)-BINAP in THF. However, when we used 4-CF3-4-propargyl benzoxazinanone 4a instead of 3a under otherwise identical conditions, we unexpectedly obtained 3-CF3-2-substituted indole 6aa in 72% with a 5/1 E/Z selectively (Scheme 2).
Scheme 2.
Two Reaction Modes for the Decarboxylative Annulation of 4-Substituted 4-Propargyl-Benzoxazinanones (3, 4) with Sulfur Ylides 2a under Cu Catalysis Conditions
Encouraged by these unprecedented preliminary results, we initially studied the enantioselective [4 + 1] cycloaddition reaction of 4-Me-propargyl benzoxazinanone 3a with sulfur ylide 2a (Scheme 3, Table 1). First, the effect of (R)-BINAP on this transformation was examined at room temperature under a variety of conditions (entries 1–4). However, the enantioselectivity of 5aa was only moderate (up to 44%; entry 2). Subsequently, we focused on the use of Pybox ligands for the improvement of the enantioselectivity in this transformation. After a careful evaluation of chiral ligands, Lewis acids, solvents, and substituents on sulfur ylides 2a (2a′) (entries 5–16; Tables S1–S7), we found that the commercially available iso-propyl-substituted Pybox ligand L3 exhibited the best performance, producing chiral indoline 5aa in 72% yield with 74% ee (entry 10). More details of the screening of other ligands such as L5 and L6 are shown in the Supplemental Information (Table S1). An investigation into the solvent effect (Table S3) revealed that dichloromethane (DCM) provided the best reaction efficiency with a slightly lower yield and improved enantiocontrol (entry 12, 69% yield, 78% ee). An evaluation of different bases showed that N-ethyl morpholine was superior to other bases (entry 13, 84% yield, 82% ee). Gratifyingly, a more favorable outcome (85% ee) was observed without a significant decrease in yield when the reaction was carried out with 1.5 equiv. of 2a’ (entry 15, 83% yield, 85% ee). In all these cases, >95:5 diastereoselectivity was confirmed by a 1H NMR analysis of the crude reaction mixture. While the amount of N-ethylmorpholine can be reduced to a catalytic amount, the corresponding yield decreased slightly (79% yield, 85% ee, entry 16). The absolute configuration of 5aa, induced by L3, was determined to be 2(S) and 3(R) by a single-crystal X-ray diffraction analysis (CCDC1971179). The 2(S), 3(R)-stereochemistry of 5aa is a surprise, as we expected the configuration of 5aa to be 2(R),3(R) or 2(S),3(S) based on a previous report (Scheme 1A) (Wang et al., 2016). Ts group on 3a is important since the reaction of Boc-protected variant of 3a with 2a′ under the same conditions resulted in a complex mixture.
Scheme 3.
Optimization of the Reaction Conditions for the Cu-Catalyzed [4 + 1] Cycloaddition of 3a with 2a
Table 1.
Optimization of the Reaction Conditions for the Cu-Catalyzed [4 + 1] Cycloaddition of 3a with 2a
| Entry | Ligand | R (2a or 2a′) | Cu | Solvent | Yield (%)a | ee (%)b |
|---|---|---|---|---|---|---|
| 1c | (R)-BINAP | Me (2a) | Cu(OAc)2 | THF | 54 | −25 |
| 2 | (R)-BINAP | Me (2a) | Cu(OAc)2 | THF | 55 | −44 |
| 3 | (R)-BINAP | p-tolyl (2a′) | Cu(OAc)2 | THF | 49 | −32 |
| 4 | (R)-BINAP | p-tolyl (2a′) | Cu(OTf)2 | THF | 31 | 0 |
| 5 | L1 | Me (2a) | Cu(OAc)2 | THF | 59 | −38 |
| 6 | L1 | Me (2a) | Cu(OTf)2 | THF | 52 | 42 |
| 7 | L1 | p-tolyl (2a′) | Cu(OAc)2 | THF | 49 | 0 |
| 8 | L1 | p-tolyl (2a′) | Cu(OTf)2 | THF | 30 | 42 |
| 9 | L2 | p-tolyl (2a′) | Cu(OTf)2 | THF | 50 | 56 |
| 10 | L3 | p-tolyl (2a′) | Cu(OTf)2 | THF | 72 | 74 |
| 11 | L4 | p-tolyl (2a′) | Cu(OTf)2 | THF | 63 | −46 |
| 12 | L3 | p-tolyl (2a′) | Cu(OTf)2 | DCM | 69 | 78 |
| 13d | L3 | p-tolyl (2a′) | Cu(OTf)2 | DCM | 84 | 82 |
| 14d | L3 | Me (2a) | Cu(OTf)2 | DCM | 79 | 63 |
| 15d,e | L3 | p-tolyl (2a′) | Cu(OTf)2 | DCM | 83 | 85 |
| 16d,e,f | L3 | p-tolyl (2a′) | Cu(OTf)2 | DCM | 79 | 85 |
Determined by a 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethoxybenzene as the internal standard.
Determined by a chiral HPLC analysis.
Using i-Pr2NEt (2.1 equiv.).
Using N-ethylmorpholine.
Using 2a’ (0.15 mmol).
Using 0.015 mmol of N-ethylmorpholine.
Substrate Scope and Synthetic Application I
With the optimal reaction conditions for the enantioselective formation of 5 in hand (Table 1, entry 15), the scope of this reaction with respect to the sulfur ylides was examined by treating 4-Me-4-propargyl benzoxazinanone 3a with 2b′–2i’ (Scheme 4). All ylide derivatives 2′ were well tolerated under the applied reaction conditions and delivered the desired products (5ab–5ai) in moderate to good yield (≤82%) with decent enantioselectivity (62%–80% ee). Substrates bearing electron-withdrawing groups such as 4-NO2 (2d′) or 4-CF3 (2f′) afforded the desired products in good yield with moderate enantioselectivity (5ad: 66%, 78% ee; 5af: 82%, 74% ee). Furthermore, both electron-donating and -withdrawing substituents are tolerated in this reaction and exert only a minimal effect on the enantioselectivity (74%–79% ee). Particularly, heteroaromatic sulfur ylide 2h′ also smoothly produces the desired product in high yield (5ah, 80%) with a good enantioselectivity (80% ee). Cyclohexyl-substituted sulfur ylide 2i′ also delivers the corresponding product (5ai) in decent yield (68%) with moderate enantioselectivity (62% ee). Next, we examined the substrate scope with respect to the 4-Me-4-propargyl benzoxazinanones by treating 3a–3f with sulfur ylide 2a’ (Scheme 4). The introduction of the substituent at different positions of the benzoxazinanone moiety resulted in higher levels of enantioselectivity (77%–86% ee). The variation of the substituent pattern exerts a subtle impact on the selectivity. For instance, substrates bearing halogen substituents such as 7-F (3b), 6-Cl (3d), or 6-Br (3f) smoothly furnish the desired products (5b, 5d, and 5f) in moderate to good yield (60%–82%) with good enantioselectivity, albeit that the product yield is lower for 6-Br substitution than for 6-Cl substitution. A substrate bearing an electron-withdrawing group (3c: 7-CF3) delivered the corresponding product in good yield with good enantioselectivity (5ca: 83%, 77% ee). Furthermore, a benzoxazinanone with an electron-donating group (3e: 7-Me) yielded the desired product in good yield with high enantioselectivity (5ea: 74%, 82% ee). To understand the effect of the 4-Me substitution of 3 on this transformation, we carried out the same reactions using 4-ethyl (Et)-4-propargyl benzoxazinanone 3g instead of 4-Me-substituted 3a. To our satisfaction, the reaction of 3g with sulfur ylides 2a′ and 2g′ under standard conditions resulted in the formation of the desired products in acceptable yield with excellent enantioselectivity (5ga: 46%, 91% ee; 5gg: 42%, 91% ee). The increased steric demand at the propargylic position (Me→Et) presumably improves the enantioselectivity under concomitant decrease of the reactivity.
Scheme 4.
Substrate Scope for 4-Propargyl Benzoxazinanones 3a-3g and Sulfur Ylides 2a′-2i′ for the Formation of 5aa-5gg via a Decarboxylative [4 + 1] Cycloaddition
Experiments were carried out using 3 (0.1 mmol), 2' (0.15 mmol), Cu(OTf)2 (10 mol %), L3 (12 mol %), and N-ethyl morpholine (0.12 mmol) in dry DCM (1.0 mL). Isolated yields are shown together with 1H NMR yields (in parenthesis; using 1,3,5-trimethoxybenzene as the internal standard). In all cases, the diastereomeric ratio of the products 5 was >95:5.
The ee values were determined based on a chiral HPLC analysis.
To demonstrate the synthetic utility of the 3-propargyl indoline products 5, we carried out two subsequent transformations (Scheme 5). Optically active indoline 5aa was smoothly converted into triazole 7 via a 1,3-dipolar cycloaddition with tosyl azide in the presence of CuTc. As expected, 7 was formed in 99% yield without any loss of enantiopurity (85% ee). Furthermore, a Sonogashira coupling of 5aa with iodobenzene afforded the disubstituted alkyne 8 in 70% yield under retention of its enantiopurity.
Scheme 5.
Derivatization of 5; Transformations of 5aa to 7 and 8
Optimization, Substrate Scope, and Synthetic Application II
Next, we focused our attention on the unexpected annulation observed for the reaction between 4-CF3-4-propargyl-benzoxazinanone 4a and 2a. As mentioned in Scheme 2, the formation of, e.g., 5a, i.e., the product of a γ-attack on the indoline, was not observed, and 2-functionalized indole 6aa was obtained instead. After an extensive screening of combinations of copper catalysts, ligands, bases, and solvents (Tables S8 and S9), we identified the optimal conditions as: dimethyl-sulfur ylide 2, Cu(OAc)2 (10 mol%), rac-BINAP (12 mol%), and i-Pr2NEt (1.6 equiv.) in DCM at rt. Ts group on 4a is again important since the reaction of Boc-protected variant of 4a with 2a under the same conditions resulted in no reaction. The substrate scope for the reaction between CF3-propargyl benzoxazinanones 4 and sulfur ylides 2 for the formation of 6 is shown in Scheme 6. A variety of substituted sulfur ylides 2 are suitable for this transformation and smoothly produce the corresponding 3-CF3-indole products 6. Sulfur ylides with either electron-donating groups (2b: 4-OMe; 2c: 4-Me) or a -withdrawing group (2d: 4-NO2) furnish the corresponding 3-CF3-indoles in good yield (6ab, 79%; 6ac, 73%; 6ad, 70%) with a good E/Z ratio (≥5.3:1). Heteroaromatic sulfur ylide 2h also smoothly produces the desired product in high yield (6ah, 80%) with a good E/Z ratio (6.9:1). Notably, cyclohexyl-substituted sulfur ylide 2i also delivers the corresponding product (6ai) in moderate yield (61%). Remarkably, sterically demanding t-Bu ester sulfur ylide 2j also provided corresponding product (6aj) with acceptable yield (44%) and E/Z ratio (1.4:1). Furthermore, we examined the reaction scope with respect to 4-CF3-4-propargyl benzoxazinanones 4 under the aforementioned reaction conditions. Substrates with electron-withdrawing groups on the benzene ring, such as 7-CF3 (4c) or 6-Cl (4d) efficiently produced the desired products in moderate yield (6ca: 58%; 6da: 60%) with a low E/Z ratio (≤2.1:1). When 6,7-di-OMe-substituted benzoxazinanone 4h was treated with sulfur ylides 2a or 2b, the corresponding products were obtained in good yield (6ha: 67%; 6hb: 60%) with an improved E/Z ratio (≥5.3:1). In addition, the reaction of 6-F-substituted 4b with sulfur ylides 2c, 2d, 2g, and 2h provided the desired products in moderate to good yield and E/Z ratio (6bc: 60%; 6bd: 60%; 6bg: 70%; 6bh: 80%). It should be noted here that the introduction of a reactive ester moiety at the 7-position of benzoxazinanone also yielded the desired products in acceptable yield (6ga: 40%; 6gd: 45%) with a moderate E/Z ratio. We further carried out a reaction of 4a with 2a on the gram scale using the optimal reaction conditions, which afforded 6aa in 73% yield. The configuration of the major isomer (E) was determined based on an X-ray diffraction analysis of single crystals of 6aa (CCDC1971178, Scheme 6). The configuration of the other indole products was accomplished by comparison.
Scheme 6.
Substrate Scope with Respect to CF3-Propargyl Benzoxazinanones 4a-4h and Sulfur Ylides 2a-2j for the Formation of 6aa-6hb via a Decarboxylative Annulation
Gram scale reaction using 4a (1.185 g, 3.0 mmol) was performed.
The E/Z ratio was determined by 19F NMR spectroscopy on the isolated products (in parenthesis).
Experiments were carried out using 4 (0.1 mmol), 2 (0.2 mmol), Cu(OAc)2 (10 mol %), rac-BINAP (12 mol %), and i-Pr2NEt (0.16 mmol) in dry DCM (2.0 mL).
While the 3-CF3-2-functionalized indoles were obtained as a mixture of E/Z isomers, the isomerization to the E isomer proceeded smoothly upon treatment of, e.g., 6aa with iodine under irradiation with blue light (96% yield; Scheme 7A). Moreover, we performed a couple of transformations of 6aa to demonstrate the utility of the functionalized CF3-indoles 6 (Scheme 7B). First, the cyclopropanation of (E)-6aa via a Corey-Chaykovsky reaction furnished cyclopropane 9 in 68% yield. A 1,2-selective trifluoromethylation of (E)-6aa with CF3-SiMe3 in the presence of a catalytic amount of tetramethylammonium fluoride (TMAF) provided trifluoromethyl-carbinol derivative 10 in 97% yield. Pd–C catalytic hydrogenation of (E)-6aa provided indole ketone 11 in 87% yield.
Scheme 7.
Transformations of 6aa
(A) Photolytic isomerization of the E/Z isomers of 6aa into predominantly the E isomer.
(B) Cyclopropanation of (E)-6aa; 1,2-chemoselective addition of CF3SiMe3; hydrogenation of (E)-6aa.
Furthermore, we examined the reaction conditions to generate the indole product 6 with major E isomer. As mentioned in Scheme 8, the formation of the indole product 6 (standard reaction condition) and E/Z isomerization were achieved in concerted manner (Scheme 8).
Scheme 8.
Single Step Formation of 6aa-6ai into Predominantly the E Isomer
Proposed Reaction Mechanisms
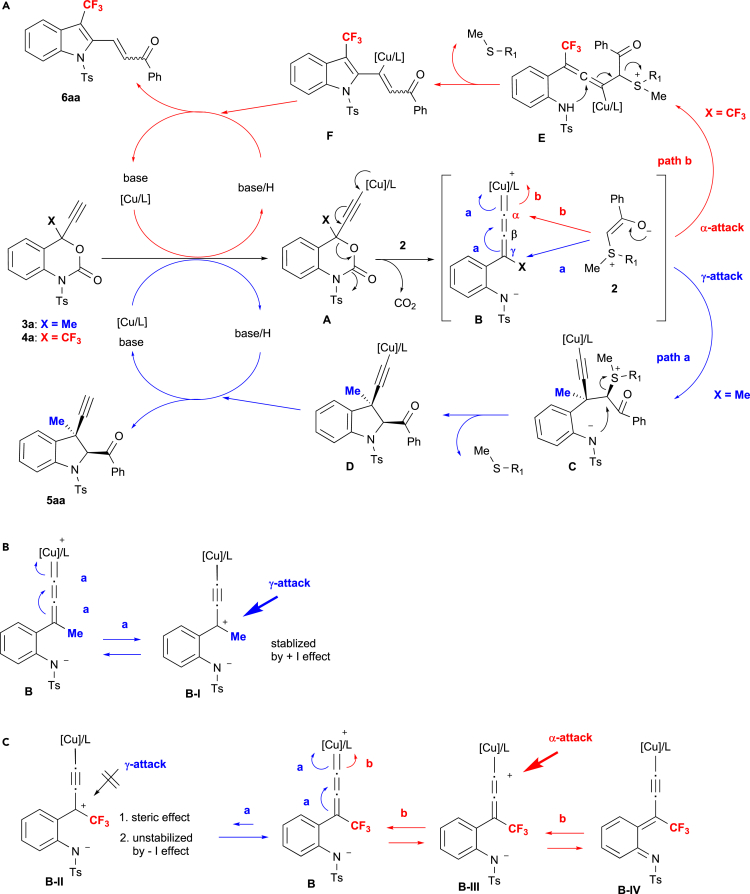
Based on the observed experimental results and previous reports (Wang et al., 2016, Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018c, Li et al., 2016, Li et al., 2017, Li et al., 2018, Song et al., 2017, Lu et al., 2017, Lu et al., 2018a, Shao and You, 2017, Chen et al., 2018, Jiang et al., 2018, Zhang et al., 2018a, Zhang et al., 2018b, Zhang et al., 2019, Ji et al., 2018, Simlandy et al., 2019, Sun et al., 2019), we would like to propose a feasible mechanism to rationalize the chemo/stereoselective formation of indolines/indoles from 4-substituted 4-propargyl benzoxazinanones (3, 4) with sulfur ylides 2 (2′) (Figure 1A). As described in Figure 1A, the Cu complex initially activates the propargyl benzoxazinanone (3a or 4a) in the presence of a base to generate Cu−acetylide A. Then, the Cu-allenylidene zwitterionic intermediate B, which is stabilized by its resonance form, is generated via an extrusion of CO2. Depending on the substitution pattern at the propargylic position of the Cu-stabilized allenylidene zwitterionic intermediate B, the sulfur ylide 2 attacks at the γ- (X = Me) or α-position (X = CF3). The Me-substitution at the propargylic position of transient species B allows sulfur ylide 2a to attack at the γ-position (propargylic position) to generate intermediate C, which further converts into copper-containing cycloadduct D via an intramolecular SN2 reaction. Finally, 3-Me-3-propargyl indoline 5aa is produced through a proton transfer under concomitant regeneration of the copper catalyst to close the catalytic cycle. The 2,3-cis-selectivity of alkyne and benzoyl groups in 5aa could be explained by the bulkiness of 4-methyl group (Csp3 group) rather than 4-alkynyl moiety (Csp group). On the other hand, in the unprecedented catalytic reaction of 4-trifluoromethyl 4-propargyl benzoxazinanone 4a with sulfur ylide 2a, the α-addition of sulfur ylide 2a to transient species B should afford intermediate E. Finally, 6aa is furnished through the subsequent intramolecular addition/sulfide elimination from E, followed by protolysis of intermediate F under regeneration of the Cu catalyst in the final stage.
Figure 1.
Feasible Reaction Mechanism
(A) Two modes of the reaction mechanism are proposed for the catalytic decarboxylative annulation via Cu-allenylidene intermediates B.
(B) Stabilization of the γ-cation of Cu-allenylidene B-I by the Me group.
(C) Destabilization of the γ-cation by the CF3 group and steric blocking of the nucleophiles in B-II, whereas α-vinyl cation intermediate B-III might be stabilized by the resonance induced by the CF3 group.
Although the reasons for the noticeable α/γ-selectivity depend on the 4-substitution in 4-propargyl benzoxazinanones 3 (Me) and 4 (CF3) remain obscure at present, the α/γ-selectivity could potentially be rationalized in terms of stabilization and steric effects of the reactive intermediates. Specifically, the Cu-stabilized allenylidene zwitterionic intermediate B, which contains a Me group, has a resonance structure B-I, in which the carbocation is stabilized by the positive inductive (+I) effect of the Me group. Thus, nucleophilic 2 approaches the γ-position of Cu-allenylidene intermediate B (Figure 1B). In the case of 4a, however, the similar intermediate carbocation B-II, generated from the Cu-stabilized allenylidene zwitterionic intermediate B with a CF3 group, is not stabilized by the strong electron-withdrawing effect of the CF3 group, whereas the vinyl cation in intermediate B-III is stabilized by the additional resonance structure B-IV induced by the electron-withdrawing effect of the CF3 substituent. Moreover, the γ-attack should also be unfavorable owing to the steric demand of the bulky CF3 group. All of the aforementioned aspects should favor the unprecedented α-attack (Figure 1C).
Conclusion
In conclusion, we have constructed optically active indolines 5, which contain an all-carbon quaternary stereocenter, in good yield with high enantioselectivity from the decarboxylative [4 + 1] annulation of Me-propargyl benzoxazinanones 3 and sulfur ylides 2. Irrespective of the substituents on 3 and 2, the reaction yielded the corresponding indoline derivatives 5 with excellent enantioselectivity (up to 91% ee) via a γ-attack on a Cu-allenylidene zwitterionic intermediate. Interestingly, the reaction between CF3-propargyl benzoxazinanones 4 and 2 delivered indole derivatives 6 in good yield via an unprecedented α-attack on the Cu-allenylidene zwitterionic intermediate. In their entirety, these results represent the first example of controlling two modes (α- versus γ-attack) of decarboxylative annulation of propargyl benzoxazinanones via Cu-allenylidenes with the same interceptors. With respect to the importance for research in the area of N-containing heterocycles, enantio-enriched indolines with all-carbon quaternary propargyl stereogenic center and CF3-substituted indoles with a 2-functional group are both extremely useful precursors in medicinal chemistry. Further investigations into unique reaction patterns that are dominated by fluorine-containing groups and non-fluorinated groups are currently in progress in our laboratories.
Limitations of the Study
The N-tosyl group of 4-propargyl benzoxazinanones (3, 4) is crucial for this two-mode of transformations, and the N-Boc-protected variants of them under the same conditions resulted in complex mixtures. Other 4-substituted benzoxazinanones such as 4-isopropyl (3h) and 4-phenyl (3i) analogs (Figure 2) were unsuccessful in generating desired annulation products. The reactions using 4-isopropyl (3h) and 4-phenyl (3i) variants gave very different products. The preliminary results were shown in Supplemental Information (Figure S1), and further extension is under consideration.
Figure 2.

Other 4-Substituted Benzoxazinanones, 4-Isopropyl (3h) and 4-Phenyl (3i) Analogues
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by JSPS KAKENHI grants JP 18H02553 (KIBAN B) and JP 18H04401 (Middle Molecular Strategy). We thank Hiroto Uno for the analysis of X-ray diffraction data.
Author Contributions
N.S. conceived the concept of this study. M.R.G. and J.Z. optimized the reaction conditions and surveyed the substrate scope. M.R.G., J.Z., and B.J. prepared the starting materials. N.S. directed the project. N.S. and M.R.G. prepared the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100994.
Data and Code Availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession numbers CCDC 1971179 (5aa) and of CCDC1971178 (6aa). Copies of the data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/.
Supplemental Information
References
- Cacchi S., Fabrizi G. Update 1 of: synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2011;111:PR215–PR283. doi: 10.1021/cr100403z. [DOI] [PubMed] [Google Scholar]
- Chen H., Lu X., Xia X., Zhu Q., Song Y., Chen J., Cao W., Wu X. Asymmetric catalytic [4 + 2] cycloaddition via Cu–allenylidene intermediate: stereoselective synthesis of tetrahydroquinolines fused with a γ-lactone moiety. Org. Lett. 2018;20:1760–1763. doi: 10.1021/acs.orglett.8b00253. [DOI] [PubMed] [Google Scholar]
- Das P., Gondo S., Punna N., Uno H., Tokunaga E., Shibata N. Access to benzo-fused nine-membered heterocyclic alkenes with a trifluoromethyl carbinol moiety via a double decarboxylative formal ring-expansion process under palladium catalysis. Chem. Sci. 2018;9:3276–3281. doi: 10.1039/c7sc05447e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza D.M., Muller T.J. Multi-component syntheses of heterocycles by transition-metal catalysis. Chem. Soc. Rev. 2007;36:1095–1108. doi: 10.1039/b608235c. [DOI] [PubMed] [Google Scholar]
- Engl P.S., Senn R., Otth E., Togni A. Synthesis and characterization of N-trifluoromethyl N-heterocyclic carbene ligands and their complexes. Organometallics. 2015;34:1384–1395. [Google Scholar]
- Giorgio C. Metal-catalyzed dehydrogenative synthesis of pyrroles and indoles from alcohols. Coord. Chem. Rev. 2017;331:37–53. [Google Scholar]
- Gulevich A.V., Dudnik A.S., Chernyak N., Gevorgyan V. Transition metal-mediated synthesis of monocyclic aromatic heterocycles. Chem. Rev. 2013;113:3084–3213. doi: 10.1021/cr300333u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Huang F., Yu L., Yu Z. Indole synthesis through transition metal-catalyzed C–H activation. Tetrahedron Lett. 2015;56:296–302. [Google Scholar]
- He X.H., Ji Y.L., Peng C., Han B. Organocatalytic asymmetric synthesis of cyclic compounds bearing a trifluoromethylated stereogenic center: recent developments. Adv. Synth. Catal. 2019;361:1923–1957. [Google Scholar]
- Huang G., Yin B. Recent developments in transition metal-catalyzed dearomative cyclizations of indoles as dipolarophiles for the construction of indolines. Adv. Synth. Catal. 2019;361:405–425. [Google Scholar]
- Huang Y.Y., Yang X., Chen Z., Verpoort F., Shibata N. Catalytic asymmetric synthesis of enantioenriched heterocycles bearing a C-CF3 stereogenic center. Chem. Eur. J. 2015;21:8664–8684. doi: 10.1002/chem.201500361. [DOI] [PubMed] [Google Scholar]
- Ishikura M., Abe T., Choshi T., Hibino S. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2015;32:1389–1471. doi: 10.1039/c5np00032g. [DOI] [PubMed] [Google Scholar]
- Ji D., Wang C., Sun J. Asymmetric [4 + 2]-cycloaddition of copper-allenylidenes with hexahydro-1,3,5-triazines: access to chiral tetrahydroquinazolines. Org. Lett. 2018;20:3710–3713. doi: 10.1021/acs.orglett.8b01584. [DOI] [PubMed] [Google Scholar]
- Jiang F., Feng X., Wang R., Gao X., Jia H., Xiao Y., Zhang C., Guo H. Asymmetric [3 + 3] annulation of copper–allenylidenes with pyrazolones: synthesis of chiral 1,4-dihydropyrano[2,3-c]pyrazoles. Org. Lett. 2018;20:5278–5281. doi: 10.1021/acs.orglett.8b02214. [DOI] [PubMed] [Google Scholar]
- Kaushik N.K., Kaushik N., Attri P., Kumar N., Kim C.H., Verma A.K., Choi E.H. Biomedical importance of indoles. Molecules. 2013;18:6620–6662. doi: 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H., Shibata N. Asymmetric synthesis of agrochemically attractive trifluoromethylated dihydroazoles and related compounds under organocatalysis. Chem. Rec. 2014;14:1024–1040. doi: 10.1002/tcr.201402023. [DOI] [PubMed] [Google Scholar]
- Kochanowska-Karamyan A.J., Hamann M.T. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010;110:4489–4497. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.R., Tan F., Lu L.Q., Wei Y., Wang Y.N., Liu Y.Y., Yang Q.Q., Chen J.R., Shi D.Q., Xiao W.J. Asymmetric trapping of zwitterionic intermediates by sulphur ylides in a palladium-catalysed decarboxylation-cycloaddition sequence. Nat. Commun. 2014;5:5500. doi: 10.1038/ncomms6500. [DOI] [PubMed] [Google Scholar]
- Li T.R., Cheng B.Y., Wang Y.N., Zhang M.M., Lu L.Q., Xiao W.J. A copper-catalyzed decarboxylative amination/hydroamination sequence: switchable synthesis of functionalized indoles. Angew. Chem. Int. Ed. 2016;55:12422–12426. doi: 10.1002/anie.201605900. [DOI] [PubMed] [Google Scholar]
- Li T.R., Lu L.Q., Wang Y.N., Wang B.C., Xiao W.J. Divergent synthesis of polycyclic indolines: copper-catalyzed cascade reactions of propargylic carbamates and indoles. Org. Lett. 2017;19:4098–4101. doi: 10.1021/acs.orglett.7b01903. [DOI] [PubMed] [Google Scholar]
- Li T.R., Zhang M.M., Wang B.C., Lu L.Q., Xiao W.J. Synthesis of 3,3′-biindoles through a copper-catalyzed friedel–crafts propargylation/hydroamination/aromatization sequence. Org. Lett. 2018;20:3237–3240. doi: 10.1021/acs.orglett.8b01100. [DOI] [PubMed] [Google Scholar]
- Li X., Han C., Huang Y., Yao H., Lin A. Copper-catalyzed [3 + 2] annulation of ethynyl epoxides with malononitrile to access highly substituted dihydrofurans with an all-carbon quaternary stereocenter. Org. Chem. Front. 2019;6:245–248. [Google Scholar]
- Liang K., Xia C. Recent advances of transition metal-mediated oxidative radical reactions in total synthesis of indole alkaloids. Chin. J. Chem. 2017;35:255–270. [Google Scholar]
- Lu X., Ge L., Cheng C., Chen J., Cao W., Wu X. Enantioselective cascade reaction for synthesis of quinolinones through synergistic catalysis using Cu-pybox and chiral benzotetramisole as catalysts. Chem. Eur. J. 2017;23:7689–7693. doi: 10.1002/chem.201701741. [DOI] [PubMed] [Google Scholar]
- Lu Q., Cembellin S., Gressies S., Singha S., Daniliuc C.G., Glorius F. Manganese(I)-catalyzed C-H (2-indolyl)methylation: expedient access to diheteroarylmethanes. Angew. Chem. Int. Ed. 2018;57:1399–1403. doi: 10.1002/anie.201710060. [DOI] [PubMed] [Google Scholar]
- Lu S., Ong J.Y., Poh S.B., Tsang T., Zhao Y. Transition-metal-free decarboxylative propargylic substitution/cyclization with either azolium enolates or acyl anions. Angew. Chem. Int. Ed. 2018;57:5714–5719. doi: 10.1002/anie.201801340. [DOI] [PubMed] [Google Scholar]
- Mancuso R., Dalpozzo R. Recent progress in the transition metal catalyzed synthesis of indoles. Catalysts. 2018;8:458. [Google Scholar]
- Meyer F. Trifluoromethyl nitrogen heterocycles: synthetic aspects and potential biological targets. Chem. Commun. 2016;52:3077–3094. doi: 10.1039/c5cc09414c. [DOI] [PubMed] [Google Scholar]
- Mo Y., Zhao J., Chen W., Wang Q. Recent advance of the application of interrupted fischer indolization toward bioactive indoline alkaloids. Res. Chem. Intermed. 2015;41:5869–5877. [Google Scholar]
- Nakamura I., Yamamoto Y. Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem. Rev. 2004;104:2127–2198. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]
- Patil N.T., Yamamoto Y. Coinage metal-assisted synthesis of heterocycles. Chem. Rev. 2008;108:3395–3442. doi: 10.1021/cr050041j. [DOI] [PubMed] [Google Scholar]
- Patil R., Patil S.A., Beaman K.D., Patil S.A. Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents, an update (2013-2015) Future Med. Chem. 2016;8:1291–1316. doi: 10.4155/fmc-2016-0047. [DOI] [PubMed] [Google Scholar]
- Punna N., Das P., Gouverneur V., Shibata N. Highly diastereoselective synthesis of trifluoromethyl indolines by interceptive benzylic decarboxylative cycloaddition of nonvinyl, trifluoromethyl benzoxazinanones with sulfur ylides under palladium catalysis. Org. Lett. 2018;20:1526–1529. doi: 10.1021/acs.orglett.8b00237. [DOI] [PubMed] [Google Scholar]
- Punna N., Harada K., Zhou J., Shibata N. Pd-Catalyzed decarboxylative cyclization of trifluoromethyl vinyl benzoxazinanones with sulfur ylides: access to trifluoromethyl dihydroquinolines. Org. Lett. 2019;21:1515–1520. doi: 10.1021/acs.orglett.9b00330. [DOI] [PubMed] [Google Scholar]
- Qiao J., Jia X., Li P., Liu X., Zhao J., Zhou Y., Wang J., Liu H., Zhao F. Gold-catalyzed rapid construction of nitrogen-containing heterocyclic compound library with scaffold diversity and molecular complexity. Adv. Synth. Catal. 2019;361:1419–1440. [Google Scholar]
- Reen G.K., Kumar A., Sharma P. Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage. Beilstein J. Org. Chem. 2019;15:1612–1704. doi: 10.3762/bjoc.15.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Marco A., Blay G., Vila C., Pedro J.R. Catalytic enantioselective conjugate alkynylation of β-aryl-β-trifluoromethyl enones constructing propargylic all-carbon quaternary stereogenic centers. Org. Lett. 2016;18:3538–3541. doi: 10.1021/acs.orglett.6b01494. [DOI] [PubMed] [Google Scholar]
- Shao W., You S.L. Highly diastereo- and enantioselective synthesis of tetrahydro-5H-indolo[2,3-b]quinolines through copper-catalyzed propargylic dearomatization of indoles. Chem. Eur. J. 2017;23:12489–12493. doi: 10.1002/chem.201703443. [DOI] [PubMed] [Google Scholar]
- Sharma V., Kumar P., Pathak D. Biological importance of the indole nucleus in recent years: a comprehensive review. J. Heterocycl. Chem. 2010;47:491–502. [Google Scholar]
- Shemet A., Carreira E.M. Total synthesis of (−)-Rhazinilam and formal synthesis of (+)-Eburenine and (+)-Aspidospermidine: asymmetric Cu-catalyzed propargylic substitution. Org. Lett. 2017;19:5529–5532. doi: 10.1021/acs.orglett.7b02619. [DOI] [PubMed] [Google Scholar]
- Silva T.S., Rodrigues M.T., Santos H., Zeoly L.A., Almeida W.P., Barcelos R.C., Gomes R.C., Fernandes F.S., Coelho F. Recent advances in indoline synthesis. Tetrahedron. 2019;75:2063–2097. [Google Scholar]
- Simlandy A.K., Ghosh B., Mukherjee S. Enantioselective [4 + 2]-annulation of azlactones with copper-allenylidenes under cooperative catalysis: synthesis of α-quaternary α-acylaminoamides. Org. Lett. 2019;21:3361–3366. doi: 10.1021/acs.orglett.9b01103. [DOI] [PubMed] [Google Scholar]
- Sole D., Fernandez I. scienceDirect; 2018. Advances in Transition-Metal Mediated Heterocyclic Synthesis. [Google Scholar]
- Song J., Zhang Z.J., Gong L.Z. Asymmetric [4 + 2] annulation of c1 ammonium enolates with copper-allenylidenes. Angew. Chem. Int. Ed. 2017;56:5212–5216. doi: 10.1002/anie.201700105. [DOI] [PubMed] [Google Scholar]
- Sun B.B., Hu Q.X., Hu J.M., Yu J.Q., Jia J., Wang X.W. Asymmetric [4 + 2] cycloaddition of azlactones with dipolar copper–allenylidene intermediates for chiral 3,4-dhydroquinolin-2-one derivatives. Tetrahedron Lett. 2019;60:1967–1970. [Google Scholar]
- Sundberg R.J. Academic Press; 1996. Indoles. [Google Scholar]
- Tsuchida K., Senda Y., Nakajima K., Nishibayashi Y. Construction of chiral tri- and tetra-arylmethanes bearing quaternary carbon centers: copper-catalyzed enantioselective propargylation of indoles with propargylic esters. Angew. Chem. Int. Ed. 2016;55:9728–9732. doi: 10.1002/anie.201604182. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li T.R., Lu L.Q., Li M.M., Zhang K., Xiao W.J. Catalytic asymmetric [4 + 1] annulation of sulfur ylides with copper–allenylidene intermediates. J. Am. Chem. Soc. 2016;138:8360–8363. doi: 10.1021/jacs.6b04414. [DOI] [PubMed] [Google Scholar]
- Wang S., Liu M., Chen X., Wang H., Zhai H. Copper-catalyzed decarboxylative propargylation/hydroamination reactions: access to C3 β-ketoester-functionalized indoles. Chem. Commun. 2018;54:8375–8378. doi: 10.1039/c8cc04499f. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhu L., Wang M., Xiong J., Chen N., Feng X., Xu Z., Jiang X. Catalytic asymmetric [4 + 3] annulation of C,N-cyclic azomethine imines with copper allenylidenes. Org. Lett. 2018;20:6506–6510. doi: 10.1021/acs.orglett.8b02828. [DOI] [PubMed] [Google Scholar]
- Wang B.C., Wang Y.N., Zhang M.M., Xiao W.J., Lu L.Q. Copper-catalyzed decarboxylative cyclization via tandem C–P and C–N bond formation: access to 2-phosphorylmethyl indoles. Chem. Commun. 2018;54:3154–3157. doi: 10.1039/c8cc00739j. [DOI] [PubMed] [Google Scholar]
- Wendlandt A.E., Vangal P., Jacobsen E.N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature. 2018;556:447–451. doi: 10.1038/s41586-018-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.W., Hu X.P. Diastereo- and enantioselective copper-catalyzed decarboxylative ring-opening [3 + 2] annulation of tertiary propargylic carbamates through regioselective α-attack of γ-butenolides. Org. Lett. 2019;21:8091–8096. doi: 10.1021/acs.orglett.9b03081. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes. Chem. Soc. Rev. 2014;43:1575–1600. doi: 10.1039/c3cs60369e. [DOI] [PubMed] [Google Scholar]
- Zeeli S., Weill T., Finkin-Groner E., Bejar C., Melamed M., Furman S., Zhenin M., Nudelman A., Weinstock M. Synthesis and biological evaluation of derivatives of indoline as highly potent antioxidant and anti-inflammatory agents. J. Med. Chem. 2018;61:4004–4019. doi: 10.1021/acs.jmedchem.8b00001. [DOI] [PubMed] [Google Scholar]
- Zhang D., Song H., Qin Y. Total synthesis of indoline alkaloids: a cyclopropanation strategy. Acc. Chem. Res. 2011;44:447–457. doi: 10.1021/ar200004w. [DOI] [PubMed] [Google Scholar]
- Zhang Y.C., Zhang Z.J., Fan L.F., Song J. Enantioselective decarboxylative propargylation/hydroamination enabled by organo/metal cooperative catalysis. Org. Lett. 2018;20:2792–2795. doi: 10.1021/acs.orglett.8b01101. [DOI] [PubMed] [Google Scholar]
- Zhang Y.C., Zhang B.W., Geng R.L., Song J. Enantioselective [3 + 2] cycloaddition reaction of ethynylethylene carbonates with malononitrile enabled by organo/metal cooperative catalysis. Org. Lett. 2018;20:7907–7911. doi: 10.1021/acs.orglett.8b03454. [DOI] [PubMed] [Google Scholar]
- Zhang Z.J., Zhang L., Geng R.L., Song J., Chen X.H., Gong L.Z. N-Heterocyclic carbene/copper cooperative catalysis for the asymmetric synthesis of spirooxindoles. Angew. Chem. Int. Ed. 2019;58:12190–12194. doi: 10.1002/anie.201907188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession numbers CCDC 1971179 (5aa) and of CCDC1971178 (6aa). Copies of the data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/.