Abstract
Background
Intraductal papillary mucinous neoplasms (IPMNs) are precursor lesions of pancreatic cancer, which is characterized by an immunosuppressive microenvironment. Yet, the spatial distribution of the immune infiltrate and how it changes during IPMN progression is just beginning to be understood.
Methods
We obtained tissue samples from patients who underwent pancreatic surgery for IPMN, and performed comprehensive immunohistochemical analyses to investigate the clinical significance, composition and spatial organization of the immune microenvironment during progression of IPMNs. Survival analysis of pancreatic cancer patients was stratified by tumour infiltrating immune cell subtypes.
Findings
The immune microenvironment evolves from a diverse T cell mixture, comprising CD8+ T cells, Th/c1 and Th/c2 as major players combined with Th9, Th/c17, Th22, and Treg cells in low-grade IPMN, to a Treg dominated immunosuppressive state in invasive pancreatic cancer. Organized lymphoid clusters formed in IPMN surrounding stroma and accumulated immunosuppressive cell types during tumour progression. Survival of pancreatic cancer patients correlated with Th2 signatures in the tumour microenvironment.
Interpretation
The major change with regards to T cell composition during IPMN progression occurs at the step of tissue invasion, indicating that malignant transformation only occurs when tumour immune surveillance is overcome. This suggests that novel immunotherapies that would boost spontaneous antitumor immunity at premalignant states could prevent pancreatic cancer development.
Funding
The present work was supported by German Cancer Aid grants (70,112,720 and 70,113,167) to S. R., and the Olympia Morata Programme of the Medical Faculty of Heidelberg University to S. R.
Keywords: Pancreatic cancer, Premalignant lesion, Intraductal papillary mucinous neoplasm, Tumour microenvironment, Tumour immunology
Research in context.
Evidence before this study
Pancreatic cancer is one of the most aggressive malignancies, which mainly arises from two types of precursor lesions, pancreatic intraepithelial neoplasias and intraductal papillary mucinous neoplasms (IPMNs). Pancreatic cancer is characterized by an immunosuppressive microenvironment and its development is strongly influenced by inflammation. Tumour-reactive T cells are limited in quantity and quality, and current immunotherapies are mostly ineffective. However, the spatial distribution of T cells subsets has not been comprehensively analysed within the pancreatic cancer microenvironment, nor how their distribution evolves during tumour progression.
Added value of this study
We herein delineate the spatial organization of the immune microenvironment, with a systematic analysis of T cell subtypes during pancreatic carcinogenesis using IPMN precursor lesions. We demonstrate that the frequency and localization of T cells drastically changes during the natural history of IPMN progression, and that the T cell orchestra in the pancreatic tumour microenvironment comprises subsets beyond the well-studied cytotoxic CD8+ T cells, Tregs, Th1, Th2, and Th17 cells.
Implications of all the available evidence
The present data lay the basis for further in-depth functional characterizations of T cell subtypes, including Th9 and Th22 cells, in IPMN progression, which might enable the design of more effective immunotherapies against pancreatic cancer.
Alt-text: Unlabelled box
1. Introduction
Pancreatic cancer, 85% of which are adenocarcinomas, is one of99 the most aggressive malignancies with an extremely poor prognosis and still rising incidence. Currently, it is the 3rd leading cause of cancer-related deaths in the Western world [1], and the 5-year survival rate is about 9% [1]. Pancreatic ductal adenocarcinomas (PDACs) mainly arise from two types of precursor lesions, pancreatic intraepithelial neoplasias (PanINs) and intraductal papillary mucinous neoplasms (IPMNs) [2], [3], [4]. While microscopic PanINs are usually undetectable by radiologic imaging, impeding their early diagnosis, IPMNs are readily identifiable cystic precursor lesions of pancreatic cancer, which are increasingly detected by abdominal cross-sectional imaging [5]. IPMNs of the pancreas are formed by intraductal proliferations of mucinous cells with papillary growth patterns and extensive mucin production leading to cystic dilatations [2,3] that communicate with the main pancreatic duct. While main duct (MD) and mixed-type (MT) IPMNs that involve the main pancreatic duct itself have a risk of malignancy of about 40–90%, IPMN cysts that are confined to secondary branches (branch duct type, BD), are associated with a much lower rate of malignancy [6,7]. IPMNs seem to progress from lesions with low-grade dysplasia (IPMN-L), to high-grade dysplasia (IPMN—H) and eventually to invasive pancreatic carcinoma (IPMN-IC) [3]. In the absence of invasive carcinoma IPMN prognosis is excellent with surgical resection, but as poor as conventional PDAC, if malignant invasion has already occurred [8]. However, the mechanisms of malignant transformation are incompletely understood.
Development and progression of pancreatic cancer is strongly influenced by intra-and peritumoral inflammation [9,10]. While early, premalignant stages of IPMN lesions were shown to contain antitumor immune components, including cytotoxic T cells, those seemed to be progressively lost during tumour progression, accompanied by the accumulation of immunosuppressive cells [10,11]. Although cytotoxic CD8+ T cells are potent mediators of antitumor immunity and exceptionally high neoantigen numbers with robust antitumor CD8+ T cell responses have been associated with long-term survival in pancreatic cancer patients [12], antitumor-reactive cytotoxic CD8+ T cells are generally limited in quantity and functional activity. T cell effector functions are orchestrated by CD4+ T helper (Th) cells. IFNγ-producing Th1 cells mediating cytotoxic T cell responses are well known for their antitumoral capacity and have been shown to impair tumour development in murine models of pancreatic cancer [13], while Th2 cells have been associated with tumour permissive immune anergy. The dichotomy of Th1 and Th2 cells has been extended during the last decade with the discovery of additional T cell subsets, which can be discriminated based on extracellular markers and lineage-specifying transcription factors that control gene-expression programs determining their fate and functional activity. Thereby, T-bet+ Th1, GATA3+ Th2, PU.1+ Th9, RORγt+ Th17, AHR+ IL-22 cells, as well as FOXP3+ regulatory T cells (Tregs) can be distinguished [14,15]. Recently it has been shown that CD8+ cytotoxic T (Tc) cells similarly separate into Tc1, Tc2, Tc9, Tc17, and CD8+ Tregs [15].
In pancreatic cancer, the immune infiltrate varies substantially within distinct compartments and T cells with potential antitumor activity seem to be predominantly present in peritumoral stroma and tertiary lymphoid structures (TLS) [16,17], while only rarely in the direct vicinity of tumour cells [18], [19], [20]. TLS are induced lymphoid aggregates that directly form in tissues upon chronic inflammation or tumour development, which are characterized by distinct B and T cell zones and mature dendritic cells presenting antigens for T cell priming and generation of specific antitumor immune responses [21]. TLS have been described in pancreatic cancer [16,17] and are mostly located at the tumour periphery [16]. The presence of TLS in pancreatic cancer was associated with higher T cell infiltration, less immunosuppressive cells and a favourable clinical outcome [16,22].
However, the spatial distribution of T cells subsets has not been comprehensively analysed within the pancreatic cancer microenvironment, nor how their distribution changes during tumour progression. Therefore, in the present study we investigated the cellular composition and spatial organization of the immune microenvironment, with a systematic analysis of T cell subtypes during pancreatic multistep carcinogenesis using IPMN precursor lesions. This might help to better understand the naturally occurring potential antitumor immune responses as well as obstacles to immunotherapies in pancreatic cancer enabling the design of more effective treatments.
2. Materials and methods
2.1. Patients and samples
Surgically resected IPMN or IPMN-associated invasive pancreatic cancer tissue specimen were obtained from patients who provided prior written-informed consent (Department of Surgery, Heidelberg University Hospital) under a research protocol approved by the Ethics Committee of the University of Heidelberg, Germany. FFPE tissue samples were provided by the Biobank of the European Pancreas Centre and the National Centre for Tumour Diseases (NCT, Heidelberg, Germany). Clinical information was acquired from the Department of Surgery of Heidelberg University Hospital.
2.2. Immunohistochemistry
The FFPE tissue samples were cut into 4 μm thin sections. IPMN was confirmed in all tissue samples by H&E staining and grade of dysplasia was evaluated by an experienced pancreatic histopathologist. For single stains tissue slides were incubation for 10 min in 0.3% hydrogen peroxide. Subsequently sections were incubated with universal blocking peptide (Biogenex Laboratories, San Ramon, USA) for 1 h prior to overnight incubation at 4 °C with the specific primary antibody. Then, slides were incubated with a secondary antibody, either EnVision+System-HRP Labelled Polymer anti-rabbit or anti-mouse (Dako, Agilent, Santa Clara, USA), or goat anti-rat IgG-HRP (Santa Cruz Biotechnology, Dallas, USA). Colour reaction was obtained by using Liquid DAB+ Substrate Chromogen System (Dako, Agilent, Santa Clara, USA). Slides were counterstained with Mayer's hematoxylin (Merck, Darmstadt, Germany). Double stains were performed with MULTIVIEW (mouse-HRP/rabbit-AP) IHC kit (Enzo, Farmingdale, NY, USA) and HighDef blue IHC chromogen (Enzo, Farmingdale, NY, USA) according to the manufacturers instructions. Weigert's hematoxylin (Merck, Darmstadt, Germany) was used as counter stain. Slides were scanned with a SCN400 Slide Scanner (Leica, Wetzlar, Germany). Distinct areas of tumours were categorized into the following compartments: IPMN or carcinoma epithelium (single cells, or ducts/islands) with a surrounding area of about 150 μm (juxtatumoral stroma), stroma without any tumour cells surrounding the juxtatumoral compartment (peritumoral stroma), TLS, and normal adjacent pancreatic tissue. Necrosis, fatty tissue, big vessels and other organs present in the tissue section were excluded. An individual cell counting algorithm for each single stain protocol was applied on the tissue scans using Tissue 1a 2.0 software (Leica, Wetzlar, Germany). Using the same software double positive cells were automatically marked depending on their colour and counted manually if more than 50% of the cell was marked. All slides were evaluated with a virtual 20x objective.
2.3. Antibodies
The primary antibodies anti-CD45 (MEM-28; RRID:AB_2,747,795) and anti-FOXP3 (236A/E7; RRID:AB_445,284) were from Abcam (Cambridge, UK), anti-CD1a (010), anti-CD3 (polyclonal, rabbit; RRID:AB_2,732,001), anti-CD3 (F7.2.38; RRID:AB_2,631,163), anti-CD8 (C8/144B; RRID:AB_2,075,537), anti-CD20cy (clone L26; RRID:AB_2,282,030), and anti-CD68 (KP1; RRID:AB_2,661,840) were purchased from Dako, Agilent (Santa Clara, USA), anti-GATA-3 (D13C9; RRID:AB_10,835,690), anti-PU.1 (9G7; RRID:AB_2,186,909), and anti-T-bet (D6N8B; RRID:AB_2,616,022) were from Cell signaling Technology (Danvers, USA). Anti-AHR (H-211; RRID:AB_633,731) was from Santa Cruz Biotechnology (Dallas, USA), anti-CD208 (1010E1.01) from Dendritics (Lyon, France), anti-CD4 (4B12; RRID:AB_10,554,438) from Leica Biosystems (Wetzlar, Germany), and anti-RORC/RORγ (polyclonal, rabbit; RRID:AB_2,301,075) from LifeSpan Biosciences (Seattle, USA).
2.4. Correlation of immune cell subtypes with overall survival of pancreatic adenocarcinoma patients
Survival analysis of pancreatic cancer patients stratified by immune cell subtypes was based on the comprehensive immunogenomics analysis by Thorsson et al. [23], which scored TCGA tumour samples, including 184 specimens from pancreatic adenocarcinoma patients, for 160 immune expression signatures. Kaplan-Meier survival estimates with 95% confidence intervals were computed using the survfit() function from the Survival R package and stratified by specific immune signature scores of the “PanImmune Feature Matrix of Immune Characteristics“ [23]. The ggsurvplot() function in the Survminer R package was used to visualize survival curves. P-values were calculated using the Log-Rank test comparing the survival curves of the lower and upper half of scores in each immune cell signature group.
2.5. Principal component analysis
An unsupervised principal component analysis (PCA) was performed on all analysed tissue samples based on the quantities of infiltrating macrophages and T cell subtypes as determined by immunohistochemistry. The principal components were calculated using the prcomp() function from the Stats R package, and the first three (PC1, PC2, PC3) were plotted in 3D projection using the scatterplot3d() function from the scatterplot3d R package. For better visualization of the three different histological grades (low-grade, high-grade, invasive carcinoma) within the plot one colour is assigned to each grade.
2.6. Statistical analysis
Statistical analyses were performed using R (version 3.4.4). For continuous variables significance was determine using Kruskal-Wallis test with Dunn‘s posthoc analysis, and categorical variables were evaluated with Pearson‘s Chi-square test. A p-value < 0.05 was considered statistically significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Quantifications of IHC are presented as box (median with interquartile ranges) and whisker plots using the ggboxplot() function of the ggpubr R package. Outliners, defined as data values beyond the 25th or 75th percentile minus and plus 1.5 times the interquartile range, respectively, are displayed as separate points. Stacked barplots were generated using the ggplot2 and RcolorBrewer R packages. To create balloonplots the balloonplot() function of the gplots R package was used. The diameter of the balloons is proportionate to the number of cells per 100 μm2 as quantified via IHC. Hmisc and corrplot R packages were used to determine and visualize correlation significance between the presence of T cell subtypes in tissue areas based on Spearman rank-based correlation coefficient.
2.7. Role of the funding source
No funding source was involved in study design, or conduct, nor in analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the paper for publication.
3. Results
3.1. Immune cell infiltration of human IPMNs occurs already at an early stage
Tissue samples from 58 patients who underwent pancreatic resection for IPMN were analysed by histopathology and immunohistochemistry (IHC). All specimens were classified by an expert pathologist according to the consensus recommendations for pancreatic cancer precursor lesions [4] into low-grade and high-grade dysplastic lesions, or IPMN-associated invasive pancreatic carcinoma. Herein, high-grade dysplasia corresponds to carcinoma in situ type lesions [4]. According to the histological grade of the 58 samples, 32 were classified as low-grade IPMN (IPMN-L), 10 as high-grade IPMN (IPMN—H), and 16 as IPMN-associated invasive pancreatic carcinoma (IPMN-IC). The clinicopathological features are summarized in Table 1. There were no significant correlations between the IPMN histological grade and age, gender, tumour location, type of surgery, IPMN subtype, tumour size, or main pancreatic duct dilatation (Table 1).
Table 1.
Univariable Analysis of demographics and clinicopathological characteristics in relation to IPMN histological grades.
| Characteristics | IPMN-L (N = 32) | IPMN-H (N = 10) | IPMN-IC (N = 16) | p-value |
|---|---|---|---|---|
| Age, years (median, IQR) | 63.63 (57.27–72.24) | 75.42 (70.16–77.3) | 64.02 (60.62–73.64) | 0.0516 |
| Gender (%) | 0.3725 | |||
| Male | 17 (53.13) | 3 (30) | 9 (56.25) | |
| female | 15 (46.88) | 7 (70) | 7 (43.75) | |
| Tumour location (%) | 0.5296 | |||
| Head | 22 (68.75) | 7 (70) | 12 (75) | |
| Body | 1 (3.13) | 0 | 2 (12.5) | |
| Tail | 1 (3.13) | 1 (10) | 0 | |
| Multiple sites | 8 (25) | 2 (20) | 2 (12.5) | |
| Type of surgery (%) | 0.4696 | |||
| PD | 21 (65.63) | 6 (60) | 10 (62.5) | |
| DP | 6 (18.75) | 2 (20) | 1 (6.25) | |
| TP | 3 (9.38) | 2 (20) | 5 (31.25) | |
| Enucleation | 2 (6.25) | 1 (10) | 0 | |
| IPMN subtype (%) | 0.1338 | |||
| MD | 0 | 0 | 0 | |
| BD | 31 (96.88) | 8 (80) | 13 (81.25) | |
| MT | 1 (3.13) | 2 (20) | 3 (18.75) | |
| Cyst diameter in cm (median, IQR) | 2.6 (1.95–3.85) | 2.4 (1.7–2.8) | 1.95 (1.625–2.725) | 0.383 |
| Cyst diameter ≥ 4 cm (%) | 7 (21.88) | 1 (10) | 2 (12.5) | 0.6595 |
| MPD dilatation (%) | ||||
| ≥ 10 mm | 0 | 1 (10) | 1 (6.25) | 0.3711 |
| > 5 and < 10 mm | 1 (3.13) | 1 (10) | 2 (12.5) | 0.5743 |
Continuous variables are presented as median (interquartile range, IQR), and categorical variables as number of cases (percentage). Significance was determined using Kruskal-Wallis test and Pearson‘s Chi-square test for ordinal and categorical data, respectively. PD, pancreaticoduodenectomy; DP distal pancreatectomy; TP, total pancreatectomy; MD, main duct; BD, branch duct; MT, mixed-type; MPD, main pancreatic duct.
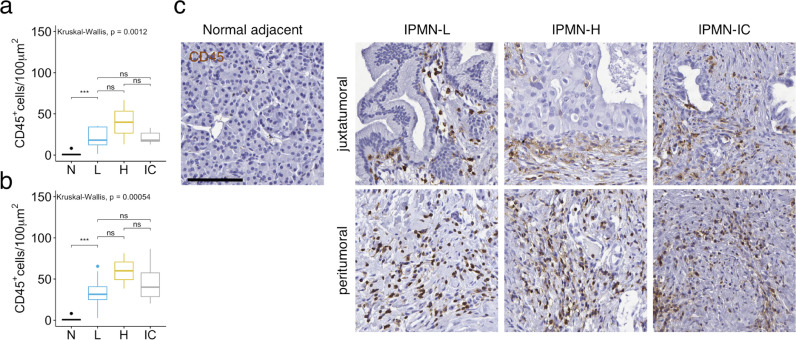
To comprehensively determine the spatial organization and cellular composition of the in situ immune reaction in the tumour microenvironment and how it evolves during pancreatic tumour progression, we systematically analysed leucocyte subsets with a special focus on T cell subtypes in defined tumour compartments at distinct stages in the disease process. Regions of the tumour containing neoplastic epithelial cells together with the directly surrounding or between single tumour cells, neoplastic ducts, or islands interspersed stroma, were marked on H&E and IHC slides as juxtatumoral areas, and separated from peritumoral stromal compartments, normal adjacent pancreatic tissue, and TLS (Fig. 1). We performed unbiased semi-automated enumerations of immune cells. Immunohistochemistry for CD45 revealed that leucocytes are already abundant in juxtatumoral and peritumoral compartments of low-grade IPMNs (Fig. 2), in line with previous reports that showed inflammatory infiltrates in early preinvasive pancreatic lesions [9,10]. Interestingly, the overall immune cell density does not seem to further increase during tumour development (Fig. 2).
Fig. 1.
Tumour microenvironment compartments. Representative image of a human low-grade IPMN tissue section stained with H&E. Immune cell populations were quantified in distinct areas, juxtatumoral (red) and peritumoral (yellow) stroma, tertiary lymphoid structures (green), and normal adjacent pancreatic tissue (purple). Scale bar represents 100 μm2. The H&E image was acquired at 20x magnification. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Immune cells accumulate in human IPMN already at an early stage. (a and b) CD45+ immune cell numbers per 100 μm2 in normal adjacent (N) pancreatic tissue, within the juxtatumoral stroma (a), or in the peritumoral stroma (b) of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) as determined by immunohistochemistry (IHC). All data are presented as box and whisker plots. ***p < 0.001, Kruskal-Wallis test with Dunn‘s posthoc analysis. (c), Representative images of CD45 IHC on indicated tissue areas. Scale bar represents 100 μm2. Images were acquired at 20x magnification.
3.2. The immune landscape evolves during progression from low-grade IPMN to invasive pancreatic cancer
Although the amount of infiltrating CD45+ leukocytes remains stable throughout tumour progression, the composition of immune cells has been shown to change and might be the key to understanding when and how immune surveillance is overcome. Yet, like total CD45+ cells, entire CD3+ T cells are similarly increased in IPMN lesions of all histological grades as compared with normal adjacent pancreatic tissue (Fig. 3a). Next we mapped immune subsets that have previously been shown to be involved in pancreatic cancer development and associated with patient survival, including CD8+ and CD4+ T cells, B cells, macrophages, and dendritic cells [9,12,19,[24], [25], [26], [27], [28], [29], [30]] (Fig. 3b, Table 2). CD20 was used to stain B cells and CD68 was used as a pan macrophage marker. Immature and mature dendritic cells (DCs) were distinguished by immunohistochemistry for CD1a and CD208 [31].
Fig. 3.
Immune cell composition changes from low-grade IPMN to invasive pancreatic cancer. (a) Box and whisker plots comparing CD3+ T cell numbers per 100 μm2 in normal adjacent (N) pancreatic tissue, within the juxtatumoral stroma (left), or in the peritumoral stroma (right) of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) as detected by IHC. Representative image of CD3 IHC on an IPMN tissue section. (b) Stacked barplots showing leucocyte subtypes as percentage of CD45+ total leucocyte counts in juxtatumoral (left) and peritumoral stroma (right) of IPMN lesions as determined by IHC for CD1a, CD4, CD8, CD20, CD68 and CD208. (c and d) CD8+ T cell (c) and CD68+ macrophage (d) numbers per 100 μm2 in juxtatumoral, or peritumoral stroma of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) based on IHC analyses. Representative images of CD8 (c) and CD68 (d) IHC on IPMN sections. e, Box and whisker plots comparing CD4+ T cell numbers per 100 μm2 in normal adjacent (N) pancreatic tissue, within the juxtatumoral stroma (left), or in the peritumoral stroma (right) of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) as detected by IHC. Representative image of CD4 IHC on an IPMN tissue section. Scale bars represent 100 μm2. Images were acquired at 20x magnification. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Kruskal-Wallis test with Dunn‘s posthoc analysis; ns, not significant.
Table 2.
Correlation of juxta- and peritumoral immune cell infiltration with IPMN histological grades.
| Immune markers |
IPMN-L (N = 32) median (IQR) |
IPMN-H (N = 10) median (IQR) |
IPMN-IC (N = 16) median (IQR) |
p-value |
|---|---|---|---|---|
| Juxtatumoral | ||||
| CD45 | 18.119 (12.3422–34.1127) | 39.8961 (26.5312–53.2611) | 18.1024 (16.6316–26.5639) | 0.87935 |
| CD3 | 16.6456 (14.1141–21.6057) | 19.5116 (15.3562–37.5473) | 10.4596 (5.3281–23.8441) | 0.10454 |
| CD4 | 5.0807 (2.356–9.4492) | 6.8511 (2.2536–10.3946) | 3.6155 (1.5966–5.127) | 0.26919 |
| CD8 | 7.5207 (4.429–9.6054) | 7.3803 (3.2352–10.3849) | 2.4447 (1.6215–4.9724) | 0.00202 |
| CD20 | 0.5386 (0.3559–1.4956) | 3.0519 (1.9465–4.1573) | 0.606 (0.2322–0.9467) | 0.41835 |
| CD68 | 1.1906 (0.5489–2.2682) | 1.6128 (0.9508–2.2749) | 4.7891 (3.5692–6.8197) | 0.00476 |
| CD1a | 0.0522 (0.0263–0.078) | 0.2779 (0.2051–0.3507) | 0.6535 (0.6296–0.7207) | 0.03910 |
| CD208 | 0.0172 (0.0069–0.0266) | 0.0397 (0.022–0.0575) | 0.0521 (0.0485–0.0654) | 0.16528 |
| FOXP3 | 0.0266 (0–0.0733) | 0.0762 (0.0233–0.1391) | 0.164 (0.1028–0.337) | 0.00112 |
| CD3 T-bet | 0.0988 (0.0317–0.2045) | 0.1894 (0.1003–0.3364) | 0.0328 (0.0075–0.0348) | 0.01776 |
| CD3 GATA3 | 0.2531 (0.056–0.4502) | 0.0715 (0.0192–0.5435) | 0.0522 (0.0113–0.1078) | 0.23722 |
| CD3 PU.1 | 0.0508 (0.02–0.0614) | 0.0806 (0.0673–0.0938) | 0.0216 (0.0041–0.0284) | 0.05653 |
| CD3 RORγt | 0.0975 (0.0098–0.1513) | 0.055 (0.0137–0.1079) | 0 (0–0) | 0.06537 |
| CD3 AHR | 0.0639 (0.0341–0.0978) | 0.0662 (0–0.1245) | 0.0059 (0–0.0244) | 0.00386 |
| Peritumoral | ||||
| CD45 | 31.4617 (25.0002–40.7373) | 59.9491 (49.2267–70.6715) | 40.1613 (28.6339–57.5185) | 0.26266 |
| CD3 | 25.8305 (17.4384–30.1838) | 44.4058 (31.8996–51.9453) | 22.6657 (17.1074–46.6558) | 0.04307 |
| CD4 | 5.3158 (3.2702–10.7912) | 9.771 (4.7845–13.6689) | 4.5797 (3.1887–6.3983) | 0.24896 |
| CD8 | 7.0467 (4.9036–10.1494) | 6.9054 (5.2204–12.0002) | 4.3425 (2.7369–10.2698) | 0.50664 |
| CD20 | 1.8964 (0.6582–3.2727) | 14.6956 (11.5009–17.8904) | 2.9753 (1.4425–10.154) | 0.10026 |
| CD68 | 3.6405 (3.0393–4.3672) | 4.4784 (2.2392–6.7176) | 8.5749 (6.8454–10.5991) | 0.01960 |
| CD1a | 0.1031 (0–0.2595) | 0.2096 (0.117–0.3022) | 0.7148 (0.4929–1.2756) | 0.09186 |
| CD208 | 0.0563 (0.0133–0.0796) | 0.3615 (0.1902–0.5329) | 0.3264 (0.1568–0.4472) | 0.05317 |
| FOXP3 | 0.0556 (0.006–0.1529) | 0.1237 (0.0917–0.1711) | 0.7705 (0.2471–0.8317) | 0.00048 |
| CD3 T-bet | 0.032 (0.0072–0.0803) | 0.0873 (0.0546–0.1343) | 0.0576 (0.0265–0.0765) | 0.12343 |
| CD3 GATA3 | 0.0796 (0.0334–0.1606) | 0.0278 (0.0033–0.1392) | 0.0287 (0.0023–0.1372) | 0.32875 |
| CD3 PU.1 | 0.0221 (0.0101–0.0344) | 0.0596 (0.0556–0.0635) | 0.0197 (0.0157–0.0278) | 0.27563 |
| CD3 RORγt | 0.0413 (0.0188–0.0861) | 0.0288 (0.0041–0.0482) | 0.0154 (0.0028–0.0213) | 0.18421 |
| CD3 AHR | 0.0287 (0.0082–0.0524) | 0.0628 (0.0305–0.1055) | 0.0151 (0.0064–0.071) | 0.53697 |
Numbers of immune marker positive cells per 100 μm [2] on indicated tissue areas as determined by immunohistochemistry are presented as median (interquartile range, IQR). Significance was determined using Kruskal-Wallis test. Bold value signifies p < 0.05.
While the density of CD8+ T cells in the peritumoral stroma is similar amongst all IPMN stages, they are diminished in the juxtatumoral compartment of invasive pancreatic cancer compared to low-grade lesions (Fig. 3b and c), indicating that potential tumour reactive CD8+ T cells are kept away from direct contact with neoplastic cells during tumour progression, consistent with previous studies on human pancreatic ductal adenocarcinoma [19,20].
Macrophages have been mainly attributed immunosuppressive and tumour promoting functions in pancreatic cancer [29,32] and shown to play an important role in development and progression of PanINs. Our results confirm that CD68+ macrophages are already recruited to low-grade IPMN lesions and further increased in the tumour microenvironment of invasive cancer (Fig. 3b and d). They not only accumulate in the peritumoral stroma, but also invade regions directly surrounding neoplastic cells, where they might restrain antitumor immune responses at the frontline.
Similar to total CD3+ T cells, immunohistochemistry for CD4, which marks all Th cell subsets, but also some antigen presenting cell populations, revealed that these cells are already present in low-grade IPMN lesions and that their overall density does not change during malignant transformation (Fig. 3b and e). To comprehensively analyse the spatial distribution of T cell subtypes in the tumour microenvironment, we performed stainings of lineage-defining intracellular transcription factors, including FOXP3, T-bet, GATA3, PU.1, RORγt, and AHR in combination with CD3. Consistent with multiple previous reports [9,27,33], our results show that the prevalence of FOXP3+ Tregs significantly increases during the carcinogenesis of IPMNs and that they constitute a major T cell subtype in invasive pancreatic cancer (Fig. 4a and b, Table 2). All other T cell subsets, including Th/c1, Th/c2, Th9, Th/c17, and Th22 cells, infiltrate low-grade lesions and relatively decrease during IPMN progression, especially in the juxtatumoral compartment (Fig. 4, Table 2). While CD3+/GATA3+ Th2/Tc2 together with CD3+/T-bet+ Th1/Tc1 cells were the relatively most abundant T cell subsets in proximity of neoplastic cells in low-grade IPMN lesions, they were overtaken by FOXP3+ Tregs at malignant states, potentially shifting immunosurveillance towards immunosuppression. Similarly, PU.1+ Th9, RORγt+ Th/c17, and AHR+ Th22 cells are already significantly increased in low-grade IPMN lesions compared to normal adjacent pancreatic tissue (Fig. 4e–g). To ascertain potential interdependencies of distinct T cell subtypes in pancreatic tumour microenvironments we assessed their quantitative correlations. Spearman's rank correlation analysis of immune cell densities in the juxtatumoral compartment illustrates the interrelation of T cell subtypes at different IPMN stages. Apart from Tregs, all T cell subsets are positively correlated, meaning that their densities change in the same direction, i.e. decrease from low-grade IPMNs to invasive cancer (Fig. 5a). To determine whether IPMNs with different histological grades can be distinguished based on their immune microenvironment we performed unsupervised principal component analysis (PCA) on immune cell density data from all IPMN groups. PCA allows the identification of underlying patterns in high dimensional data by reducing a large set of variables, i.e. densities of numerous immune cell subtypes, to a small number of principal components. Since our main focus was on distinct T cell subtypes and our analysis revealed that T cells and macrophages together constitute the major fraction of tumour infiltrating immune cells we restricted the immunohistochemical data to these cell types. Reassuringly, along the first three principal components (PC1, PC2, PC3), which explain most of the variance in the data, the tissue samples seem to segregate into two distinct clusters (Fig. 5b), demonstrating strong intragroup correlation, but differences in intergroup immune signatures. While low-grade IPMNs conglomerate with high-grade lesions, invasive pancreatic cancer specimen formed a separate cluster, although partially overlapping with precursor lesions (Fig. 5b). These findings indicate that preinvasive IPMN lesions have specific T cell compositions that are distinct from invasive pancreatic cancer and dramatically change at malignant transformation.
Fig. 4.
Distribution of T cell subtypes varies between distinct compartments of IPMN lesions and evolves during tumour progression. (a) Balloonplots showing relative abundance of indicated T cell subtypes in juxtatumoral stroma (left), or in the peritumoral stroma (right) of IPMN lesions. The size of the balloons correlates with cell densities as determined by IHC for FOXP3, CD3/T-bet, CD3/GATA3, CD3/PU.1, CD3/RORγt, and CD3/AHR. b, FOXP3+ cell numbers per 100 μm2 in juxta-, or peritumoral stroma of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) based on IHC analyses. Representative image of FOXP3 IHC in the peritumoral stroma. (c and d) Box and whisker plots comparing CD3+T-bet+ (c), and CD3+GATA3+ (d) cell numbers per 100 μm2 in normal adjacent (N) pancreatic tissue, within the juxtatumoral stroma (left), or in the peritumoral stroma (right) of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) as detected by IHC. Representative images of CD3/T-bet (c), and CD3/GATA3 (d) IHC on IPMN sections. (e–g) Box and whisker plots comparing numbers of CD3+PU.1+ (e), CD3+RORγt+ (f), and CD3+AHR+ (g) T cells per 100 μm2 in normal adjacent (N) pancreatic tissue and in the juxtatumoral stroma of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) as detected by IHC. Representative images of CD3/PU.1 (e), CD3/RORγt (f), and CD3/AHR (g) IHC on juxtatumoral stroma of IPMN sections. Examples of CD3+T-bet+, CD3+GATA3+, CD3+PU.1+, CD3+RORγt+, and CD3+AHR+ cells are denoted with arrows. b-g, Scale bars represent 50 μm2. Images were acquired at 20x magnification. *p < 0.05, ***p < 0.001, ****p < 0.0001, Kruskal-Wallis test with Dunn‘s posthoc analysis; ns, not significant.
Fig. 5.
Correlation of T cell subtypes in the tumour microenvironment with IPMN histological grade. (a) Correlation matrix comparing the densities of T cell subtypes as determined by IHC in juxtatumoral areas of all IPMN lesions. Scale refers to the rank-based Spearman correlation coefficient. (b) Principal component analysis (PCA) of all IPMN tissue samples based on immunohistochemical quantification of juxtatumoral macrophages and T cell subtypes. The first three principal components (PC1, PC2, PC3), which explain most of the data variation, are shown in 3D. Each data point represents one sample, colours correspond to the three different histological grades (blue, low-grade IPMN; yellow, high-grade IPMN; grey, IPMN-associated invasive pancreatic cancer). *p < 0.05, **p < 0.01, ***p < 0.001, p-values are approximated by using the t distribution based on Spearman correlations. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Together, our data demonstrate that T cell subtypes beyond CD8+ T cells and Tregs participate in the immune orchestra in the pancreatic tumour microenvironment and evolve during IPMN progression.
3.3. Peritumoral TLS increase in frequency and accumulate immunosuppressive cell types during IPMN progression
Recently, lymphocyte aggregates with immune cell composition and spatial organization similar to secondary lymphoid organs have been identified in pancreatic cancer [16,17,19]. In line with these studies, our histological analysis revealed that these lymphocyte clusters are frequently formed in peritumoral regions of IPMN-associated invasive pancreatic carcinoma, and that they might develop already at preinvasive states (Fig. 6a). Immunohistochemistry confirmed that these lymphoid follicles were TLS, organized in distinct B cell zones in the centre and surrounding T-cell zones with interspersed mature CD208+ DCs in most clusters (Fig. 6a). While only a minor proportion of low-grade IPMN lesions contained TLS, they were present in the majority of analysed pancreatic cancer samples (Fig. 6b). The absence of TLS in normal pancreatic tissue suggests that their formation is dependant on tumour related immune responses.
Fig. 6.
Tertiary lymphoid structures (TLS) evolve from low-grade IPMN to invasive pancreatic cancer. (a) Representative images of human low-grade IPMN (IPMN-L) and IPMN-associated pancreatic cancer (IPMN-IC) specimen containing TLS stained with H&E, or by IHC for CD3, CD20, or CD208, showing B cell follicles and T cell zones in lymphoid aggregates. (b) Percentage of low-grade IPMN (L), high-grade IPMN (H), and IPMN-associated invasive pancreatic cancer (IC) specimen with at least one tertiary lymphoid structure present in the peritumoral area. (c), Box and whisker plots comparing numbers of CD8 (left) and CD4 (right) T cells per 100 μm2 in normal adjacent (N) pancreatic tissue and in TLS of low-grade IPMN (L) and IPMN-associated invasive pancreatic cancer (IC) as detected by IHC. (d) Stacked barplots showing relative densities of indicated T cell subtypes. The height of the bars is proportionate to the cell densities after normalization of cell counts as determined by IHC for FOXP3, CD3/T-bet, CD3/GATA3, CD3/PU.1, CD3/RORγt, and CD3/AHR. (e-j) FOXP3+ (e), CD3+T-bet+ (f), CD3+GATA3+ (g), CD3+PU.1+ (h), CD3+RORγt+ (i), and CD3+AHR+ (j) T cell numbers per 100 μm2 in normal adjacent (N) pancreatic tissue and tertiary lymphoid structures of low-grade IPMN (L) and IPMN-associated invasive pancreatic cancer (IC) based on IHC analyses. (k) Stacked barplots showing relative densities of immature (CD1a) and mature (CD208) dendritic cells in juxtatumoral stroma (left), peritumoral stroma (middle), and TLS (right) of low-grade IPMN (L) and IPMN-associated invasive pancreatic cancer (IC). The height of the bars correlates with cell densities after normalization of cell counts as determined by IHC for CD1a and CD208. Scale bars represent 100 μm2. Images were acquired at 20x magnification. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Kruskal-Wallis test with Dunn‘s posthoc analysis; ns, not significant.
B cells have been shown to strategically localize to TLS or scatteringly infiltrate pancreatic cancer and are already present in preinvasive PanIN lesions [24,26]. Consistently, besides their accumulation in TLS (Fig. 6a), we identified CD20+ B cells scattered in juxta-and peritumoral compartments of IPMN-L, IPMN-H and IPMN-IC, in which their densities did not significantly differ amongst all histological grades (Fig. 3b, Table 2).
TLS have been suggested to be important for T cell priming and regulation of local immune responses within the tumour [17,34]. Immunohistochemical analysis showed that CD8+ and CD4+ cells are present in the T cell zones of IPMN and pancreatic cancer associated TLS. To comprehensively assess the composition of T cells within these TLS we evaluated the expression of the lineage-defining transcription factors in combination with their surface antigen CD3 by IHC. All analysed T cell subtypes, including Tregs, Th/c1, Th/c2, Th9, Th/c17, and Th22 cells, were detected in peritumoral TLS of IPMN lesions. Whereas CD3+/GATA3+ Th/c2 cells were the relatively most abundant specific T cell subset in TLS associated with low-grade IPMNs, they were surpassed by FOXP3+ Tregs at the malignant state. The infiltration of Th/c2 cells significantly decreased in tumour associated TLS during pancreatic cancer progression, while Treg densities significantly increased, indicating that negative regulatory signals could also limit the generation of specific antitumor responses within local privileged areas of adaptive immune regulation.
Solid tumours contain dendritic cells that can take up tumour-derived antigens, migrate to secondary or tertiary lymphoid organs and activate antigen-specific lymphocytes. Tumour infiltrating immature DCs expressing CD1a have been associated with poor prognosis, whereas DCs expressing the maturation marker lysosomal-associated membrane protein 3 (DC-LAMP, CD208) [31] correlated with beneficial outcomes [35]. Immature CD1a+ DCs scatteringly infiltrate the tumour microenvironment of IPMN lesions already at an early stage and further increase in the juxtatumoral stroma during tumour development. In contrast, mature CD208+ DCs mainly localize to T cell rich areas of TLS (Fig. 3b, Fig. 6a and k, Table 2). Notably, TLS in invasive pancreatic cancer also contained sparse immature CD1a+ DCs (Fig. 6k).
These data demonstrate that bona fide TLS naturally form in the surrounding stroma of human IPMN and evolve during progression to invasive pancreatic cancer, promoting an immunosuppressive microenvironment.
3.4. Correlation of the immune infiltrate with pancreatic cancer prognosis
To determine a potential association of tumour infiltrating immune cells that evolve during IPMN progression with pancreatic cancer patient prognosis we performed survival analyses of pancreatic cancer patients stratified by levels of immune cell subtypes in the tumour microenvironment. Since meaningful survival analysis requires large patient cohorts and the number of IPMN associated invasive cancer samples, which were immunohistochemically characterized in-depth within the present study, was limited, we made use of a previously published dataset of immune expression signatures of 184 pancreatic cancer specimen based on transcriptomic analyses of bulk tumour samples by the PanCancer Atlas consortium [23]. 33 samples with incomplete data were excluded from the analysis. The remaining 151 specimens were stratified into two groups based on the predetermined score for each immune signature (Fig. 7a). We then performed univariable survival analyses comparing groups with low vs. high signature scores of CD8+ T cells, Tregs, Th1, Th2, and Th17 cells, B cells, macrophages and dendritic cells. Signatures for Th9 and Th22 cells were not present in the published dataset [23]. Kaplan-Meier survival analysis revealed a significant association of Th2 signatures with overall survival of pancreatic cancer patients (Fig. 7a, b). The median survival time of patients with high Th2 signature scores was significantly lower compared to patients with low Th2 infiltrates (498 days (95% confidence interval (CI) 393–607) vs. 695 days (627 - NA); p = 0.0046).
Fig. 7.
Association of T cell subtypes with pancreatic cancer prognosis. (a) Illustration of included samples and design of the Kaplan-Meier survival analysis based on the immune signature scores of the “PanImmune Feature Matrix“ published by Thorsson et al. [23] (b), Survival plots of pancreatic cancer patient groups stratified by Th2 signatures. Overall survival time is shown in days. TCGA, The Cancer Genome Atlas; CD8, CD8+ T cells; B, B cells; MP, macrophages; DC, dendritic cells; **p < 0.01, Log-Rank test; ns, not significant.
4. Discussion
Multiple clinical trials concerning immune-based therapies in pancreatic cancer have recently been conducted or are currently under way - so far with limited success. Mostly T cell effector functions have been targeted in the development of cancer immunotherapies, but endogenous reactive T cells are limited in quantity and quality in pancreatic cancer, and single agent immunotherapies with immune checkpoint inhibitors are mostly ineffective. Thus, for the design of more effective immunotherapies a better understanding of the immune microenvironment, especially the mechanisms by which the tumour disables potent tumor-specific T cell responses during carcinogenesis is urgently needed.
In the present study we demonstrate that the frequency and localization of T cells drastically changes during the natural history of IPMN progression, and that the T cell orchestra in the pancreatic tumour microenvironment comprises subsets beyond the well-studied cytotoxic CD8+ T cells, Tregs, Th1, Th2, and Th17 cells.
Recently, an in-depth analysis of the cellular composition of IPMN lesions with distinct histological grades based on single-cell RNA sequencing has shown that cytotoxic T cells, activated Th cells and DCs infiltrate low-grade IPMNs, but are replaced by immune components with immunosuppressive phenotypes during dysplastic progression [10]. However, T cell subsets have not been investigated in detail and the spatial distribution of cells within the tumour microenvironment still remains unclear. Since the generation of immune responses largely depends on cellular interactions amongst immune cells, as well as their target cells, the spatial organization of the immune landscape, which cannot be revealed from gene expression studies of disaggregated tissues, needs to be further elucidated.
Multiple previous studies investigated the abundance and localization of CD8+ T cells and Tregs in pancreatic cancer, and suggested that CD8+ lymphocytes are trapped in peritumoral compartments, while Tregs invade intraepithelial regions of neoplastic cells during the progression of premalignant lesions [19,20,27,33]. Within the present study, we find similar trends in the distribution of these cells from low-grade IPMN lesions to invasive pancreatic carcinoma, indicating that potential antitumor-reactive cytotoxic T cells are ineffective in pancreatic cancer, at least partially because they are hampered in getting into direct contact with the tumour target cells and limited in their activity by accompanying Tregs.
Similar to Tregs, macrophages have been shown to infiltrate the stroma at the earliest premalignant states and contribute to the development and progression of pancreatic cancer precursor lesions [27,29,32]. Our immunohistochemical data confirm that macrophages are already present in low-grade IPMN lesions and further accumulate during IPMN progression, potentially sustaining tumour immune evasion.
Tumour immune surveillance has also been shown to be critically determined by Th cell subsets [36], [37], [38], which play a crucial role in coordinating immune responses through their ability to tailor the functions of other immune cell types [14]. In pancreatic cancer, previous studies showed that Th1-polarized CD4+ T cells are less frequent at the tumour site compared to Th2-polarized CD4+ T cells, and that their ratio correlates with patient survival [25]. Our results corroborate a preponderance of Th/c2 over Th/c1 cells in the pancreatic cancer microenvironment, and further demonstrate that the Th2 skew is already evident in early premalignant IPMN.
Th17 cells have been detected in human pancreatic cancer precursor lesions, and in mouse models of pancreatic cancer Th17 cells infiltrating the pancreatic stroma have been shown to promote the development and progression of premalignant lesions and invasive PDAC [39], [40], [41]. Consistently, we show that CD3+/RORγt+ Th/c17 cells infiltrate already low-grade IPMNs.
Over the last decade, an important role of Th9 and Th22 cells in tumour immunology has been discovered. In mice, Th9 cells induced protective antitumor immune responses via the generation of tumor-reactive cytotoxic T cells [42], and intrinsic cytolytic activity [37]. IL-22 and Th22 cells controlled tissue regeneration and inflammation-associated carcinogenesis in the colon via STAT3 signalling [38]. In pancreatic cancer, high expression of the IL-22 receptor, and increased frequencies of IL-22 producing T cells correlated with tumour progression and poor prognosis [43,44]. Although IL-9 seems to promote pancreatic cancer cell proliferation [45], a potential role of Th9 cells in pancreatic cancer has not been investigated. In the present study, we observed scattered infiltrations of Th9 and Th22 cells in the pancreatic tumour microenvironment, particularly in juxtatumoral compartments. Similar to all other investigated T cell subtypes, except Tregs, Th9 and Th22 cells showed a tendency to decrease from low-grade IPMN to invasive pancreatic cancer. To our knowledge, this is the first in situ spatial analysis of Th9 and Th22 cells in human pancreatic cancer and its precursor lesions. Further functional studies now need to evaluate a potential causal role of these T cell subtypes in pancreatic cancer development and progression.
While it was initially assumed that distinct master regulator transcription factors mediate the development of stable T cell subtypes, recent evidence demonstrates that the co-expression and interplay between lineage-defining transcription factors creates the diversity and plasticity of T cells. Thus, the distinction of T cell phenotypes based on single transcription factors is somewhat oversimplified and does not allow the precise discrimination of overlapping cell populations [46]. In addition, these lineage-defining transcription factors also determine the development and fate of innate lymphoid cells (ILCs) [46], which share immune regulatory functions with their adaptive counterparts. To distinguish T cells from ILCs we performed co-stainings of intracellular transcription factors and the T cell specific surface antigen CD3. However, while CD3 allows the separation from ILCs, a distinction of CD4+ Th cell subsets from corresponding CD8+ Tc cells is not possible based on our data.
Recently, several studies have reported the presence of TLS within the pancreatic cancer microenvironment [16,17,19,22]. We identified bona fide peritumoral TLS with separate T and B cell zones already in IPMN lesions, with increasing frequencies from low-grade dysplasia to invasive carcinoma. Consistent with the notion that antitumor specific immune responses might be generated within local TLS via presentation of tumour derived-antigens by mature DCs to T cells [21], our data show that mature CD208+ DCs predominantly localize to T cell zones of TLS, while immature CD1a+ DCs scatteringly infiltrate the microenvironment of IPMN lesions. Remarkably, while DC maturation usually occurs during migration to secondary or tertiary lymphoid organs following antigen engulfment, TLS in invasive pancreatic cancer also contained immature CD1a+ DCs, indicating that proper DC activation and maturation might be impaired during pancreatic carcinogenesis, which might hamper the induction of tumor-reactive cytotoxic T cell responses. Within TLS, T cell effector functions can also be restrained by Tregs [34]. While CD8+ T cells are abundant in TLS of low-grade IPMN and invasive pancreatic cancer, we observed increased densities of Tregs in TLS of carcinoma compared to low-grade IPMN lesions, which potentially further impedes specific antitumor immune responses at the malignant state. In parallel, Th/c2 cells are lost from TLS during IPMN progression.
The complex tumour immune microenvironment, especially its T cell infiltrate, has not only been shown to be involved in pancreatic cancer development, but also associated with patient prognosis [9,12,19,[24], [25], [26], [27], [28], [29], [30]]. Higher levels of tumor-infiltrating Tregs and macrophages were identified as a negative prognostic factor [27,28], while abundant CD8+ T cells in the presence of high neoantigen numbers correlated with longer overall survival [12]. In addition, the ratio of tumour infiltrating GATA3+/T-bet+ lymphocytes was demonstrated to be a predictor of patient survival after pancreatic cancer surgery. A Th2 (GATA3+) over Th1 (T-bet+) predominant lymphoid infiltrate was associated with a shortened overall survival [25]. In line with these previously published data [25], we show that higher Th2 immune expression signatures in the tumour microenvironment correlate with diminished patient survival. A recent study accurately demonstrated that only the combination of high levels of tumour infiltrating T cells and high neoantigen numbers, but neither alone, is associated with long-term survival [12]. Consistently, our analysis did not reveal survival differences of pancreatic cancer patients stratified by CD8+ T cell immune infiltrate signatures only. However, in contrast to previous survival analyses based on immunohistochemical FOXP3 stainings of pancreatic cancer samples [27,28], stratification of patients by Treg immune expression signatures was not associated with overall survival. This discrepancy might be due to differences in analysed patient cohorts, including tumour stage and grade, resection status, or demographic and clinical characteristics, which were not addressed in our immune signature based survival analysis. In addition, in silico prediction of cell types from bulk tissue transcriptomic data is limited to the precision of predefined specific gene expression signatures, and signatures of rare cell types might be obscured by transcripts from highly abundant cells.
The herein corroborated heterogeneous distribution of immune components in the pancreatic tumour microenvironment, including lymphoid aggregates, illustrates the importance of assessing distinct cell types in their spatial context, which likely greatly affects their functionality. Despite several important observations we describe in our present study based on primary human pancreatic tissue samples, our analysis has limitations, including the retrospective design and the bias towards early-stage, resectable pancreatic cancer.
Overall, our study represents the most comprehensive analysis of T cell subtypes and their spatial distribution in the tumour microenvironment of IPMN and associated invasive pancreatic cancer lesions to date. In addition, we describe for the first time the presence of potential tumor-reactive Th9 cells in the pancreatic tumour stroma. The immune microenvironment evolves from a diverse T cell orchestra, comprising CD8+ T cells, Th/c1 and Th/c2 cells as major players in concert with Th9, Th/c17, Th22, and Treg cells in low-grade IPMN, to a Treg dominated immunosuppressive state in invasive pancreatic cancer. The major change with regards to T cell composition during IPMN progression occurs at the step of tissue invasion from high-grade IPMN to invasive pancreatic cancer, which was reinforced by an unbiased PCA separating low-and high-grade dysplasia from invasive carcinoma, suggesting that malignant invasion only occurs when tumour immune surveillance is overcome. Additional studies are required to refine the T cell composition, distinguishing Th from Tc subtypes and determine their functional role during pancreatic tumour development and progression. This might provide important insights, which could pave the way for the design of novel immunotherapies that might even boost spontaneous antitumor immunity at premalignant states and prevent pancreatic cancer development.
Declaration of competing interest
The authors declare no potential conflicts of interest.
Acknowledgments
Acknowledgments
We thank Domenic Hartmann for excellent technical assistance.
Funding sources
This work was supported by grants from German Cancer Aid (70112720 and 70113167 to Susanne Roth) and the Medical Faculty of Heidelberg University (Olympia Morata Programme to Susanne Roth).
Author contributions
S. R. conceived and designed the study, designed and performed experiments, analysed and interpreted data, and wrote the manuscript. K. Z. Designed and performed experiments, analysed and interpreted data and contributed to writing the manuscript. M. M. G. histopathologically evaluated all IPMN sections, analysed data and critically revised the manuscript. M. H. helped to develop automated immunohistochemical stainings and analysis, analysed and interpreted data. C. T. provided and analysed clinical data from IPMN patients. U. H, provided and analysed clinical data from IPMN patients, and critically revised statistical analyses. P. B. performed survival analyses. C. W. M. conceived and supervised the study. T. H. supervised the study, and critically revised and finalized the manuscript.
REFERENCES
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Biankin A.V., Kench J.G., Dijkman F.P., Biankin S.A., Henshall S.M. Molecular pathogenesis of precursor lesions of pancreatic ductal adenocarcinoma. Pathology. 2003;35(1):14–24. [PubMed] [Google Scholar]
- 3.Hruban R.H., Takaori K., Klimstra D.S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28(8):977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 4.Basturk O., Hong S.M., Wood L.D. A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39(12):1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong K., Nio C.Y., Hermans J.J. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8(9):806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Hackert T., Fritz S., Klauss M. Main-duct intraductal papillary mucinous neoplasm: high cancer risk in duct diameter of 5 to 9 mm. Ann Surg. 2015;262(5):875–880. doi: 10.1097/SLA.0000000000001462. discussion 80-1. [DOI] [PubMed] [Google Scholar]
- 7.Marchegiani G., Mino-Kenudson M., Sahora K. IPMN involving the main pancreatic duct: biology, epidemiology, and long-term outcomes following resection. Ann Surg. 2015;261(5):976–983. doi: 10.1097/SLA.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crippa S., Bassi C., Salvia R. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2017;66(3):495–506. doi: 10.1136/gutjnl-2015-310162. [DOI] [PubMed] [Google Scholar]
- 9.Clark C.E., Hingorani S.R., Mick R., Combs C., Tuveson D.A., Vonderheide R.H. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 10.Bernard V., Semaan A., Huang J. Single-Cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin Cancer Res. 2019;25(7):2194–2205. doi: 10.1158/1078-0432.CCR-18-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraoka N., Yamazaki-Itoh R., Ino Y. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140(1):310–321. doi: 10.1053/j.gastro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Balachandran V.P., Luksza M., Zhao J.N. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551(7681):512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukunaga A., Miyamoto M., Cho Y. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Annunziato F., Romagnani C., Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Mousset C.M., Hobo W., Woestenenk R., Preijers F., Dolstra H., van der Waart A.B. Comprehensive phenotyping of t cells using flow cytometry. Cytometry A. 2019 doi: 10.1002/cyto.a.23724. [DOI] [PubMed] [Google Scholar]
- 16.Hiraoka N., Ino Y., Yamazaki-Itoh R., Kanai Y., Kosuge T., Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112(11):1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz E.R., Wu A.A., Bigelow E. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2(7):616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blando J., Sharma A., Higa M.G. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights vista as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116(5):1692–1697. doi: 10.1073/pnas.1811067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stromnes I.M., Hulbert A., Pierce R.H., Greenberg P.D., Hingorani S.R. T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2017;5(11):978–991. doi: 10.1158/2326-6066.CIR-16-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ene-Obong A., Clear A.J., Watt J. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145(5):1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieu-Nosjean M.C., Giraldo N.A., Kaplon H., Germain C., Fridman W.H., Sautes-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271(1):260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 22.Wartenberg M., Cibin S., Zlobec I. Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin Cancer Res. 2018;24(18):4444–4454. doi: 10.1158/1078-0432.CCR-17-3401. [DOI] [PubMed] [Google Scholar]
- 23.Thorsson V., Gibbs D.L., Brown S.D. The immune landscape of cancer. Immunity. 2018;48(4):812–830. doi: 10.1016/j.immuni.2018.03.023. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castino G.F., Cortese N., Capretti G. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology. 2016;5(4) doi: 10.1080/2162402X.2015.1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Monte L., Reni M., Tassi E. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunderson A.J., Kaneda M.M., Tsujikawa T. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6(3):270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiraoka N., Onozato K., Kosuge T., Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 28.Ino Y., Yamazaki-Itoh R., Shimada K. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stromnes I.M., Brockenbrough J.S., Izeradjene K. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63(11):1769–1781. doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjomsland V., Niklasson L., Sandstrom P. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin Dev Immunol. 2011;2011 doi: 10.1155/2011/212810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Saint-Vis B., Vincent J., Vandenabeele S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9(3):325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 32.Di Caro G., Cortese N., Castino G.F. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 2016;65(10):1710–1720. doi: 10.1136/gutjnl-2015-309193. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi N., Kubota K., Kato S. FOXP3+ regulatory T cells and tumoral indoleamine 2,3-dioxygenase expression predicts the carcinogenesis of intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2010;10(5):631–640. doi: 10.1159/000308966. [DOI] [PubMed] [Google Scholar]
- 34.Joshi N.S., Akama-Garren E.H., Lu Y. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor t cell responses. Immunity. 2015;43(3):579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaput N., Conforti R., Viaud S., Spatz A., Zitvogel L. The janus face of dendritic cells in cancer. Oncogene. 2008;27(45):5920–5931. doi: 10.1038/onc.2008.270. [DOI] [PubMed] [Google Scholar]
- 36.Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y., Wang Q., Xue G. Th9 cells represent a unique subset of CD4(+) T cells endowed with the ability to eradicate advanced tumors. Cancer Cell. 2018;33(6):1048–1060. doi: 10.1016/j.ccell.2018.05.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kryczek I., Lin Y., Nagarsheth N. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2014;40(5):772–784. doi: 10.1016/j.immuni.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alam M.S., Gaida M.M., Bergmann F. Selective inhibition of the p38 alternative activation pathway in infiltrating T cells inhibits pancreatic cancer progression. Nat Med. 2015;21(11):1337–1343. doi: 10.1038/nm.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barilla R.M., Diskin B., Caso R.C. Specialized dendritic cells induce tumor-promoting IL-10(+)IL-17(+) FoxP3(neg) regulatory CD4(+) T cells in pancreatic carcinoma. Nat Commun. 2019;10(1):1424. doi: 10.1038/s41467-019-09416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAllister F., Bailey J.M., Alsina J. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25(5):621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Y., Hong S., Li H. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122(11):4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He W., Wu J., Shi J. IL22RA1/STAT3 signaling promotes stemness and tumorigenicity in pancreatic cancer. Cancer Res. 2018;78(12):3293–3305. doi: 10.1158/0008-5472.CAN-17-3131. [DOI] [PubMed] [Google Scholar]
- 44.Xu X., Tang Y., Guo S. Increased intratumoral interleukin 22 levels and frequencies of interleukin 22-producing CD4+ t cells correlate with pancreatic cancer progression. Pancreas. 2014;43(3):470–477. doi: 10.1097/MPA.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 45.Hu B., Qiu-Lan H., Lei R.E., Shi C., Jiang H.X., Qin S.Y. Interleukin-9 promotes pancreatic cancer cells proliferation and migration via the miR-200a/Beta-Catenin axis. Biomed Res Int. 2017;2017 doi: 10.1155/2017/2831056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang D., Zhu J. Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets. J Exp Med. 2017;214(7):1861–1876. doi: 10.1084/jem.20170494. [DOI] [PMC free article] [PubMed] [Google Scholar]