Significance
In many animals, males deceive females into mating using traits that mimic cues of food, predators, preferred habitats, or offspring in need of care. However, if and how these deceptive signals guide reliable communication without females confusing the mimic and the model remain unclear. We discovered that female sea lamprey discriminate a nonsexual cue of productive habitat from the deceptive male sex pheromone that mimics it and identify a pheromone antagonist as the underlying mechanism. Our results implicate a means by which females can detect and benefit from male deceit and could have applications for control of destructive populations of sea lamprey in the Laurentian Great Lakes.
Keywords: sexual selection, signal evolution, mimicry, receiver bias, chemical ecology
Abstract
The evolution of male signals and female preferences remains a central question in the study of animal communication. The sensory trap model suggests males evolve signals that mimic cues used in nonsexual contexts and thus manipulate female behavior to generate mating opportunities. Much evidence supports the sensory trap model, but how females glean reliable information from both mimetic signals and their model cues remains unknown. We discovered a mechanism whereby a manipulative male signal guides reliable communication in sea lamprey (Petromyzon marinus). Migratory sea lamprey follow a larval cue into spawning streams; once sexually mature, males release a pheromone that mimics the larval cue and attracts females. Females conceivably benefit from the mimetic pheromone during mate search but must discriminate against the model cue to avoid orienting toward larvae in nearby nursery habitats. We tested the hypothesis that spawning females respond to petromyzonol sulfate (PZS) as a behavioral antagonist to avoid attraction to the larval cue while tracking the male pheromone despite each containing attractive 3-keto petromyzonol sulfate (3kPZS). We found 1) PZS inhibited electrophysiological responses to 3kPZS and abated preferences for 3kPZS when mixed at the same or greater concentrations, 2) larvae released more PZS than 3kPZS whereas males released more 3kPZS than PZS, and 3) mixtures of 3kPZS and PZS applied at ratios measured in larval and male odorants resulted in the discrimination observed between the natural odors. Our study elucidates how communication systems that arise via deception can facilitate reliable communication.
In 1860, Darwin famously decried the perplexity of elaborate male displays by claiming the sight of a peacock’s tail made him sick (1); today, the evolution of sexual signals continues to agitate the curiosity of biologists but also has implications for understanding and conserving biodiversity in a time of dramatic environmental change (2, 3). As Darwin proposed (4), exaggerated male traits are often the result of sexual selection via female choice (5). Classic models of sexual selection suggest female preferences for male signals arise per the benefits they provide or indicate (6). Alternatively, receiver bias models propose males manipulate female behavior using signals that target preexisting nonsexual responses (7). For example, some male signals mimic nonsexual cues of food (8), predation (9), preferred habitats (10), or offspring in need of parental care (11). These signals, known as sensory traps (12, 13), elicit out-of-context reactions that give males opportunity to mate females and are inherently deceptive as females respond to the male sex signal as if it were a cue associated with nonsexual information (14). A deluge of empirical data indicates that receiver biases explain the origin of many signals (15). However, if and how females adapt to resist manipulation by males (16, 17) or incorporate deceptive signals into reliable sexual communication remain the focus of much debate and theory (14, 18–20). As male signals and female preferences represent an intertwined coevolutionary unit, the narrow focus on signal origin but not subsequent preference evolution precludes a full understanding of how receiver biases shape communication.
Sea lamprey (Petromyzon marinus) are a useful model to track the evolutionary trajectory of communication that originated via a sensory trap. Chemical cues and pheromones guide sea lamprey behavior throughout their complex life history, especially during the terminal reproductive phase (21). After parasitizing fish in lakes or the Atlantic Ocean, prespawning sea lamprey migrate into streams following chemical cues released by larvae residing in nursery habitats near spawning grounds. The larval cue consists, in part, of the steroidal compound 3-keto petromyzonol sulfate (3kPZS), which is released by larvae into the water (22) at rates sufficient to create detectable plumes (23) and attracts prespawning males and females during their migration (24, 25). Several weeks after entering streams, sea lamprey become sexually mature (spermiated or ovulated) and exhibit behaviors markedly distinct from those of prespawning migrants. At this point, males construct nests in gravel beds and release a multicomponent sex pheromone that attracts females over long distances (26, 27). Interestingly, several lines of evidence implicate 3kPZS as the major component of the male sex pheromone: 1) Spermiated males release 3kPZS at high rates via specialized gill cells (28), 2) ovulated females orient toward 3kPZS in 2-choice flumes and large-scale in-stream assays (26, 27), and 3) 3kPZS replicates the behavioral effect of the complete male sex pheromone in attracting ovulated females to nests over long distances (26). Therefore, males signal with a compound that is a component of the larval cue females orient toward during their prespawning migration. Interestingly, the nonsexual migratory preference for 3kPZS likely originated before male signaling with 3kPZS, as some other lamprey species respond to it as a component of the larval cue but not as a sex pheromone (10). Taken together, these observations support the hypothesis that males signal with a sex pheromone that partially mimics the nonsexual larval cue.
We postulated that female sea lamprey discriminate between the model larval cue and the mimetic male pheromone to enable reliable sexual communication. A long-distance signal for locating mates conceivably benefits females, as they bear the onus of mate search and have only a few days to a week to reproduce before they die (29, 30). Indeed, evidence that communication with 3kPZS is mutually adaptive comes from observations that females evolved a sexual preference for 3kPZS in response to sexual signaling by males; in sea lamprey and other lamprey species, the nonsexual response to 3kPZS is nontargeted upstream movement fit for navigation into streams, but the sexual response is a robust attraction to the point source fit for orientation to a male’s nest (10, 22, 31). However, females tracking 3kPZS from males are likely to also encounter 3kPZS from larvae, given that males nest in gravel patches above or interspersed with larval nursery habitat (29). Consequently, females that fail to discriminate between larval odor (the model) and the male pheromone (the mimic) risk orienting toward larvae residing in soft sediments when searching for a mate nesting in gravel patches. Therefore, reliable communication—in which the signal consistently correlates with an attribute of the signaler and receivers benefit from information about that attribute (32)—requires females to discern the larval cue from the male sex pheromone and respond appropriately according to the context. Here, we report evidence that a pheromone antagonist allows females to distinguish the larval cue from the male pheromone that mimics it and thus enables reliable mate search.
Results
Ovulated Females Discriminate between Larval Odor and the Male Pheromone That Mimics It.
We used odor-choice assays to test our prediction that ovulated females discriminate between larval odor and its partial mimic released by males. First, we used an established in-stream bioassay to test whether females track the male pheromone when it is offered alongside larval odor (26, 33). This assay creates a scenario similar to that females face when navigating to males on spawning nests near patches of larval populations. As predicted, females released in a natural spawning stream strongly preferred artificial spawning nests baited with the male pheromone over those baited with larval odor when each was applied to reach a concentration of 5 × 10−14 M 3kPZS (male: 92%; χ21 = 10.89, P < 0.001, n = 12).
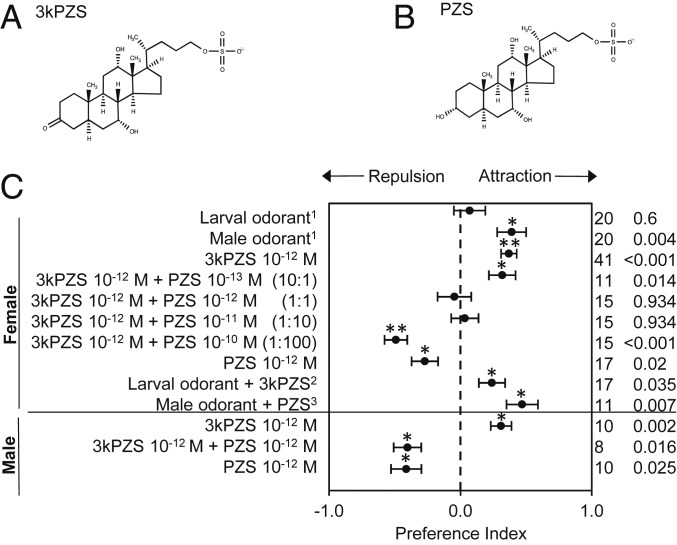
Next, we questioned whether ovulated females prefer male odor solely due to its minor components (26) or avoid orienting toward larval odor by discriminating against additional components released by larvae along with 3kPZS. Females in their natural spawning habitat encounter 3kPZS from larvae and males simultaneously and from each source alone; therefore, we reasoned that females searching for mates should not only prefer male pheromone over larval odor but also stop orienting toward larval odor altogether. Indeed, females in a 2-choice flume (34) showed no responses to larval odor (P = 0.6) even though it was applied at an attractive concentration of 3kPZS (Fig. 1). Evidently, larval odor includes at least one additional component that nulls ovulated female attraction to 3kPZS.
Fig. 1.
Petromyzonol sulfate abated preference for 3-keto petromyzonol sulfate in a 2-choice flume. (A and B) 3kPZS (A) and PZS (B) differ only in the hydrogenation of the oxygen on carbon 3. (C) Odor mixtures with equal or more PZS than 3kPZS elicited neutral or repulsive behavioral responses in ovulated females and spermiated males. The preference index (mean ± SE) was calculated as the value of [Ae/(Ae + Be) − Ac/(Ac + Bc)], where Bc is the cumulative amount of time spent in the control channel before odorant application, Be is the cumulative amount of time spent in the experimental channel before odorant application, Ac is the cumulative amount of time spent in the control channel after odorant application, and Ae is the cumulative amount of time spent in the experimental channel after odorant application in a 2-choice flume (34). A positive index value indicates attraction and a negative index value indicates repulsion. Significance was evaluated using a Wilcoxon signed-rank test. *P < 0.05, **P < 0.001. n, sample size, followed by the P value, is displayed (Right). 1Applied to reach a concentration of 3kPZS at 5 × 10−13 M. 2Added to reach the male ratio of 3kPZS to PZS. 3Added to reach the larval ratio of 3kPZS to PZS.
Petromyzonol Sulfate Abates Olfactory and Behavioral Responses to 3kPZS.
As we began our search for the mechanism underlying ovulated female indifference to larval odorants which include 3kPZS, we noted previous indications that the larval bile acid petromyzonol sulfate (PZS) is a potent odorant that reduces olfactory responses to 3kPZS (35–37). Therefore, we hypothesized that PZS in larval odor nulls ovulated female preference for 3kPZS to prevent misguided orientation toward larvae during mate search. Accordingly, we predicted that PZS nulls the olfactory and behavioral responses to 3kPZS.
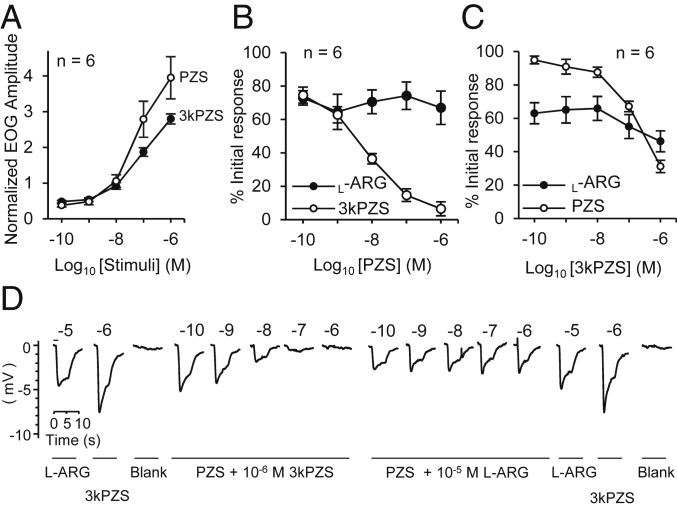
PZS reduced olfactory responses to 3kPZS across a wide range of concentrations (Fig. 2). We perfused the olfactory epithelium with PZS and recorded the response to 3kPZS using electroolfactogram (EOG) recordings. The olfactory response to 3kPZS at 10−6 M decreased as the concentration of the PZS increased from 10−10 to 10−6 M (P < 0.001; Fig. 2B), whereas the response to l-arginine (10−5 M) did not change across PZS exposure concentrations (P = 0.773; Fig. 2B). The olfactory response to 3kPZS at 10−6 M during exposure to PZS at 10−6 M was 6.5 ± 4.2% (mean ± SE, n = 6) of the response to 3kPZS prior to exposure to PZS. At equal concentrations, saturating the olfactory epithelia with PZS resulted in a greater suppression of the 3kPZS responses than did saturating with 3kPZS to the PZS responses; exposure to 7.5 × 10−9 M PZS suppressed 50% of the response to 3kPZS (Fig. 2B), whereas the concentration of 3kPZS needed to suppress 50% of the response to PZS was 2 orders of magnitude higher (1.8 × 10−7 M; Fig. 2C).
Fig. 2.
PZS reduced olfactory responses to 3kPZS. Responses were measured by electroolfactogram in adult sea lamprey (mean ± SE). Blank, vehicle solution; l-ARG, l-arginine. (A) Both 3kPZS and PZS induced concentration-dependent responses. The response amplitude was normalized to the response amplitude of 10−5 M l-ARG. (B) Exposure to PZS (10−10 to 10−6 M) reduced responses to 10−6 M 3kPZS, but not 10−5 M l-ARG, in a concentration-dependent manner. The response amplitude was expressed as a percent of the response amplitude of 10−5 M l-ARG or 10−6 M 3kPZS. (C) Exposure to 3kPZS (10−10 to 10−6 M) reduced responses to 10−6 M PZS in a concentration-dependent manner. The response amplitude was expressed as a percent of the response amplitude of 10−5 M l-ARG or 10−6 M PZS. (D) Representative EOG traces to 10−6 M 3kPZS, 10−5 M l-ARG, and increasing concentrations of stimuli from B. The number above each trace is the logarithmic value of the molar concentration of each stimulant. The bar above the l-ARG trace (Left) represents the duration of stimuli exposure.
As predicted, PZS also abated behavioral preferences of ovulated females for 3kPZS in a 2-choice flume. Females were attracted to 3kPZS at 10−12 M (P < 0.001) but were neutral to a 1:1 mixture of 3kPZS and PZS (P = 0.934) and avoided PZS alone (P = 0.02; Fig. 1). Therefore, the larval odorant PZS reduces females’ olfactory and behavioral response to 3kPZS—a component of both the larval cue and male pheromone.
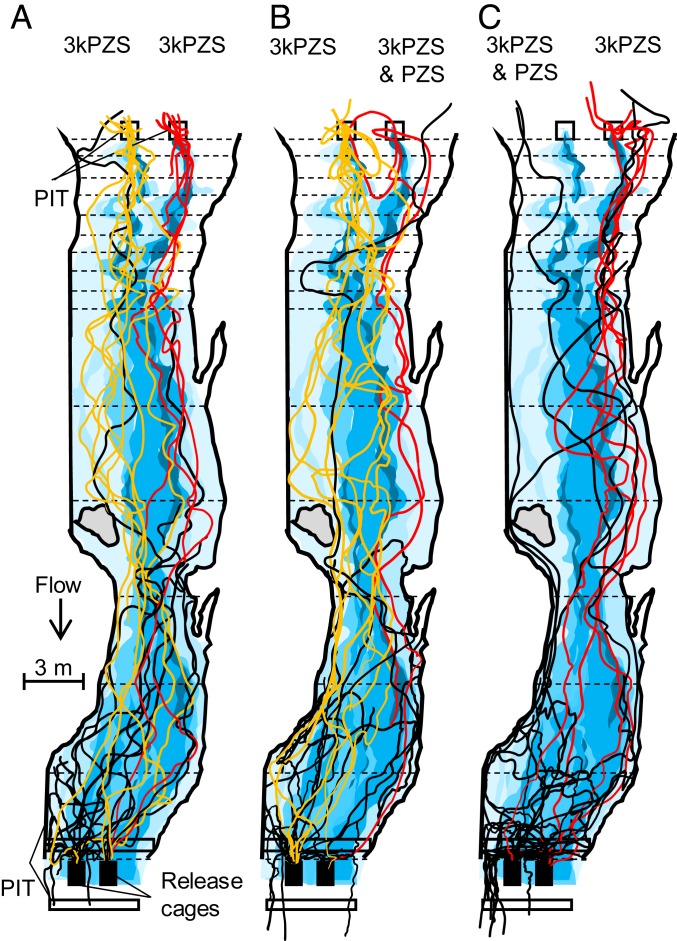
Next, we studied ovulated female responses to PZS and 3kPZS in a natural stream to determine if PZS can guide females to males nesting near patches of larvae as they traverse long stretches of stream. Returning to our 45-m in-stream bioassay, we found fewer ovulated females entered the nest baited with a 1:1 mixture of 3kPZS and PZS than the adjacent nest baited with 3kPZS alone (5 × 10−13 M; P < 0.001; Table 1). Females also spent less time in the nest baited with the mixture (mixture: 61.9 ± 25.9 s; 3kPZS alone: 148.5 ± 36.3 s; mean ± SE, t = −3.34, degrees of freedom [df] 27, P = 0.002; paired t test). Furthermore, fewer females exited the release cage (P < 0.001) and moved upstream (P = 0.001) during trials in which one nest was baited with 3kPZS and PZS compared with control trials in which only 3kPZS was applied to both nests (5 × 10−13 M; Table 1). Females entered the left and right nests at approximately equal proportions when 3kPZS was applied to each (P = 0.864; Table 1) and remained at each for comparable durations of time (115.4 ± 47.5 s and 114.3 ± 54.6 s; t = 0.04, df 18, P = 0.968; paired t test), indicating there was no relevant side bias. Swim tracks from visual observations overlain onto an odorant plume map indicated PZS modified the path females swam upstream (Fig. 3); the mean sinuosity of swim tracks (track length/shortest connecting line) was greater in 3kPZS and PZS mixture trials (1.45 ± 0.12, mean ± SE, n = 19) compared with 3kPZS alone (1.25 ± 0.06, n = 7; t = −3.462, df 24, P = 0.002; 2-tailed independent sample t test). Previous studies found more sinuous swimming is characteristic of females’ movement when they lose access to pheromone plumes (26, 38, 39). Taken together, the above studies indicate PZS nulls ovulated female preference for 3kPZS, and is a likely cue used to avoid orienting toward 3kPZS released by larvae residing near nesting males.
Table 1.
Behavioral responses of ovulated females to 3kPZS and PZS in a natural stream
| Odorant A | Odorant B | Trials | Released | Cage | Down | Up | Enter A | Enter B | χ2 | df | P |
| 3kPZS | 3kPZS | 5 | 46 | 15% (7) | 7% (3) | 65% (30) | 52% (11) | 48% (10) | 0.03 | 1 | 0.864 |
| 3kPZS and PZS | 3kPZS | 20 | 179 | 53% (94) | 7% (12) | 37% (66) | 19% (6) | 81% (25) | 18.25 | 1 | <0.001 |
| χ2 | 20.91 | 0.0007 | 10.29 | ||||||||
| df | 1 | 1 | 1 | ||||||||
| P | <0.001 | 0.978 | 0.001 |
3kPZS (5 × 10−13 M, final in-stream concentration) versus 3kPZS (5 × 10−13 M) was applied at the odorant sources for control trials. A mixture of 3kPZS and PZS (1:1, each at 5 × 10−13 M) versus 3kPZS (5 × 10−13 M) was applied during experimental trials. Treatments applied to odorant sources were alternated each trial. Cage: the percentage (count) of released ovulated females that remained in the release cage at the end of the trial. Downstream movement: the percentage (count) of the released ovulated females that moved at least 3 m downstream of the release cages. Upstream movement: the percentage (count) of the released females that moved at least 3 m upstream of the release cages. Enter: the percentage (count) of the females that moved upstream that then entered the 1-m2 odorant source A or B. Movement responses were evaluated with a logistic regression with a binomial distribution. Representative swim tracks from these trials are displayed in Fig. 3. Experiments were conducted in a 45-m section of the upper Ocqueoc River (Presque Isle County, MI).
Fig. 3.
PZS modified the path of ovulated females swum in a 45-m section of the upper Ocqueoc River. Females were exposed to (A) 3kPZS (5 × 10−13 M, final in-stream concentration) vs. 3kPZS (5 × 10−13 M) (control trials, n = 46) or (B and C) 3kPZS (5 × 10−13 M) vs. a mixture of 3kPZS and PZS (1:1, each at 5 × 10−13 M) (experimental trials, n = 179). The movement of females from the release cages (solid black boxes downstream) was monitored with 4 passive integrated transponder (PIT) antennas (locations shown) and visual observations. Each solid line indicates the swim track of an individual female mapped. A red solid line indicates the female entered the right odorant source first, yellow indicates the left, and black indicates neither. Rhodamine dye was administered in the center of the odorant source and sampled at 10 points along each transect (transecting dashed lines) to map the odorant plume. The shading of the odorant plume corresponds to the relative molar concentration of the odorant. Darker blue indicates a higher relative odorant concentration.
PZS Guides Discrimination between Larval Odor and the Male Pheromone That Mimics It.
In our final set of experiments, we directly tested the hypothesis that PZS allows sea lamprey to discriminate between larval odor and the male pheromone.
First, we used ultra–high-performance liquid chromatography-tandem mass spectrometry to quantify the release of 3kPZS and PZS by males and larvae. Groups of larvae (19, 23, and 26 per group) held in a natural stream released 37.97 ± 33.19 ng⋅larva−1⋅h−1 3kPZS and 149.38 ± 86.37 ng⋅larva−1⋅h−1 PZS at a 3kPZS:PZS ratio of 0.17 ± 0.05 (mean ± SE, n = 3; Fig. 4). Populations of larval lamprey in small habitat patches (1 m2) can number up to the thousands (40) and therefore easily produce 3kPZS concentrations that attract females (ovulated females respond to 3kPZS at concentrations as low as 10−14 M) (26). In contrast, males sampled off spawning nests released 815.91 ± 106.60 µg⋅male−1⋅h−1 3kPZS and 10.16 ± 1.05 µg⋅male−1⋅h−1 PZS at a 3kPZS:PZS ratio of 81.43 ± 9.64 (mean ± SE, n = 7). Therefore, males and larvae both release 3kPZS and PZS but at opposite proportions; 3kPZS is the more abundant component in the male pheromone whereas PZS is the more abundant component in the larval cue.
Fig. 4.
Females discriminated a nonsexual larval cue from the male pheromone that mimics it using a pheromone antagonist. (A) In a natural stream, females chose the artificial nest treated with natural male pheromone (n females = 11) over an adjacent nest treated with natural larval odor (n females = 1) when each was applied to reach 5 × 10−14 M 3kPZS. (B) The ratio of 3kPZS and PZS (mean ± SE) released by male (light gray, n = 7) and larva (dark, n = 3 groups of 19, 23, and 26 individuals) measured with ultra–high-performance liquid chromatography-tandem mass spectrometry. (C) In another natural stream assay, females chose the nest treated with synthesized mixtures of 3kPZS and PZS at a ratio typical of the male pheromone (100:1, n females = 11) over an adjacent nest with the mixture at a ratio typical of larval odor (10:1, n females = 0) when each was applied to reach 5 × 10−14 M 3kPZS.
Returning to our 2-choice flume, we tested ovulated female responses to mixtures of 3kPZS and PZS at various ratios spanning the range observed in natural odorants. Indeed, ovulated females were attracted to a 10:1 mixture of 3kPZS and PZS (P = 0.014), neutral to a 1:10 mixture (P = 0.934), and avoided a 1:100 mixture (P < 0.001; Fig. 1).
Knowing the larval cue and male pheromone each comprises a suite of odorants (21), we next evaluated the importance of 3kPZS and PZS ratios within the complete natural mixtures. We predicted that altering the ratios in natural odorant mixtures with synthesized 3kPZS and PZS would disrupt ovulated female neutrality to larval odor and attraction to male odor. As expected, larval odorants mixed with synthesized 3kPZS to reach the male ratio of 3kPZS to PZS evoked significant preference in ovulated females (P = 0.035; Fig. 1). Interestingly, female preference for male odor was not abated by adding synthesized PZS to reach the larval ratio of 3kPZS to PZS (P = 0.007; Fig. 1), indicating that PZS inhibits responses to 3kPZS but not all other components of the male pheromone (26).
We then repeated our in-stream behavioral assay using mixtures of synthesized 3kPZS and PZS that approximated the ratios found in larval (1:10) and male (100:1) odors, standardized to reach an in-stream concentration of 5 × 10−13 M 3kPZS. All ovulated females chose the nest treated with a mixture of 3kPZS and PZS typical for males over that of larvae (P < 0.001; Fig. 4). Indeed, mixtures of 3kPZS and PZS at ratios typical of male and larval odorants were sufficient to replicate the effects of the natural odorants.
Lastly, we predicted that spermiated males also respond to PZS as a behavioral antagonist to prevent orienting toward larvae during spawning. Like females, sexually immature males track larval odor and 3kPZS when navigating into tributaries that support reproduction (24, 25, 41). Although spermiated males do not exhibit a strong attraction to the complete male odor (27), we observed that they preferred the channel of our 2-choice flume that was activated with 3kPZS alone (P = 0.002; Fig. 1). That males also orient toward 3kPZS supports the hypothesis that preference for 3kPZS originated outside of mate choice (42). However, males, like females, conceivably incur costs when they track larva-released 3kPZS into nursery habitats unsuitable for spawning. Using our 2-choice flume, we found males avoided the channel activated with PZS alone and a 1:1 mixture of 3kPZS and PZS (P = 0.025 and 0.016; Fig. 1).
Discussion
Despite widespread evidence that exaggerated male displays originate as sensory traps, if and how these inherently deceptive signals can guide reliable communication remain unclear (15, 16). Here, we report a series of behavioral, electrophysiological, and biochemical studies focused on female attraction to a mimetic sex pheromone and its model cue in the sea lamprey. Male sea lamprey signal with a sex pheromone that appears to mimic a nonsexual larval cue used during migration into spawning streams (10). The mimetic signal, albeit likely beneficial to females (10, 22), presumably confuses mate search as ovulated females encounter the main component 3kPZS from both males and larvae while traversing streams interspersed with spawning and nursery habitats. First, we confirmed that ovulated females appropriately orient toward the male pheromone but not toward the nonsexual larval cue it mimics. We then tested the hypothesis that the larval odorant PZS antagonizes the response of ovulated females to 3kPZS to prevent orientation toward larval odor during mate search. In support of this hypothesis, we found 1) PZS reduced olfactory responses to 3kPZS across a range of concentrations and abated female preference for 3kPZS when added to reach the same or greater concentrations, 2) larvae released more PZS than 3kPZS whereas males released more 3kPZS than PZS, and 3) mixtures of synthesized 3kPZS and PZS at ratios in larval odor (1:10) and the male pheromone (100:1) resulted in the same behavioral discrimination observed between the natural larval cue and the male pheromone that mimics it. We conclude that the antagonistic role of PZS constitutes a mechanism by which ovulated females discriminate the model larval cue from the male pheromone that mimics it, thus enabling reliable communication with a signal of a deceptive origin.
Our results provide evidence that females incorporate a sensory trap into reliable sexual communication without sacrificing their response to the model cue. Terms such as “exploit,” which are commonly used in the receiver bias literature, could imply females’ reactions to manipulative male signals cause maladaptive mating (16, 17), but deceptive signals might not impose any costs on females and may even benefit females by guiding mate search (19). However, deceptive signals that guide adaptive mating behavior should often impose costs on females that confuse the model cue and the mimetic male trait, whether in the nonsexual or sexual context (16, 20). Despite decades of theory (15–17, 19, 20), only one set of empirical data provides information on how females adapt to deceptive male signaling (43, 44). In some species of splitfin fishes (Goodeidae), males signal to females with a yellow band that exploits a feeding response to damselfly larvae but appears to guide adaptive mate choice; however, the costs of responding similarly to prey and the male trait that mimics prey drove females to reduce their feeding response to damselfly larvae and use the sensory attraction solely for mate choice (43, 44). Sea lamprey also appear to benefit from the mimetic male signal in mate choice (10, 22) and respond to it as a reliable indicator of male location, but do so without abandoning or attenuating their response to the model larval cue that continues to guide initial upstream migration. Rather, PZS acts as a behavioral antagonist to distinguish the model larval cue from the mimetic male pheromone and allow females to appropriately orient only to the male pheromone during mate search. Importantly, a previous study indicates PZS does not null the nonsexual migratory response to 3kPZS (22), allowing sexually immature females to navigate using 3kPZS in the larval cue during the prespawning migration. We suggest that the evolution of context-appropriate responses to both the model cue and the mimetic male signal might be a widespread result of sensory traps because 1) the stimulus often benefits females in both the nonsexual and sexual contexts, 2) the optimal response often differs between contexts, and 3) animals are well-adapted to recognize the broader context when attending to individual stimuli (20).
Sea lamprey exemplify how multiple cues and contextual nuances can allow reliable communication using mimetic signals. First, mimetic signals might convey reliable information when detected alongside other cues (20). Navigating upstream searching for mates, ovulated females are likely to first encounter larva-released 3kPZS, as larvae drift downstream from nests after hatching (40). We found that ovulated females were not attracted to larval odor or mixtures of 3kPZS and PZS at ratios found in larval odorants, indicating that PZS is a critical cue that prevents orientation toward 3kPZS released by larvae while females move upstream to nesting males. Continuing upstream, females likely encounter 3kPZS from both larvae and males simultaneously where nursery and spawning habitats are in close proximity (29). Our in-stream behavioral assay recreated the choice females face in this scenario and indicated mixtures of 3kPZS and PZS are sufficient to guide females to males over larvae; 100% of ovulated females chose the mixture at the male-typical ratio over the mixture at the larva-typical ratio. Although not directly tested here, minor components of the male pheromone may also help females track plumes of 3kPZS from males over those from larvae (26, 45). Indeed, ovulated females in our 2-choice flume, which positions females in close proximity to odorants, were still attracted to natural male odorant mixed with PZS to reach the ratio in natural larval odorants. The function of minor components of the male pheromone remains poorly understood (21) and may have evolved through mechanisms different from those underlying communication with 3kPZS (46). Notably, 3kPZS completely replicates the natural male sex pheromone at a long distance (26), so minor components could only guide discrimination between larval and male odors in close proximity. Similarly, some, but not all, larvae reside in eddies on stream edges that are unlikely to have sufficient flow to stimulate the odor-conditioned rheotaxis by which females track 3kPZS plumes (39). Therefore, in some scenarios, females might not orient toward larva-released 3kPZS as a result of the habitat larvae occupy and the behavioral mechanisms underlying attraction to 3kPZS. However, eddies will also produce a tractable plume where they seep into the main stream channel, making velocity cues, as minor pheromone components, only relevant in close proximity to the odor source. Second, females might adjust their response to better match the mating context. In migratory sea lamprey, 3kPZS elicits nontargeted upstream movement fit for large-scale navigation into tributaries that support larval populations (22, 24). However, spawning females of species which respond to 3kPZS as a sex pheromone exhibit honed preferences suited to guide them over long distances to males on ∼0.5-m2 nests (22, 30, 31). Indeed, context-appropriate responses to mimetic male signals seem likely given the hormonal changes that occur during reproduction and control the reactions to external stimuli such as pheromones (20).
Our discovery of a pheromone antagonist in a vertebrate allows comparisons with odorants that cause similar behavioral antagonism in insects. Many moths and beetles use heterospecific components as pheromone antagonists—compounds that reduce or eliminate the attractive effects of pheromone components—to mediate reproductive isolation between sympatric species with similar pheromone blends (47, 48). Furthermore, one recent study on cotton bollworms reports that a pheromone precursor acts as an antagonist to deter males from mating with sexually immature females (49). In contrast, our experiments indicate sea lamprey respond to PZS, which, interestingly, is also a likely pheromone precursor (50), as a behavioral antagonist to discriminate a mimetic pheromone from its model cue. Despite the difference in ecological functions, the behavioral mechanisms of PZS and other pheromone antagonists appear remarkably similar; female sea lamprey and male moths exposed to antagonists are less likely to initiate movement upstream or upwind toward sex pheromones and exhibit more frequent lateral casting while tracking the plume (51, 52). The olfactory mechanism by which PZS nulls females’ response to 3kPZS remains unclear. In insects, behavioral antagonism may be the result of unbalanced input between the various pheromone components (47). Unbalanced olfactory input may explain PZS antagonism as the nonreciprocal interaction of 3kPZS and PZS in our EOG experiments is consistent with the 2 compounds being detected by 2 receptor types. For example, one receptor type may predominantly bind 3kPZS and the other bind both 3kPZS and PZS. However, our observation that PZS did not null female attraction to natural male odor indicates the necessary balance involves 3kPZS and PZS but not all other components, and supports previous suggestions that constituents of the male pheromone act as distinct components rather than a collective blend (26).
In conclusion, our studies implicate a pheromone antagonist as a mechanism by which a sensory trap guides reliable communication in the sea lamprey. Along with providing basic insights into the evolution and underlying mechanisms of communication, our results offer potential applications for disrupting mate search for sea lamprey in the Laurentian Great Lakes, where they are a destructive invader (53).
Methods
All behavioral assays used ovulated females or spermiated males. Procedures for 2-choice flume and in-stream behavioral assays were slightly modified from previous studies (33, 34, 45) and are described in SI Appendix. Natural odorants for behavior tests were collected from a captive population of ∼25,000 larvae as previously described (24) and from a group (14 or 20) of males held in 40 L water for 4 h. Synthesized 3kPZS and PZS were prepared by Bridge Organics. The methods for EOG recordings are described in previous reports (34, 54) and in SI Appendix. Release of 3kPZS and PZS by larvae and spermiated males was quantified using methods described by Li et al. (55) and in SI Appendix.
Michigan State University’s Institutional Animal Use and Care Committee approved all procedures involving sea lamprey (animal use forms 03/12-063-00, 12/14-223-00, 03/11-053-00, 05/09-088-00, and 02/17-031-00).
Data Availability Statement.
All data discussed in the paper are available in SI Appendix and Dataset S1.
Supplementary Material
Acknowledgments
The US Fish and Wildlife Service’s Ludington and Marquette Biological Stations and Fisheries and Oceans Canada provided sea lamprey. Brandon Blasius, Ethan Buchinger, Camryn Bullock, Autumn Idalski, Ellery Marano, Joseph Riedy, Aaron Slater, Zachary Smilie, Joe Waker, and Gloria Yarandi assisted with behavioral experiments and pheromone sampling. Two anonymous reviewers provided valuable suggestions on an earlier version of the paper. This study was funded primarily by grants from the Great Lakes Fishery Commission. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921394117/-/DCSupplemental.
References
- 1.Darwin C. R., C. R. Darwin to A. Gray, Darwin Correspondence Project, Letter 2743, 3 April 1860. https://www.darwinproject.ac.uk/. Accessed 23 September 2019.
- 2.Candolin U., Wong B. B., Mate choice in a polluted world: Consequences for individuals, populations and communities. Phil. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180055 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer H. M., Ruxton G. D., Signal diversity, sexual selection, and speciation. Annu. Rev. Ecol. Evol. Syst. 46, 573–592 (2015). [Google Scholar]
- 4.Darwin C., The Descent of Man and Selection in Relation to Sex (Murray, 1871). [Google Scholar]
- 5.Andersson M. B., Sexual Selection (Princeton University Press, 1994). [Google Scholar]
- 6.Andersson M., Simmons L. W., Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Endler J. A., Basolo A. L., Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415–420 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Kolm N., Amcoff M., Mann R. P., Arnqvist G., Diversification of a food-mimicking male ornament via sensory drive. Curr. Biol. 22, 1440–1443 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Nakano R., Takanashi T., Surlykke A., Skals N., Ishikawa Y., Evolution of deceptive and true courtship songs in moths. Sci. Rep. 3, 2003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchinger T. J., Wang H., Li W., Johnson N. S., Evidence for a receiver bias underlying female preference for a male mating pheromone in sea lamprey. Proc. Biol. Sci. 280, 20131966 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stålhandske P., Nuptial gifts of male spiders function as sensory traps. Proc. Biol. Sci. 269, 905–908 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christy J. H., Mimicry, mate choice, and the sensory trap hypothesis. Am. Nat. 146, 171–181 (1995). [Google Scholar]
- 13.West-Eberhard M., “Sexual selection, competitive communication and species-specific signals in insects” in Insect Communication: 12th Symposium of the Royal Entomological Society of London, Lewis T., Ed. (Academic Press, 1984), pp 283–324. [Google Scholar]
- 14.Stuart-Fox D., Deception and the origin of honest signals. Trends Ecol. Evol. 20, 521–523 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Ryan M. J., Cummings M. E., Perceptual biases and mate choice. Annu. Rev. Ecol. Evol. Syst. 44, 437–459 (2013). [Google Scholar]
- 16.Arnqvist G., Sensory exploitation and sexual conflict. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 375–386 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland B., Rice W. R., Perspective: Chase‐away sexual selection: Antagonistic seduction versus resistance. Evolution 52, 1–7 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Basolo A. L., The phylogenetic distribution of a female preference. Syst. Biol. 45, 290–307 (1996). [Google Scholar]
- 19.Dawkins M. S., Guilford T., Sensory bias and the adaptiveness of female choice. Am. Nat. 148, 937–942 (1996). [Google Scholar]
- 20.Rodríguez R. L., Trait duplication by means of sensory bias. Behav. Ecol. 20, 1376–1381 (2009). [Google Scholar]
- 21.Buchinger T. J., Siefkes M. J., Zielinski B. S., Brant C. O., Li W., Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front. Zool. 12, 32–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brant C. O., Johnson N. S., Li K., Buchinger T. J., Li W., Female sea lamprey shift orientation toward a conspecific chemical cue to escape a sensory trap. Behav. Ecol. 27, 810–819 (2016). [Google Scholar]
- 23.Polkinghorne C. N., Olson J. M., Gallaher D. G., Sorensen P. W., Larval sea lamprey release two unique bile acids** to the water at a rate sufficient to produce detectable riverine pheromone plumes. Fish Physiol. Biochem. 24, 15–30 (2001). [Google Scholar]
- 24.Brant C. O., Li K., Johnson N. S., Li W., A pheromone outweighs temperature in influencing migration of sea lamprey. R. Soc. Open Sci. 2, 150009–150016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson N. S., et al. , A synthesized mating pheromone component increases adult sea lamprey (Petromyzon marinus) trap capture in management scenarios. Can. J. Fish. Aquat. Sci. 70, 1101–1108 (2013). [Google Scholar]
- 26.Johnson N. S., Yun S.-S., Thompson H. T., Brant C. O., Li W., A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc. Natl. Acad. Sci. U.S.A. 106, 1021–1026 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W., et al. , Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 296, 138–141 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Siefkes M. J., Scott A. P., Zielinski B., Yun S.-S., Li W., Male sea lampreys, Petromyzon marinus L., excrete a sex pheromone from gill epithelia. Biol. Reprod. 69, 125–132 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Applegate V. C., “Natural history of the sea lamprey, Petromyzon marinus, in Michigan” (Spec. Sci. Rep., Fisheries 55, US Fish and Wildlife Service, 1950).
- 30.Johnson N. S., Buchinger T. J., Li W., “Reproductive ecology of lampreys” in Lampreys: Biology, Conservation and Control, Docker M. F., Ed. (Springer, 2015), pp. 265–303. [Google Scholar]
- 31.Buchinger T. J., et al. , Phylogenetic distribution of a male pheromone that may exploit a nonsexual preference in lampreys. J. Evol. Biol. 30, 2244–2254 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Searcy W. A., Nowicki S., The Evolution of Animal Communication (Princeton University Press, 2005). [Google Scholar]
- 33.Buchinger T. J., et al. , Increased pheromone signaling by small male sea lamprey has distinct effects on female mate search and courtship. Behav. Ecol. Sociobiol. 71, 155 (2017). [Google Scholar]
- 34.Scott A. M., et al. , Spermine in semen of male sea lamprey acts as a sex pheromone. PLoS Biol. 17, e3000332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine J. M., Sorensen P. W., Isolation and biological activity of the multi-component sea lamprey migratory pheromone. J. Chem. Ecol. 34, 1259–1267 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Raschka S., et al. , Enabling the hypothesis-driven prioritization of ligand candidates in big databases: Screenlamp and its application to GPCR inhibitor discovery for invasive species control. J. Comput. Aided Mol. Des. 32, 415–433 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Siefkes M. J., Li W., Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lampreys (Petromyzon marinus). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 190, 193–199 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Johnson N. S., Luehring M. A., Siefkes M. J., Li W., Mating pheromone reception and induced behavior in ovulating female sea lampreys. N. Am. J. Fish. Manage. 26, 88–96 (2006). [Google Scholar]
- 39.Johnson N. S., Muhammad A., Thompson H., Choi J., Li W., Sea lamprey orient toward a source of a synthesized pheromone using odor-conditioned rheotaxis. Behav. Ecol. Sociobiol. 66, 1557–1567 (2012). [Google Scholar]
- 40.Dawson H. A., Quintella B. R., Almeida P. R., Treble A. J., Jolley J. C., “The ecology of larval and metamorphosing lampreys” in Lampreys: Biology, Conservation and Control, Docker M. F., Ed. (Springer, 2015), pp. 75–137. [Google Scholar]
- 41.Bjerselius R., et al. , Direct behavioral evidence that unique bile acids released by larval sea lamprey (Petromyzon marinus) function as a migratory pheromone. Can. J. Fish. Aquat. Sci. 57, 557–569 (2000). [Google Scholar]
- 42.Rodd F. H., Hughes K. A., Grether G. F., Baril C. T., A possible non-sexual origin of mate preference: Are male guppies mimicking fruit? Proc. Biol. Sci. 269, 475–481 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia C. M.,Lemus Y. S., Foraging costs drive female resistance to a sensory trap. Proc. Biol. Sci. 279, 2262–2268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia C. M., Ramirez E., Evidence that sensory traps can evolve into honest signals. Nature 434, 501–505 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Brant C. O., Huertas M., Li K., Li W., Mixtures of two bile alcohol sulfates function as a proximity pheromone in sea lamprey. PLoS One 11, e0149508 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchinger T. J., et al. , Evidence for partial overlap of male olfactory cues in lampreys. J. Exp. Biol. 220, 497–506 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Baker T. C., Balanced olfactory antagonism as a concept for understanding evolutionary shifts in moth sex pheromone blends. J. Chem. Ecol. 34, 971–981 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Zhang D.-D., Löfstedt C., Moth pheromone receptors: Gene sequences, function, and evolution. Front. Ecol. Evol. 3, 105 (2015). [Google Scholar]
- 49.Chang H., et al. , A pheromone antagonist regulates optimal mating time in the moth Helicoverpa armigera. Curr. Biol. 27, 1610–1615.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Brant C. O., Chung-Davidson Y.-W., Li K., Scott A. M., Li W., Biosynthesis and release of pheromonal bile salts in mature male sea lamprey. BMC Biochem. 14, 30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bau J., Martínez D., Renou M., Guerrero A., Pheromone-triggered orientation flight of male moths can be disrupted by trifluoromethyl ketones. Chem. Senses 24, 473–480 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Linn C. E. Jr, Domingue M. J., Musto C. J., Baker T. C., Roelofs W. L., Support for (Z)-11-hexadecanal as a pheromone antagonist in Ostrinia nubilalis: Flight tunnel and single sensillum studies with a New York population. J. Chem. Ecol. 33, 909–921 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Li W., Twohey M., Jones M., Wagner M., Research to guide use of pheromones to control sea lamprey. J. Great Lakes Res. 33, 70–86 (2007). [Google Scholar]
- 54.Li K., et al. , Fatty-acid derivative acts as a sea lamprey migratory pheromone. Proc. Natl. Acad. Sci. U.S.A. 115, 8603–8608 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li K., Wang H., Brant C. O., Ahn S., Li W., Multiplex quantification of lamprey specific bile acid derivatives in environmental water using UHPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 3879–3886 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are available in SI Appendix and Dataset S1.