Abstract
Cassia seed is the dried ripe seed of Cassia obtusifolia L. or Cassia tora L., which is widely used as a food or traditional Chinese medicine. The aim of the present study was to detect the components and metabolites in the culture of human or rat intestinal microflora suspension with the water decoction of cassia seed in vitro, using an ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry system equipped with a negative ion scan mode. Initially, ellagic acid was identified in the cassia seed decoction. Subsequently, six different metabolites, including urolithin (uro)-A, uro-B, uro-D, uro-M6, uro-M7 and uro-B-glucuronide (glur), were detected after co-culture of the cassia seed decoction with intestinal microflora, but not in the cassia seed decoction alone. Uro-M6, uro-M7, uro-A and uro-B were common metabolites in the culture of human or rat intestinal microflora suspension with the water decoction of cassia seed. However, uro-D was only detected in the culture of rat intestinal microflora suspension with the water decoction of cassia seed, and uro-B-glur was only detected in the culture of human intestinal microflora with the water decoction of cassia seed. The uro and intermediate metabolites were produced by ellagic acid in the cassia seed decoction under the action of the intestinal microflora. The production of metabolites might be related to the abundance and diversity of the intestinal microflora in humans and rats. The present study provided rationale for further pharmacological and clinical studies on the mechanisms of action of cassia seeds.
Keywords: cassia seed, ellagic acid, intestinal microflora, ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry, urolithin
Introduction
The chemical composition of compounds, including prototypes and metabolites, is the basis of the effect of traditional Chinese medicine (1,2). The majority of Chinese medicines enter into the body via oral administration and thus, interact with intestinal microbes (3). A number of Chinese herbal medicines play a therapeutic role primarily via intestinal flora metabolism, which has become a popular research topic (2,3).
The intestinal microflora is an extremely complex microbial system, and is associated with the health of the individual or animal it exists within (4). Certain chemical components of traditional Chinese medicine can inhibit or promote the growth of certain bacteria, altering the composition of the intestinal microflora (5). Conversely, intestinal microflora can alter the metabolism of Chinese herbal medicine directly, by reductive or hydrolytic reactions, or indirectly, by producing a variety of enzymes, amino acids and vitamins, such as N-acyl-3-hydroxyglycines and β-glucuronidases, β-glucosidase and dioxygenase (3,6,7). Therefore, investigating the role of intestinal microflora in the transformation and metabolism of the components of Chinese medicines may aid in further understanding the metabolic pathways of intestinal microflora and the potential mechanisms of action of Chinese medicines in the body.
Cassia seed is the dried ripe seed of the leguminous plant Cassia obtusifolia L. or Cassia tora L. (8). It was first recorded in ‘Shen Nong's Herbal Classic’ (9). The seed displays extensive pharmacological actions, for example, modern pharmacological research has reported that the cassia seed possesses antibacterial activity (10,11). Beyond the purgative action of the cassia seed, it has also been reported to decrease blood pressure and alleviate hyperlipidemia (12), protect the liver (13), improve immunity (14) and display anti-inflammatory (15) and neuroprotective effects in models of Parkinson's disease (16,17). The cassia seed is commonly used in the treatment of hypertension, fatty liver and constipation (12,13). At present, research into the pharmacodynamic properties of the cassia seed has primarily focused on the anthraquinone compounds of the seed, which are related to its effect on diarrhea (18,19). However, based on the complex characteristics of Chinese medicine, it is speculated that the role of the cassia seed in protecting the liver and cardio-cerebral vessels, as well as improving eyesight may be related to other unknown components, in particular the components that are transformed by the intestinal microflora (20,21). Based on the aforementioned understanding, the present study cultured human or rat intestinal microflora suspensions with cassia seeds in vitro, to clarify the mechanism of action of the cassia seed. The present study focused on the transformation of active ingredients by the intestinal microflora and examined the differences in the transformation of active ingredients between humans and laboratory rats. The water decoction of cassia seeds was placed into culture medium containing human or rat intestinal microflora suspension under an anaerobic and sterile environment in vitro. Subsequently, the metabolites were analyzed using an ultra-high-performance liquid chromatography (UPLC)-quadrupole time-of-flight (QTOF) mass spectrometry (MS) system. The present study hypothesized that if the water decoction of cassia seeds was transformed by the intestinal microflora, a number of novel compounds could be detected. Alternatively, if no novel compounds were identified, the composition of the cassia seed would be as has previously been described (20).
Materials and methods
Ethics
The present study was approved by the Ethics Committee of Henan University of Chinese Medicine. Written informed consent was received from all participants.
Materials and reagents
Raw cassia seeds were collected from the Medicine Botanical Garden of Henan University of Chinese Medicine in September 2016. The samples were identified by Professor Suiqing Chen (College of Pharmacy, Henan University of Chinese Medicine) as the seeds of the legume C. obtusifolia L. Ellagic acid (batch no. 1013A022) was obtained from Beijing Solarbio Science & Technology Co., Ltd. The broth medium (batch no. HB0384-1) and agar powder (batch no. 01-023) were purchased from Haibo Biotechnology Co., Ltd. Methanol was purchased from Tianjin Siyou Fine Chemicals Co., Ltd. Acetonitrile (UPLC/MS grade) and formic acid (high performance liquid chromatography grade) with a purity of 99% were purchased from Kareo. Purified water was acquired from an ESW-1-30 system (Easywell Water System, Inc.). All the other reagents were obtained from Huayu Biotech Co., Ltd.
Preparation of the cassia seed decoction
The cassia seeds were broken into pieces and weighed using an electronic balance. Subsequently, 20.0 g of seeds were soaked in 200 ml water for 30 min at 25˚C. The suspension was boiled for at least 30 min and then the filtrate was collected using a 0.22 µm microporous filter. The filter residue was mixed with water in the ratio of 1:6 and boiled for 20 min. The resulting filtrate was collected. The two filtrates were combined and concentrated to 20 ml at 60˚C under vacuum, using a rotary evaporator. The concentration of the resulting solution was 1.0 g/ml crude cassia seed, and the solution was stored at 4˚C until further analysis.
Collection of human fecal samples
Human fecal samples were obtained from three healthy males (aged 21, 22 and 23 years; body mass 60-70 kg; height 172-178 cm) in July 2018 from Jinshui, Zhengzhou, Henan. Each subject provided two fecal samples to allow culture experiments to be performed in duplicate. Samples were stored at 4˚C and were processed within 1 h of donation. No differences in the microbial concentrations of the samples were observed between fresh samples before and after processing. The samples were maintained in anoxic conditions using the YQX-II anaerobic workstation with 5% CO2, 5% hydrogen and 90% nitrogen (Shanghai Longyue Equipment Co., Ltd.).
Collection of rat fecal samples
Rat fecal samples were obtained from three Sprague-Dawley rats (male; body mass ~250 g; age, 6-7 weeks; Jinan Pengyue Experimental Animal Breeding Co., Ltd.). Rats were maintained on a 12 light/dark cycle in a 22±2˚C room with 40% relative humidity. Food and water were provided ad libitum. To allow for culture experiments to be performed in duplicate, two fecal samples were collected from each rat. All procedures regarding the processing of the rat fecal samples were the same as for the human fecal samples.
Culture of human or rat intestinal microflora suspension with cassia seed decoction
The individual human or rat fecal samples were mixed to an even consistency in a germ-free and anoxic environment. Subsequently, ~1 g of fecal sample was mixed with 100 ml 0.1 M PBS to produce an intestinal microflora suspension. Then, 200 µl human or rat intestinal microflora suspension was placed into solid broth medium (18 mg/ml broth medium, 16.7 mg/ml agar; sterilization at 121˚C for 20 min) with the water decoction of cassia seeds (0.3, 0.15 or 0.075 g/ml). Additionally, 200 µl human or rat intestinal microflora suspension was placed into liquid broth medium (18 mg/ml broth medium; sterilization at 121˚C for 20 min) with the water decoction of cassia seeds (0.17 g/ml). The human or rat intestinal microflora suspension was not added to the broth medium for the control group. Each group had duplicate samples. The culture protocol was performed in a germ-free laminar flow cabinet. The culture was incubated in a YQX-II anoxic workstation (Shanghai Longyue Equipment Co., Ltd.) for 48 h.
Preparation of samples
The culture medium solution was centrifuged at 11,342 x g at 4˚C for 10 min. Subsequently, 1 ml supernatant was mixed with 1 ml 99.9% of methanol and the mixture was incubated at 4˚C for 10 min. The mixture was then centrifuged at 11,342 x g and 4˚C for 10 min. The resulting solution was filtered through a 0.22-mm membrane filter and then injected into the UPLC-QTOF/MS system for analysis.
Liquid chromatography
The chromatographic analysis was performed on an Acquity I-Class UPLC system (Waters Corporation). The separation was performed using an Acquity BEH C18 column (100x2.1 mm2; particle size, 1.7 µm; Waters Corporation) maintained at 25˚C with a flow rate of 0.4 ml/min. The injection volume was 5 µl. The optimal mobile phase consisted of A (acetonitrile) and B (HCOOH/H2O; 0.1:100). The optimized UPLC gradient elution conditions were as follows: 0-1 min, 2% A and 98.0% B; 1-10 min, 98.0% A and 2.0% B; and 11-13 min, 2.0% A and 98.0% B. The detection wavelength was 256 nm.
MS
MS detections were performed on a Xevo-G2-XS QTOF tandem mass spectrometer (Waters Corporation) with negative and positive electrospray ionization (ESI) modes. The sensitivity of the system ensured the identification of as high of a number of putative compounds as possible. QTOF-MS was performed for the mass ranges of 100-1,200 m/z, and the experiments were run with 200 msec accumulation time. Positive and negative ionization modes were tested and the negative ionisation mode was selected for improved sensitivity. The conditions used for the ESI source were as follows: Ion source injection voltage, 4 kV; capillary voltage, 3.0 kV; sampling cone, 40 V; source temperature, 120˚C; and desolvation temperature, 500˚C. Nitrogen was used as a cone and desolvation gas with a flow rate of 50 and 800 l/h, respectively. The nebulizer pressure of the nitrogen gas was 100 psi. Argon was used as the collision gas with a coll ision energy of 10-30 V, a scan time of 0.5 sec and an interval scan time of 0.02 sec. Acquiring data in this manner allowed for information regarding the precursor and fragment ions to be collected. Mass tolerance was set at <5 ppm to reduce the number of options used to determine the elemental compositions of both the precursor and the product ions.
Data processing and analysis strategy
For data processing, Masslynx software (version 4.1; Waters Corporation) was used for qualitative analyses. Extracted ion chromatograms and the MS Library made by identification for unknown components of the elucidation tool in the Masslynx software were used to identify the target compounds. A formula database of target compounds, including names, molecular formulas, accurate molecular weights and chemical structures, was established for the target compounds. This database was prepared using previously reported information (22,23). Subsequently, the names of the target compounds were imported into the extracted ion chromatograms in the UNIFI Scientific Information System (Water Corporation) to finalize the screening of the target compounds. After screening, the compounds that matched the names, molecular formulas, accurate molecular weights and chemical structures of the target compounds in the formula database were extracted. Then, the target compound information was compared with that of standard compounds (Beijing Solarbio Science & Technology Co., Ltd.) whose spectra were obtained by matching the MS/MS fragments. Hence, the common compounds existing in the water decoction of cassia seeds were identified. The structures of the metabolites were presumed primarily based on accurate mass and mass fragmentation using the UNIFI Scientific Information System. Finally, the fragment ions were used to further confirm the chemical structures by making comparisons with previously reported data (24-31).
Results
Discovery and identification of ellagic acid in cassia seeds
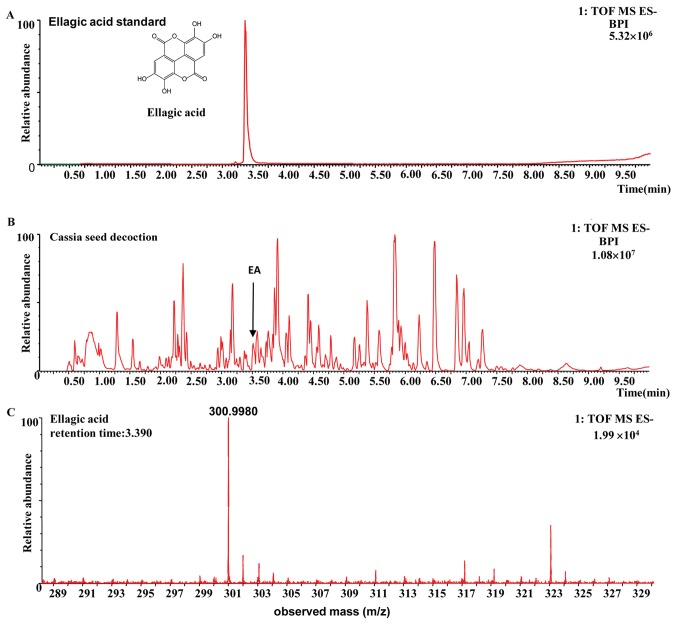
The compound was identified as ellagic acid on the basis of its retention time and the m/z of characteristic ions, compared with those of the standard compounds and data obtained from the literature (22,23). The fragmentation pathway of ellagic acid was recorded using a collision energy of 10-30 V and the collision-induced dissociation MS/MS spectrum for ellagic acid is shown in Fig. 1. In the MS/MS spectrum, the [M-H]- ion at m/z 300.9980 was selected as a precursor ion to provide fragmentation information. The precursor ions produced were an [M-H-OH]- ion at m/z 283.9951 (C14H4O7) and an [M-H-CO2]- ion at m/z 257.0080 (C13H5O7). The [M-H-CO2]- ion yielded ions at m/z 229.01328 (C12H5O5), 201.01843 (C11H5O4), 173.02396 (C10H5O3) and 145.02948 (C9H5O2), consecutively by the sequential loss of CO, and were identified as the characteristic ions of ellagic acid (22,23). The [M-H-CO2-CO]- ion at m/z 229.01328 underwent direct elimination of CO2 and yielded the product [M-H-2CO2-CO]- ion at m/z 185.02388 (C11H5O3). The structures, retention times and MS data of compounds are summarized in Fig. 1 and Table I.
Figure 1.
Identification of EA in the cassia seed decoction. Total ion current chromatogram of (A) the EA reference standard and (B) the cassia seed decoction. (C) Negative mass spectrometry spectrum of the EA in the cassia seed decoction. (D) Negative tandem mass spectrometry spectrum of EA (blue diagrams are EA fragment ion symbols). EA, ellagic acid; TOF, time of flight; MS, mass spectrometry; ES, electrospray; BPI, base peak ion chromatogram.
Table I.
Mass spectrometry data and proposed fragmentation pathways of ellagic acid.
| tR (min) | Ion (m/z) | Diff (ppm) | Chemical formula | Fragmentation pathways | Diff (ppm) | Identification |
|---|---|---|---|---|---|---|
| 3.39 | 300.9980 | -3.29 | C14H5O8 | 283.99510 [M-H-OH]- | -4.052 | Ellagic acid |
| 257.00800 [M-H-CO2]- | -4.518 | |||||
| 229.01328 [M-H-CO2-CO]- | -4.220 | |||||
| 201.01843 [M-H-CO2-2CO]- | -4.478 | |||||
| 185.02388 [M-H-2CO2-CO]- | -2.904 | |||||
| 173.02396 [M-H-CO2-3CO]- | -2.643 | |||||
| 157.02930 [M-H-2CO2-2CO]- | -1.291 | |||||
| 145.02948 [M-H-CO2-4CO]- | -0.157 |
tR, retention time; Diff, the error between the observed mass-to-charge ratio and the theoretical mass-to-charge ratio; ppm, parts per million.
General metabolites in the culture of human or rat intestinal microflora suspension with the water decoction of cassia seeds
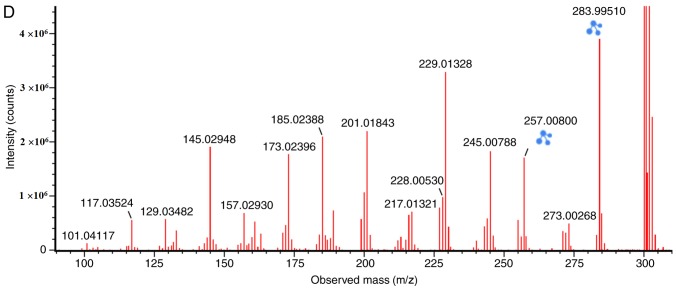
The [M-H]- ion at m/z 227.0329, with a retention time of 4.25 min, gave rise to a fragment ion at m/z 198.0357 by the loss of an hydrogen ion and a carbonyl ion. The fragment ion then gave rise to an [M-CO-O-H]- ion at m/z 182.0398 by the loss of an oxygen ion. It was speculated that the [M-CO-O-H]- ion was an aromatic acid ester compound. The characteristics of the [M-CO-O-H]- ion were the same as those of the urolithin (uro)-A (C13H8O4) metabolite of ellagic acid (24). Therefore, the [M-H]- ion was identified as uro-A. The structures, retention times and MS data of the compounds are summarized in Fig. 2 and Table II.
Figure 2.
Discovery and identification of urolithin A (C13H8O4) in the co-culture of the human or rat intestinal microflora suspension and the cassia seed decoction. The spectrum presented was prepared from rat microflora and is representative of the result from both human and rat microflora. TOF, time of flight; MS, mass spectrometry; ES, electrospray.
Table II.
Metabolites in the co-culture of the human or rat intestinal microflora suspension and the cassia seed decoction.
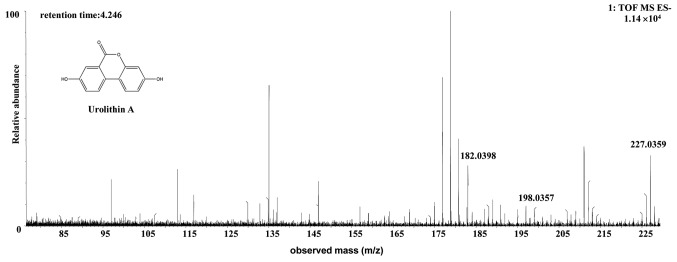
| Metabolites | Source | Chemical formula | Retention time (min) | Parent ion (m/z) | Error (mda) | Molecular ion | MS/MS m/z (error/ppm) |
|---|---|---|---|---|---|---|---|
| Uro-A | Human or rat | C13H8O4 | 4.25 | 227.0329 | -0.8 (human)/0.3 (rat) | [C13H7O4]- | MS2[227.0329]: 198.0357, 182.0398 |
| Uro-B | Human or rat | C13H8O3 | 5.92 | 211.0381 | -0.3 (human)/0.3 (rat) | [C13H7O3]- | MS2[211.0381]: 167.0509 (3.962), 139.0531(-15.991) |
| Uro-M6 | Human or rat | C13H8O6 | 3.64 | 259.0249 | 0.1 (human)/0.5 (rat) | [C13H7O6]- | MS2[259.0249]: 213.0155 (-17.9898), 187.0428(14.690), 159.0453 (0.925) |
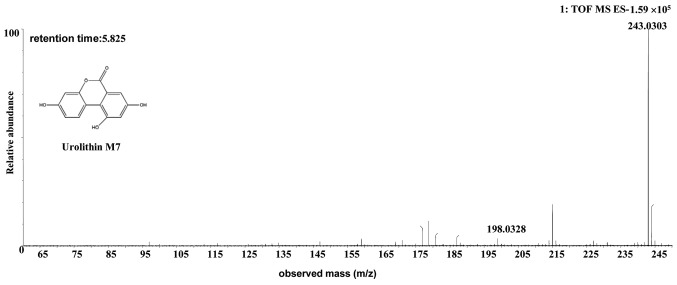
| Uro-M7 | Human or rat | C13H8O5 | 5.82 | 243.0303 | -0.7 (human)/-0.5 (rat) | [C13H7O5]- | MS2[243.0303]: 198.0328 |
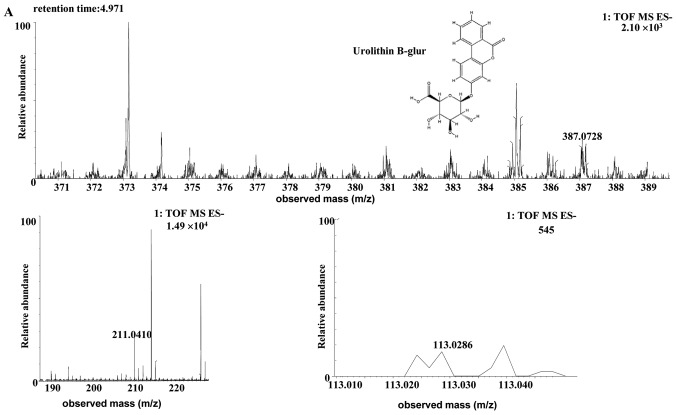
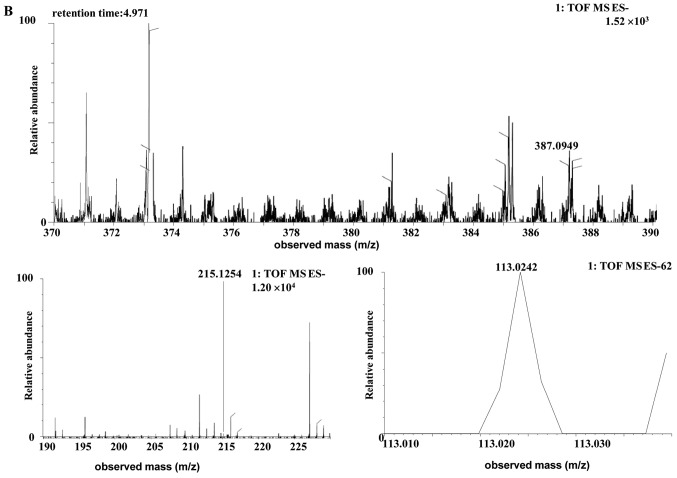
| Uro-B glur | Human | C19H16O9 | 4.97 | 387.0728 | 0.4 (human) | [C19H15O9]- | MS2[387.0728]: 211.0410 (4.419), 113.0286 |
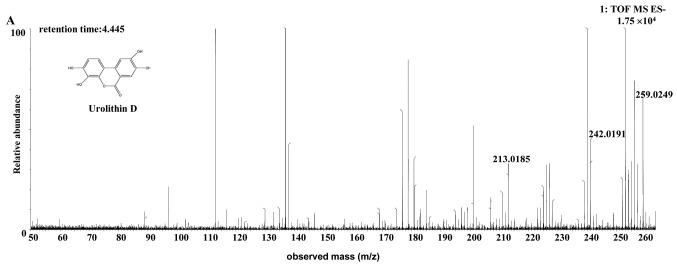
| Uro-D | Rat | C13H8O6 | 4.45 | 259.0249 | 1.1 (rat) | [C13H7O6]- | MS2[259.0249]: 242.0191, 213.0185 (-3.906) |
m/z, mass-to-charge ratio; mda, millidalton; ppm, parts per million; uro, urolithin.
The [M-H]- ion at m/z 211.0381, with a retention time of 5.92 min, gave rise to a fragment ion at m/z 167.0509, by the loss of an hydrogen ion and a carbonyl ion. The fragment ion then gave rise to an [M-H-COO-CO]- fragment ion at m/z 139.0531 by the loss of a carbonyl ion. The characteristics of this ion were the same as those of the uro-B (C13H8O3) metabolite of ellagic acid (25). Therefore, the product ion was identified as uro-B. The structures, retention times and MS data of the compounds are summarized in Fig. 3 and Table II.
Figure 3.
Discovery and identification of urolithin B (C13H8O3) in the co-culture of the human or rat intestinal microflora suspension and the cassia seed decoction. The spectrum was prepared from rat microflora and is representative of the result from both human and rat microflora. TOF, time of flight; MS, mass spectrometry; ES, electrospray.
The [M-H]- ion at m/z 259.0249, with a retention time of 3.64 min, gave rise to a fragment ion at m/z 213.0155, 187.0428 and 159.0453, by MS/MS analyses. The characteristics of the [M-H]- ion were the same as those of the uro-M6 (C13H8O6) metabolite of ellagic acid (26,27). Therefore, the product ion was identified as uro-M6. The structures, retention times and MS data of the compounds are summarized in Fig. 4 and Table II.
Figure 4.
Discovery and identification of urolithin M6 (C13H8O6) in the co-culture of the human or rat intestinal microflora suspension and the cassia seed decoction. The spectrum was prepared from rat microflora and is representative of the result from both human and rat microflora. TOF, time of flight; MS, mass spectrometry; ES, electrospray.
The [M-H]- ion at m/z 243.0303, with a retention time of 5.82 min, gave rise to a fragment ion at m/z 198.0328 [M-H-COOH]-, by MS/MS analyses. The characteristics of this [M-H]- ion were the same as those of the uro-M7 (C13H8O5) metabolite of ellagic acid (28,29). Therefore, the product ion was identified as uro-M7. The structures, retention times and MS data of the compounds are summarized in Fig. 5 and Table II.
Figure 5.
Discovery and identification of urolithin M7 (C13H8O5) in the co-culture of the human or rat intestinal microflora suspension and the cassia seed decoction. The spectrum was prepared from rat microflora and it is representative of the result from both human and rat microflora. TOF, time of flight; MS, mass spectrometry; ES, electrospray.
Metabolites in the culture of human intestinal microflora suspension with the water decoction of cassia seeds
The [M-H]- ion at m/z 387.0728, with a retention time of 4.97 min, gave rise to a fragment ion at m/z 211.0410 and 113.0286, by MS/MS analyses. The characteristics of this [M-H]- ion were the same as those of the metabolite uro-B-glucuronide (glur; C19H16O9) of ellagic acid (30). Therefore, the product ion was identified as uro-B-glur. The structures, retention times and MS data of the compounds are summarized in Fig. 6 and Table II.
Figure 6.
Discovery and identification of the urolithin B-glur (C19H16O9) in the co-culture of (A) the human or (B) the rat intestinal microflora suspension and the cassia seed decoction. Glur, glucuronide; TOF, time of flight; MS, mass spectrometry; ES, electrospray.
Metabolites in the culture of rat intestinal microflora suspension with the water decoction of cassia seeds
The [M-H]- ion at m/z 259.0249, with a retention time of 4.45 min, gave rise to an [M-OH-H]- fragment ion at m/z 242.0191, by the loss of an hydroxide ion. The fragment ion then gave rise to an [M-OH-CHO-H]- ion at m/z 213.0185, by the loss of a CHO. The characteristics of this [M-H]- ion were the same as those of the uro-D (C13H8O6) metabolite of ellagic acid (31). Therefore, the product ion was identified as uro-D. The structures, retention times and MS data of the compounds are summarized in Fig. 7 and Table II.
Figure 7.
Discovery and identification of the urolithin D (C13H8O6) in the co-culture of (A) the rat or (B) the human intestinal microflora suspension and the cassia seed decoction. TOF, time of flight; MS, mass spectrometry; ES, electrospray.
Metabolic pathways of ellagic acid
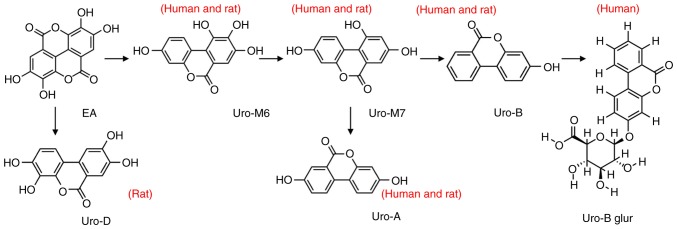
Urolithin was generated from ellagic acid via three metabolic pathways (Fig. 8). The metabolites were uro-A, uro-B and uro-D. Moreover, the metabolic pathways were different for human or rat intestinal microflora. uro-B was further transformed into uro-B-glur by human intestinal microflora and uro-D was generated only by the rat intestinal microflora.
Figure 8.
Metabolic pathways of ellagic acid that generate urolithins in the co-culture of the human or rat intestinal microflora suspension and the cassia seed decoction. Uro, urolithin; glur, glucuronide.
Discussion
In the present study, the interaction between the intestinal micoflora and the cassia seed decoction was investigated. UPLC-MS/MS technology is a combination of high-resolution UPLC and highly sensitive and selective MS. The use of this technology does not require the measured samples to be subjected to purification or derivatization with complicated separation and enrichment processes prior to analysis (32). A large amount of reliable qualitative and quantitative information can be quickly obtained following a simple pre-treatment with UPLC-MS/MS technology (32). The present study described a novel, rapid and effective UPLC-MS/MS method for the discovery and identification of ellagic acid in the cassia seed decoction. Ellagic acid is a derivative of gallic acid, which is a tannin with antioxidant, anticancer and anti-inflammatory effects (33). Ellagic acid also regulates the intestinal microflora and performs other functions, including its anti-atherogenic and neuroprotective effects (33). Despite displaying a wide range of biological activities, the permeability of ellagic acid is too low for direct absorption into the body. Synthesis and transformation of ellagic acid are still widely used today. In general, ellagic acid is obtained in two main forms. One form can be produced by the oxidative polymerization of gallic acid or gallic acid ester under the action of peroxidase. The other form is prepared by hydrolyzing ellagitannin, which requires a high temperature and is performed under acidic conditions (33). Extraction methods using hot water reflux in this experiment do not provide the correct conditions for the conversion of other components into ellagic acid (34). Therefore, the present study identified ellagic acid as one of the natural components and parent compounds in the cassia seed.
Previous studies have reported that ellagic acid is metabolized to the more easily absorbed urolithin by the intestinal microflora (28,30). Compared with traditional methods such as HPLC or UPLC, UPLC-MS/MS can identify a number of metabolites in a short time, reduce the use of animals and facilitate the metabolite identification process (23). The environmental factors influencing the metabolic process are easy to control in the UPLC-MS/MS system (28,32,35). Moreover, certain active metabolites in the biological samples were likely to be generated at relatively low levels and UPLC-MS/MS is able to detect low levels of metabolites. A number of studies have used UPLC-MS/MS to detect novel compounds in the co-culture of human or rat intestinal microflora and the cassia seed decoction (20,35). The cassia seed contains a number of chemical components, including anthraquinones and tannins (12). Although the cassia seed contains a large amount of anthraquinones, anthraquinones are also present in a number of other traditional Chinese medicines, including rhubarb (Rheum palmatum L.) (12,35). The biotransformation of anthraquinone metabolites under the action of the intestinal microflora has been previously investigated using tUPLC-QTOF/MS (35).
The present study identified ellagic acid as a novel compound in the cassia seed decoction. To further investigate the presence of ellagic acid in the cassia seed decoction, the biotransformation metabolites of ellagic acid were detected using a UPLC-Q-TOF/MS system. The six types of urolithin, which are the metabolites of ellagic acid under the action of the intestinal microflora, were detected and identified. To the best of our knowledge, the present study was the first to identify ellagic acid as a component of the cassia seed. Furthermore, the present study suggested that the urolithin metabolites of ellagic acid may be the active component of cassia seeds.
The therapeutic effect of urolithin has been widely recognized (36-40). Urolithin can enhance the anti-inflammatory effects of neutrophils, alleviate the symptoms of inflammation (36) and protect against prostate cancer (37). Uro-A and uro-B increase cell viability and protect cells, reduce malondialdehyde content and enhance superoxide dismutase activity (38). Uro-A regulates the expression of intercellular adhesion factor-1 via the ERK/peroxisome proliferator activated receptor-γ signaling pathway, which exerts anti-inflammatory effects (39). Uro-A also induces mitophagy, prolongs the lifespan of Caenorhabditis elegans and increases muscle function in rodents (40,41). In the present study, it was speculated that uro-A was the active component that induced the antioxidant effect of cassia seeds (12,42).
Furthermore, the present study identified a difference between the presence of urolithins in human and rat intestinal microflora. Uro-B-glur was only identified in human intestinal microflora and uro-D only in rat intestinal microflora. This finding indicated that the differences between metabolic products in human and rat intestinal microflora might be related to the abundance and diversity of the intestinal microflora. A significant difference between human and rat intestinal microflora has been previously reported. For example, the proportion of Bacteroides and Variovorax was the highest in human and rat intestinal microflora, respectively (43,44). Furthermore, ellagic acid was metabolized to urolithin and its derivatives under the action of lactonase (45) and high concentrations of G. urolithinfaciens DSM 27213T (≥107 cfu/g feces) (46). Moreover, the degradation of ellagic acid by the intestinal microflora leads to the formation of different urolithin metabolites in fecal cultures in vitro (20). The intestinal microflora is a micro-ecological system that is important in the interaction between the animal, medicine and disease. However, in the present study it was difficult to understand the complexity of this interaction because the individual differences between the three human or animal samples were large. This is a potential limitation of the present study. Furthermore, additional drug metabolism systems, including the liver, are present in whole organisms. Therefore, cassia seed treatment in vivo could reveal different results compared with in vitro treatment. The effect of the intestinal microbiota on drug metabolism has gained importance in clinical research because it can influence the hepatic drug metabolism of the host (21,47). In the present study, although ellagic acid was not a key or main ingredient of the crude cassia seed, it could be identified in the chemical profiles of the cassia seed. Therefore, further investigation into ellagic acid and the components of the cassia seed is required.
In summary, the cassia seed has a complex chemical composition and a wide range of pharmacological activities (12). The seed is a natural botanical drug, which in recent years has been widely used in research (12). In the present study, ellagic acid was identified in the cassia seed decoction and subsequently, ellagic acid and its metabolite urolithin were studied. The present study suggested that urolithin and the intermediate metabolites were produced by ellagic acid in the cassia seed decoction, under the action of the intestinal microflora by hydrolysis and glucuronidation. The mechanism of action of the intestinal microflora might be related to the abundance and diversity of the human or rat intestinal microflora. The metabolism of ellagic acid involved the biotransformation of urolithin into a number of final products, including uro-B-glur. However, with regards to uro-B-glur, the related mass spectrometry signal was buried in the baseline noise, potentially due to the low content of the product in the sample. The present study provided rationale for further pharmacological and clinical investigation into the mechanisms of action of the cassia seed and to clarify the pathways and mechanisms of action of phytochemicals containing ellagic acid.
Acknowledgements
The authors would like to thank Dr Wei-Xia Li and Dr Ying-Jie Cao of the Pharmaceutical Department of the First Affiliated Hospital of the Henan University of Chinese Medicine for their technical guidance.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81503363 and 81473435) and the Science and Technology Innovation Talents Support Project of the Henan University of Chinese Medicine (grant no. 2015XCXRC02).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SHW and HBL conceived and designed the study. NL and YJQ performed the LC-MS analysis and collected the data. GLL prepared the samples. SHW wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics committee of Henan University of Chinese Medicine (approval no. DWLL2018030060). Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yang C, Yin X, Dong X Zhang X, You L, Wang W, Wang J, Chen Q, Ni J. Determination of the phytochemical composition of Jingning fang and the in vivo pharmacokinetics of its metabolites in rat plasma by UPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1067:71–88. doi: 10.1016/j.jchromb.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Wu S, Xu W, Wang FR, Yang XW. Study of the biotransformation of tongmai formula by human intestinal flora and its intestinal permeability across the caco-2 cell monolayer. Molecules. 2015;20:18704–18716. doi: 10.3390/molecules201018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng W, Ao H, Peng C, Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Goodman AL, Gordon JI. Our unindicted coconspirators: Human metabolism from a microbial perspective. Cell Metab. 2010;12:111–116. doi: 10.1016/j.cmet.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang RF, Yuan M, Yang XB, Xu W, Yang XW. Intestinal bacterial transformation-a nonnegligible part of Chinese medicine research. J Asian Nat Prod Res. 2013;15:532–549. doi: 10.1080/10286020.2013.783573. [DOI] [PubMed] [Google Scholar]
- 6.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen LJ, Esterhazy D, Kim SH, Lemetre C, Aguilar RR, Gordon EA, Pickard AJ, Cross JR, Emiliano AB, Han SM, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. State Pharmacopoeia Commission of the PRC: Pharmacopoeia of the People's Republic of China. Vol. Ⅰ. Beijing. People's Medical Publishing House, 2015. [Google Scholar]
- 9.Xin T, Zhang Y, Pu X, Gao R, Xu Z, Song J. Trends in herbgenomics. Sci China Life Sci. 2019;62:288–308. doi: 10.1007/s11427-018-9352-7. [DOI] [PubMed] [Google Scholar]
- 10.Huang YL, Chow CJ, Tsai YH. Composition, characteristics, and in-vitro physiological effects of the water-soluble polysaccharides from Cassia seed. Food Chem. 2012;134:1967–1972. doi: 10.1016/j.foodchem.2012.03.127. [DOI] [PubMed] [Google Scholar]
- 11.Sahu J, Koley KM, Sahu BD. Attribution of antibacterial and antioxidant activity of Cassia tora extract toward its growth promoting effect in broiler birds. Vet World. 2017;10:221–226. doi: 10.14202/vetworld.2017.221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong X, Fu J, Yin X, Yang C, Zhang X, Wang W, Du X, Wang Q, Ni J. Cassiae semen: A review of its phytochemistry and pharmacology (Review) Mol Med Rep. 2017;16:2331–2346. doi: 10.3892/mmr.2017.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Q, Guo FF, Zhou W. Protective effects of cassia seed ethanol extract against carbon tetrachloride-induced liver injury in mice. Acta Biochim Pol. 2012;59:265–270. [PubMed] [Google Scholar]
- 14.Kim M, Lim SJ, Lee HJ, Nho CW. Cassia tora seed extract and its active compound aurantio-obtusin inhibit allergic responses in IgE-mediated mast cells and anaphylactic models. J Agric Food Chem. 2015;63:9037–9046. doi: 10.1021/acs.jafc.5b03836. [DOI] [PubMed] [Google Scholar]
- 15.Yi JH, Park HJ, Lee S, Jung JW, Kim BC, Lee YC, Ryu JH, Kim DH. Cassia obtusifolia seed ameliorates amyloid β-induced synaptic dysfunction through anti-inflammatory and Akt/GSK-3β pathways. J Ethnopharmacol. 2016;178:50–57. doi: 10.1016/j.jep.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Ju MS, Kim HG, Choi JG, Ryu JH, Hur J, Kim YJ, Oh MS. Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson's disease models. Food Chem Toxicol. 2010;48:2037–2044. doi: 10.1016/j.fct.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Drever BD, Anderson WG, Riedel G, Kim DH, Ryu JH, Choi DY, Platt B. The seed extract of Cassia obtusifolia offers neuroprotection to mouse hippocampal cultures. J Pharmacol Sci. 2008;107:380–392. doi: 10.1254/jphs.08034fp. [DOI] [PubMed] [Google Scholar]
- 18.Shi BJ, Zhang WD, Jiang HF, Zhu YY, Chen L, Zha XM, Lu YY, Zhang WM. A new anthraquinone from seed of Cassia obtusifolia. Nat Prod Res. 2016;30:35–41. doi: 10.1080/14786419.2015.1032280. [DOI] [PubMed] [Google Scholar]
- 19.Xu YL, Tang LY, Zhou XD, Zhou GH, Wang ZJ. Five new anthraquinones from the seed of Cassia obtusifolia. Arch Pharm Res. 2015;38:1054–1058. doi: 10.1007/s12272-014-0462-x. [DOI] [PubMed] [Google Scholar]
- 20.García-Villalba R, Beltrán D, Espín JC, Selma MV, Tomás-Barberán FA. Time course production of urolithins from ellagic acid by human gut microbiota. J Agric Food Chem. 2013;61:8797–8806. doi: 10.1021/jf402498b. [DOI] [PubMed] [Google Scholar]
- 21.Koppel N, Maini Rekdal V, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356 doi: 10.1126/science.aag2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen W, Yokota T, Lean ME, Crozier A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry. 2003;64:617–624. doi: 10.1016/s0031-9422(03)00281-4. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Johnson JV, Talcott ST. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J Agric Food Chem. 2005;53:6003–6010. doi: 10.1021/jf050468r. [DOI] [PubMed] [Google Scholar]
- 24.Cerdá B, Periago P, Espín JC, Tomás-Barberán FA. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. 2005;53:5571–5576. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- 25.Lucas R, Alcantara D, Morales JC. A concise synthesis of glucuronide metabolites of urolithin-B, resveratrol, and hydroxytyrosol. Carbohydr Res. 2009;344:1340–1346. doi: 10.1016/j.carres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Rupiani S, Guidotti L, Manerba M, Di Ianni L, Giacomini E, Falchi F, Di Stefano G, Roberti M, Recanatini M. Synthesis of natural urolithin M6, a galloflavin mimetic, as a potential inhibitor of lactate dehydrogenase A. Org Biomol Chem. 2016;14:10981–10987. doi: 10.1039/c6ob01977c. [DOI] [PubMed] [Google Scholar]
- 27.Nuñez-Sánchez MA, García-Villalba R, Monedero-Saiz T, García-Talavera NV, Gómez-Sánchez MB, Sánchez-Álvarez C, García-Albert AM, Rodríguez-Gil FJ, Ruiz-Marín M, Pastor-Quirante FA, et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res. 2014;58:1199–1211. doi: 10.1002/mnfr.201300931. [DOI] [PubMed] [Google Scholar]
- 28.González-Barrio R, Truchado P, Ito H, Espin JC, Tomás-Barberán FA. UV and MS identification of Urolithins and Nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals. J Agric Food Chem. 2011;59:1152–1162. doi: 10.1021/jf103894m. [DOI] [PubMed] [Google Scholar]
- 29.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 30.García-Villalba R, Espín JC, Tomás-Barberán FA. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J Chromatogr A. 2016;1428:162–175. doi: 10.1016/j.chroma.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 31.Giorgio C, Mena P, Del Rio D, Brighenti F, Barocelli E, Hassan-Mohamed I, Callegari D, Lodola A, Tognolini M. The ellagitannin colonic metabolite urolithin D selectively inhibits EphA2 phosphorylation in prostate cancer cells. Mol Nutr Food Res. 2015;59:2155–2167. doi: 10.1002/mnfr.201500470. [DOI] [PubMed] [Google Scholar]
- 32.Fan M, Qin K, Ding F, Huang Y, Wang X, Cai B. Identification and differentiation of major components in three different ‘Sheng-ma’ crude drug species by UPLC/Q-TOF-MS. Acta Pharm Sin B. 2017;7:185–192. doi: 10.1016/j.apsb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vattem DA, Shetty K. Biological functionality of ellagic acid: A review. J Food Biochem. 2005;29:234–266. [Google Scholar]
- 34.Jadhav PD, Laddha KS. Synthesis of new ellagic acid derivatives. Indian J Chem B. 2006;45:1551–1553. [Google Scholar]
- 35.Huang ZH, Xu Y, Wang Q, Gao XY. Metabolism and mutual biotransformations of anthraquinones and anthrones in rhubarb by human intestinal flora using UPLC-Q-TOF/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1104:59–66. doi: 10.1016/j.jchromb.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Piwowarski JP, Granica S, Zwierzyńska M, Stefańska J, Schopohl P, Melzig MF, Kiss AK. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J Ethnopharmacol. 2014;155:801–809. doi: 10.1016/j.jep.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 37.Stolarczyk M, Piwowarski JP, Granica S, Stefanska J, Naruszewicz M, Kiss AK. Extracts from Epilobium sp. herbs, their components and gut microbiota metabolites of Epilobium ellagitannins, urolithins, inhibit hormone-dependent prostate cancer cells-(LNCaP) proliferation and PSA secretion. Phytother Res. 2013;27:1842–1848. doi: 10.1002/ptr.4941. [DOI] [PubMed] [Google Scholar]
- 38.Haddad EH, Gaban-Chong N, Oda K, Sabaté J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr J. 2014;13(4) doi: 10.1186/1475-2891-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han QA, Yan C, Wang L, Li G, Xu Y, Xia X. Urolithin A attenuates ox-LDL-induced endothelial dysfunction partly by modulating microRNA-27 and ERK/PPAR-γ pathway. Mol Nutr Food Res. 2016;60:1933–1943. doi: 10.1002/mnfr.201500827. [DOI] [PubMed] [Google Scholar]
- 40.Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Félix AA, Williams EG, Jha P, Lo Sasso G, Huzard D, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–893. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 41.Chen P, Chen F, Lei J, Li Q, Zhou B. Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin A attenuates D-galactose-induced brain aging in mice. Neurotherapeutics. 2019;16:1269–1282. doi: 10.1007/s13311-019-00753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar RS, Narasingappa RB, Joshi CG, Girish TK, Prasada Rao UJ, Danagoudar A. Evaluation of Cassia tora Linn. against oxidative stress-induced DNA and cell membrane damage. J Pharm Bioallied Sci. 2017;9:33–43. doi: 10.4103/0975-7406.206215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. Microbiology. Animal behavior and the microbiome. Science. 2012;338:198–199. doi: 10.1126/science.1227412. [DOI] [PubMed] [Google Scholar]
- 45.Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: The evidence so far. Evid Based Complement Alternat Med. 2013;2013(270418) doi: 10.1155/2013/270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selma MV, Beltrán D, García-Villalba R, Espín JC, Tomás-Barberán FA. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014;5:1779–1784. doi: 10.1039/c4fo00092g. [DOI] [PubMed] [Google Scholar]
- 47.Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP. Gut reactions: Breaking down xenobiotic-microbiome interactions. Pharmacol Rev. 2019;71:198–224. doi: 10.1124/pr.118.015768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.