Abstract
Aim
The aim of this study is to assess the safety and efficacy of the new bidirectional rotational mechanical sheath TightRail™ (Spectranetics) for lead extraction.
Methods and results
This is a bicentric prospective study that included patients who underwent a transvenous lead extraction (TLE) in two Italian centers (San Raffaele Hospital and Humanitas Gavazzeni Hospital). From November 2016 to December 2018, 26 patients underwent a TLE procedure in which the TightRail™ was used. The new TightRail Sub‐C was used in 20 (76%) patients to overcome the fibrosis between the vessel and the first rib. Median age was 69 (IQR 60.7‐79.5) years. The indication for TLE were infection (57.7%) or lead dysfunction (42.3%). A total of 57 leads (range 1‐4), 40 of which using the TightRail (range 1‐4), were extracted. Overall mean implant duration was 98.2.0 ± 66.5 months. Mean age of the lead extracted with the TightRail sheath was 99.1 ± 70.2 months and was higher compared to that of the leads extracted manually (84.4 ± 60.3 months, P = .001). The overall clinical success was 100% and complete procedural success without the use of a snare was achieved in 98.3%. There were no cases of death or major complications and only two minor complications occurred. All patients were event‐free at 6‐month follow‐up.
Conclusion
This initial experience using the TightRail™ suggests a high safety and efficacy profile for extractions in a wide range of lead age.
Keywords: lead extraction, rotational sheath
The bidirectional rotational mechanical sheath TightRail™ (Spectranetics) is a novel tool for transvenous lead extraction (TLE). This is the first multicentric study evaluating the safety and efficacy of this new extraction system. This is the first experience with the new TightRail Sub‐C sheath to overcome the fibrous between the vessel and the first rib.
1. INTRODUCTION
In recent years, the use of cardiovascular implantable electronic devices has increased in parallel with the number of transvenous lead extraction (TLE) procedures due to either lead malfunction, infection, or system upgrade.1 A recent large European observational registry showed that TLE can be safely and effectively performed in high‐volume centers,2 but it can also be associated to serious and potentially life‐threatening complications.3 However, the removal of chronically implanted leads, which develop fibrous adhesions with surrounding vessel walls and tissues, could be challenging. To address this issue many extraction techniques have been developed including laser sheaths, mechanical sheaths, or electrosurgical dissection sheaths.4, 5, 6, 7 A bidirectional rotational mechanical sheath (Evolution RL, Cook medical) has proved to be extremely safe and effective with long implanted leads.8, 9 However, powered sheaths have been related to higher complication rates,2 and concerns can be raised about their use in case of recently implanted leads. The treated metal distal tip of Evolution RL allows the system to pass through adhesions but could also damage surrounding tissue. A new bidirectional rotational mechanical sheath TightRail (Spectranetics), with a more flexible shaft that provides high co‐axiality with the lead and a dilating blade that remains shielded until activated, is now available. Multicentric data about its safety and efficacy still lacks. The aim of this study was to report our preliminary experience with the TightRail (Spectranetics) sheath for lead extractions at two tertiary care Italian centers.
2. METHODS
2.1. Patients
This is a prospective study that included all patients who underwent a TLE in which the TightRail was used, either alone or in association with other extraction sheath, at two tertiary referral centers: San Raffaele Hospital (Milan, Italy) and Humanitas Gavazzeni Hospital (Brescia, Italy). Indication for TLE included infection and lead malfunction. For each lead, the underlying type (active/passive fixation, single double coil catheters), duration of implant, and fixation modality were recorded. All patients provided a written informed consent before the procedure and the study was approved by the Institutional Committee of Human Research at our Hospital.
2.2. Extraction procedure
All TLE procedures were conducted in the electrophysiology laboratory by two skilled operators (PM and GM), with an experience of more than one thousand of extracted catheters each and ability to perform TLE with a wide range of tools, including laser and rotational sheaths, and performed under general anesthesia or sedation and ECG, pulse oximetry, and arterial blood pressure monitoring, with a cardiothoracic surgeon on standby.10 A subclavian approach was initially preferred; if the patient was pacemaker (PM) dependent, a temporary right ventricular active fixation lead was placed from the femoral vein. All patients underwent TLE with a standard stepwise approach, as previously described.11 At first, the leads were dissected free from the scar in the pocket, then simple manual traction or traction on a locking stylet (Liberator Universal locking stylet, Cook Vascular Inc and/or LLD® Lead Locking Device; Spectranetics), was attempted, avoiding disrupting the lead integrity. If unsuccessful, the new TightRail™ (Spectranetics) dilator sheath was used. In cases of excessive fibrous adhesions or calcification between the vessel and first rib the TightRail Sub‐C (Spectranetics), a shorter mechanical dilator, was used at the discretion of the physician. The bidirectional mechanical dilator sheath Evolution RL (Cook Medical) was used depending on the operator choice, when the targeted leads included chronically implanted leads (>10 years), considering that long‐standing leads (>10 years) are independent predictors of clinical failure.2 Free floating leads, remnants after extraction, were retrieved using a snare such as AndraSnare (Andramed GmbH) or Needle's Eye Snare (Cook Vascular) via either a right jugular or a femoral approach.
The TightRail dilator sheath is composed by an inner and outer shaft and a handled drive mechanism (Figure 1). The inner shaft (drive shaft) is available in French sizes of 9, 11, and 13, and is able to rotate within the outer shaft. The rotary dilating feature at the tip remains shielded until activated, allowing a safe progression through the vessel. The stationary outer shaft is contained within a polymer jacket and is extremely flexible, enabling to remain coaxial to the lead. The handheld drive mechanism attached to the proximal end of the device is used to rotate the inner shaft. Rotation of the distal cam of the inner shaft causes dilation of the tissue and fibrous attachments surrounding the lead facilitating its removal. The bidirectional rotational mechanism should avoid the lead wrapping phenomenon, a possible complication of unidirectional rotational sheaths. An outer sheath can be used in conjunction with the device to support the device shaft facilitating an additional tissue dilation effect and serve as a conduit for reimplant.
Figure 1.
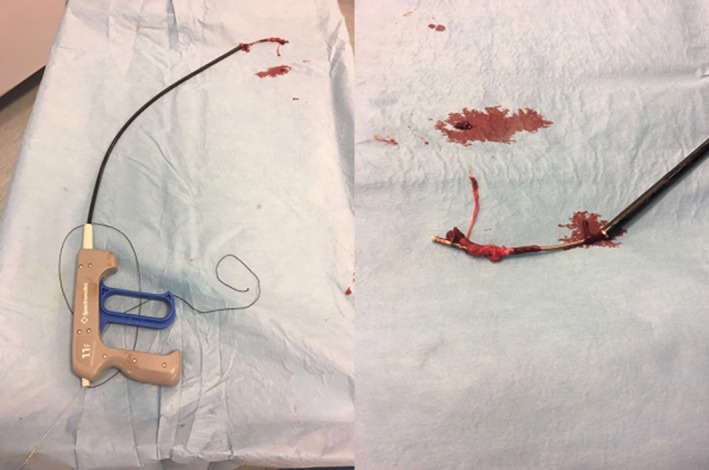
The TightRail dilator sheath is composed by an inner and outer shaft and a handled drive mechanism used to rotate the inner shaft
A shorter, stiffer, and more aggressive version of the TightRail, the so‐called Sub‐C, is available to overcome heavy calcifications under the clavicle, which are not uncommon.
2.3. Outcomes
All patients were monitored for procedure‐related complications in the operating room and during hospital stay. Clinical outcomes and adverse events were prospectively monitored at 30 days and 6 months by ambulatory direct visit or phone interview.
2.4. Definitions
Complete procedural success corresponded to the removal of all targeted leads and all lead material from the vascular space, without complication or procedure‐related death. Clinical success was defined as the removal of all targeted leads and all lead material from the vascular space or retention of a small portion of the lead that does not negatively impact the outcome goals of the procedure. Any event occurring while the patient was in the operating room and all events related to the procedure occurring within 30 days were classified as intraprocedural complications or postprocedural complications, respectively.12, 13
2.5. Statistical analysis
Categorical variables are presented as absolute rates and percentages, continuous variables are presented as mean ± standard deviation (SD) or median (and interquartile range [IQR]), and compared with Student's t test or Mann‐Whitney or Wilcoxon tests, according to the normality of the data, verified by Kolmogorov‐Smirnov goodness‐of‐fit test. All data were analyzed with the IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp.).
3. RESULTS
The TightRail™ (Spectranetics) was first used at San Raffaele Hospital in November 2016 and at Humanitas Gavazzeni Hospital in November 2017. From November 2016 to November 2018, 26 patients (26.9% females) underwent a TLE procedure in which the TightRail™ was used. Baseline population characteristics and complete patient details are provided in Tables 1 and 2, respectively.
Table 1.
Population and explanted leads characteristics
| Patients, n | 26 |
| Clinical variables | |
| Age, y, median (IQR) | 69 (60.7‐79.5) |
| Female sex, n (%) | 7 (26.9) |
| Chronic kidney disease, n (%) | 7 (26.9) |
| Creatinine clearance, ml/min, median (IQR) | 71.7 (55.5‐92.0) |
| Indication for implantation, n (%) | |
| Bradyarrhythmias | 11 (41.6) |
| Ventricular tachycardia | 15 (58.4) |
| Indication for explantation, n (%) | |
| Malfunction | 11 (42.3) |
| Endocarditis | 8 (30.7) |
| Pocket infection | 7 (26.9) |
| Extracted leads (TightRail), n | 40 |
| Extracted leads (overall), n | 57 |
| Generator, n (%) | |
| Pacemaker | 10 (38.5) |
| ICD | 7 (26.9) |
| CRT‐D | 8 (30.8) |
| CRT‐P | 1 (3.8) |
| Fixation (TightRail), n (%) | |
| Active | 10 (25) |
| Passive | 30 (75) |
| Mean time from implant (TightRail), mo, mean ± SD | 99.1 ± 70.2 |
| Mean time from implant (overall), mo, mean ± SD | 98.2 ± 66.5 |
| Number of leads per patient (TightRail), n, mean ± SD | 1.6 ± 0.7 |
| Number of leads per patient (overall), n, mean ± SD | 2.2 ± 0.7 |
| Lead type (TightRail), n (%) | |
| RA | 15 (37.5) |
| RV | 8 (20.0) |
| RV ICD | 13 (32.5) |
| LV | 4 (10.0) |
| Dilator sheath diameter (TightRail), n (%) | |
| 11 mm | 22 (61.1) |
| 13 mm | 16 (38.9) |
Table 2.
Patients list and clinical characteristic
| Patient | Age (y) | Disease | CrCl (ml/min) | Device | Reason for extraction | Reimplant | Reimplant site |
|---|---|---|---|---|---|---|---|
| #1 | 53 | Complete AV block after surgical aortic valve replacement | 45 | Dual chamber PM | Endocarditis | Yes | Contralateral |
| #2 | 66 | IDCM | 69 | CRT‐D | Pocket infection | Yes | Contralateral |
| #3 | 74 | IDCM | 73 | CRT‐D | Lead dysfunction | Yes | Contralateral |
| #4 | 85 | Sinus node dysfunction | 30 | Dual chamber PM | Endocarditis | No | / |
| #5 | 62 | AV block | 80 | Dual chamber PM | Lead malfunction | Yes | Ipsilateral |
| #6 | 77 | Idiopathic VT | 29 | Dual chamber ICD | Pocket infection | Yes | Contralateral |
| #7 | 84 | IDCM | 65 | CRT‐D | Endocarditis | Yes | Ipsilateral (external PM) |
| #8 | 69 | Valvular heart disease | 82 | CRT‐D | Lead malfunction | Yes | Contralateral |
| #9 | 68 | IDCM | 87 | Dual chamber ICD | Lead malfunction | Yes | Ipsilateral |
| #10 | 87 | IDCM | 33 | CRT‐D | Pocket infection | Yes | Contralateral |
| #11 | 37 | AV block | 110 | Dual chamber PM | Pocket infection | No | / |
| #12 | 68 | Valvular heart disease | 36 |
Dual chamber ICD |
Endocarditis | Yes | Contralateral |
| #13 | 82 | AV Block | 31 | CRT‐P | Infection | No | / |
| #14 | 58 | NIDCM | 100 | CRT‐D | Infection | Yes | Contralateral |
| #15 | 81 | AV block | 66 | Dual chamber PM | Infection | Yes | Contralateral |
| #16 | 44 | AV block | 90 | Dual chamber PM | Lead malfunction | Yes | Ipsilateral |
| #17 | 38 | Ventricular tachycardia | 107 | Dual chamber ICD | Lead malfunction | Yes | Ipsilateral |
| #18 | 79 | IDCM | 75 | CRT‐D | Lead malfunction | Yes | Ipsilateral |
| #19 | 73 | Sinus node dysfunction | 61 | Dual chamber PM | Succlavian obstruction | Yes | Contralateral |
| #20 | 86 | Ventricular tachycardia | 68 | Dual chamber ICD | Infection | Yes | Contralateral |
| #21 | 69 | AV block | 71 | Dual chamber PM | Lead malfunction | Yes | Ipsilateral |
| #22 | 63 | Av Block | 110 |
Dual chamber PM |
Infection | No | / |
| #23 | 61 | IDCM | 59 | Dual chamber ICD | Lead malfunction | Yes | Ipsilateral |
| #24 | 60 | IDCM | 98 | CRT‐D | Lead malfunction | Yes | Ipsilateral |
| #25 | 72 | AV block | 105 | Dual chamber PM | Infection | No | / |
| #26 | 72 | IDCM | 85 | ICD | Infection | No | / |
Abbreviations: AV, atrioventricular; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance, calculated using the MDRD equation; CRT‐D, cardiac resynchronization therapy – defibrillator; IDCM, ischemic dilated cardiomyopathy; LVEF, left ventricular ejection fraction; NIDCM, non‐ischemic dilated cardiomyopathy; PM, pacemaker; T2DM, type 2 diabetes mellitus; VT, ventricular tachycardia.
Median age was 69 (IQR 60.7‐79.5) years. Seven patients (26.9%) were affected by chronic kidney disease (median creatinine clearance was 71.7 [IQR 55.5‐92.0] ml/min). At preoperative transthoracic echocardiography evaluation, mean left ventricular ejection fraction was 45.5 ± 12.5%.
The indication for TLE were infection (57.7%), either endocarditis (30.8%) or pocket infection (26.9%), or lead dysfunction (42.3%).
Characteristics of extracted leads and complete procedural details are provided in Tables 1 and 3, respectively.
Table 3.
Procedural characteristics
| Patient | Number of leads | Total lead extracted | Leads extracted with TightRail | Type of lead | Fixation | Coils | Other techniques used | Leads age (mo) | TightRail sheath (French) | Use of snare | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 2 | 2 | 2 (RA, RV) | RA | Passive | / | / | 77 | 13 | No | No |
| RV | Passive | / | / | 77 | No | ||||||
| #2 | 2 | 2 | 1 (RV ICD) | RV ICD | Passive | Single | / | 34 | 13 | No | No |
| RA | Passive | / | Traction | 34 | No | ||||||
| #3 | 3 | 3 | 1 (LV) | LV | Passive | / | / | 129 | 13 | No | Yes (minor pneumothorax) |
| RA | Passive | / | Evolution RL | 129 | No | ||||||
| RV ICD | Passive | Single | Evolution RL | 129 | Yes | ||||||
| #4 | 3 | 3 | 1 (RA) | RA | Active | / | / | 41 | 11 | No | No |
| RV | Active | / | Locking stylet | 41 | No | ||||||
| RV | Active | / | Traction | 29 | No | ||||||
| #5 | 2 | 2 | 2 (RA, RV) | RA | Active | / | / | 49 | 11 | No | No |
| RV | Active | / | / | 49 | No | ||||||
| #6 | 3 | 3 | 2 (RA, RV) | RA | Passive | / | / | 89 | 13 | No | No |
| RV ICD | Passive | Dual | / | 89 | 13 | No | |||||
| RV ICD | Active | Single | Traction | 3 | No | ||||||
| #7 | 2 | 2 | 2 (RV ICD, LV) | RV ICD | Active | Single | / | 24 | 11 | No | No |
| LV | Passive | / | / | 24 | 11 | No | |||||
| #8 | 4 | 4 | 3 (RA, RV ICD, RV, LV) | RA | Passive | / | Evolution RL | 157 | No | Yes | |
| RV | Passive | / | / | 157 | 11 | No | (hematoma) | ||||
| RV ICD | Active | Single | / | 25 | 11 | No | |||||
| LV | Passive | / | / | 25 | 11 | No | |||||
| #9 | 2 | 2 | 2 (RA, RV ICD) | RA | Passive | / | / | 62 | 13 | No | No |
| RV ICD | Active | Single | / | 62 | 13 | No | |||||
| #10 | 3 | 3 | 3 (RA, RV ICD, LV) | RA | Passive | / | / | 51 | 13 | No | No |
| RV ICD | Passive | Dual | / | 51 | 13 | No | |||||
| LV | Passive | / | / | 51 | 13 | No | |||||
| #11 | 2 | 2 | 1 (RA) | RA | Passive | / | / | 164 | 11 | No | No |
| RV | Passive | / | Evolution RL | 164 | 11 | No | |||||
| #12 | 2 | 2 | 2 (RA, RV ICD) | RA | Passive | / | / | 58 | 11 | No | No |
| RV ICD | Active | Single | / | 58 | 13 | No | |||||
| #13 | 3 | 3 | 1 (RA) | RA | Passive | / | / | 196 | 11 | No | No |
| RV | Passive | / | Manual | 196 | No | ||||||
| LV | Passive | / | Manual | 196 | No | ||||||
| #14 | 3 | 3 | 1 (RV ICD) | RV ICD | Active | Single | / | 68 | 11 | No | |
| RA | Active | / | Manual | 68 | No | ||||||
| LV | Passive | / | Manual | 68 | No | No | |||||
| #15 | 2 | 2 | 1 (RV) | RV | Passive | / | / | 81 | 11 | No | No |
| RA | Passive | / | Manual | 81 | No | ||||||
| #16 | 2 | 1 | 1 (RA) | RA | Passive | / | / | 291 | 11 | No | No |
| #17 | 2 | 2 | 1 (RV ICD) | RV ICD | Passive | Single | / | 227 | 11 | No | No |
| RA | Passive | / | Manual | 100 | No | ||||||
| #18 | 3 | 2 | 1 (RA) | RA | Passive | / | / | 109 | 11 | No | No |
| LV | Passive | / | Manual | 109 | No | ||||||
| #19 | 2 | 2 | 1 (RA) | RA | Passive | / | / | 328 | 11 | No | No |
| RV | Active | / | Manual | 45 | No | ||||||
| #20 | 2 | 2 | 2 (RA, RV ICD) | RA | Passive | / | / | 170 | 11 | No | No |
| RV ICD | Passive | Single | / | 170 | 11 | No | |||||
| #21 | 2 | 2 | 2 (RA, RV) | RA | Active | / | / | 84 | 13 | No | No |
| RV | Active | / | / | 84 | 13 | No | |||||
| #22 | 2 | 2 | 2 (RA, RV) | RA | Passive | / | / | 113 | 11 | No | No |
| RV | Passive | / | / | 113 | 11 | No | |||||
| #23 | 1 | 1 | 1 (RV ICD) | RV ICD | Passive | Single | / | 157 | 13 | No | No |
| #24 | 3 | 1 | 1 (RV ICD) | RV ICD | Passive | Single | / | 36 | 11 | No | No |
| #25 | 2 | 2 | 1 (RV) | RV | Passive | / | / | 84 | 11 | No | No |
| RA | Passive | / | Manual | 84 | |||||||
| #26 | 2 | 2 | 2 (RA, RV ICD) | RV ICD | Passive | Single | / | 103 | 13 | No | No |
| RA | Passive | / | / | 103 | 13 | No | No |
Abbreviations: ICD, implantable cardioverter defibrillator; LV, left ventricular lead; RA, right atrial lead; RV, right ventricular lead.
The extracted device was a dual chamber PM in 10 (38.5%) cases, dual chamber ICD in 7 (26.9%) cases, CRT‐D in 8 (30.8%), and CRT‐P in the remaining 1 case.
A total of 57 leads (range 1‐4), 40 of which using the TightRail (range 1‐4), were extracted; of these, 42 (73.7%) were pacing leads and 15 leads were ICD (13 single coil vs 2 dual coil). Among leads extracted with TightRail, 15 (37.5%) were right atrium leads, 8 (20.0%) were right ventricular pacing leads, 13 (32.5%) were right ventricular ICD leads and the remaining 4 (10.0%) were resynchronizing left ventricular lead.
Overall mean implant duration was 98.2.0 ± 66.5 (range 24‐328) months. Mean age of the lead extracted with the TightRail sheath was 99.1 ± 70.2 (range 24‐328) months and was higher compared to that of the leads extracted manually (84.4 ± 60.3 months, P = .001). Twenty‐two patients underwent device reimplant in a second procedure during the same hospitalization; after collegial discussion, reimplantation was not performed in four patients. All the leads were extracted using initially the subclavian approach. Twenty patients (76%), because of adherence between the vessel and the first rib, were initially approached with the TightRail Sub‐C.
The overall clinical success was 100%. Complete procedural success without the use of a snare was achieved in 98.3%. In one case (patient #11), the Evolution RL was needed to complete the extraction of a right ventricular pacing lead after a first ineffective attempt with the TightRail, while in other two (#3, #8) the Evolution RL was used to complete the extraction under operator's choice. There were no cases of death or major complications and only two minor complications occurred: small pneumothorax treated conservatively in patient #3 and a hematoma of the device pocket in patient #8 who was on anticoagulant therapy for atrial fibrillation. No patient required admission to the internal care unit after the procedure.
Complete follow‐up was available for all patients. The 30‐day and 6‐month survival rate were 100%. Patient #1 underwent successful aortic valve substitution 1 month after TLE. All patients were event‐free at 6‐month follow‐up.
4. DISCUSSION
To the best of our knowledge, this is the first prospective multicentric study assessing the safety and efficacy of the new bidirectional rotating dilator sheath TightRail™ (Spectranetics). In our preliminary experience, we demonstrated that it is an effective tool for extracting chronic implanted leads (up to 227 months for right ventricular leads, 129 months for left ventricular leads) when both manual traction or the use of a locking stylet are ineffective, with excellent results at 6‐month follow‐up.
Only two single‐center experiences with the TightRail™ have been reported: Aytemir used it in 23 patients with a median time from implantation of 72 months14; compared to this report, in our multicentric experience the mean lead age was higher; Sawhney described successful TLE with TightRail™ in 3 patients.15 Compared to these previous reports, in our multicentric experience the median lead age was higher, further confirming the possibility to use the TightRail™ also in long‐standing, chronically implanted leads.
Moreover, we are the first to describe the use of TightRail Sub‐C to exceed the excessive fibrous adhesions or calcification between the vessel and first rib that was previously considered a critical issue about the use of TightRail for lead extraction.
Amount of evidence is available about the new Evolution RL mechanical sheath that has proven to be safe and effective for long‐standing lead extraction in single and multicentric registries.8, 16 The use of powered sheaths has been shown to be associated with higher procedure related major complications.2 However, the TightRail, because of its technical characteristics, has a theoretically safer profile compared to other mechanical extraction sheaths, as Evolution. The extreme flexibility of the outer shaft should allow a higher coaxiality to the lead, reducing the risk of lead fracture. The dilating blade that remains hidden until activated, should guarantee minor possibility of damage to vessel walls and surrounding structures during catheter advancement. The Laser system, differently from mechanical sheath (Evolution and TightRail) uses an excimer laser that acts by breaking intracellular tissue bonds, vaporizing fibrotic sheaths surrounding targeted leads, while not damaging other leads. Compared to laser sheath in a study on 121 patients, the Evolution exhibited acceptably high levels of procedural and clinical success, although additional use of snare was required.11 However, the two techniques may be considered equally effective for TLE. Actually, direct comparison between the three techniques are not available yet and further studies are warranted to provide insights into this setting and determine if there may be one or more technique suitable in a specific subset of patients.
Safety is a main issue in TLE, and the TightRail, with his potentially safe profile, allowed us to achieve a successful and safe extraction in patients with a wide range of lead age. In only one case (patient #11), the TightRail was ineffective in extracting a long‐standing lead (164 months) due to excessive fibrosis and the Evolution RL was needed to complete the extraction of a right ventricular pacing lead.
As shown in a recent large European observational study, lead extraction procedure attempted in low‐volume centers (<30 procedures/y) was an independent predictor of clinical failure and all‐cause mortality.2 It is therefore advisable that TLE procedures be performed in centers with suitable volume and operators' experience. However, considering the increasing need for TLE procedures in the near future, the request could overcome the volume capacity of high‐experienced centers. It would be appropriate for less‐experienced centers to refer high‐risk patients with potentially complicated TLE to high‐experienced centers and operators and attempt at first TLE in low‐risk patients. The safety and feasibility of this strategy warrant further investigations. Our study is a first step toward this opportunity, showing that TightRail™ (Spectranetics), with its extremely safe profile, is a valid additional tool that could be used for TLE with excellent outcomes at midterm follow‐up.
4.1. Limitations
The main limitations are represented by the small size of the population studied and by the lack of comparison with other mechanical dilator sheaths. Larger scale randomized studies should be conducted in the future to identify appropriate decision‐making and therapeutic strategies to deal with the increasing request of TLE procedures.
5. CONCLUSIONS
This initial experience using the TightRail suggests a high safety and efficacy profile for lead extraction when initial fibrous adherence is present.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this article.
INFORMED CONSENT
All patients provided informed consent for data collection and the study was in accordance with the ethical standards of the institutional research committee.
ACKNOWLEDGMENTS
We thank Terenzia Natale for helping in data collection.
Mazzone P, Melillo F, Radinovic A, et al. Use of the new rotating dilator sheath TightRail™ for lead extraction: A bicentric experience. J Arrhythmia. 2020;36:343–350. 10.1002/joa3.12310
REFERENCES
- 1. Maytin M, Epstein LM. Lead extraction is preferred for lead revisions and system upgrades when less is more. Circ Arrhythm Electrophysiol. 2010;3(4):413–24. [DOI] [PubMed] [Google Scholar]
- 2. Bongiorni MG, Kennergren C, Butter C, Deharo JC, Kutarski A, Rinaldi CA, et al. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J. 2017;28:180–4. [DOI] [PubMed] [Google Scholar]
- 3. Hauser RG, Katsiyiannis WT, Gornick CC, Almquist AK, Kallinen LM. Deaths and cardiovascular injuries due to device‐assisted implantable cardioverter‐defibrillator and pacemaker lead extraction. Europace. 2010;12(3):395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R, et al. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33(6):1671–6. [DOI] [PubMed] [Google Scholar]
- 5. Kennergren C, Bucknall CA, Butter C, Charles R, Fuhrer J, Grosfeld M, et al. Laser‐assisted lead extraction: the European experience. Europace. 2007;9(8):651–6. [DOI] [PubMed] [Google Scholar]
- 6. Scott PA, Chow W, Ellis E, Morgan JM, Roberts PR. Extraction of pacemaker and implantable cardioverter defibrillator leads: a single‐centre study of electrosurgical and laser extraction. Europace. 2009;11(11):1501–4. [DOI] [PubMed] [Google Scholar]
- 7. Starck CT, Rodriguez H, Hurlimann D, Grunenfelder J, Steffel J, Salzberg SP, et al. Transvenous lead extractions: comparison of laser vs. mechanical approach. Europace. 2013;15(11):1636–41. [DOI] [PubMed] [Google Scholar]
- 8. Mazzone P, Migliore F, Bertaglia E, Facchin D, Daleffe E, Calzolari V, et al. Safety and efficacy of the new bidirectional rotational Evolution® mechanical lead extraction sheath: results from a multicentre Italian registry. Europace. 2018;20(5):829–34. [DOI] [PubMed] [Google Scholar]
- 9. Migliore F, Carretta D, Piazzi E, Zorzi A, Marzi A, Gerosa G, et al. Multicenter experience with the Evolution RL mechanical sheath for lead extraction using a stepwise approach: safety, effectiveness, and outcome. Pacing Clin Electrophysiol. 2019;42(7):989–97. [DOI] [PubMed] [Google Scholar]
- 10. Monaco F, Di Tomasso N, Landoni G, Nardelli P, Radinovic A, Melillo F, et al. Predictors of intensive care unit admission in patients undergoing lead extraction: a 10‐year observational study in a high‐volume center. J Cardiothorac Vasc Anesth. 2019;33(7):1845–51. [DOI] [PubMed] [Google Scholar]
- 11. Mazzone P, Tsiachris D, Marzi A, Ciconte G, Paglino G, Sora N, et al. Advanced techniques for chronic lead extraction: heading from the laser towards the evolution system. Europace. 2013;15(12):1771–6. [DOI] [PubMed] [Google Scholar]
- 12. Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH, et al. Transvenous lead extraction: heart rhythm society expert consensus on facilities, training, indications, and patient management. Hear Rhythm. 2009;6(7):1085–104. [DOI] [PubMed] [Google Scholar]
- 13. Deharo JC, Bongiorni MG, Rozkovec A, Bracke F, Defaye P, Fernandez‐Lozano I, et al. Erratum: Pathways for training and accreditation for transvenous lead extraction: a European Heart Rhythm Association position paper (Europace (2012) 14, (124–134) DOI: 10.1093/europace/eur338). Europace. 2012;14(4):460. [DOI] [PubMed] [Google Scholar]
- 14. Aytemir K, Yorgun H, Levent MS. Initial experience with the TightRail™ Rotating Mechanical Dilator Sheath for transvenous lead extraction. Europace. 2016;18(7):1043–8. [DOI] [PubMed] [Google Scholar]
- 15. Sawhney V, Breitenstein A, Sporton S, Dhinoja M. Percutaneous lead extraction and venous recanalisation using spectranetics TightRail: a single centre experience. Indian Pacing Electrophysiol J. 2016;16(4):134–8. [Google Scholar]
- 16. Witte OA, Adiyaman A, Smit JJJ, Ramdat Misier AR, Elvan A, Ghani A, et al. Success and complication rates of lead extraction with the first‐ vs. the second‐generation evolution mechanical sheath. Europace. 2017;19(10):1717–22. [DOI] [PubMed] [Google Scholar]


