Abstract
Correlation of vitamin D with inflammatory factors, oxidative stress and T cell subsets in patients with autoimmune hepatitis were investigated. Patients with liver diseases (n=635) treated in The Sixth People's Hospital of Qingdao City from March 2015 to January 2018 were selected, among which 80 cases diagnosed with autoimmune hepatitis were included into observation group, and 80 healthy cases were included into control group. Patients with autoimmune hepatitis were further divided into normal 25-hydroxyvitamin D [25-(OH) D] group (n=40) and abnormal 25-(OH) D group (n=40) according to the level of 25-(OH) D, and divided into normal liver function group (n=40) and abnormal liver function group (n=40). 25-(OH) D, liver function, inflammatory factors, oxidative stress level and T cell subsets were compared. In the observation group, levels of 25-(OH) D, superoxide dismutase (SOD), total antioxidant capacity, and T cell subsets were lower than those in the control group (P<0.05), while levels of total bilirubin (TBIL), indirect BIL (IBIL), direct BIL (DBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), inflammatory factors and malondialdehyde (MDA) were higher than those in the control group (P<0.05). In the normal 25-(OH) D group, levels of inflammatory factors and oxidative stress factor were lower than those in the abnormal 25-(OH) D group (P<0.05), while the SOD level, total antioxidant capacity and T cell subset counts were higher than those in the abnormal 25-(OH) D group (P<0.05). Moreover, the 25-(OH) D level in patients with autoimmune hepatitis was negatively correlated with hs-CRP, tumor necrosis factor-α (TNF-α), ALT and MDA, but positively correlated with CD3+ and CD4+ counts, SOD and total antioxidant capacity. Patients with autoimmune hepatitis, especially those with decreased level of vitamin D, are more prone to enhanced inflammatory and stress responses, decreased levels of T cell subsets and decline in immunity.
Keywords: autoimmune hepatitis, vitamin D, inflammatory factors, oxidative stress, T cell subsets
Introduction
Autoimmune hepatitis is an autoimmune response mainly due to antibodies against hepatocytes in the body, which often leads to hepatic parenchymal inflammation. Positive autoantibody and immunoglobulin G (IgG)-globulinemia can be observed in clinical biochemical examination (1), while inflammatory changes in hepatic tissue border can be seen under the microscope. Autoimmune hepatitis will develop into cirrhosis and hepatic failure if not treated in time. Moreover, autoimmune hepatitis has complex clinical manifestations with chronic hidden onset, and few patients have acute onset (2). With the prolongation of course of disease, hepatic failure will occur, threatening the life of the patients. Since autoimmune hepatitis attracted attention in the clinic in the 1960s (3), a variety of clinical treatment methods have been adopted, among which immunosuppressors are of important value in improving biochemical indexes and clinical symptoms of patients, and even in reversing hepatic tissue fibrosis (4). With progress made in diagnostic techniques, the detection rate of autoimmune hepatitis has remarkably increased, and autoimmune hepatitis has become the second major disease following virus-induced liver diseases in clinic, attracting increasingly more attention from clinicians and patients (5).
It has been confirmed in previous studies (6) that there is significant enhancement in the inflammatory response and oxidative stress response of patients with autoimmune hepatitis, and patients are complicated with immune dysfunction. According to a recent study (7), changes in the vitamin D level are closely correlated with the occurrence and development of autoimmune hepatitis. However, correlations of the vitamin D level with inflammatory response, oxidative stress response and immune function of patients with autoimmune hepatitis have rarely been studied. Therefore, this study investigated the above correlations.
Patients and methods
General data
A total of 635 patients with liver diseases treated in The Sixth People's Hospital of Qingdao City from March 2015 to January 2018 were selected. Eighty cases meeting diagnostic criteria for autoimmune hepatitis of the American Association for the Study of Liver Diseases in 2002 were included in the observation group. They were aged 18-60 years with normal mental state and educational level of primary school and above. Each patient signed an informed consent before enrollment, and the study was approved by the Medical Ethics Committee of The Sixth People's Hospital of Qingdao City. Subjects with alcoholism, a history of long-term drug therapy, diagnosed with hepatolenticular degeneration, genetic metabolic liver-related diseases, complicated with viral hepatitis, liver tumor or other autoimmune diseases, or who took immunosuppressors and/or glucocorticoids within 15 days before enrollment were excluded. Additionally, 80 healthy cases receiving physical examination in The Sixth People's Hospital of Qingdao City during the same period were included in the control group, and subjects with known liver diseases were excluded. Eighty patients in the observation group were further divided into normal 25-hydroxyvitamin D [25-(OH) D] group (n=40) and abnormal 25-(OH) D group (n=40) according to the examined level of 25-(OH) D, and divided into normal liver function group (n=40) and abnormal liver function group (n=40) according to whether the liver function of patients was normal. Levels of inflammatory factors and oxidative stress factors, and related indexes to T cell subsets in all patients are shown in Tables I-III.
Table I.
Comparison of 25-(OH) D, liver function, inflammatory factors, oxidative stress level and T cell subsets between the two groups (mean ± SD).
| Groups | 25-(OH) D (µg/l) | TBIL (µmol/l) | IBIL (µmol/l) | DBIL (µmol/l) | AST (U/l) | ALT (U/l) |
|---|---|---|---|---|---|---|
| Observation group | 6.3±0.1 | 23.1±0.3 | 15.1±0.3 | 8.9±0.3 | 65.5±2.3 | 68.9±3.4 |
| Control group | 53.2±5.6 | 8.1±0.1 | 6.1±0.2 | 3.3±0.2 | 32.1±1.7 | 25.6±1.3 |
| t | 59.960 | 300.000 | 140.001 | 98.230 | 73.858 | 75.233 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Groups | hs-CRP (ml/l) | IL-6 (ng/l) | TNF-α (ng/l) | MDA (nmol/ml) | SOD (µU/ml) | |
| Observation group | 11.6±0.6 | 0.7±0.1 | 136.5±6.3 | 7.1±0.3 | 135.0±13.1 | |
| Control group | 5.0±0.3 | 0.4±0.1 | 63.2±2.6 | 3.1±0.1 | 308.3±20.5 | |
| t | 62.225 | 13.416 | 68.021 | 80.000 | 45.053 | |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| Groups | Total antioxidant capacity (µU/ml) | CD3+ (ng/µl) | CD4+ (ng/µl) | CD8+ (ng/µl) | ||
| Observation group | 4.1±0.2 | 2,356.1±25.5 | 1,146.3±23.9 | 1,050.3±15.3 | ||
| Control group | 23.1±1.9 | 463.5±8.3 | 236.2±15.3 | 155.4±7.6 | ||
| t | 62.898 | 446.357 | 202.834 | 331.302 | ||
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
25-(OH) D, 25-hydroxyvitamin D; TBIL, total bilirubin; IBIL, indirect BIL; DBIL, direct BIL; AST, aspartate aminotransferase; ALT, alanine aminotransferase; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; MDA, malondialdehyde; SOD, superoxide dismutase.
Table II.
Comparison of inflammatory factors, oxidative stress level and T cell subsets when 25-(OH) D was normal or not (mean ± SD).
| Groups | hs-CRP (ml/l) | IL-6 (ng/l) | TNF-α (ng/l) | MDA (nmol/ml) | SOD (U/ml) |
|---|---|---|---|---|---|
| Normal 25-(OH) D group | 4.6±0.3 | 0.4±0.1 | 65.1±2.3 | 3.0±0.1 | 298.3±23.5 |
| Abnormal 25-(OH) D group | 12.1±0.5 | 0.8±0.1 | 141.3±6.1 | 7.3±0.4 | 126.5±11.3 |
| t | 81.349 | 17.889 | 73.925 | 65.959 | 41.669 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Groups | Total antioxidant capacity (µU/ml) | CD3+ (ng/µl) | CD4+ (ng/µl) | CD8+ (ng/µl) | |
| Normal 25-(OH) D group | 24.3±1.6 | 2,446.3±21.5 | 1,155.1±21.9 | 1,131.3±12.3 | |
| Abnormal 25-(OH) D group | 3.9±0.3 | 451.5±9.1 | 241.2±13.3 | 144.5±6.6 | |
| t | 79.257 | 540.390 | 225.586 | 447.104 | |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
25-(OH) D, 25-hydroxyvitamin D; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; MDA, malondialdehyde; SOD, superoxide dismutase.
Table III.
Comparison of 25-(OH) D, inflammatory factors, oxidative stress level and T cell subsets when the liver function was normal or not (mean ± SD).
| Groups | hs-CRP (ml/l) | IL-6 (ng/l) | TNF-α (ng/l) | MDA (nmol/ml) | SOD (U/ml) |
|---|---|---|---|---|---|
| Normal liver function group | 5.1±0.3 | 0.4±0.1 | 63.0±2.8 | 3.0±0.2 | 311.2±21.5 |
| Abnormal liver function group | 11.8±0.5 | 0.8±0.1 | 138.3±6.1 | 7.2±0.4 | 136.3±13.3 |
| t | 72.672 | 17.886 | 70.954 | 59.397 | 43.754 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Groups | Total antioxidant capacity (µU/ml) | 25-(OH) D (µg/l) | CD3+ (ng/µl) | CD4+ (ng/µl) | CD8+ (ng/µl) |
| Normal liver function group | 24.1±1.9 | 56.2±5.6 | 2,360.1±24.5 | 1,163.1±23.9 | 1,061.6±14.3 |
| Abnormal liver function group | 4.3±0.2 | 6.1±0.1 | 465.1±8.0 | 233.5±16.1 | 156.4±7.3 |
| t | 65.546 | 56.573 | 465.021 | 204.023 | 356.574 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
25-(OH) D, 25-hydroxyvitamin D; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; MDA, malondialdehyde; SOD, superoxide dismutase.
Research methods
Related indexes of patients were retrospectively analyzed. Based on different groups, 25-(OH) D, liver function, inflammatory factors, oxidative stress level and T cell subsets were compared between patients diagnosed with autoimmune hepatitis and healthy people. Inflammatory factors, oxidative stress level and T cell subsets were detected when 25-(OH) D was normal or not, and changes in 25-(OH) D, inflammatory factors, oxidative stress level and T cell subsets were detected when the liver function was normal or not. Moreover, correlation of the 25-(OH) D level with inflammatory factors, liver function-related indexes, T cell subsets and oxidative stress factors in patients with autoimmune hepatitis were analyzed.
Evaluation criteria
Serum 25-(OH) D (liquid chromatography-tandem mass spectrometry, 10-80 µg/l), detection indexes of liver function include total bilirubin (TBIL, bilirubin oxidase method, 2-20 µmol/l), indirect BIL (IBIL, bilirubin oxidase method, 0-14 µmol/l), direct BIL (DBIL, bilirubin oxidase method, 0-7 µmol/l), aspartate aminotransferase (AST, colorimetric method, 8-40 U/l) and alanine aminotransferase (ALT, colorimetric method, 5-40 U/l), inflammatory factors include high-sensitivity C-reactive protein (hs-CRP, immunoturbidimetry, <10 ml/l), interleukin-6 (IL-6, ELISA, 0.37-0.46 ηg/l) and tumor necrosis factor-α (TNF-α, ELISA, 5-100 ηg/l), oxidative stress factors include malondialdehyde (MDA, TBA method, 3.52-4.78 ηmol/ml) and superoxide dismutase [superoxide dismutase (SOD), WST method, 0.242-0.620 µU/ml], total antioxidant capacity of femoral venous blood (FRAP method, 2.34-26.96 µU/ml), and detection indexes of T cell subsets include cluster of differentiation (CD)3+ count (955-2860/µl), CD4+ count (450-1,440/µl) and CD8+ count (320-1,250/µl), and the FRCSCalibur flow cytometer (BD Biosciences) was used for detection.
Statistical processing
Statistical Product and Service Solutions (SPSS) 13.0 software was used for statistical processing. Measurement data are presented as the mean ± standard deviation (SD). t-test was used for the comparison of mean between two groups, and Chi-square test was used for the comparison of ratio between two groups. The correlation coefficient method was adopted for the correlation analysis. P<0.05 indicates that the difference was statistically significant.
Results
Comparison of 25-(OH) D, liver function, inflammatory factors, oxidative stress level and T cell subsets between the two groups
In observation group, levels of 25-(OH) D, SOD, total antioxidant capacity, and CD3+, CD4+ and CD8+ counts were lower than those in control group (P<0.05), while levels of TBIL, IBIL, DBIL, AST, ALT, hs-CRP, IL-6, TNF-α and MDA were higher than those in the control group (P<0.05) (Table I).
Comparison of inflammatory factors, oxidative stress level and T cell subsets when 25-(OH) D was normal or not
In normal 25-(OH) D group, levels of inflammatory factors (hs-CRP, IL-6 and TNF-α) and oxidative stress factor (MDA) were lower than those in abnormal 25-(OH) D group (P<0.05), while in the SOD level, total antioxidant capacity and CD3+, CD4+ and CD8+ counts were higher than those in abnormal 25-(OH) D group (P<0.05) (Table II).
Comparison of 25-(OH) D, inflammatory factors, oxidative stress level and T cell subsets when the liver function was normal or not
In normal liver function group, levels of hs-CRP, IL-6, TNF-α and MDA were lower than those in abnormal liver function group (P<0.05), while the SOD level, total antioxidant capacity and CD3+, CD4+ and CD8+ counts were higher than those in abnormal liver function group (P<0.05) (Table III).
Correlation analyses of 25-(OH) D level with inflammatory factors, related indexes to liver function and T cell subsets in patients with autoimmune hepatitis
The 25-(OH) D level in patients with autoimmune hepatitis was negatively correlated with hs-CRP, TNF-α and ALT (r=-0.9264, -0.9095 and -0.9165, P<0.05), but positively correlated with CD3+ and CD4+ counts (r=0.9489 and 0.8271, P<0.05) (Figs. 1-5).
Figure 1.
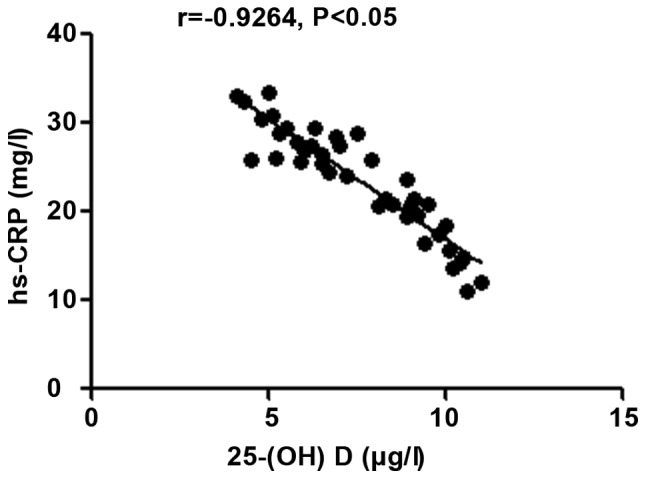
Correlation analysis between 25-(OH) D level and inflammatory factor hs-CRP. 25-(OH) D, 25-hydroxyvitamin D.
Figure 2.
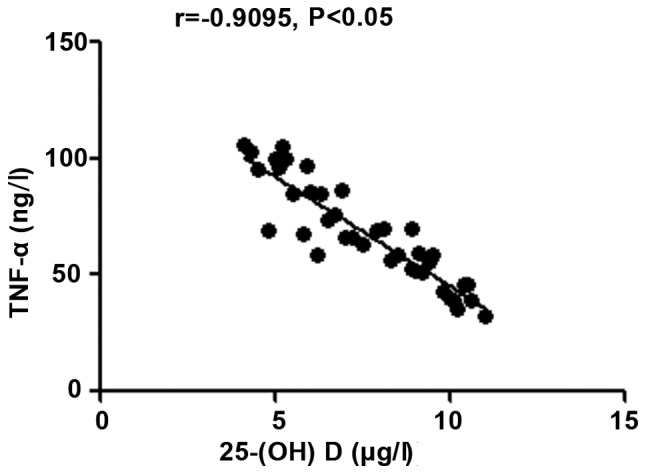
Correlation analysis between 25-(OH) D level and inflammatory factor TNF-α. 25-(OH) D, 25-hydroxyvitamin D; TNF-α, tumor necrosis factor-α.
Figure 3.
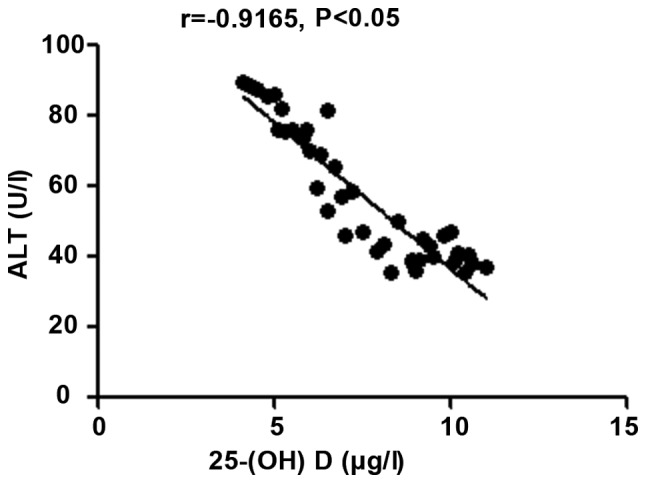
Correlation analysis between 25-(OH) D level and ALT. 25-(OH) D, 25-hydroxyvitamin D; ALT, alanine aminotransferase.
Figure 4.
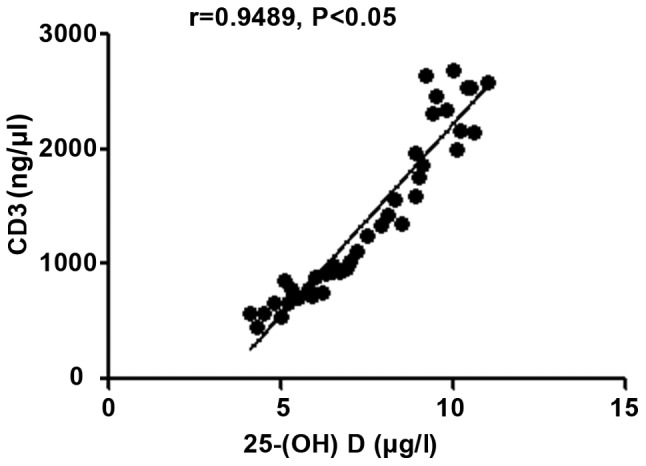
Correlation analysis between 25-(OH) D level and CD3+ count. 25-(OH) D, 25-hydroxyvitamin D.
Correlation analyses of 25-(OH) D level with oxidative stress factors in patients with autoimmune hepatitis
The 25-(OH) D level in patients with autoimmune hepatitis was negatively correlated with MDA (r=-0.8818, P<0.05), but positively correlated with SOD and the body's total antioxidant capacity (r=0.7523 and 0.9338, P<0.05) (Figs. 6-8).
Figure 6.
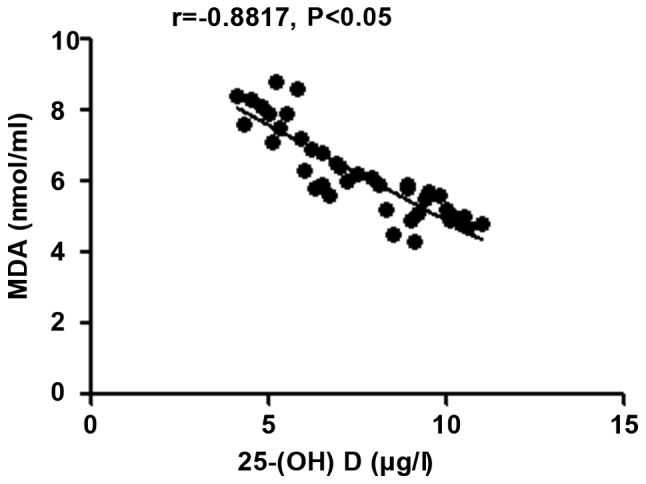
Correlation analysis between 25-(OH) D level and MDA in patients with autoimmune hepatitis. 25-(OH) D, 25-hydroxyvitamin D; MDA, malondialdehyde.
Figure 7.
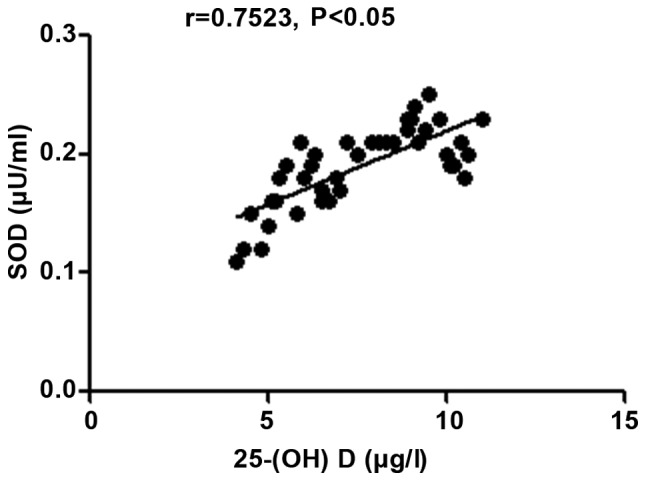
Correlation analysis between 25-(OH) D level and SOD in patients with autoimmune hepatitis; 25-(OH) D, 25-hydroxyvitamin D; SOD, superoxide dismutase.
Figure 8.
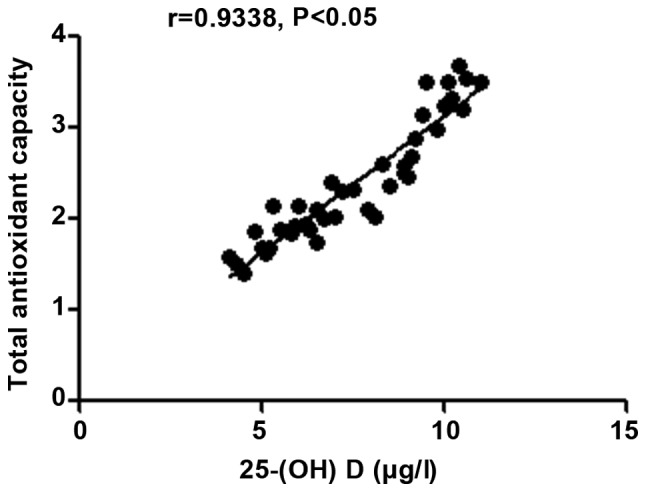
Correlation analysis between 25-(OH) D level and the body's total antioxidant capacity in patients with autoimmune hepatitis. 25-(OH) D, 25-hydroxyvitamin D.
Discussion
Currently, the etiology and pathogenesis of autoimmune hepatitis remain unclear, and genetic susceptibility, virus infection mechanism, and abnormal autoimmunity, are considered to be involved in its development (8). Besides drug damage, virus effect and other common precipitating factors, changes in cytokine levels, immune dysfunction and dysregulation in the body and genetic factors are related factors causing hepatocyte damage (9). The basic pathological changes of autoimmune hepatitis lie in the piecemeal necrosis around the hepatic lobules accompanied by the acute and chronic inflammatory cell infiltration of lymphocytes, monocytes and plasmocytes (10). Autoimmune hepatitis frequently occurs in women of all ages, often complicated with autoimmune diseases except liver disease (11). Satisfactory therapeutic effect can be obtained through using immunosuppressors, and the mortality rate of autoimmune hepatitis is up to 40% (12). Therefore, it is extremely important to effectively understand the clinical features of disease and realize early detection, early diagnosis and early treatment (13).
In this study, patients diagnosed with autoimmune hepatitis were studied, and changes in vitamin D level and liver function in subjects enrolled were investigated. It was found that in patients diagnosed with autoimmune hepatitis, the inflammatory level in the body was significantly increased, the oxidative stress capacity was significantly enhanced, and T cell subset and vitamin D levels were obviously decreased. At the same time, patients with autoimmune hepatitis with declined vitamin D level were studied, and it was found that inflammatory and stress responses were more significant and T cell subset levels were decreased compared with those in patients with autoimmune hepatitis whose vitamin D level was normal. Moreover, inflammatory and stress responses were increased remarkably, T cell subset levels were decreased obviously and the vitamin D level declined in patients with autoimmune hepatitis whose liver function was abnormal compared with those in patients whose liver function was normal. Finally, correlation analysis of 25-(OH) D level with inflammatory factors, related indexes to liver function and related indexes to T cell subsets in patients with autoimmune hepatitis manifested that the 25-(OH) D level in patients with autoimmune hepatitis was negatively correlated with hs-CRP, TNF-α, ALT and MDA, but positively correlated with CD3+ and CD4+ counts, SOD and total antioxidant capacity.
Results of this study indicate that in patients with autoimmune hepatitis, there are significantly decreased level of vitamin D, enhanced inflammatory and oxidative stress responses in the body, and decreased level of T cell subsets. Autoimmune hepatitis patients complicated with decreased level of vitamin D are more prone to aseptic inflammatory changes and peroxidation in the body. At the same time, the decline in T cell subset levels in the body leads to further damage to the immune function in the body. The decline in the vitamin D level is mainly manifested in the change in the level of its metabolite 25-(OH) D (14). The mutation of the FokI gene in the initiation codon of vitamin D receptor exon 2 affects the mRNA transcription and replication in the vitamin D receptor (15), thereby inhibiting the function of vitamin D receptor and leading to immunosuppression, so that the autoantigen tolerance is decreased, causing autoimmune hepatitis (16). Moreover, damage is caused to the body's immunity, especially resistance to virus infection, maintenance of homeostasis and immune regulatory function (17). Immunopathological cascade reaction is also further mediated, autoantibody levels are increased (18), and a large number of antigen-antibody complexes are formed, leading to further damage to hepatic tissues (19) and enhanced inflammatory and stress response in the body (20).
In conclusion, patients with autoimmune hepatitis, especially those with decreased level of vitamin D, are more prone to enhanced inflammatory and stress responses, decreased levels of T cell subsets and decline in immunity.
Figure 5.
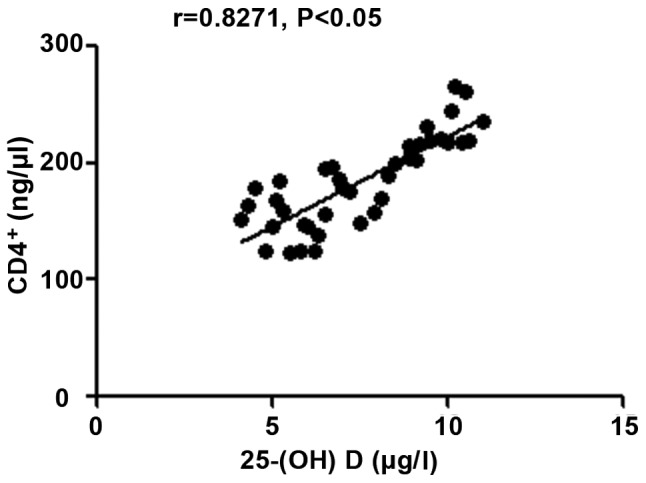
Correlation analysis between 25-(OH) D level and CD4+ count. 25-(OH) D, 25-hydroxyvitamin D.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ST and HZ collected and analyzed the general data of patients. QZ, HB and HW detected the liver function and the levels of inflammatory factors. HG was responsible for the oxidative stress factors and T cell subset analysis. ST wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Sixth People's Hospital of Qingdao City and informed consents were signed by the patients and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lee WS, Jalaludin MY, Wong SY, Ong SY, Foo HW, Ng RT. Vitamin D non-sufficiency is prevalent in children with chronic liver disease in a tropical country. Pediatr Neonatol. 2019;60:12–18. doi: 10.1016/j.pedneo.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Czaja AJ. Epigenetic changes and their implications in autoimmune hepatitis. Eur J Clin Invest. 2018;48:899–912. doi: 10.1111/eci.12899. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari SM, Fallahi P, Antonelli A, Benvenga S. Environmental issues in thyroid diseases. Front Endocrinol (Lausanne) 2017;8:50–58. doi: 10.3389/fendo.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arooj M, Malik A, Basit A, Husain Qazi M, Asif M, Sarwar Jamal M, Mostafa Mahmoud M, Choudhry H, Natesan Pushparaj , Rasool M. Implications of prognostic variables in the assessment of autoimmunity in hepatitis C patients receiving interferon therapy. Bioinformation. 2016;12:131–134. doi: 10.6026/97320630012131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montano-Loza AJ, Thandassery RB, Czaja AJ. Targeting hepatic fibrosis in autoimmune hepatitis. Dig Dis Sci. 2016;61:3118–3139. doi: 10.1007/s10620-016-4254-7. [DOI] [PubMed] [Google Scholar]
- 6.Kaji H. Treatment for hepatic osteodystrophy. Clin Calcium. 2015;25:1689–1694. (In Japanese) [PubMed] [Google Scholar]
- 7.Ahmed SZ, Jaleel A, Hameed K, Qazi S, Suleman A. Does vitamin D deficiency contribute to the severity of asthma in children and adults? J Ayub Med Coll Abbottabad. 2015;27:458–463. [PubMed] [Google Scholar]
- 8.Purohit T, Cappell MS. Primary biliary cirrhosis: Pathophysiology, clinical presentation and therapy. World J Hepatol. 2015;7:926–941. doi: 10.4254/wjh.v7.i7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter FP, Hohenester S, Nagel JM, Wimmer R, Artmann R, Wottke L, Makeschin MC, Mayr D, Rust C, Trauner M, et al. 1,25-(OH)2-vitamin D3 prevents activation of hepatic stellate cells in vitro and ameliorates inflammatory liver damage but not fibrosis in the Abcb4(-/-) model. Biochem Biophys Res Commun. 2015;459:227–233. doi: 10.1016/j.bbrc.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 10.Beyazit Y, Kocak E, Tanoglu A, Kekilli M. Oxidative stress might play a role in low serum vitamin D associated liver fibrosis among patients with autoimmune hepatitis. Dig Dis Sci. 2015;60:1106–1108. doi: 10.1007/s10620-015-3526-y. [DOI] [PubMed] [Google Scholar]
- 11.Chen EQ, Shi Y, Tang H. New insight of vitamin D in chronic liver diseases. Hepatobiliary Pancreat Dis Int. 2014;13:580–585. doi: 10.1016/s1499-3872(14)60295-2. [DOI] [PubMed] [Google Scholar]
- 12.Petta S, Grimaudo S, Tripodo C, Cabibi D, Calvaruso M, Di Cristina A, Guarnotta C, Macaluso FS, Minissale MG, Marchesini G, et al. The hepatic expression of vitamin D receptor is inversely associated with the severity of liver damage in genotype 1 chronic hepatitis C patients. J Clin Endocrinol Metab. 2015;100:193–200. doi: 10.1210/jc.2014-2741. [DOI] [PubMed] [Google Scholar]
- 13.Boglione L, Cusato J, De Nicolò A, Cariti G, Di Perri G, D'Avolio A. Role of CYP27B1+2838 promoter polymorphism in the treatment of chronic hepatitis B HBeAg negative with PEG-interferon. J Viral Hepat. 2015;22:318–327. doi: 10.1111/jvh.12288. [DOI] [PubMed] [Google Scholar]
- 14.Efe C, Kav T, Aydin C, Cengiz M, Imga NN, Purnak T, Smyk DS, Torgutalp M, Turhan T, Ozenirler S, et al. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig Dis Sci. 2014;59:3035–3042. doi: 10.1007/s10620-014-3267-3. [DOI] [PubMed] [Google Scholar]
- 15.Borella E, Nesher G, Israeli E, Shoenfeld Y. Vitamin D: A new anti-infective agent? Ann N Y Acad Sci. 2014;1317:76–83. doi: 10.1111/nyas.12321. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Rehim AS, Sheha DS, Mohamed NA. Vitamin D level among Egyptian patients with chronic spontaneous urticaria and its relation to severity of the disease. Egypt J Immunol. 2014;21:85–90. [PubMed] [Google Scholar]
- 17.Luong KV, Nguyen LT. The role of vitamin D in autoimmune hepatitis. J Clin Med Res. 2013;5:407–415. doi: 10.4021/jocmr1505w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zúñiga S, Firrincieli D, Housset C, Chignard N. Vitamin D and the vitamin D receptor in liver pathophysiology. Clin Res Hepatol Gastroenterol. 2011;35:295–302. doi: 10.1016/j.clinre.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Ramagopalan SV, Goldacre R, Disanto G, Giovannoni G, Goldacre MJ. Hospital admissions for vitamin D related conditions and subsequent immune-mediated disease: Record-linkage studies. BMC Med. 2013;11:171–178. doi: 10.1186/1741-7015-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyk DS, Orfanidou T, Invernizzi P, Bogdanos DP, Lenzi M. Vitamin D in autoimmune liver disease. Clin Res Hepatol Gastroenterol. 2013;37:535–545. doi: 10.1016/j.clinre.2013.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


