Abstract
Long noncoding RNAs (lncRNAs) and microRNAs (miRs) serve critical roles in various cellular processes and can be used as noninvasive biomarkers in human diseases. The present study aimed to investigate the effects of lncRNA plasmacytoma variant translocation 1 (PVT1) and miR-190a-5p on vascular endothelial cell (EC) proliferation and assess their clinical value in the diagnosis of chronic heart failure (CHF). The expression of PVT1 and miR-190a-5p was investigated using reverse transcription-quantitative PCR. The interaction between PVT1 and miR-190a-5p was confirmed using a luciferase reporter assay. A Cell Counting Kit-8 assay was performed to examine EC proliferation. A receiver operating characteristic (ROC) curve was plotted to evaluate the diagnostic value of PVT1 and miR-190a-5p. PVT1 directly decreased the expression of miR-190a-5p in ECs. Overexpression of miR-190a-5p in ECs led to inhibited cell proliferation and miR-190a-5p antagonized the promotive effect of PVT1 on EC proliferation. Serum expression of PVT1 increased, while serum expression of miR-190a-5p decreased in patients with CHF compared with healthy controls (all P<0.001). The ROC curves indicated that PVT1 and miR-190a-5p were two diagnostic biomarkers of CHF, and the combination of PVT1 and miR-190a-5p showed better diagnostic accuracy compared with using PVT1 or miR-190-5p alone. In conclusion, the present study demonstrated that PVT1 promoted EC proliferation by directly suppressing miR-190a-5p. Circulating PVT1 and miR-190a-5p are possible two candidate diagnostic biomarkers of CHF, and the combined detection of the two indicators may provide a novel approach for CHF diagnosis.
Keywords: chronic heart failure, endothelial cell, long noncoding RNA plasmacytoma variant translocation 1, microRNA-190a-5p, proliferation, diagnosis
Introduction
Heart failure (HF) is characterized by abnormal cardiac structure or function, which leads to the failure of oxygen delivery or oxygen delivery with increased filling pressure (1). Chronic HF (CHF) is considered a progressive syndrome and represents one of the leading causes of global disability and death (2). Changes in dietary habits and the increase in the aging population contribute to the prevalence of CHF (3). Despite progress in the management of cardiovascular diseases, the morbidity and mortality of CHF continue to increase (4). Diagnosis of CHF mainly depends on clinical manifestations, echocardiography and several circulating biomarkers, such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP). However, the increased levels of these established biomarkers are also detected in patients with primary aldosteronism, renal failure, thyroid disease, lung disease and cirrhosis, and are influenced by the age and body mass index of patients (5-7). Therefore, novel reliable biomarkers with high sensitivity and specificity are necessary for CHF diagnosis. Vascular endothelial cells (ECs) are critical in maintaining vascular homeostasis via their roles in regulation of vascular growth, remodeling and permeability, cell immune response, cell adhesion and angiogenesis (8). Thus, impairments in the function of ECs are major causes of cardiovascular diseases (9). Dysregulation of ECs, such as abnormal cell proliferation, was determined to be an important event involved in the development and progression of CHF (10). Thus, novel therapeutic approaches for CHF need to be investigated based on the methods to improve EC function, such as studying biomarkers that are involved in the regulation of EC function.
Emerging studies have shown that long noncoding RNAs (lncRNAs) play important functional roles in various human diseases, including cardiovascular diseases (11). LncRNAs are a group of RNAs >200 nucleotides in length and have regulatory roles in a number of cellular processes, such as cell proliferation, migration, invasion and apoptosis (12). Aberrant expression of lncRNAs has been reported in several cardiovascular diseases, including CHF, and could be involved in disease initiation and development (13,14). Increased expression of lncRNA plasmacytoma variant translocation 1 (PVT1) was found in several heart-related abnormalities, such as cardiac hypertrophy (15) and atrial fibrosis (16). In addition, Zheng et al (17) showed the promotive effects of PVT1 on the angiogenesis of ECs. However, to the best of our knowledge, the role of PVT1 in CHF has rarely been investigated.
MicroRNAs (miRs/miRNAs) are another group of noncoding RNAs of 18-22 nucleotides in length. Accumulatingevidence indicates the pivotal roles of miRNAs in the diagnosis, prognosis and therapy of various diseases (18,19). miRNAs are involved in disease progression by regulating diverse cellular processes (20). An increasing number of studies reported that miRNAs act as direct targets of lncRNAs, thus mediating the function of lncRNAs (21). miR-190a-5p was identified as a target of PVT1 in glioma and mediated the effects of PVT1 on tumor cell proliferation (22). Decreased circulating levels of miR-190a-5p werereported in HF cases (23). However, the precise role of miR-190-5p in CHF remains to be elucidated.
Considering the important effect of EC function on the development of CHF, the present study sought to investigate the regulatory effects of PVT1 and miR-190a-5p on EC proliferation. Furthermore, clinical research was performed to evaluate the expressional patterns and diagnostic value of circulating PVT1 and miR-190a-5p in the ECs of patients with CHF. The results of the present study might provide a novel insight into the mechanisms underlying the promotive effect of PVT1 on EC function and as diagnostic biomarkers for CHF.
Materials and methods
Cell culture and transfection
Human umbilical vein endothelial cells (HUVECs) were obtained from the American Type Culture Collection. Cells were cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 5% CO2 at 37˚C.
Cell transfection was performed using Lipofectamine™ 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. miR-190a-5p mimic (50 nM; 5'-UGAUAUGUUUGAUAUAUUAGGU-3') and miR-190a-5p inhibitor (50 nM; 5'-ACCUAAUAUAUCAAACAUAUCA-3') were used to regulate the in vitro expression of miR-190a-5p, while small interfering RNA (siRNA)-PVT1 (100 nM; 5'-CCCAACAGGAGGACAGCUUTT-3') was used to knock down the expression of PVT1. miRNA negative control (miR-NC; 50 nM; 5'-UCACAACCUCCUAGAAAGAGUAGA-3') and siRNA NC (100 nM; 5'-UUCUCCGAACGUGUCACGUTT-3') were used as controls. Untreated cells served as the mock group. All sequences were synthesized by Shanghai GenePharma Co., Ltd. Subsequent cell experiments were performed 48 h after transfection.
Patients and blood collection
A total of 92 CHF patients were recruited from Yidu Central Hospital of Weifang between January 2015 and February 2017. The patients were enrolled with the following inclusion criteria: i) All patients were diagnosed with CHF in accordance with the guidelines of the American Heart Association (24); ii) patients had good compliance and could cooperate to complete the present study; and iii) had no infectious diseases, history of myocardial infarction or other cardiac diseases. The CHF patients included 58 males and 34 females with a mean age of 62.5±12.6 years (age range of 35-85 years). In addition, 60 healthy volunteers who underwent physical examination were enrolled in the present study during the same time period, including 38 males and 22 females with a mean age of 62.2±12.1 years (age range of 38-82 years). No significant difference in age and gender was found between the CHF patients and healthy controls. Venous blood was collected from all participants and centrifuged at 3,000 x g for 10 min at 4˚C for serum isolation. The experimental procedures were approved by the Ethics Committee of Yidu Central Hospital of Weifang. Written informed consent was obtained from each participant.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from cells and serum using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Single-stranded cDNA was synthesized from 2 µg RNA using SuperScript III Reverse Transcriptase (cat. no 18080044; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The expression levels of PVT1 and miR-190a-5p were examined by RT-qPCR, which was performed using a SYBR Green PCR Master Mix kit (cat. no 4364344; Applied Biosystems; Thermo Fisher Scientific, Inc.) on a 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following thermocycling conditions were used for the PCR: Initial denaturation at 95˚C for 5 min; 40 cycles of 95˚C for 10 sec, and 60˚C for 1 min; and a final extension at 72˚C for 10 min. GAPDH and U6 were used as endogenous controls for PVT1 and miR-190a-5p, respectively. The following primer pairs were used for the PCR: PVT1 forward, 5'-GGGGAATAACGCTGGTGGAA-3' and reverse, 5'-CCCATGGACATCCAAGCTGT-3'; miR-190a-5p forward, 5'-GCCGAGTGATATGTTTGATAT-3' and reverse, 5'-CTCAACTGGTGTCGTGGA-3'; GAPDH forward, 5'-AGCTGAACGGGAAGCTCACT-3' and reverse, 5'-TGCTTAGCCAAATTCGTTG-3'; and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'. Relative expression levels of mRNA were calculated using the 2-ΔΔCq method (25).
Luciferase reporter assay
According to TargetScan (version 7.1; www.targetscan.org/vert_71/) analysis, the sequence of PVT1 contains a target complementary sequence for miR-190a-5p. PVT1 wild-type (WT) or mutant type (MT) fragments were cloned into the pMIR-REPORT™ luciferase vector (Ambion; Thermo Fisher Scientific, Inc.) to construct the luciferase reporter vectors. HUVECs were seeded and co-transfected with reporter vector and miR-190a-5p mimic or reporter vector and miR-190a-5p inhibitor using Lipofectamine™ 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Following 48 h of transfection, luciferase activity was measured using a Dual Luciferase Reporter Assay system (Promega Corporation). Renilla luciferase activity was detected for normalization.
Cell proliferation assay
Cell proliferation was analyzed using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). HUVECs were seeded into 96-well plates at a density of 2x103 cells/well and cultured at 37˚C with 5% CO2. CCK-8 reagent was added to the cells at time points of 0, 24, 48 and 72 h with a further 2-h incubation. The absorbance was measured at a wavelength of 450 nm using a microplate reader (Omega Bio-Tek, Inc.).
Statistical analysis
Data are presented as the mean ± SD and were analyzed using SPSS 18.0 (SPSS Inc.) and GraphPad Prism 5.0 (GraphPad Software, Inc.). Comparisons between parameters were performed using Student's t-test or one-way ANOVA followed by Tukey's post hoc test. Correlation between parameters was assessed using the Pearson's correlation coefficient. A receiver operating characteristic (ROC) curve was plotted to evaluate the diagnostic value of PVT1 and miR-190a-5p in CHF, and logistics regression analysis was conducted to obtain the ROC analysis results based on the combination of PVT1 and miR-190a-5p. P<0.05 was considered to indicate a statistically significant difference.
Results
PVT1 directly inhibits miR-190a-5p expression in ECs
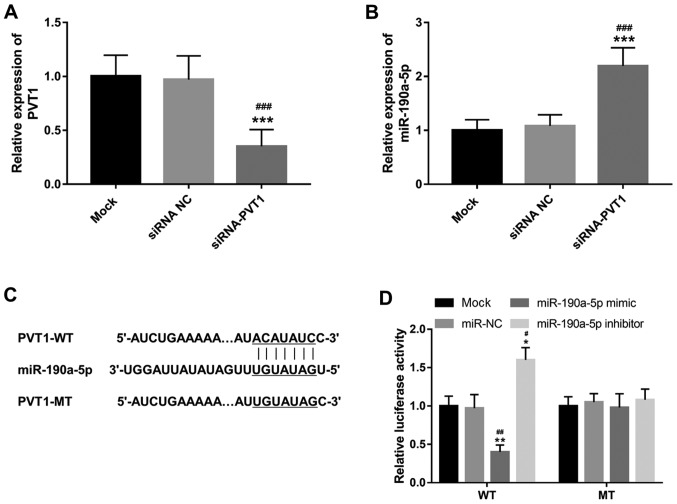
The present study investigated the effects of PVT1 on miR-190a-5p expression in ECs. The relative expression of PVT1 was downregulated in ECs in vitro following transfection with siRNA-PVT1 compared with both mock and siRNA NC groups (P<0.001; Fig. 1A). As shown in Fig. 1B, knockdown of PVT1 in ECs resulted in a significant increase in the expression of miR-190a-5p compared with mock and siRNA NC groups (P<0.001). PVT1 was demonstrated to contain a complementary sequence for miR-190a-5p (Fig. 1C), suggesting the potential for PVT1 to directly regulate miR-190a-5p. Thus, a luciferase reporter assay was performed to confirm this interaction in ECs. As shown in Fig. 1D, the luciferase activity of the WT group was reduced by the overexpression of miR-190a-5p (P<0.01) but promoted by the reduction of miR-190a-5p (P<0.05). However, the luciferase activity of the MT group did not show significant difference among different experimental conditions. The data indicated that miR-190a-5p served as a direct target of PVT1 and could be inhibited by PVT1 in ECs.
Figure 1.
PVT1 directly regulates the expression of miR-190a-5p in HUVECs. (A) Expression of PVT1 was suppressed by siRNA-PVT1 in HUVECs. ***P<0.001, vs. the mock group; ###P<0.001 vs. siRNA NC group. (B) Expression of miR-190a-5p was promoted by the knockdown of PVT1 in HUVECs. ***P<0.001, vs. the mock group; ###P<0.001 vs. siRNA NC group. (C) PVT1 contains a complementary sequence to miR-190a-5p. (D) Luciferase reporter assay results confirmed the direct interaction between PVT1 and miR-190a-5p. *P<0.05 and **P<0.01, vs. the mock group; #P<0.05 and ##P<0.01 vs. the miR-NC group. HUVEC, human umbilical vein endothelial cells; PVT1, plasmacytoma variant translocation 1; siRNA-PVT1, small interfering RNA targeting PVT1, NC, negative control; WT, wild-type; MT, mutant-type; miR, microRNA.
miR-190a-5p suppresses cell proliferation of HUVECs
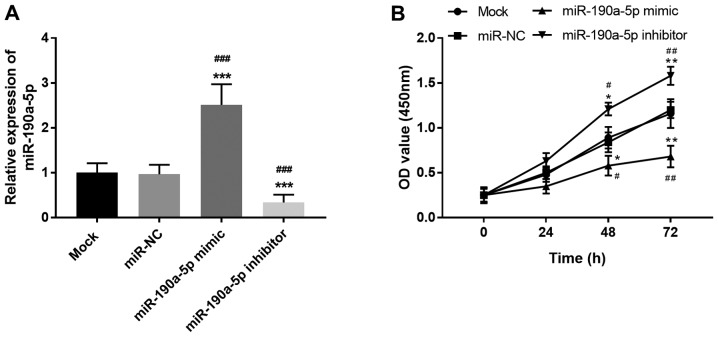
Considering the critical regulatory role of PVT1 in the angiogenesis of ECs (17), the present study further focused on the effects of miR-190a-5p on the proliferation of ECs. miR-190a-5p expression in HUVECs was regulated by cell transfection. miR-190a-5p mimic transfection led to the overexpression of miR-190a-5p, while miR-190a-5p inhibitor resulted in the knockdown of miR-190a-5p expression compared with both mock and miR-NC groups (all P<0.001; Fig. 2A). Based on the CCK-8 assay results, cell proliferation of HUVECs was markedly suppressed by the upregulation of miR-190a-5p but enhanced by the downregulation of miR-190a-5p at both 48 h (P<0.05) and 72 h (P<0.01; Fig. 2B). The results demonstrated that miR-190a-5p exerted an opposite effect on EC proliferation compared with PVT1, specifically inhibiting the proliferation of HUVECs.
Figure 2.
Effect of miR-190a-5p on HUVEC proliferation. (A) Expression of miR-190a-5p was upregulated by miR-190a-5p mimic and downregulated by miR-190a-5p inhibitor in HUVECs. (B) Overexpression of miR-190a-5p suppressed cell proliferation, while knockdown of miR-190a-5p promoted the proliferation of HUVECs. *P<0.05, **P<0.01 and ***P<0.001 vs. the mock group; #P<0.05, ##P<0.01 and ###P<0.001 vs. the miR-NC group. HUVEC, human umbilical vein endothelial cells; miR, microRNA; NC, negative control.
PVT1 facilitates EC proliferation via regulation of miR-190a-5p
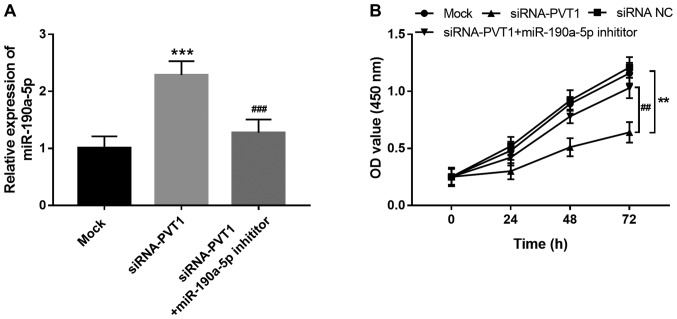
PVT1 was previously determined to be a promoter of EC angiogenesis (17). Considering the direct interaction between PVT1 and miR-190a-5p in ECs, the present study further investigated the role of miR-190a-5p in the regulatory effects of PVT1 on EC proliferation. As shown in Fig. 3A, increased expression of miR-190a-5p induced by the knockdown of PVT1 was reversed by miR-190a-5p inhibitor transfection (P<0.001). Cell proliferation assay results showed that the proliferation of HUVECs was suppressed by the downregulation of PVT1 (P<0.01), but this inhibitory effect was rescued by inhibiting miR-190a-5p (P<0.01; Fig. 3B), which suggested that the promotive effect of PVT1 on EC proliferation might be mediated by downregulating miR-190a-5p.
Figure 3.
PVT1 promotes HUVEC proliferation via suppressing miR-190a-5p expression. (A) Increase in miR-190a-5p expression induced by the silencing of PVT1 was inhibited by a miR-190a-5p inhibitor. (B) Suppressed HUVEC proliferation caused by the reduction in PVT1 was rescued by the downregulation of miR-190a-5p. **P<0.01 and ***P<0.001 vs. mock; ##P<0.01 and ###P<0.001 vs. siRNA-PVT1. HUVEC, human umbilical vein endothelial cells; PVT1, plasmacytoma variant translocation 1; siRNA-PVT1, small interfering RNA targeting PVT1, NC, negative control; miR, microRNA.
Expression of PVT1 and miR-190a-5p in CHF patients
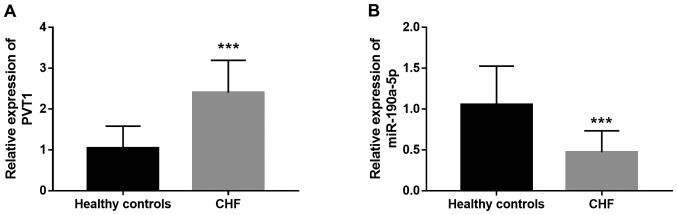
Circulating lncRNAs and miRNAs were previously identified as a group of non-invasive clinical biomarkers for disease diagnosis (18). In the present study, serum expression of PVT1 and miR-190a-5p was measured in patients with CHF. As shown in Fig. 4A, circulating PVT1 expression was significantly higher in CHF cases compared with healthy individuals (P<0.001). By contrast, the serum expression of miR-190a-5p significantly decreased in CHF patients compared with healthy controls (P<0.001; Fig. 4B). Dysregulation of circulating PVT1 and miR-190a-5p levels might be a potential biomarker for CHF diagnosis.
Figure 4.
Serum expression of (A) PVT1 and (B) miR-190a-5p in CHF patients. ***P<0.001 vs. healthy controls. PVT1, plasmacytoma variant translocation 1; miR, microRNA; CHF, chronic heart failure.
Clinical significance of PVT1 and miR-190a-5p in the diagnosis of CHF
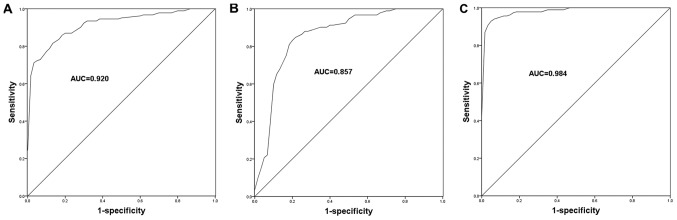
Considering the dysregulation of PVT1 and miR-190a-5p in the serum specimens of CHF patients, the current study aimed to further assess the diagnostic potential of these molecules in CHF. The ROC curves for CHF patients were plotted based on the expression levels of PVT1 and miR-190a-5p, and the area under the curve (AUC) was calculated to reflect diagnostic accuracy. As shown in Fig. 5, the ROC curves showed that the AUCs were 0.920 and 0.857 for PVT1 (Fig. 5A) and miR-190a-5p (Fig. 5B), respectively. The best cut-off values for CHF diagnosis were 2.015 and 0.730 for PVT1 and miR-190a-5p, respectively. These data indicated the relatively high diagnostic accuracy of PVT1 and miR-190a-5p. Furthermore, the study assessed the diagnostic value of the combination of PVT1 and miR-190a-5p in CHF. As shown in Fig. 5C and Table I, a higher AUC of 0.984 was observed when the two indicators were combined, with improved sensitivity and specificity. This suggested that the combined detection of PVT1 and miR-190a-5p might be an efficient novel strategy for the diagnosis of CHF.
Figure 5.
ROC curves based on serum PVT1 and miR-190a-5p expression levels. (A) ROC curve of PVT1. (B) ROC curve of miR-190a-5p. (C) ROC curve of the combined PVT1 and miR-190a-5p. ROC, receiver operating characteristic; AUC, area under the curve; PVT1, plasmacytoma variant translocation 1; miR, microRNA.
Table I.
Diagnostic value of PVT1 and miR-190a-5p in chronic heart failure.
| Indicator | AUC | Sensitivity, % | Specificity, % |
|---|---|---|---|
| PVT1 | 0.920 | 71.7 | 96.7 |
| miR-190a-5p | 0.857 | 83.7 | 80.0 |
| PVT1 and miR-190a-5p | 0.984 | 92.4 | 96.7 |
PVT1, plasmacytoma variant translocation 1; miR, microRNA; AUC, area under the ROC curve.
Discussion
The present study focused on the treatment of CHF by investigating the roles of PVT1 and miR-190a-5p in the regulation of vascular EC proliferation and disease diagnosis. Knockdown of PVT1 in ECs led to increased miR-190a-5p expression and PVT1 suppressed cell proliferation by targeting miR-190a-5p. The present study demonstrated that miR-190a-5p antagonized the effect of PVT1 on ECs proliferation, which provided a novel insight into the mechanisms underlying the role of PVT1 in the regulation of EC angiogenesis. The clinical study showed that the expression of PVT1 was upregulated, while the expression of miR-190a-5p was downregulated in serum samples collected from CHF patients compared with healthy controls. Furthermore, the ROC curves indicated the high diagnostic accuracy of PVT1 and miR-190a-5p, particularly when the two indicators were combined, in patients with CHF. Thus, the combined detection of PVT1 and miR-190a-5p might be a novel efficient diagnostic approach for CHF.
Vascular ECs are basic components in the innermost layer of blood vessels and are considered a pivotal vascular barrier (26). The dysfunction of ECs contributes to the occurrence of various cardiovascular diseases, such as thrombus formation, atherosclerosis, hypertension and CHF (27-29). Accumulating evidence indicated that numerous molecules are involved in the progression of cardiovascular diseases through the regulation of abnormal cell proliferation of ECs. For example, Zheng et al (30) demonstrated that overexpression of miR-155 could suppress the proliferation of ECs, leading to increased vascular endothelial permeability and contributing to the progression of atherosclerosis. Another study by Schober et al (31) also showed the regulatory role of miR-126-5p in the proliferation of ECs, thereby improving atherosclerosis. In CHF, miR-214 expression was increased in patients with this disease and served as a potential therapeutic target by regulating EC proliferation (32). The aforementioned studies indicated that the treatment of cardiovascular diseases, including CHF, should focus on the improvement of impaired EC function.
The present study found suppressed EC proliferation induced by the reduction in PVT1 expression. Zheng et al (17) demonstrated the promotive effect of PVT1 on the proliferation of ECs, consistent with the present results. PVT1 regulates the expression of miR-190a-5p during the tumorigenesis of glioma, miR-190a-5p is identified as a target of PVT1 in the regulation of glioma cell proliferation (22). However, whether miR-190a-5p is also a target of PVT1 in ECs remains to be elucidated. In the present study, ECs with knocked down PVT1 were constructed, and the results showed that the expression of miR-190a-5p was enhanced. The luciferase activity data further confirmed the direct interaction between PVT1 and miR-190a-5p in ECs. miR-190a-5p was reported to suppress cell proliferation in glioma (22). Similarly, overexpression of miR-190a-5p inhibited the proliferation of ECs, indicating the role of miR-190a-5p in the regulation of EC biological function. Inhibition of EC proliferation induced by PVT1 knockdown was rescued by downregulation of miR-190a-5p. The results implied that PVT1 contributes to the cell proliferation of ECs through targeting miR-190a-5p. Thus, the PVT1/miR-190a-5p axis may be a novel therapeutic target for the treatment of cardiovascular diseases.
A study by Wong et al (23) reporteddecreased circulating miR-190a-5p in HF patients. However, thestudy did not investigate the precise clinical role of miR-190a-5p in HF. Considering the role of the PVT1/miR-190a-5p axis in EC proliferation, the expressional patterns and clinical significance of the two RNAs were further assessed. Increased expression of PVT1 and decreased expression of miR-190a-5p were found in the serum specimens of CHF patients, and the aberrant expression of the two molecules had relatively high diagnostic accuracy. Currently, the diagnosis of CHF is limited by the cost of the ultrasound examination and lowspecificity of established circulating biomarkers, such as BNP and NT-proBNP (6,7,33). In the current study, the combination of PVT1 and miR-190a-5p presented a diagnostic potential for CHF with considerable sensitivity and specificity, providing a novel potential approach for the diagnosis of CHF. Aberrant expression levels of lncRNAs and miRNAs are considered valuable diagnostic biomarkers in various human diseases, including CHF (34,35). Increased expression of lncRNAcolorectal neoplasia differentially expressed-h was identified as a diagnostic and prognostic indicator in colorectal carcinoma (34). Decreased plasma lncRNAHOX transcript antisense intergenic RNA serves as an efficient biomarker for the diagnosis of acute myocardial infarction (35). The two circulating lncRNAs NRON and myosin heavy chain associated RNA transcripts actas two candidate diagnostic markers, with the ability to distinguish HF patients from healthy controls (36). In a previous study, circulating upregulated miR-195-3p was described as a potential biomarker for the diagnosis of HF (7). To further improve the diagnosis of CHF, the present study provided evidence for PVT1 and miR-190a-5p as two candidate diagnostic biomarkers, and the combination of PVT1 and miR-190a-5p as markers suggested a more effective clinical significance for CHF diagnosis.
Although the present study demonstrated the regulatory effect of the PVT1/miR-190a-5p axis on EC proliferation, the biological function of the PVT1/miR-190a-5p axis in ECs warrants further investigation. In addition, the present study found aberrant expression of PVT1 and miR-190a-5p in CHF patients. Thus, the PVT1/miR-190a-5p axis might be involved in CHF development via regulation of EC function. However, this hypothesis needs to be verified in future studies, such as investigation into the role of the PVT1/miR-190a-5p axis in EC apoptosis and angiogenesis.
Taken together, the present study provided evidence for the inhibitory effect of miR-190a-5p on the proliferation of ECs and showed that PVT1 promoted EC proliferation by directly suppressing miR-190a-5p, implying that the PVT1/miR-190a-5p axis may serve as a therapeutic target in diseases with EC dysfunction. Additionally, increased circulating PVT1 levels and decreased miR-190a-5p expression in CHF patients may serve as two candidate diagnostic biomarkers, and the combined detection of PVT1 and miR-190a-5p may be a novel effective approach to diagnose CHF cases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the currnt study are available from the corresponding author on reasonable request.
Authors' contributions
BS made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. MM and JW contributed to acquisition of data and statistical analysis. SW designed the study, analysed the data and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experimental procedures were approved the Ethics Committee of Yidu Central Hospital of Weifang. Written informed consent was obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6:187–214. doi: 10.1002/cphy.c140055. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman TM. Chronic heart failure. Pediatr Crit Care Med. 2016;17 (8 Suppl 1):S119–S123. doi: 10.1097/PCC.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 3.Ford I, Robertson M, Komajda M, Böhm M, Borer JS, Tavazzi L, Swedberg K. SHIFT Investigators: Top ten risk factors for morbidity and mortality in patients with chronic systolic heart failure and elevated heart rate: The SHIFT Risk Model. Int J Cardiol. 2015;184:163–169. doi: 10.1016/j.ijcard.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Maggioni AP. Epidemiology of heart failure in Europe. Heart Fail Clin. 2015;11:625–635. doi: 10.1016/j.hfc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Heil B, Tang WH. Biomarkers: Their potential in the diagnosis and treatment of heart failure. Cleve Clin J Med. 2015;82 (12 Suppl 2):S28–S35. doi: 10.3949/ccjm.82.s2.05. [DOI] [PubMed] [Google Scholar]
- 6.Schaub JA, Coca SG, Moledina DG, Gentry M, Testani JM, Parikh CR. Amino-Terminal Pro-B-type natriuretic peptide for diagnosis and prognosis in patients with renal dysfunction: A systematic review and meta-analysis. JACC Heart Fail. 2015;3:977–989. doi: 10.1016/j.jchf.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Ji J, Wang T, Wang MB, Chen XL. Upregulation of Circulating miR-195-3p in Heart Failure. Cardiology. 2017;138:107–114. doi: 10.1159/000476029. [DOI] [PubMed] [Google Scholar]
- 8.Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev. 2018;98:3–58. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieve I, Munster-Kuhnel AK, Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul Pharmacol. 2018;100:26–33. doi: 10.1016/j.vph.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–864. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 12.Kong Q, Qiu M. Long noncoding RNA SNHG15 promotes human breast cancer proliferation, migration and invasion by sponging miR-211-3p. Biochem Biophys Res Commun. 2018;495:1594–1600. doi: 10.1016/j.bbrc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Dangwal S, Schimmel K, Foinquinos A, Xiao K, Thum T. Noncoding RNAs in heart failure. Handb Exp Pharmacol. 2017;243:423–445. doi: 10.1007/164_2016_99. [DOI] [PubMed] [Google Scholar]
- 14.Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res. 2014;114:1569–1575. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 15.Yu YH, Hu ZY, Li MH, Li B, Wang ZM, Chen SL. Cardiac hypertrophy is positively regulated by long non-coding RNA PVT1. Int J Clin Exp Pathol. 2015;8:2582–2589. [PMC free article] [PubMed] [Google Scholar]
- 16.Cao F, Li Z, Ding WM, Yan L, Zhao QY. LncRNA PVT1 regulates atrial fibrosis via miR-128-3p-SP1-TGF-β1-Smad axis in atrial fibrillation. Mol Med. 2019;25(7) doi: 10.1186/s10020-019-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J, Hu L, Cheng J, Xu J, Zhong Z, Yang Y, Yuan Z. lncRNA PVT1 promotes the angiogenesis of vascular endothelial cell by targeting miR26b to activate CTGF/ANGPT2. Int J Mol Med. 2018;42:489–496. doi: 10.3892/ijmm.2018.3595. [DOI] [PubMed] [Google Scholar]
- 18.Qiu Z, Li H, Wang J, Sun C. MiR-146a and miR-146b in the diagnosis and prognosis of papillary thyroid carcinoma. Oncol Rep. 2017;38:2735–2740. doi: 10.3892/or.2017.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Ponnusamy M, Zhang L, Zhang Y, Liu C, Yu W, Wang K, Li P. The role of miR-214 in cardiovascular diseases. Eur J Pharmacol. 2017;816:138–145. doi: 10.1016/j.ejphar.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014;15:1–19. doi: 10.1093/bib/bbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Han T, Li Y, Sun J, Zhang S, Liu Y, Shan B, Zheng D, Shi J. The lncRNA SNHG5/miR-32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4. FASEB J. 2017;31:893–903. doi: 10.1096/fj.201600994R. [DOI] [PubMed] [Google Scholar]
- 22.Xue W, Chen J, Liu X, Gong W, Zheng J, Guo X, Liu Y, Liu L, Ma J, Wang P, et al. PVT1 regulates the malignant behaviors of human glioma cells by targeting miR-190a-5p and miR-488-3p. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1783–1794. doi: 10.1016/j.bbadis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Wong LL, Armugam A, Sepramaniam S, Karolina DS, Lim KY, Lim JY, Chong JP, Ng JY, Chen YT, Chan MM, et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2015;17:393–404. doi: 10.1002/ejhf.223. [DOI] [PubMed] [Google Scholar]
- 24. doi: 10.1161/CIR.0b013e31829e8776. WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, et al: 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: e240-e327, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Aman J, Weijers EM, van Nieuw Amerongen GP, Malik AB, van Hinsbergh VW. Using cultured endothelial cells to study endothelial barrier dysfunction: Challenges and opportunities. Am J Physiol Lung Cell Mol Physiol. 2016;311:L453–L466. doi: 10.1152/ajplung.00393.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jufri NF, Mohamedali A, Avolio A, Baker MS. Mechanical stretch: Physiological and pathological implications for human vascular endothelial cells. Vascular Cell. 2015;7(8) doi: 10.1186/s13221-015-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyama T, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Shindo T. Adrenomedullin-RAMP2 system in vascular endothelial cells. J Atheroscler Thromb. 2015;22:647–653. doi: 10.5551/jat.29967. [DOI] [PubMed] [Google Scholar]
- 29.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, Jin LS, Zhan H, Zhang H, Li JS, Wen JK. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol Ther. 2017;25:1279–1294. doi: 10.1016/j.ymthe.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan Q, Yang L, Gong W, Chaugai S, Wang F, Chen C, Wang P, Zou MH, Wang DW. MicroRNA-214 Is upregulated in heart failure patients and suppresses xbp1-mediated endothelial cells angiogenesis. J Cell Physiol. 2015;230:1964–1973. doi: 10.1002/jcp.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill SA, Booth RA, Santaguida PL, Don-Wauchope A, Brown JA, Oremus M, Ali U, Bustamam A, Sohel N, McKelvie R, et al. Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: A systematic review of the evidence. Heart Fail Rev. 2014;19:421–438. doi: 10.1007/s10741-014-9447-6. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L, Zheng G, Li P, Li C, Wang C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–85563. doi: 10.18632/oncotarget.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, Liu Y, Guo S, Yao R, Wu L, Xiao L, Wang Z, Liu Y, Zhang Y. Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cell Physiol Biochem. 2017;44:1497–1508. doi: 10.1159/000485588. [DOI] [PubMed] [Google Scholar]
- 36.Xuan L, Sun L, Zhang Y, Huang Y, Hou Y, Li Q, Guo Y, Feng B, Cui L, Wang X, et al. Circulating long non-coding RNAs NRON and MHRT as novel predictive biomarkers of heart failure. J Cell Mol Med. 2017;21:1803–1814. doi: 10.1111/jcmm.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the currnt study are available from the corresponding author on reasonable request.