Abstract
Purpose
To describe a case of rapid keratitis and corneal perforation after epithelium off collagen cross-linking.
Observations
We report a case of a 17-year-old male who underwent collagen cross-linking with the protocol and device approved by the United States Food and Drug Administration (FDA) that developed a corneal infiltrate 3 days after the procedure. He later developed corneal thinning and perforation on day 5 requiring the use of cyanoacrylate glue and a Kontur lens. Despite initial improvement in the infiltrate with fortified antibiotics he later had leakage of aqueous around the glue and a flat chamber requiring an emergent penetrating keratoplasty on postoperative day 16.
Conclusion and importance
While collagen cross-linking has been very effective for treating keratoconus and is being recommended more frequently since FDA approval in the United States, severe complications such as corneal perforation requiring early transplant can still occur.
Keywords: Cross-linking, Cornea, Keratoconus, Perforation, Infectious keratitis
1. Introduction
Corneal ectasia is a disorder characterized by abnormal thinning and steepening of the cornea resulting in a decrease in vision due to irregular astigmatism and higher order aberrations. This disorder may be unilateral or bilateral – and occur spontaneously (i.e. keratoconus, pellucid marginal degeneration) or after having had refractive procedures.1,2 Generally this is seen in patients from late teenage years, up to the age of 30. The etiology for progression is unclear but may be linked to mechanical stress such as eye-rubbing, genetic factors, and chromosomal or enzymatic abnormalities.3
While there is no cure for corneal ectasia, in 1997 researchers at the University of Dresden introduced the concept of corneal collagen cross-linking (CXL) using ultraviolet (UV) light to induce collagen cross-linking in riboflavin soaked porcine and rabbit corneas.4 These corneas were found to be stiffer and more resistant to enzymatic digestions. By 2003 Dresden investigators began human protocols which were found to be promising, leading to the use of cross-linking outside the United States to treat progressive corneal ectasia.5
The United States Food and Drug Administration (FDA) approved Avedro's (Avedro, Inc, Waltham, MA) CXL system for treatment of patients with progressive keratoconus and post-refractive surgery ectasia in April 2016 using Photrexa Viscous (0.1% riboflavin ophthalmic solution/20% dextran) and Photrexa (0.1% riboflavin ophthalmic solution). The Phase III trials leading to this approval noted that adverse side effects included corneal opacity (haze), punctate keratitis, corneal striae, corneal epithelium defect, eye pain, reduced visual acuity, and blurred vision.6,7 We report a patient who underwent CXL using the FDA approved Avedro protocol that experienced rapidly progressive infiltration and necrosis of his cornea followed by perforation.
2. Case report
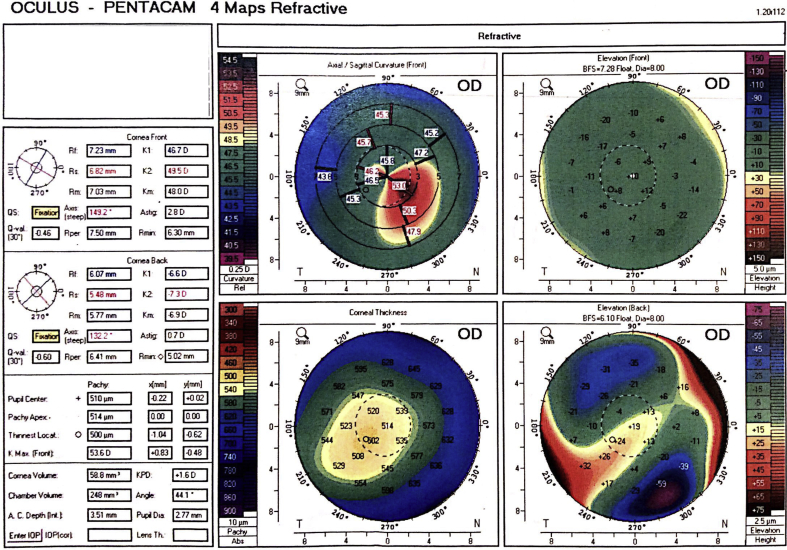
The patient was a 17-year-old male with a past medical history significant for childhood asthma and Scheuermann's disease that was referred for a rapid decline in vision in his right eye over the past year. His ophthalmologist noted that he was developing an increasing amount of irregular astigmatism and could not correct his vision with glasses. He denied being on any medications or eye drops but did have a prior history of using Accutane (isotretinoin) “months” before for acne. He could not elaborate on duration of use. Pentacam (Oculus GmbH, Wetzlar, Germany) scans were done which revealed significant posterior elevation of his right cornea consistent with keratoconus (Fig. 1). His right refraction was −4.50 + 3.00 × 139 with k's of 46.75 × 50.50 at 142 while his left cornea was −2.50 + 0.75 × 94 with k's of 46.25/47.25 at 94. His right cornea's thinnest point by ultrasound pachymetry was 502 μm.
Fig. 1.
Initial pentacam prior to cross-linking.
After informed consent with his family the patient elected to undergo corneal collagen cross-linking using the FDA approved epithelium off Avedro (Dresden protocol) given his progressive keratoconus of the right eye. After a 9 mm epithelial defect was created by an Amoils brush, 1 drop of Photrexa Viscous (0.1% riboflavin ophthalmic solution/20% dextran) was then applied every 2 minutes for 30 minutes topically to the cornea. The corneal thickness at this point was found to be greater than 400 μm so no additional administering of Photrexa Viscous (0.1% riboflavin ophthalmic solution/20% dextran) was needed. Following this, the patient underwent UVA irradiation at 365 mW at an intensity of 3 mW/cm2. Throughout UVA exposure, administration of the riboflavin/dextran solution was continued every 2 minutes. At the end of the case, 1 drop of Ofloxacin 0.3% and Predforte (prednisolone acetate 1%) was applied to the cornea. One drop of Diclofenac 0.1% was given over a bandage contact lens which was placed after the antibiotic and steroid drop was given. The patient was sent home on Ofloxacin 0.3% and Predforte four times a day.
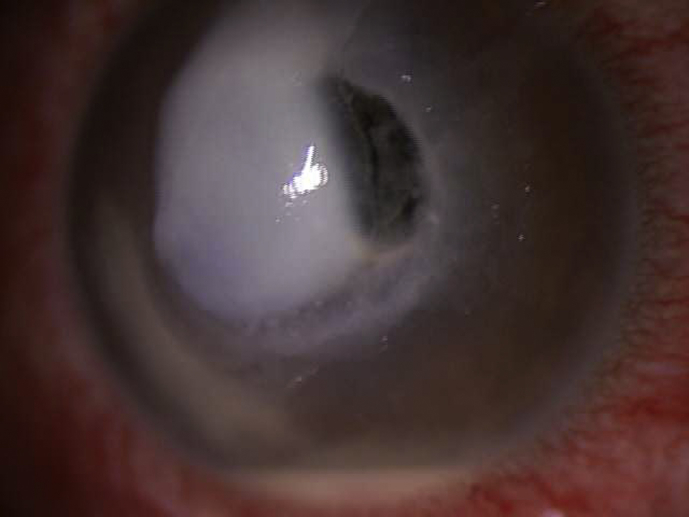
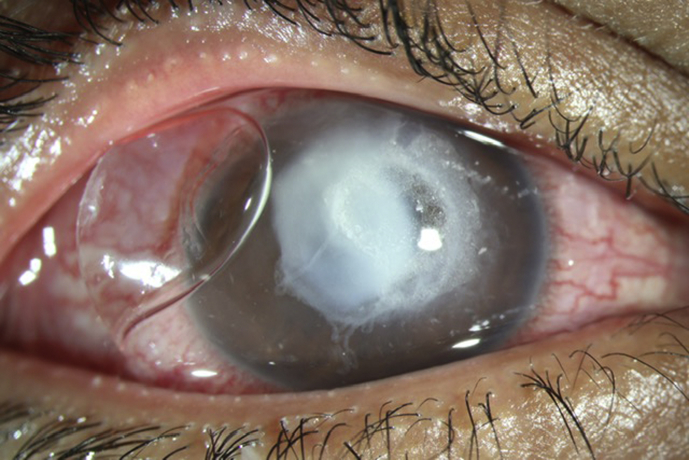
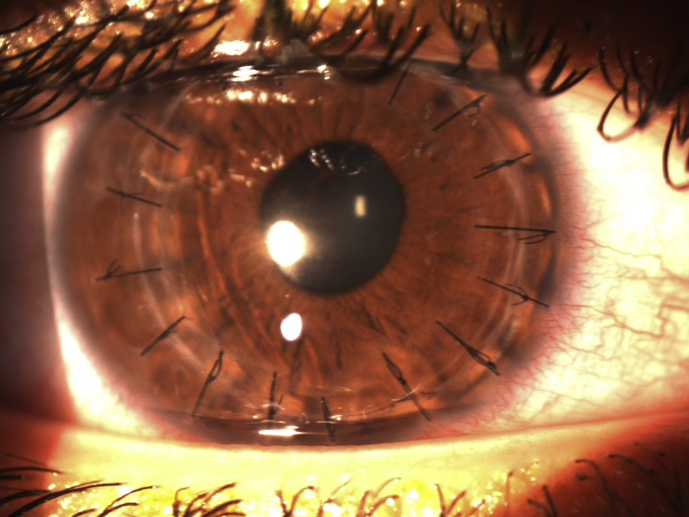
Postoperative day one, the patient was noted to have uncorrected visual acuity of 20/60, pinholing to 20/50. Slit lamp examination revealed a 8 mm epithelial defect, mild haze consistent with having had cross-linking, and no infiltrate. His bandage contact lens was in place. Postoperative day 3, the patient called to say that he noted a “white spot” on his cornea (which he noted seeing on day 2 but never called) was enlarging. He was asked to come in immediately. He noted that his pain which had been 8/10 postoperative day 1 was now 0/10. On examination his vision was Hand Motions and his bandage contact lens was not present. He had a central 5 × 5.5 mm stromal necrotic ulcer with associated 1 mm hypopyon (Fig. 2). Cultures were taken and the patient was started on topical fortified vancomycin 25mg/ml and tobramycin 15mg/ml every 1 hour around the clock, Doxycycline 100 mg orally bis in die (BID) and Acyclovir 800 mg orally BID. An autoimmune workup was initiated to rule out any diseases which may predispose the patient to corneal melting; these tests all came back negative. On postoperative day 5 the patient was noted to have an increasing amount of stromal thinning, approximately 2 × 3 mm in diameter temporally, with a mildly positive seidel test. He was started on oral Prednisone 30 mg once a day and cyanoacrylate glue was placed over the area of thinning along with a Kontur lens. Over the next week it was noted that his necrosis was resolving and his hypopyon was also lessening. However, on postoperative day 16 it was noted that while he was healing, the corneal glue began to loosen and his anterior chamber shallowed as a result (Fig. 3). Due to persistent leakage, he was taken to the operating room where he underwent an emergent penetrating keratoplasty (Fig. 4). The patient tolerated the procedure well without complication.
Fig. 2.
Initial presentation of infiltrate, corneal thinning and hypopyon.
Fig. 3.
Photograph taken before penetrating keratoplasty with glue and kontur lens.
Fig. 4.
Postoperative appearance after penetrating keratoplasty.
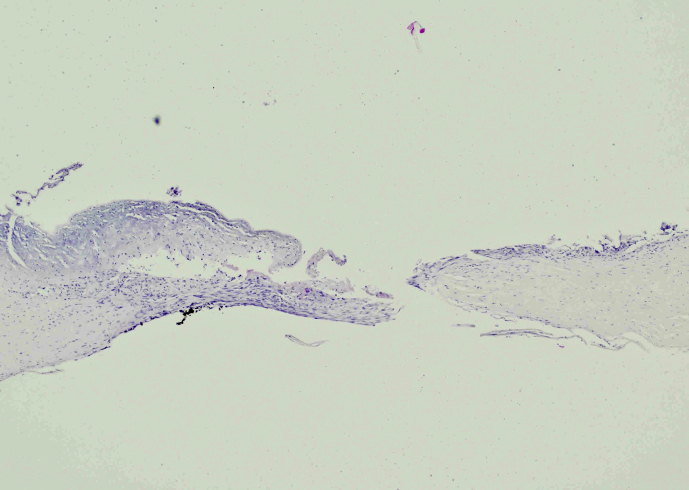
Postoperatively the patient's course has been uneventful and his vision was 20/20-2 postoperative month 3. Of note, on pathology there were no organisms noted within the corneal button with special stains – just extensive keratolysis (Fig. 5). The cultures taken on day 3 after CXL were negative however the broth revealed “unidentifiable gram-negative rods.” This was further sent to the New York State department of health who noted “gram-positive spore-forming bacillus – unable to identify further, Streptococcus parasanguinis, Streptococcus cristatus, and Neisseria species.” These organisms were sensitive to fluoroquinolones. Cultures taken at the time of surgery were all negative.
Fig. 5.
Corneal button with extensive keratolysis, no organisms.
3. Discussion
Corneal collagen cross-linking was approved by the FDA in 2016. Results of the phase III clinical trials in both keratoconus patients and post-refractive surgery ectasia patients demonstrated few significant complications.5,6 For the past year and half since FDA approval many corneal providers across the United States have been performing CXL. The goal of CXL is to stabilize the cornea and maintain good vision to prevent the need for potential keratoplasty in the future. However, in this report we demonstrate that despite following the approved protocol visually debilitating complications can occur. To our knowledge this is the first case of corneal perforation following FDA-approved CXL in the United States.
CXL has been performed outside the United States and off-label since 2003. There have been some reported cases of infectious keratitis and corneal perforation. A recent report from the Cornea society suggest the estimated incidence of infection was 0.0017%.8 A prior literature review noted 17 cases of post-CXL keratitis with 11 isolated case reports and 1 case series.9 In our patient the time to corneal infiltrate was 3 days versus 5 days in the literature review. The most common pathogen was bacterial, specifically Staph aureus with some cases of polymicrobial infection. There was an association with vernal kerato-conjunctivitis in 57% of cases.
Rapid keratolysis causing melt and perforation after CXL is extremely rare. Upon literature review there were 5 prior reported cases. 80% were done with the epi-off protocol, 1 case was done trans epithelial. The first case perforated on day 8 and was positive for Alternaria spp. and required a therapeutic penetrating keratoplasty.9 Rana et al. reported 2 cases of post-CXL microbial keratitis that ultimately perforated despite optimal therapy requiring corneal gluing. These cases perforated on postoperative day 2 and 7 and were associated with Staph aureus and methicillin-resistant Staph aureus respectively.10 Additionally, a case of post-CXL Acanthamoeba keratitis was reported that underwent penetrating keratoplasty for corneal perforation on postoperative day 11.11 There was also an isolated case of corneal melt requiring a penetrating keratoplasty with no known pathogen occurring postoperative month 2.12
Our patient had no underlying inflammatory or atopic condition. Laboratory workup remained negative and cultures were inconclusive. He did have a remote history of isotretinoin use, but use was several months prior to the procedure. The etiology as to the cause of the rapid keratolysis and perforation in our case is unclear. The etiology is likely multifactorial secondary to a large epithelial defect and concomitant use of a BCL with topical steroids and nonsteroidal anti-inflammatory drugs (NSAIDs) in the immediate postoperative period. However, the NSAID was unlikely to precipitate given only one drop was given after CXL over the bandage lens. The patient was not prescribed any postoperative NSAID drops. It is also possible that patient hygiene plays a role, with more difficult to manage hygiene in younger aged patients. Those with underlying inflammatory conditions, atopy or those on certain medications that may affect corneal wound healing may play a role which tends to be more common in those with keratoconus. To our knowledge in reviewing the literature we have found nothing to implicate isotretinoin or Scheuermann's disease as a contributing factor to corneal infection, melting or perforation after CXL.
4. Conclusion
This case report highlights a severe complication that may occur after CXL. Patients must be carefully counseled about the risk of corneal melt and perforation.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interests
Authors have no financial disclosures or conflicts of interest.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2020.100658.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Rabinowitz Y.S. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Krachmer J.H., Feder R.S., Belin M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 3.Gordon-Shaag A., Millodot M., Shneor E. The epidemiology and etiology of keratoconus. Int J Keratoconus Ectatic Corneal Dis. 2012;70(1) [Google Scholar]
- 4.Spörl E., Huhle M., Kasper M., Seiler T. Increased rigidity of the cornea caused by intrastromal cross-linking. Ophthalmologe. 1997;94(12):902–906. doi: 10.1007/s003470050219. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 6.Hersh P.S., Stulting R.D., Muller D. US multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology. 2017 Oct 1;124(10):1475–1484. doi: 10.1016/j.ophtha.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Hersh P.S., Stulting R.D., Muller D. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017 Sep 1;124(9):1259–1270. doi: 10.1016/j.ophtha.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 8.Belin M.W., Lim L., Rajpal R.K., Hafezi F., Gomes J.A., Cochener B. Corneal cross-linking: current USA status report from the Cornea society. Cornea. 2018 Oct 1;37(10):1218–1225. doi: 10.1097/ICO.0000000000001707. [DOI] [PubMed] [Google Scholar]
- 9.Maharana P.K., Sahay P., Sujeeth M. Microbial keratitis after accelerated corneal collagen cross-linking in keratoconus. Cornea. 2018 Feb 1;37(2):162–167. doi: 10.1097/ICO.0000000000001439. [DOI] [PubMed] [Google Scholar]
- 10.Rana M., Lau A., Aralikatti A., Shah S. Severe microbial keratitis and associated perforation after corneal crosslinking for keratoconus. Contact Lens Anterior Eye. 2015;38(2):134–137. doi: 10.1016/j.clae.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Rama P., Di Matteo F., Matuska S., Paganoni G., Spinelli A. Acanthamoeba keratitis with perforation after corneal crosslinking and bandage contact lens use. J Cataract Refract Surg. 2009;35(4):788–791. doi: 10.1016/j.jcrs.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Labiris G., Kaloghianni E., Koukoula S., Zissimopoulos A., Kozobolis V.P. Corneal melting after collagen cross-linking for keratoconus: a case report. J Med Case Rep. 2011;5(1):152. doi: 10.1186/1752-1947-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.