Abstract
Background
Patients with relapsed or refractory (R/R) high-risk chronic lymphocytic leukaemia (CLL) or mantle cell lymphoma (MCL) often do not derive durable benefit from ibrutinib monotherapy. We hypothesized that dual B-cell receptor pathway blockade would be tolerable and efficacious. We conducted a phase 1/1b, investigator-initiated, multicentre study of a next-generation phosphoinositol-3-kinase-δ inhibitor (PI3K-δi) umbralisib (TGR-1202) plus ibrutinib in R/R CLL and MCL.
Methods
The intent-to-treat population consisted of patients ≥18 years with R/R CLL or MCL with an ECOG PS ≤2 were treated daily with umbralisib (400, 600, or 800 mg) and ibrutinib (420 mg CLL/560 mg MCL) until progression or unacceptable toxicity. The primary endpoints were MTD, safety, and DLTs. This trial is ongoing and is registered on clinicaltrials.gov (NCT02268851).
Findings
Forty-two patients were treated on study (21 CLL/21 MCL), median age 68 and 2 prior therapies (range 1–6). No DLTs were observed, and the MTD of umbralisib was not reached. The recommended phase 2 dose (RP2D) of umbralisib with ibrutinib was 800 mg daily. The most frequent AEs were diarrhoea (22 [52%] patients, [9·5% grade 3]), infection (21 [50%] patients [17% grade 3/4]), and transaminitis (10 [24%] patients, [2·4% grade 3]). Serious AEs occurred in 29% (12/42) of patients, and included lipase elevation, atrial fibrillation, hypophosphataemia, adrenal insufficiency, transaminitis, and infections.
Interpretation
Umbralisib plus ibrutinib is well-tolerated and active in R/R CLL and MCL, with a RP2D of umbralisib 800 mg daily. To the best of our knowledge, these represent the first clinical data on a BTKi/PI3K-δi doublet in B-cell malignancies, and the results suggest that this approach is feasible and worthy of further study.
Keywords: Chronic Lymphocytic Leukemia, Mantle Cell Lymphoma, Clinical Trial
Introduction
The Bruton tyrosine kinase inhibitor (BTKi) ibrutinib is an effective therapy for many patients with chronic lymphocytic leukaemia and mantle cell lymphoma; however, few patients with relapsed or refractory (R/R) chronic lymphocytic leukaemia or mantle cell lymphoma will achieve complete remission on ibrutinib monotherapy.1,2 Moreover, the selective pressure of ibrutinib monotherapy can lead to resistance mechanisms, such as the C481S binding-site mutation in BTK, which decreases the ability of ibrutinib to inhibit BTK, as well as mutations in downstream proteins like PLCγ−2.3 These mutations are likely the predominant mechanism of resistance in high-risk chronic lymphocytic leukaemia,4 and they have also been described in mantle cell lymphoma.5 Thus, although ibrutinib has a high overall response rate (ORR), the median progression-free survival (PFS) following ibrutinib monotherapy in patients with R/R mantle cell lymphoma or high-risk forms of R/R chronic lymphocytic leukaemia, such as those patients who harbour del(17p), are a modest 13·9 months and 26 months, respectively.6,7 As such, novel agent combination strategies are being explored to improve the durability of therapy for these patient populations.
One such approach is dual inhibition of key kinases in the B-cell receptor (BCR) signaling pathway. Preclinical studies showed the potential for synergy of the combination of BTKi with phosphoinositide-3-kinase delta isoform inhibition (PI3K-δi), first with in vitro models of chronic lymphocytic leukaemia and MCL, and later in vivo in a chronic lymphocytic leukaemia mouse model.8,9 An early attempt to combine a PI3K-δi with a spleen tyrosine kinase inhibitor (SYKi)led to unexpectedly high rates of immune-mediated toxicities, particularly severe pneumonitis.10 Based on that trial, it was unclear whether dual BCR pathway blockade would be prohibitively toxic, or whether it may be a feasible strategy if better tolerated agents targeting this same pathway were utilised.
Umbralisib is a next-generation oral PI3K-δ inhibitor with a differentiated safety profile and a chemical structure distinct from other PI3K-δ inhibitors. The drug has a unique kinome inhibition profile, with excellent specificity for PI3K-δ and only one significant known off-target effect on casein-kinase 1 epsilon (CK-1ε).11 In a phase 1 study of umbralisib monotherapy in R/R chronic lymphocytic leukaemia and R/R mantle cell lymphoma, the drug was well tolerated, and a recommended phase 2 dose (RP2D) of 800 mg daily was defined.11 Immune-mediated toxicities were observed at a lower rate than was seen in earlier studies with similar patient populations treated with the currently approved PI3K-δ inhibitors idelalisib and duvelisib.12,13 Moreover, umbralisib monotherapy was active, with disease reductions observed in all 20 patients with R/R chronic lymphocytic leukaemia and in five of six patients with R/R mantle cell lymphoma. The favorable safety profile of umbralisib has been observed across several trials,14 although the mechanism underlying this difference remains uncertain. Recent preclinical data suggest that CK-1ε inhibition may lead to augmentation of regulatory T-cell activity,15,16 which could hypothetically lead to immune suppression and thereby lower the risk of immune-mediated toxicities, though its effects on efficacy are uncertain.
We hypothesized that dual blockade of PI3K-δ and BTK with umbralisib in combination with ibrutinib would be a well-tolerated regimen. We designed this phase 1/1b trial to determine the maximum tolerated dose (MTD) of umbralisib in combination with ibrutinib and to explore the safety and efficacy of these two novel, oral, once-daily, targeted agents in patients with R/R chronic lymphocytic leukaemia and R/R mantle cell lymphoma.
Methods
Study Design and Participants
Between December 5, 2014 and March 7, 2018, eligible patients with R/R chronic lymphocytic leukaemia or mantle cell lymphoma were enrolled at five trial sites in this investigator-initiated, multicentre study of umbralisib + ibrutinib. Nine patients were enrolled into each of the two phase 1 dose escalation cohorts in chronic lymphocytic leukaemia and mantle cell lymphoma. Subsequently, 12 patient phase 1b expansion cohorts were enrolled for each chronic lymphocytic leukaemia and mantle cell lymphoma. This study was approved by local institutional review boards, and all patients provided written informed consent. The study was designed according to Good Clinical Practice guidelines and the Declaration of Helsinki. Study oversight was provided with support from the Blood Cancer Research Partnership (BCRP), funded by the Leukemia & Lymphoma Society.
Patients ≥18 years with a confirmed diagnosis of chronic lymphocytic leukaemia or mantle cell lymphoma were eligible if they had received ≥1 prior standard therapy and required therapy according to standard critera,17,18 had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≤2, and had adequate organ function (ANC ≥0·5K/uL, bilirubin ≤1·5xULN), ALT and AST≤2x ULN or ≤4x ULN with liver involvement, creatinine ≤2·5mg/dL). Patients with prior BTK/PI3Ki therapy were eligible and could be on ibrutinib monotherapy for up to 6 months prior to starting umbralisib. Patients were excluded from the study if they had an autologous stem cell transplantation (autoSCT) < 3 months or an allogeneic hematopoietic cell transplantation <12 months from study entry, active hepatitis, HIV, central nervous system involvement, or required warfarin (other anticoagulants were allowed).
Procedures
The phase 1 study cohorts included parallel chronic lymphocytic leukaemia and mantle cell lymphoma arms, which escalated independently in a standard 3 × 3 design. Umbralisib (TG Therapeutics, Inc., New York, NY, USA) was given orally daily at 400 mg (dose level 1), 600 mg (dose level 2), or 800 mg (dose level 3). Ibrutinib (commercially available)was given at the US Food and Drug Administration-approved doses of 420 or 560 mg orally daily for chronic lymphocytic leukaemia and mantle cell lymphoma, respectively. Antimicrobial prophylaxis for pneumocystis and herpes viruses was required. Dose reductions and interruptions were applied as per protocol. Patients continued on both agents in 28-day cycles until time of progression or unacceptable toxicity and were followed after discontinuing protocol therapy for up to 2 years or until next line of therapy.
Toxicity was assessed during treatment and for 30 days following discontinuation using Common Terminology Criteria for Adverse Events v4.03. The DLT observation period was the first cycle (28 days). Efficacy and haematological toxicity were assessed by 2008 International Workshop on chronic lymphocytic leukaemia (IW-CLL) criteria for chronic lymphocytic leukaemia patients17 and the 2014 Lugano criteria for mantle cell lymphoma patients.18 Response assessments were performed approximately 8 weeks after cycle 1/day 1 and just prior to cycles 6, 10, and 15. After cycle 15, efficacy assessments occurred every 6 cycles.
Outcomes
The primary endpoints were the MTD for umbralisib when given in combination with ibrutinib, as well as safety, and dose-limiting toxicities (DLTs) for this combination (See Appendix, page 6 of Supplemental). Secondary endpoints included ORR (CR+PR), complete response (CR), partial response (PR), PR with lymphocytosis (PR-L), PFS, duration of remission, overall survival (OS), as well as association of chronic lymphocytic leukaemia prognostic factors, including fluorescence in situ hybridization (FISH) cytogenetics and immunoglobulin heavy chain variable region sequence (IGHV) with response.
Statistical Analysis
The statistical analysis included assessment of response rates and duration of response, which were summarised descriptively. The Kaplan-Meier method was used to estimate PFS and OS and the log-rank test was used for group comparison. PFS was defined as time from study entry to tumour progression or death from any cause. OS was defined as time from study entry to death from any cause. Where feasible, association between clinical response and chronic lymphocytic leukaemia prognostic markers was explored in a descriptive analysis. Unless otherwise stated, all statistical analyses were performed using a two-sided hypothesis test at an overall significance level of 5%. Multiplicity was not considered. The intent-to-treat population consisted of all patients who were enrolled and had at least one dose of study drug, and the primary efficacy and safety analyses were performed on this intent-to-treat population. There was no pre-planned interim analysis. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), and R version 3.2.2 (the CRAN project, www.cran.r-project.org). This trial is registered on clinicaltrials.gov (NCT02268851).
Role of the Funding Source
The study was designed by the trial investigators with input from the funding sources. Trial investigators were responsible for the collection, analysis, and interpretation of the data. All authors met International Committee of Medical Journal Editors criteria, had full access to the data, and vouch for its completeness and accuracy. Devon Roll, PhD, a medical writer supported by TG Therapeutics, assisted with manuscript revisions with input from the authors, after the first author drafted the initial manuscript. All authors reviewed and provided feedback on subsequent manuscript versions, and the corresponding author had final responsibility for the decision to submit for publication.
Results
Patient Characteristics
Forty-four patients were enrolled in the study and two patients never started therapy due to medical complications (Figure 1). A total of 42 patients (21 chronic lymphocytic leukaemia / 21 mantle cell lymphoma) were treated on study between December 5, 2014 and March 7, 2018. Eighteen patients were included in the phase 1 portion of the study, including six patients (3 chronic lymphocytic leukaemia /3 mantle cell lymphoma) each who received umbralisib at 400 mg, 600 mg, and 800 mg in combination with ibrutinib (420 mg for chronic lymphocytic leukaemia /560 mg for mantle cell lymphoma). Twenty-four patients (12 chronic lymphocytic leukaemia /12 mantle cell lymphoma) received umbralisib 800 mg in combination with ibrutinib in the phase 1b expansion cohorts. Among all treated patients (N=42), the median age was 68 years (range, 48–85), and the median number of prior therapies was two (range, 1–6) (Table 1). In the chronic lymphocytic leukaemia patients, 4 (20%) out of 20 harbored a deletion at 17p and 7 (37%) of 19 had a deletion at 11q; 12 (63%) of 19 had unmutated IGHV; four patients had TP53 mutation, including three of the patients with del(17p), and two patients had NOTCH1 mutation. None of the mantle cell lymphoma patients had blastoid histology, del(17p) was noted in one patient, and the median Ki-67% was 40% (range 15%−60%). Twenty-nine percent (6/21) of the mantle cell lymphoma patients had relapsed post autoSCT. Seventeen percent (7/42) of all patients had prior BTKi therapy, and 19% (4/21) of the chronic lymphocytic leukaemia patients had prior PI3Ki therapy. The patients with prior ibrutinib were all started on study within 6 months of starting ibrutinib, and none had shown signs of progression prior to enrolling on study. Of the 4 patients who had prior PI3Ki therapy, 1 patient had responded for 5 years on idelalisib monotherapy prior to eventually progressing, 1 patient had to discontinue duvelisib due to a toxicity, and 2 patients, both in response, had to discontinue frontline idelalisib due to a study closing, and both subsequently had disease progression off therapy.
Figure 1.
Trial profile.
Table 1.
Baseline Patient Characteristics
| Characteristic | MCL n=21 |
CLL n=21 |
Total N=42 |
|---|---|---|---|
| Age, years, median (range) | 68 (50–83) | 67 (48–85) | 68 (48–85) |
| Sex, male, n (%) | 16 (76) | 12 (57) | 28 (67) |
| ECOGPS | |||
| 0, n (%) | 11 (52) | 7 (33) | 18(43) |
| 1, n (%) | 9 (43) | 14 (67) | 23 (55) |
| 2, n (%) | 1 (5) | 0 | 1 (2) |
| Prior therapy, median (range) | 2 (1–5) | 1 (1–6) | 2 (1–6) |
| 1 | 3 (14) | 11 (52) | 14 (33) |
| 2 | 7 (33) | 5 (24) | 12 (29) |
| 3 or more | 9 (43) | 4 (19) | 13 (31) |
| Prior autoSCT, n (%) | 6/21 (29) | 0 | 4/42 (10) |
| Prior ibrutinib, n (%) | 2/21 (10) | 2/21 (10) | 4/42 (10) |
| Prior PI3K inhibitor, n (%) | 0 | 4/21 (19) | 4/42 (10) |
| WBC (K/uL), median (range) | 5·2 (2·2–338) | 28.4 (3·9–119·7) | 9·6 (2·2–338) |
| Hgb (g/dL), median (range) | 13 (7·8–15–9) | 11.1 (7·7–15·1) | 11·7 (7·7–15·9) |
| Platelets (K/uL), median (range) | 140 (38–290) | 175 (45–316) | 155 (38–316) |
| Beta-2M (mg/L), median (range) | 3·1 (0·6–19·7) | 4.3 (2·2–9·2) | 4.0 (0·6–19·7) |
| Ki-67 (%), median (range) | 40% (15–60%) | N/A | N/A |
| Del(17p), n (%) | 1/19 (5) | 4/20 (20) | 5/29 (13) |
| Del(11q), n (%) | 7/19 (37) | ||
| Unmutated IGHV, n (%) | 12/19 (63) | ||
| TP53 mutation (%), n (%) | 4/21 (19) | ||
| NOTCH1 mutation, n (%) | 2/21 (10) |
Safety
All 42 patients were included in the safety analysis. In the 18 patients (9 chronic lymphocytic leukaemia / 9 mantle cell lymphoma) in the phase 1 portion of the study, no DLTs occurred, and an MTD for umbralisib was not identified. The maximum administered dose/RP2D of umbralisib when given in combination with ibrutinib was 800 mg for both the chronic lymphocytic leukaemia and mantle cell lymphoma cohorts.
Toxicities of umbralisib and ibrutinib were manageable, consistent with the expected toxicities of both agents, and did not appear to be dose dependent (Table 2) or disease-specific (Supplemental Table 1, page 2). With regard to haematological toxicities, as expected in this patient population, cytopenias were relatively frequent in both cohorts. All-grade haematological toxicities included neutropenia [chronic lymphocytic leukaemia: 9/21 (43%), mantle cell lymphoma: 8/21 (38%)], thrombocytopenia [(chronic lymphocytic leukaemia: 6/21 (29%), mantle cell lymphoma: 10/21 (48%)], and anaemia [chronic lymphocytic leukaemia: 4/21 (19%), mantle cell lymphoma: 9/21 (43%)]. Grade 3/4 hematological toxicities were uncommon, and included neutropenia [chronic lymphocytic leukaemia: 4/21 (19%), mantle cell lymphoma: 1/21 (4·8%)], thrombocytopenia [chronic lymphocytic leukaemia: 0/21, mantle cell lymphoma: 2/21 (9·5%)], and anaemia [chronic lymphocytic leukaemia: 0/21, mantle cell lymphoma: 2/21 (9·5%)]. Febrile neutropenia was only observed in 1 patient (grade 3, MCL patient). None of these haematological toxicities were deemed by the investigators to be likely related to the study drugs.
Table 2.
All Grade Adverse Events Occurring in ≥ 10% of Patients and Grade 3/4 Adverse Events Occurring in All Patients by Umbralisib Dose Level
| Adverse Event | All Grades n (%) |
Grade 3/4 n (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| 400 mg (n=6) |
600 mg (n=6) |
800 mg (n=30) |
Total (n=42) |
400 mg (n=6) |
600 mg (n=6) |
800 mg (n=30) |
Total (n=42) |
|
| Non-h ematologie | ||||||||
| Diarrhoea, n (%) | 5 (83) | 3 (50) | 14 (47) | 22 (52) | 1 (17) | 0 | 3 (10) | 4 (9·5) |
| Infection, n (%) | 4 (67) | 1 (17) | 16 (53) | 21 (50) | 1 (17) | 1 (17) | 5 (17) | 7 (17) |
| Nausea, n (%) | 1 (17) | 3 (50) | 14 (47) | 18 (43) | 1 (17) | 0 | 0 | 1 (2.4) |
| Fatigue, n (%) | 2 (33) | 5 (83) | 9 (30) | 16 (38) | 0 | 0 | 2 (6·7) | 2 (4·8) |
| Hyperglycemia, n (%) | 1 (17) | 2 (33) | 9 (30) | 12 (29) | 0 | 0 | 1 (3·3) | 1 (2.4) |
| Transaminitis, n (%) | 4 (67) | 1 (17) | 5 (17) | 10 (24) | 0 | 0 | 1 (3·3) | 1 (2·4) |
| Dizziness, n (%) | 2 (33) | 3 (50) | 4 (13) | 9 (21) | 0 | 0 | 0 | 0 |
| Bruising, n (%) | 2 (33) | 0 | 5 (17) | 7 (17) | 0 | 0 | 5 (17) | 5 (12) |
| Cough, n (%) | 1 (17) | 2 (33) | 4 (13) | 7 (17) | 0 | 0 | 0 | 0 |
| Headache, n (%) | 2 (33) | 1 (17) | 4 (13) | 7 (17) | 0 | 0 | 0 | 0 |
| Anorexia, n (%) | 0 | 3 (50) | 3 (10) | 6 (14) | 0 | 0 | 0 | 0 |
| Myalgia, n (%) | 2 (33) | 0 | 4 (13) | 6 (14) | 0 | 0 | 0 | 0 |
| Rash, n (%) | 0 | 0 | 6 (20) | 6 (14) | 0 | 0 | 1 (3·3) | 1 (2·4) |
| Hypertension, n (%) | 0 | 1 (17) | 4 (13) | 5 (12) | 0 | 1 (17) | 0 | 1 (2·4) |
| Hematologic | ||||||||
| Neutropenia, n (%) | 4 (67) | 1 (17) | 12 (40) | 17 (40) | 2 (33) | 1 (17) | 2 (6·7) | 5 (12) |
| Thrombocytopenia, n (%) | 3 (50) | 3 (50) | 10 (33) | 16 (38) | 1 (17) | 0 | 1 (3·3) | 2 (4·8) |
| Anaemia, n (%) | 2 (33) | 3 (50) | 8 (27) | 13 (31) | 0 | 1 (17) | 1 (3·3) | 2 (4·8) |
excludes asymptomatic laboratory values that resolved promptly
The most common all grade non-haematological AEs included diarrhoea [chronic lymphocytic leukaemia: 8/21 (38%), mantle cell lymphoma: 14/21 (67%)], infection [chronic lymphocytic leukaemia: 14/21 (67%), mantle cell lymphoma: 7/21 (33%)], nausea [chronic lymphocytic leukaemia and mantle cell lymphoma: 9/21 (43%) each], fatigue [chronic lymphocytic leukaemia: 5/21 (24%), mantle cell lymphoma: 11/21 (52%)], and transaminitis [chronic lymphocytic leukaemia: 4/21 (19%), mantle cell lymphoma: 6/21 (29%)]. Most of these non-haematological AEs were considered by the investigators to be at least possibly related to the study drugs. Grade 3/4 infections occurred in 7 (17%) of 42 patients. Serious adverse events occurred in 12 (29%) of 42 patients, including lipase elevation (n=2, 1 grade 3 and 1 grade 4), atrial fibrillation (n=2, both grade 3), hypophosphataemia (n=2, grade 3), adrenal insufficiency (n=1, grade 3), transaminitis (n=1 grade 3), and infections (n=2 C. difficile (grade 3), and n=1 each of cellulitis (grade 3), encephalitis (grade 3), influenza A (grade 3), central nervous system and pulmonary aspergillus (grade 3), and sepsis (grade 4). No deaths on study were attributed to toxicity from the study drugs. One sudden death occurred in a chronic lymphocytic leukaemia patient with adrenal insufficiency who was off study drugs at the time of death. With regard to immune-mediated toxicities, with a median follow-up of 24 months, one patient had transient grade 3 transaminitis, and one patient had transient grade 1 pneumonitis. Two of the four cases of grade 3 diarrhoea were thought to be infectious in etiology, both due to Clostridium difficile, and the other two cases were thought probably due to the study drugs and resolved with brief drug holds.
Umbralisib dose reduction occurred in one chronic lymphocytic leukaemia and three mantle cell lymphoma patients (two for diarrhea and one each for dizziness and fatigue), and ibrutinib dose reduction occurred in four patients due to atrial fibrillation, palpitations, vitreous haemorrhage, and the need for -azole–based anti-fungal therapy (n=1 each). At least one umbralisib dose was held in 10 chronic lymphocytic leukaemia and 11 mantle cell lymphoma patients, and ibrutinib dosing was held electively for procedures as per standard of care.
Efficacy
All 42 patients were evaluable for efficacy (Table 3); 30 of these received the RP2D of umbralisib 800 mg daily in combination with ibrutinib. As of the cutoff July 3, 2018, the median number of cycles for chronic lymphocytic leukaemia patients was 21 (range 3–41) was for mantle cell lymphoma was 6 (range 1–26), and the median follow-up time among survivors in both arms was 26 months (range 3–42). A waterfall plot indicating the best decrease in the percentage of lymph node involvement is depicted in Figure 2.
Table 3.
Best Overall Response by Histology and Dose of Umbralisib
| Best Response n (%) |
CLL | All CLL n=21 |
MCL | All MCL n=21 |
All Patients n=42 |
||||
|---|---|---|---|---|---|---|---|---|---|
| 400 n=3 |
600 n=3 |
800 n=15 |
400 n=3 |
600 n=3 |
800 n=15 |
||||
| ORR* | 3(100) | 2 (67) | 14 (93) | 19 (90) | 1 (33) | 3(100) | 10 (67) | 14 (67) | 33 (79) |
| CR | 1 (33) | 1 (33) | 4 (27) | 6 (29) | 0 | 0 | 4 (27) | 4 (19) | 10 (24) |
| PR | 2 (67) | 1 (33) | 10 (67) | 13 (62) | 1 (33) | 3(100) | 6 (40) | 10 (48) | 23 (55) |
| SD | 0 | 1 (33) | 1 (7) | 2 (10) | 2 (67) | 0 | 2 (13) | 4 (19) | 6 (14) |
| PD | 0 | 0 | 0 | 0 | 0 | 0 | 3 (20)# | 3 (14) | 3 (7) |
Abbreviations: CLL: chronic lymphocytic leukaemia, MCL: mantle cell lymphoma, ORR: overall response rate, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease
ORR = CR+PR+PR-L
Includes 2 patients who started treatment but came off study prior to the first response evaluation and were counted as progression events
Figure 2.
Response by Histology – Best Decrease in the Percentage of Lymph Node Involvement by Dose Cohort*
*Hatching within each bar represents different dose levels within each cohort
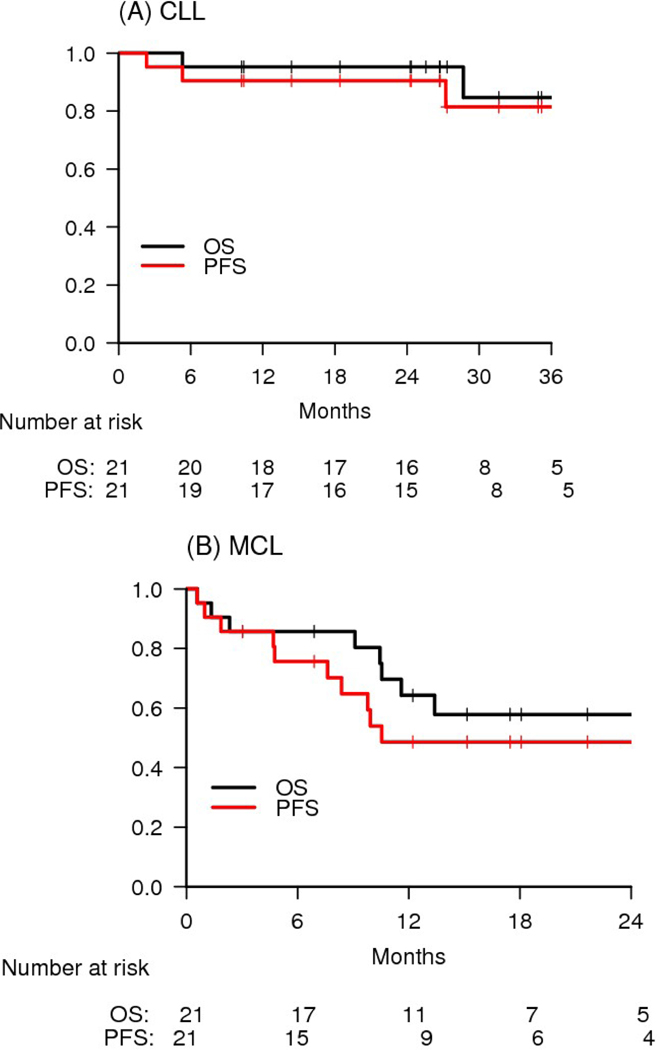
For patients with chronic lymphocytic leukaemia, the ORR was 90% (19/21). The PR/PR-L rate was 62% (13/21), and the chronic lymphocytic leukaemia rate was 29% (6/21). The median time to best response was 2 months (range 1·4 – 38·1), and median time to CR was 18·4 months (range 4·7–37·5). All of the patients in CR had detectable minimal residual disease (MRD) in the bone marrow by flow cytometry. Two partial responders of note who did not meet CR criteria included one with morphologic clearance of chronic lymphocytic leukaemia from the marrow but one residual lymph node slightly larger than 1·5 cm in long axis, and another with radiographic CR but residual low-level marrow disease. Two patients achieved radiographic CR but have not yet had marrow evaluation to assess for CR. Two of the three chronic lymphocytic leukaemia patients with del(17p) achieved response (both PR), and six of seven (86%) patients with del(11q) achieved response (2 CR/4 PR). Four of the 13 patients with unmutated IGHV (31%) achieved CR. All four chronic lymphocytic leukaemia patients with prior PI3Ki and two of three patients with prior BTKi responded, although none of these patients on prior BCRi monotherapy were considered refractory to that therapy at time of study entry. For the chronic lymphocytic leukaemia patients with prior PI3Ki exposure, responses on this study were as follows: CR (DOR 28 mo., ongoing), CR (DOR 28 mo., ongoing), PR (DOR 30 mo., ongoing), and PR (DOR 27 mo., ongoing). Both of the chronic lymphocytic leukaemia patients with prior BTKi exposure who responded on study achieved PR, with DOR of 29, ongoing, and 28 mo. The median PFS and OS were not reached, and the 2-year PFS and OS were 90% and 95%, respectively (Figure 3A). Only two chronic lymphocytic leukaemia patients have died to date, including the one patient who was in remission and experienced sudden death while off study therapy and one patient who died due to progressive chronic lymphocytic leukaemia. PFS and OS did not differ based on IGHV mutation status or FISH status, although the sample size is small (Supplemental Figures 1 and 2, pages 3–5). Notably, no chronic lymphocytic leukaemia patients treated on study have developed Richter’s Syndrome to date.
Figure 3.
Survival by Histologic Type. (A) CLL and (B) MCL
For patients with mantle cell lymphoma, the ORR was 67% (14/21). The PR rate was 48% (10/21), and the CR rate was 19% (4/21). One of the patients in PR achieved radiographic CR but had residual marrow involvement that precluded reaching CR. The median time to best response was 2 months (range 1·7–18·3 months). Clinical benefit was observed in three patients who did not meet formal criteria for response, including two patients without baseline lymphadenopathy, one of whom had stable disease for 10 months, and the other who had reduction of WBC from 342K to 25K and of spleen from 31 cm to 23 cm and was progression free for 8 months. Another mantle cell lymphoma patient had an initial 45% reduction in lymph node disease but progressed after 5 months. The 2-year PFS and OS were 49% and 58%, respectively (Figure 3B). The median PFS and OS were 10·5 months and 29·7 months, respectively. Eight mantle cell lymphoma patients died due to progressive disease, and one patient died due to toxicity from subsequent therapy.
Discussion
To our knowledge, this study represents the first clinical data on the doublet combination of a BTK inhibitor with a PI3K-δ inhibitor in patients with B-cell malignancies and the first successful combination of two agents targeting the B-cell receptor pathway. We found umbralisib in combination with ibrutinib to be well tolerated, with no DLTs observed, and we identified a RP2D of 800 mg daily of umbralisib when given in combination with standard dose ibrutinib in both chronic lymphocytic leukaemia and mantle cell lymphoma. In general, the toxicities of this combination were manageable, and consistent with the additive toxicity profiles of the two drugs individually. For example, rates of all grade diarrhea and infection were somewhat higher than has been observed with ibrutinib or umbralisib monotherapy, but in most cases these were low-grade events that improved rapidly with intervention, and our 17% rate of grade 3 or higher infection compares favorably with the rates seen in ibrutinib monotherapy studies.1–2 Importantly, we did not see increased rates of immune-mediated toxicities as had previously been seen with the PI3K-δ plus SYKi combination.10 In fact, the rates of immune-mediated toxicities on our study were relatively low, with only one case of transient grade 3 transaminitis and no grade 3/4 colitis or pneumonitis. This may be due in part to the favorable single-agent toxicity profile of umbralisib, although vigilance for the potential risk of immune-mediated toxicities may also have led to a low threshold for dose holding or dose reduction in patients with low-grade toxicities to prevent such toxicities from worsening in severity.
The outcomes for patients with B-cell malignancies have improved significantly over the last few years with the development of small molecule, novel oral agents targeting a diverse array of mechanisms crucial to the survival of the malignant cell, including inhibitors of BCR pathway kinases and the anti-apoptotic protein BCL-2.19,20 Though relatively well tolerated and efficacious, when used as monotherapy, these agents rarely provide long-term survival for patients with high-risk R/R chronic lymphocytic leukaemia and R/R mantle cell lymphoma. Moreover, novel agent monotherapy requires continuous therapy, which can be challenging in terms of adherence, cost, and chronic toxicities. A monotherapy approach may also encourage the development of resistance mutations, such as BTK C481S, which leads to ibrutinib resistance. As such, numerous combination studies are now exploring the safety and efficacy of novel agent combination approaches, with the hope of achieving higher CR rates to allow for effective time-limited regimens in these populations.
One unanticipated benefit of doublet BCR-pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held. For example, ibrutinib was temporarily held in two patients (6%) who experienced grade 3 atrial fibrillation, but disease control was maintained by continuing umbralisib. Ibrutinib was also frequently held before and after elective procedures, but continuing umbralisib throughout the perioperative period prevented disease flare that can be observed in patients following an interruption of ibrutinib monotherapy.21 Similarly, ibrutinib was continued in patients with toxicities that resulted in a temporary hold of umbralisib. For example, umbralisib was held in two patients who experienced grade 2 diarrhoea to avoid the development of colitis. Ongoing ibrutinib helped maintain disease control during the interruption in umbralisib treatment, and both patients were able to successfully restart umbralisib with a dose reduction.
In the efficacy analysis, the ORR of 90% (19/21) in R/R chronic lymphocytic leukaemia is similar to what one might expect from ibrutinib monotherapy; however, the depth of response with the umbralisib/ibrutinib doublet is promising. Whereas the CR rates with this length of follow-up are typically in the 2% range with ibrutinib monotherapy in a high-risk chronic lymphocytic leukaemia population,1,2 in our study 29% (6/21) met IW-CLL criteria for CR on the umbralisib/ibrutinib doublet. In addition, 58% (7/12) of the patients with PR had residual lymphadenopathy less than 20 mm in maximum long axis dimension. These deep responses have translated into promising durability, with 90% (19/21) of chronic lymphocytic leukaemia patients progression free at 2 years. Of note, we designed our regimen to be continuous given the prior experience with long PFS in chronic lymphocytic leukaemia patients treated with BCRi therapy, recognizing that few patients achieve MRD undetectability, yet have long PFS nonetheless.
It is also notable that with a median follow-up of 26 months, none of these R/R chronic lymphocytic leukaemia patients have developed Richter’s Syndrome. This is in contrast to earlier studies of ibrutinib monotherapy, where Richter’s Syndrome was a common cause of disease progression.1 Interestingly, unexpectedly low rates of Richter’s Syndrome have also been seen in studies in R/R chronic lymphocytic leukaemia of the approved PI3K inhibitors idelalisib and duvelisib, raising the possibility that PI3K inhibition itself may lower the risk of Richter’s Syndrome in this population.13,22
In the mantle cell lymphoma arm, the ORR of 67% (14/21) and the CR rate of 19% (4/21) are similar to what has previously been reported with ibrutinib monotherapy, and this is reflected in the modest median PFS of 10·5 months; however, the 2-year PFS and OS of 49% and 58% suggest that patients who made it to 1-year progression free had few events during the second year on therapy. Notably, this was a high-risk mantle cell lymphoma population, with a median of three prior therapies and with 29% (6/21) of patients having relapsed after autoSCT prior to enrollment. Nonetheless, we cannot conclude from our study that this doublet is advantageous in R/R mantle cell lymphoma compared with ibrutinib alone, and further data would be needed to clarify the potential benefit of the doublet in this disease.
Several other promising novel agent-based combination regimens are now being explored in R/R chronic lymphocytic leukaemia and mantle cell lymphoma. The combination of ibrutinib with the BCL-2 inhibitor venetoclax has a strong preclinical justification, suggesting the potential for synergy.23,24 Recent data from early phase clinical trials suggest that this combination is also well tolerated and highly efficacious in both R/R chronic lymphocytic leukaemia 25,26 and mantle cell lymphoma.27 Given the higher likelihood that a venetoclax-based regimen will lead to MRD-undetectability, such approaches are more likely than our regimen to allow for time-limited therapy. As such, it is likely that regimens such as venetoclax plus anti-CD20 antibody will increasingly be utilized in earlier lines of chronic lymphocytic leukaemia therapy;28,29however, our dual BCRi regimen could be used following disease progression after such regimens or in patients who are not suitable candidates for venetoclax-based therapies due to comorbidities like renal dysfunction, which can increase the risk of developing clinical sequelae of tumor lysis syndrome. Additionally, the tumor lysis syndrome risk with venetoclax initiation may complicate the logistics of starting venetoclax-based therapies outside of an academic setting, even in combination regimens where patients are partially cytoreduced before beginning the venetoclax dose ramp-up. Thus, for patients who are not good candidates for venetoclax, or for those who progress after venetoclax-based combination regimens, a BTKi/PI3Ki doublet regimen is an efficacious all oral option that is convenient to initiate and well-tolerated with long-term administration.
Limitations of this study include the relatively short follow-up, making it difficult to know at the present time how the durability of response in the chronic lymphocytic leukaemia arm compares with ibrutinib monotherapy, where mature 5-year follow-up data are now available.7 Additionally, patients on our study were not evaluated for pharmacokinetics or BTK or PLCγ−2 resistance mutations. Although all four chronic lymphocytic leukaemia patients with prior PI3K-δ inhibitor and two of the three chronic lymphocytic leukaemia patients with prior ibrutinib achieved response, none of these patients were truly refractory to those agents. As such, the ability of this doublet to overcome resistance to ibrutinib or umbralisib monotherapy remains unknown. The dosing cohorts in the phase I portion of the study were not large enough to draw conclusions about any differences in efficacy with umbralisib dose. Lastly, because this combination regimen was designed as a continuous therapy, it remains unknown whether durable response could be achieved with a time-limited doublet-based regimen, although this seems unlikely given that all patients who achieved CR still had detectable MRD.
In conclusion, we report for the first time on clinical data for a BTKi/PI3K-δi doublet in B-cell malignancies. We found that ibrutinib can be combined safely with umbralisib, with promising efficacy results in chronic lymphocytic leukaemia patients and with the convenience of an all-oral, non–chemotherapy-based regimen. This combination is also being explored in R/R B-cell malignancies as a triplet, in combination with the type II CD20 antibody ublituximab (NCT02006485). These novel agent-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies.
Supplementary Material
Research in Context.
Evidence before this study
Since its initial approval in 2013, the Bruton Tyrosine Kinase (BTK) inhibitor ibrutinib has transformed the management of patients with chronic lymphocytic leukaemia and mantle cell lymphoma through targeting the B-cell receptor (BCR) pathway. Nevertheless, as is evident from an NCBI literature search of clinical trials in this space from the last 5 years, for patients with relapsed higher risk chronic lymphocytic leukaemia and relapsed mantle cell lymphoma, durable and complete responses are uncommon, and most patients will eventually develop resistance to ibrutinib. Inhibiting multiple targets in the BCR pathway could in theory improve the depth and durability of response; however, a prior attempt to target the delta isoform of phosphoinositide-3-kinase (PI3K-δ) and spleen tyrosine kinase resulted in substantial toxicity, calling into question the feasibility of this approach. Umbralisib is a next generation PI3K-δ inhibitor with a differentiated safety profile, which suggested that it could more feasibly be combined with ibrutinib to safely achieve dual BCR blockade in patients.
Added value of this study
To the best of our knowledge, our trial is the first to investigate a BTK/PI3K-δ inhibitor doublet in B-cell malignancies. Particularly in our chronic lymphocytic leukaemia cohort, the depth and durability of responses are encouraging and warrant further study of this drug combination.
Implications of all the available evidence
Dual inhibition of BTK and PI3K-δ showed encouraging efficacy and a manageable adverse event profile. These findings have already informed the development of a triplet combination study that added the anti-CD20 monoclonal antibody ublituximab (NCT02006485), and support the idea that a BTK inhibitor plus umbralisib warrants further study as a backbone on which to build future combination regimens in chronic lymphocytic leukaemia and potentially other B-cell malignancies.
ACKNOWLEDGMENTS
The authors wish to thank Nexus GG Science LLC for medical writing support, in particular Tom Renau, PhD, Devon Roll, PhD, and Emily Hale.
Funding
TG Therapeutics, Leukemia and Lymphoma Society Therapy Accelerator Program
MSD received institutional research funding from TG Therapeutics, Genentech, Pharmacyclics, BMS, MEI, Surface Oncology, Verastem and Acerta Pharma; consultant or advisory board member for TG Therapeutics, Abbvie, Genentech, Janssen, Merck, Pharmacyclics, Gilead, MEI, Verastem, Sunesis, Syros, and Acerta Pharma. AMB serves on the speakers bureau for Takeda and Celgene. JRB serves as a consultant or advisory board member for TG Therapeutics, AbbVie, Acerta, AstraZeneca, BeiGene, Celgene, Genentech, Gilead, Janssen, Loxo, Novartis, Pfizer, Pharmacyclics, Sun, Sunesis, and Verastem, and has received grant funding from Sun, Gilead, Loxo, and Verastem. EJ serves as a consultant or advisory board member for AstraZeneca, Merck, and Seattle Genetics. CAJ serves as a consultant for Kite, Pfizer, Precision Bioscience, Bayer, and Humanigen. HM and PS are employed by and may have equity in TG Therapeutics.
Footnotes
DECLARATION OF INTEREST
The other authors declared no conflict of interest.
DATA SHARING STATEMENT
Individual participant data that underlie the results reported in this article will be shared after de-identification (text, tables, figures, and appendices). The study protocol will also be made available. These data will become available beginning 9 months following article publication with no end date to investigators whose proposed use of the data has been approved by researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to matthew_davids@dfci.harvard.edu. To gain access, data requestors will need to sign a data access agreement.
REFERENCES
- 1.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369(1): 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371(3): 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014; 370(24): 2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woyach JA, Ruppert AS, Guinn D, et al. BTK(C481S)-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017; 35(13): 1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016; 127(12): 1559–63. [DOI] [PubMed] [Google Scholar]
- 6.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. The New England journal of medicine 2013; 369(6): 507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien S, Furman RR, Coutre S, et al. Single-Agent Ibrutinib in Treatment-Naive and Relapsed/Refractory Chronic Lymphocytic Leukemia: A 5-Year Experience. Blood 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Rooij MF, Kuil A, Kater AP, Kersten MJ, Pals ST, Spaargaren M. Ibrutinib and idelalisib synergistically target BCR-controlled adhesion in MCL and CLL: a rationale for combination therapy. Blood 2015; 125(14): 2306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niemann CU, Mora-Jensen HI, Dadashian EL, et al. Combined BTK and PI3Kdelta Inhibition with Acalabrutinib and ACP-319 Improves Survival and Tumor Control in CLL Mouse Model. Clin Cancer Res 2017; 23(19): 5814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood 2016; 127(20): 2411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burris HA, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. The Lancet Oncology 2018; 19(4): 486–96. [DOI] [PubMed] [Google Scholar]
- 12.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014; 123(22): 3390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flinn IW, O’Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-delta,gamma, is clinically active in advanced hematologic malignancies. Blood 2018; 131(8): 877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davids MS, Flinn IW, Mato AR, et al. An Integrated Safety Analysis of the Next Generation PI3Kδ Inhibitor Umbralisib (TGR-1202) in Patients with Relapsed/Refractory Lymphoid Malignancies. Blood 2017; 130(Suppl 1): 4037-. [Google Scholar]
- 15.Maharaj K, Powers JJ, Achille A, et al. Differential Regulation of T Cells By PI3K Delta Inhibitors in a CLL Murine Model. Blood 2017; 130(Suppl 1): 3009-. [Google Scholar]
- 16.Deng C, Lipstein MR, Scotto L, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kdelta and CK1epsilon in hematological malignancies. Blood 2017; 129(1): 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111(12): 5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014; 32(27): 3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davids MS, Brown JR. Targeting the B cell receptor pathway in chronic lymphocytic leukemia. Leukemia & lymphoma 2012; 53(12): 2362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davids MS. Targeting BCL-2 in B-cell lymphomas. Blood 2017; 130(9): 1081–8. [DOI] [PubMed] [Google Scholar]
- 21.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA oncology 2015; 1(1): 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furman RR, Cheng S, Lu P, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med 2014; 370(24): 2352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and Protein Profiling Suggests Venetoclax (ABT-199) as Optimal Partner with Ibrutinib in Chronic Lymphocytic Leukemia. Clin Cancer Res 2015; 21(16): 3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 2017; 31(10): 2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain N, Thompson PA, Ferrajoli A, et al. Combined Venetoclax and Ibrutinib for Patients with Previously Untreated High-Risk CLL, and Relapsed/Refractory CLL: A Phase II Trial. Blood 2017; 130(Suppl 1): 429-. [Google Scholar]
- 26.Hillmen P, Munir T, Rawstron A, et al. Initial Results of Ibrutinib Plus Venetoclax in Relapsed, Refractory CLL (Bloodwise TAP CLARITY Study): High Rates of Overall Response, Complete Remission and MRD Eradication after 6 Months of Combination Therapy. Blood 2017; 130(Suppl 1): 428-. [Google Scholar]
- 27.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med 2018; 378(13): 1211–23. [DOI] [PubMed] [Google Scholar]
- 28.Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumab in chronic lymphocytic leukemia. Blood 2017; 129(19): 2702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med 2018; 378(12): 1107–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.