Abstract
The aim of the present study was to evaluate the anti-photoaging effect of neferine upon exposure of mice to ultraviolet (UV) radiation. An in vivo photoaging model was established by repeatedly exposing mouse dorsal skin to UV-A and UV-B radiation for 12 weeks. Through skin photographs, hematoxylin and eosin staining, Masson's trichrome staining, and scanning and transmission electron microscopy, skin wrinkles, epidermal thickness and dermal collagen were analyzed in the UV-irradiated mouse skin. Furthermore, the levels of endogenous antioxidants, namely superoxide dismutase (SOD) and glutathione peroxidase (GPx), were measured to determine the extent of UV-induced oxidative stress that was associated with photoaging. The results demonstrated that the topical application of neferine following UV irradiation reduced oxidative stress by increasing SOD and GPx activities, and attenuated the photoaging process. Histological and ultrastructural examination revealed that neferine delayed skin wrinkle formation by inhibiting epidermal hypertrophy and collagen loss and degradation. In conclusion, the results of the present study indicated that neferine effectively prevents UV-induced skin photoaging and photodamage.
Keywords: neferine, antioxidants, ultraviolet radiation, skin photoaging, mice
Introduction
Plants and associated derivatives are used widely in skincare products. In particular, the lotus plant is native to China and has been used in Chinese traditional medicine for >1,000 years (1). According to popular belief, lotus confers youthfulness and longevity, whereas previous studies have reported that lotus extract possesses hypoglycemic, antimicrobial, antipyretic (2), psychopharmacological (3) and anti-inflammatory properties (4). Lotus extract has also been used for skin whitening and anti-wrinkle treatment (5). Previous research has demonstrated the antioxidant properties of various parts of the lotus plant, including the leaf (6), stamen (7) and rhizome (8). Lotus seed extract has been indicated to exhibit anti-ischemic (9) and antioxidant properties (10). Furthermore, lotus seed embryos are commonly consumed as a tea ingredient or ingested raw, as they are considered to exhibit antiaging properties.
Neferine (Fig. 1) is one of the major bisbenzylisoquinoline alkaloids, along with liensinine and isoliensinine, and is derived from the green seed embryo of the lotus plant (11). Some of the pharmacological activities of neferine include antiarrhythmic, antihypertensive (12), sedative (13) and anti-diabetic properties (14). A previous study has demonstrated the anti-inflammatory and antioxidant actions of neferine (15). Neferine is being increasingly investigated for its potential clinical applications; however, its anti-photoaging effects have not yet been elucidated.
Figure 1.
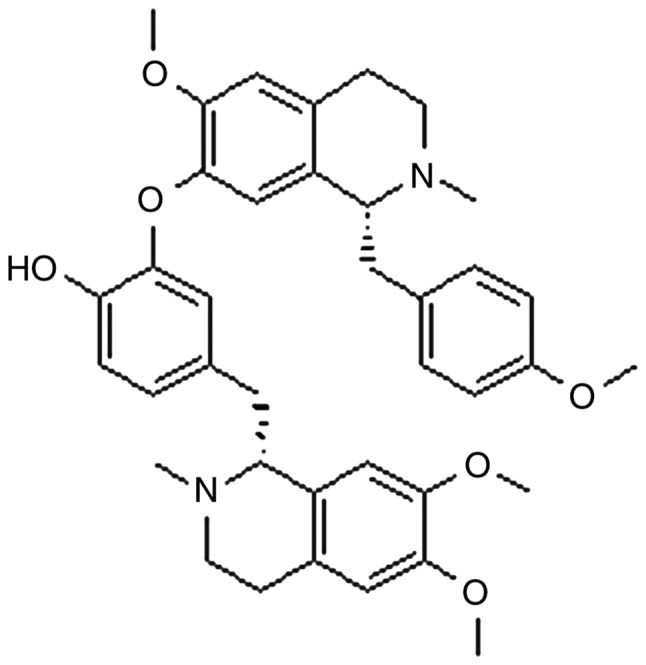
Chemical structure of neferine.
The skin is one of the most susceptible organs to ultraviolet radiation (UVR), which is the main cause of photoaging (16). Loss of skin tone, roughness, resilience and dryness, irregular pigmentation and deep wrinkle formation are the main characteristics of photoaged skin (17). It is well known that UV-B (280-315 nm) and UV-A (315-400 nm) can cause skin photoaging and photodamage (18). The principal mode of action by UVR is reactive oxygen species (ROS) generation (19), which is one of the main contributors to UV-induced photoaging. ROS damage biological macromolecules, including DNA, carbohydrates, lipids and proteins, and deplete antioxidants, including superoxide dismutase (SOD) and glutathione peroxidase (GPx) (20,21). Antioxidants scavenge free radicals and aid the repair of cellular damage. A variety of antioxidants, including vitamins C and E, selenium, soy isoflavones and polyphenolic compounds, have been revealed to attenuate UV-induced photoaging (22,23).
The aim of the present study was to analyze the anti-photoaging activity of neferine by creating an in vivo model of UV irradiation, in order to determine whether neferine could replenish skin antioxidants including SOD and GPx, and minimize skin wrinkles and collagen content loss upon UV exposure.
Materials and methods
Reagents
Neferine was purchased from Pure One Biotechnology Co., Ltd. SOD (cat. no. A001-1); GPx (cat. no. A005) and bicinchoninic acid (BCA; cat. no. A045-3) assay kits were purchased from the Nanjing Jiancheng Bioengineering Institute.
Animals
A total of 72 female Kunming mice (weight, 25-28 g; age, 6-8 weeks) were purchased from the Experimental Animal Center, School of Medicine, Xian Jiaotong University. The mice had access to food and water ad libitum and were maintained in a 12-h dark/light cycle with a controlled temperature of 24±2˚C and humidity of 55±10%. All mice were allowed to acclimatize to their new surroundings for 1 week prior to the experiments. The housing conditions were maintained constant throughout the entirety of the experimental study period. All animal experiments were approved and conducted under the guidance of the International Association for the Protection of Animal and Experimental Medicine and Laboratory Animal Ethics Committee of Xian Jiaotong University School of Medicine (Xian, China).
Preparation of neferine moisture base cream
Two types of moisture base creams were prepared for use in the current study.
Moisture base cream with neferine
Neferine (0.1%), ethanol (EtOH; 58.5%), propanediol (20.0%), water (20%) and hydroxypropyl methyl cellulose (1.5%).
Moisture base cream without neferine
EtOH (58.5%), propanediol (20.0%), water (20%), hydroxypropyl methyl cellulose (1.5%).
UV exposure and neferine topical treatment
UV irradiation was provided by Philips ACTINIC BL TL-K 40W/10R and Philips TL 20W/01RS bulbs (Philips Lighting), which were used to provide UV-A (315-400 nm; peak wavelength, 365 nm) and UV-B (280-315 nm; peak wavelength, 311 nm) using an array of six lamps adjusted at a distance of 30 cm from the skin of the mice. The minimal erythema dose (MED) was measured using a Lutron-UV-340a meter (Lutron Electronic Enterprise Co., Ltd.). The MED for UV-A and UV-B was calculated as 1,200 and 180 mJ/cm2, respectively. This dose was considered to be 1 MED. The dose was then increased from 1 to 4 MED towards week 12 of the study. This method was applied as previously described by Li et al (24). The skin on the dorsal surface (1.5x1.5 inches) of all the mice was shaved three times a week and depilated twice a week with Veet (Reckitt Benckiser Group plc) for 12 weeks. Vehicle- and neferine (0.1%)-treated groups were exposed to UVR five times a week (not on Saturday or Sunday) for 12 weeks. The mice in the control group were shaved and depilated twice a week with Veet (Reckitt Benckiser Group, plc), but received no UV irradiation. As presented in Table I, mice were divided into three groups (n=6 mice/group): The neferine (0.1%) and vehicle (without neferine) groups were topically treated with moisture base cream with a brush applicator 30 min prior to every UV irradiation treatment. The control group was left untreated.
Table I.
Experimental design of the study.
| Groups | Shave | UV irradiation | Vehicle | Neferine (0.1%) |
|---|---|---|---|---|
| Control | + | - | - | - |
| Vehicle | + | + | + | - |
| Neferine | + | + | + | + |
+, treatment; -, no treatment; UV, ultraviolet.
Evaluation of wrinkled appearance
One day prior to the end of the study period, mice were anaesthetized and the dorsal skin was photographed using a digital camera (Canon SX200 IS; Canon, Inc.). The photodamage was assessed using visual scoring (Table II), which was performed by two observers who were blinded to the grouping, which was based on the protocols previously described by Kong et al (20) and Bissett et al (25). The skin wrinkles of each mouse were further evaluated by the capture of images using a light microscope (magnification, x100; Nikon SM21500; Nikon Corporation).
Table II.
Grading scale for evaluation of skin wrinkles.
| Grade | Description |
|---|---|
| 0 | Smooth skin without any wrinkles; fine striations running down the length of the body |
| 1 | Fine striations |
| 2 | A few shallow wrinkles; disappearance of all fine striations |
| 3 | Shallow wrinkles across the dorsal skin |
| 4 | Deep and coarse wrinkles with laxity |
| 5 | Increased wrinkle depth |
| 6 | Surface marked by severe wrinkles; development of lesions |
Hematoxylin and eosin (H&E) staining and epidermal thickening analysis
At the end of experimental study, the mice were anesthetized and sacrificed, following which the dorsal skin tissues were collected. The tissues were treated with 10% phosphate-buffered formaldehyde (pH 7.4) at room temperature for 24 h prior to staining. H&E staining was performed according to standard protocols (26). Following deparaffinization with 100% xylene for 3 min and 50:50 xylene/100% EtOH for 3 min, and rehydration with 100% EtOH for 3 min and 95% EtOH for 3 min, 4-µm skin sections were stained with hematoxylin (Gill's IX) for 5 min at room temperature and subsequently rinsed with water for 5 min. Slides were then treated with 5 dips in acid alcohol (1% HCl in 70% EtOH) and subsequently rinsed with water. Following treatment with ammonia (1 ml NH4OH in 1 l water) and rinsing with water, the sections were stained with Eosin Y solution for 1 min at room temperature, followed by dehydration with graded alcohol (95% EtOH for 2 min; 100% EtOH for 2 min) and clearing in xylene (50:50 xylene/100% EtOH for 2 min; 100% xylene for 2 min). Stained slides were observed under a light microscope (magnification, x200 and x400; Nikon DS-Ri1; Nikon Corporation) and photographs of each specimen were captured at three randomly selected locations. Epidermal thickness was evaluated by measuring the distance from the stratum corneum to the basement membrane in the interfollicular epidermis at five randomly selected positions. Epidermal thickening was analyzed and quantified using Image J 1.36 analysis software (National Institutes of Health).
Masson's trichrome staining and collagen content quantification
Mice were anesthetized and sacrificed at the end of experimental study. Skin tissue samples (4 µm), after deparaffinization and rehydration, were treated with Bouin's solution at 56˚C for 15 min. After cooling the slides with water at 18-26˚C for 5 min, samples were stained using working Weigert's iron hematoxylin solution for 5 min at room temperature. Subsequently, slides were rinsed in deionized water and stained in Biebrich scarlet-acid fuchsin for 5 min at room temperature and rinsed in deionized water for 1 min. Slides were then treated with working phosphotungstic/phosphomolybdic acid solution for 5 min at room temperature and placed in Aniline Blue solution for 5 min at room temperature. Slides were treated with acetic acid (1%) for 2 min at room temperature and dehydrated through a graded alcohol series (70, 80, 90 and 100%), and cleared in xylene for 5 min. Slides were observed under a light microscope (magnification, x200 and x400; Nikon DS-Ri1; Nikon Corporation) and each specimen was photographed at three randomly selected locations. To evaluate the dermal collagen density, quantification was conducted using Image J 1.36 software (National Institutes of Health) (27,28). Collagen density was evaluated by measuring the distance from the papillary layer to the upper layer of subcutaneous tissue at five randomly selected positions. The relative % of collagen density was then calculated using the following equation:
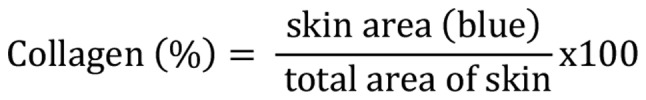 |
Scanning and transmission electron microscopy
The dorsal skin samples were cut into small pieces (~2x2x2 mm) and immediately fixed with 2.5% glutaraldehyde and 4% paraformaldehyde for 2 h at 4˚C, washed with 0.1 M phosphate buffer (PB) for 30 min at 4˚C, followed by post-fixation with 1% osmium tetroxide for 2 h at 4˚C and washing 2 or 3 times with 0.1 M PB. The skin fragments were dehydrated using a graded EtOH series (30% EtOH in water 10 min, 50% EtOH in water 10 min, 70% EtOH in water 10 min, 90% EtOH in water 10 min and 100% EtOH). The samples were immersed in a mixture of propylene oxide and Epon 812 overnight at 37˚C followed by polymerization embedding for 48 h at 60˚C. The tissues were then cut into 1-3-µm sections and stained with Azure ΙΙ or toluidine blue for 1 min on a 60˚C hotplate to identify and observe the area of interest. The blocks were sectioned at 50-70 nm using an LKB Ultratome. The ultra-thin sections were then stained with uranyl acetate and lead hydroxide at room temperature for 10 min and observed using scanning electron microscopy (Hitachi TM-1000; Hitachi, Ltd.) by probing the images with focused electron beams. For transmission electron microscopy, mice dorsal skin tissue samples were dehydrated through an ethanol series at 37˚C and then transferred to propylene oxide followed by embedded in epoxy resin at 60˚C overnight. Samples were observed using transmission electron microscope (Hitachi H-7650; Hitachi, Ltd.).
Assays of SOD and GPx activity
Dorsal skin samples (80 mg) were homogenized in physiological saline at 4˚C and centrifuged at 13,000 x g for 15 min. The collected supernatant was used to measure the total SOD and GPx activities using SOD and GPx assay kits according to manufacturers' protocols. The absorbance of SOD and GPx was measured spectrophotometrically (Multiskan; Thermo Fisher Scientific, Inc.) at 512 and 405 nm, respectively.
Assay of protein concentration
Total protein concentration was measured according to the BCA protein assay kit protocol. Briefly, the supernatant obtained by the abovementioned method was centrifuged at 13,000 x g for 15 min at 4˚C. The homogenate was then incubated for 30 min at 37˚C and the absorbance of SOD or GPx was measured at 560 nm spectrophotometrically (Multiskan; Thermo Fisher Scientific, Inc.).
Statistical analysis
Experimental values were analyzed using GraphPad Prism 5 (GraphPad Software, Inc.). Analysis of data was carried out by one-way ANOVA followed by Bonferroni's multiple comparisons test. All data are expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Neferine inhibits wrinkle formation
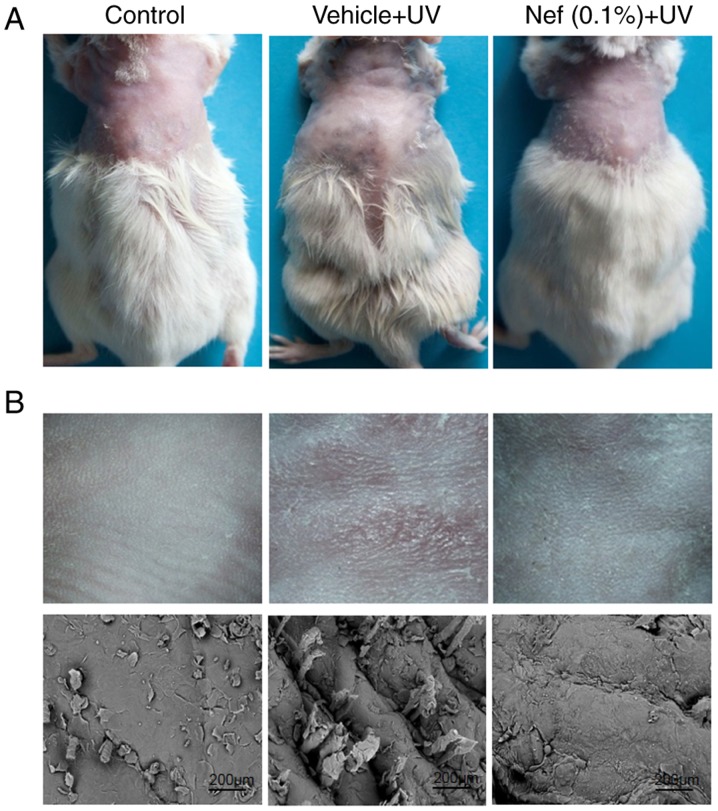
During the 12-week duration of the current study, the control group mice did not exhibit any skin changes, including deep wrinkles, desquamation, and erythema or scaling. However, there was a marked difference in the appearance of skin wrinkles between the UV irradiated vehicle and neferine-treated groups (Fig. 2). Mice in the vehicle-treated group displayed dry, scaly skin with the formation of deep wrinkles (visual scoring, 5). By contrast, the neferine-treated group exhibited improved skin appearance and significantly diminished wrinkles (visual scoring, 3; Fig. 2). Scanning electron microscopy analysis revealed deep wrinkles in the vehicle-treated group, whereas less wrinkling was observed in mice treated with neferine (Fig. 2B).
Figure 2.
Nef application inhibits skin wrinkles in mice upon UV exposure. (A) Representative images showing wrinkles on the dorsal skin of mice at the end of the 12-week study. (B) Photographic images (upper panels) and scanning electron microscopy images (lower panels) of wrinkles in mice dorsal skin. Nef, neferine; UV, ultraviolet.
Neferine inhibits epidermal hyperplasia
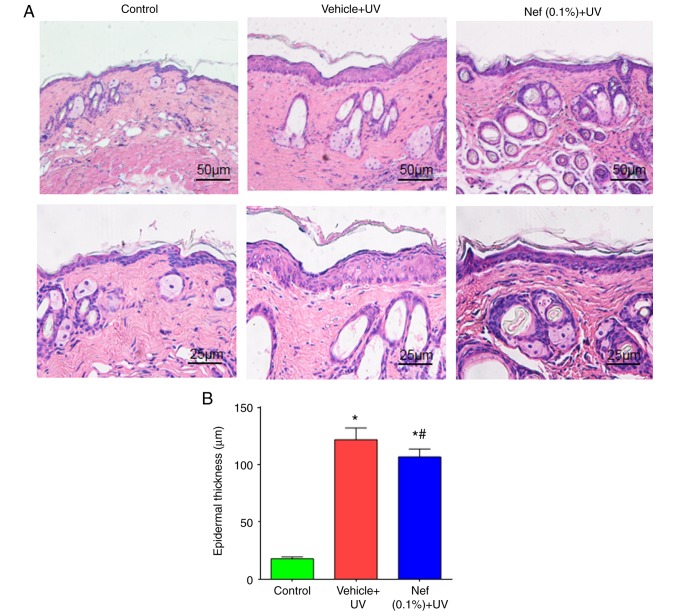
Epidermal hyperplasia is a histological characteristic of photoaged skin and can be used as one of the parameters to reflect the extent of UV-induced skin damage, since epidermal thickening contributes to skin roughness (29-31). As presented in Fig. 3, the control group exhibited a low epidermal thickness compared with the vehicle and neferine-treated groups (Fig. 3A). The vehicle-treated and Nef + UV groups exhibited significantly increased epidermal thickening after 12 weeks of UV irradiation when compared with the control group (P<0.05). In mice treated with neferine, the epidermal thickness was significantly reduced when compared with that in the vehicle-treated group (P<0.05; Fig. 3B).
Figure 3.
Nef inhibits epidermal thickening in UV-irradiated mice. (A) Hematoxylin and eosin staining showing epidermal thickness. (B) Epidermal thickness expressed as the mean ± standard deviation (n=6). *P<0.05 vs. control group; #P<0.05 vs. the vehicle + UV group. Nef, neferine; UV, ultraviolet.
Neferine prevents dermal collagen loss
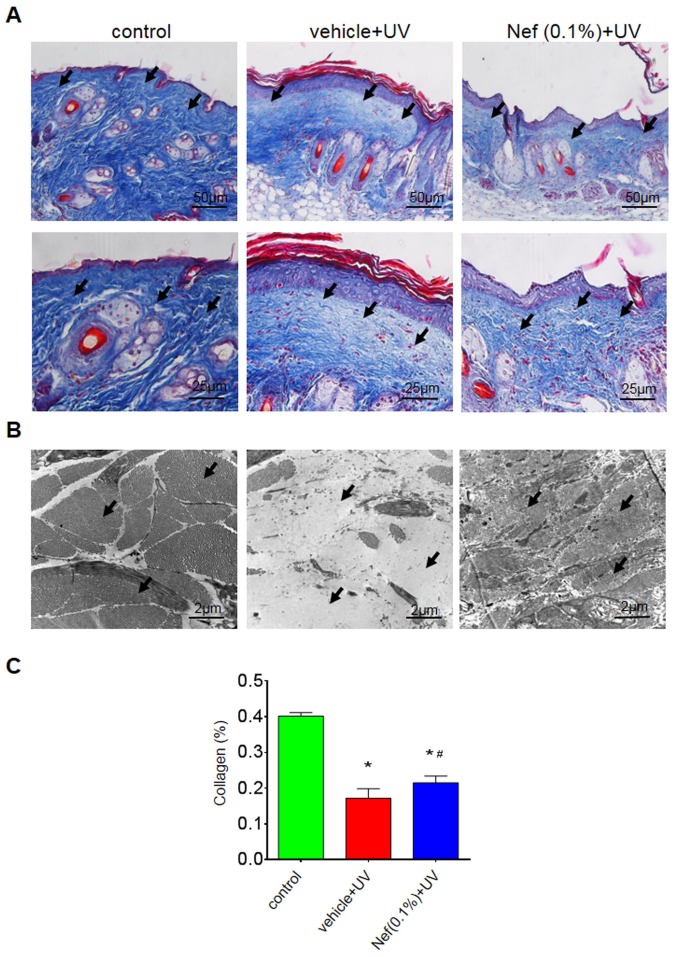
The vehicle-treated group exhibited a marked reduction in dermal collagen density compared with the control group. However, skin pretreatment with neferine inhibited this dermal collagen loss (Fig. 4). Furthermore, the collagen fibers were less dense and somewhat disorganized in the vehicle-treated group. In the neferine-treated group, however, the collagen fibers were denser and more organized (Fig. 4A).
Figure 4.
Nef application reduces dermal collagen loss following 12 weeks of UV irradiation. (A) Masson's trichrome staining: Upper panels. (B) Transmission electron microscopy. (C) Quantification of dermal collagen density. Data are expressed as the mean ± standard deviation (n=6). *P<0.05 vs. control group; #P<0.05 vs. the vehicle + UV group. Nef, neferine; UV, ultraviolet.
Transmission electron microscopy revealed disarrayed, broken and a reduced number of collagen fibers in the vehicle-treated group compared with the control group, in which the collagen fibers were intact and organized in bundles (Fig. 4B). By contrast, the neferine-treated group displayed minimal collagen loss and structural alterations (Fig. 4B). There was a significantly reduced amount of collagen in the vehicle-treated group compared with the control (*P<0.05); however, the UV-induced reduction of collagen was significantly attenuated in the neferine-treated group (#P<0.05; Fig. 4C).
Neferine increases SOD and GPx levels
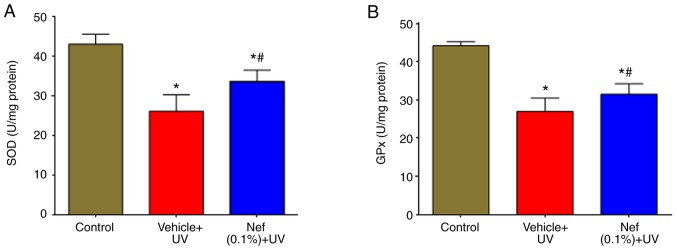
The effects of neferine on UV-induced oxidative stress were examined by measuring SOD and GPx levels. As presented in Fig. 5, SOD and GPx activities were significantly reduced in the UV-irradiated vehicle-treated group compared with the control group (*P<0.05). However, the decreased activities of SOD (Fig. 5A) and GPx (Fig. 5B) were significantly increased by the topical application of neferine (#P<0.05).
Figure 5.
Nef treatment increased antioxidant levels in mouse skin following 12 weeks of UV irradiation. (A) SOD and (B) GPx levels in mouse skin. Data are expressed as the mean ± standard deviation (n=6). *P<0.05 vs. control group; #P<0.05 vs. the vehicle + UV group. Nef, neferine; SOD, superoxide dismutase; GPx, glutathione peroxidase; UV, ultraviolet.
Discussion
Natural phytochemicals are currently used in cosmetics to replenish antioxidants, suppress collagen degradation and reduce the harmful impact of UVR on the skin. The antioxidant activity of certain natural compounds and their derivatives has been reported to prevent skin aging (32). Natural compounds contain bioactive substances, including anthocyanins, isoflavones and catechins, which may exhibit antioxidant properties (33). It has also been previously suggested that lotus seed embryos possess antiaging properties (34). Therefore, exploring the therapeutic potential of the lotus seed embryo-derived compound neferine in UV-induced photoaging may be beneficial. The present study demonstrated the anti-photoaging effects of neferine on the skin of mice that were exposed to UVR.
All cells have an antioxidant defence system. SOD, GPx and catalase are naturally occurring antioxidants that serve a crucial role in oxidative balance. SOD converts superoxide anions into the substantially less toxic H2O2 and O2. Its activity is an indirect measure of ROS scavenging, which is essential for the dynamic balance of ROS in the body. Glutathione (GSH) is the primary intracellular non-enzymatic antioxidant defense against ROS and serves a central role in the reduction of oxidative stress. GPx uses GSH as a hydrogen donor to reduce H2O2 and hydroperoxides to H2O and O2 (35). The imbalance between antioxidants and free radicals, which is either caused by a decrease in antioxidants or an increase in ROS, leads to cell damage and cell death (36). A study has previously demonstrated the photo-protective effect of neferine in UVB-irradiated human epidermal keratinocytes, in which neferine application increased SOD and GPx activities, and reduced ROS-induced oxidative stress (37). In the present study, it is indicated that the topical application of neferine significantly increased SOD and GPx activities in mice skin. However, a comparison between known antioxidants and neferine was not performed in the present study, as the focus was on neferine antioxidant activity. The antioxidant activity of neferine may be associated with the presence of the hydroxyl group in its structure. The photoprotective and anti-photoaging effects of neferine were demonstrated to be associated with an increase in antioxidant levels, which was associated with a reduction in UV-induced oxidative stress and skin photoaging. However, the effects of neferine and vehicle treatment alone were not evaluated on non-irradiated mouse skin.
Photoaging is associated with an increase in epidermal thickness and alterations in connective tissue organization (38,39). In the present study, the topical application of neferine reduced these effects, and was revealed to minimize skin photo damage and the presence of wrinkles.
Atrophy of the dermal connective tissue, particularly collagen fibers, is also associated with skin photoaging. It has been demonstrated that collagen is mainly responsible for maintaining the tensile strength and resistance of the skin, and comprises up to 75% of the dry weight of the dermis (29,40). Collagen is one of the major components of the extracellular matrix and can be directly degraded by UVR (41), which is an important early event in the progression of wrinkle formation and skin sagging (42). A number of studies have investigated the role of matrix metalloproteinases (MMPs) in skin aging. UV exposure has been demonstrated to induce MMP-1 expression in the mouse epidermis (39). Neferine has also been revealed to reduce the expression of MMP-1 in UV-A-irradiated human dermal fibroblasts (34). In the present study, collagen density was indicated to be significantly decreased after 12 weeks of UV irradiation. Observations from the present study are consistent with the previous study, in which ICR mice irradiated with UV light exhibited reduced collagen content and skin photoaging (42). In addition, neferine application to mice skin reduced UV-induced collagen density loss in the present study, and it is hypothesized that this may be associated with a reduction of MMP-1 expression. The mechanisms underlying the anti-photoaging effects of neferine on UV irradiation are associated with the inhibition of collagen degradation and increased collagen synthesis, thereby causing the reduction of wrinkles and skin sagging.
In conclusion, to the best of our knowledge, the current study is the first to investigate the anti-photoaging properties of neferine in vivo. Neferine application reduced oxidative stress by restoring antioxidant levels, reducing collagen degradation and the formation of skin wrinkles. However, further studies are required to evaluate its anti-inflammatory properties and underlying molecular pathways.
Acknowledgements
The authors would like to thank Dr Quanbao Wang, Department Histopathology, First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China), for his expert assistance in producing histological slides and Professor Mingxia Chen, Department of Electron Microscopy, Xi'an Jiaotong University (Xi'an, China), for scanning and transmission electron microscopy.
Funding
The present study was supported by grants from the Shaanxi Provincial Natural Science Foundation research project (grant no. 2014JQ4151) and the Science and Technology Commission of Shaanxi Province (grant no. 2018SF-160).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
AbK designed the study, performed experiments and wrote the manuscript. AmK, HB and ZB contributed to designing the study and revising the manuscript critically for important intellectual content. The final version of the manuscript has been read and approved by all authors.
Ethics approval and consent to participate
The study was approved by the Committee of International Association for the Protection of Animal and Experimental Medicine and Laboratory Animal Ethics Committee of Xian Jiaotong University, School of Medicine (Xian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Poornima P, Weng CF, Padma VV. Neferine, an alkaloid from lotus seed embryo, inhibits human lung cancer cell growth by MAPK activation and cell cycle arrest. Biofactors. 2014;40:121–131. doi: 10.1002/biof.1115. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee PK, Das J, Saha K, Giri SN, Pal M, Saha BP. Antipyretic activity of Nelumbo nucifera rhizome extract. Indian J Exp Biol. 1996;34:275–276. [PubMed] [Google Scholar]
- 3.Mukherjee PK, Saha K, Balasubramanian R, Pal M, Saha BP. Studies on psychopharmacological effects of Nelumbo nucifera gaertn. rhizome extract. J Ethnopharmacol. 1996;54:63–67. doi: 10.1016/s0378-8741(96)01455-9. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee PK, Saha K, Das J, Pal M, Saha BP. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med. 1997;63:367–369. doi: 10.1055/s-2006-957705. [DOI] [PubMed] [Google Scholar]
- 5.Kim T, Kim HJ, Cho SK, Kang WY, Baek H, Jeon HY, Kim B, Kim D. Nelumbo nucifera extracts as whitening and anti-wrinkle cosmetic agent. Korean J Chem Engineering. 2011;28:424–427. [Google Scholar]
- 6.Wu MJ, Wang L, Weng CY, Yen JH. Antioxidant activity of methanol extract of the lotus leaf (Nelumbo nucifera Gertn.) Am J Chin Med. 2003;31:687–698. doi: 10.1142/S0192415X03001429. [DOI] [PubMed] [Google Scholar]
- 7.Jung HA, Kim JE, Chung HY, Choi JS. Antioxidant principles of Nelumbo nucifera stamens. Arch Pharm Res. 2003;26:279–285. doi: 10.1007/bf02976956. [DOI] [PubMed] [Google Scholar]
- 8.Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC. Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine. 2003;10:544–551. doi: 10.1078/094471103322331520. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kang M, Cho C, Chung HS, Kang CW, Parvez S, Bae H. Effects of nelumbinis semen on contractile dysfunction in ischemic and reperfused rat heart. Arch Pharm Res. 2006;29:777–785. doi: 10.1007/bf02974079. [DOI] [PubMed] [Google Scholar]
- 10.Rai S, Wahile A, Mukherjee K, Saha BP, Mukherjee PK. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol. 2006;104:322–327. doi: 10.1016/j.jep.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Wang B, Li XZ, Qi LF, Liang YZ. Preparative separation and purification of liensinine, isoliensinine and neferine from seed embryo of Nelumbo nucifera GAERTN using high-speed counter-current chromatography. J Sep Sci. 2009;32:2476–2481. doi: 10.1002/jssc.200800766. [DOI] [PubMed] [Google Scholar]
- 12.Qian JQ. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol Sin. 2002;23:1086–1092. [PubMed] [Google Scholar]
- 13.Sugimoto Y, Furutani S, Itoh A, Tanahashi T, Nakajima H, Oshiro H, Sun S, Yamada J. Effects of extracts and neferine from the embryo of Nelumbo nucifera seeds on the central nervous system. Phytomedicine. 2008;15:1117–1124. doi: 10.1016/j.phymed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, Cai B, Wang K, Wang S, Zhou S, Yu X, Xu B, Chen L. Neferine enhances insulin sensitivity in insulin resistant rats. J Ethnopharmacol. 2009;124:98–102. doi: 10.1016/j.jep.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Ling ZQ, Xie BJ, Yang EL. Isolation, characterization, and determination of antioxidative activity of oligomeric procyanidins from the seedpod of Nelumbo nucifera Gaertn. J Agric Food Chem. 2005;53:2441–2445. doi: 10.1021/jf040325p. [DOI] [PubMed] [Google Scholar]
- 16.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 17.Kligman LH, Kligman AM. The nature of photoaging: Its prevention and repair. Photo Dermatol. 1986;3:215–227. [PubMed] [Google Scholar]
- 18.de Gruijl FR, van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 19.Tyrrell RM, Keyse SM. New trends in photobiology. The interaction of UVA radiation with cultured cells. J Photochem Photobiol B. 1990;4:349–361. doi: 10.1016/1011-1344(90)85014-n. [DOI] [PubMed] [Google Scholar]
- 20.Kong SZ, Shi XG, Feng XX, Li WJ, Liu WH, Chen ZW, Xie JH, Lai XP, Zhang SX, Zhang XJ, Su ZR. Inhibitory effect of hydroxysafflor yellow a on mouse skin photoaging induced by ultraviolet irradiation. Rejuvenation Res. 2013;16:404–413. doi: 10.1089/rej.2013.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharffetter-Kochanek K, Brenneisen P, Wenk J, Herrmann G, Ma W, Kuhr L, Meewes C, Wlaschek M. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol. 2000;35:307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 22.Jurkiewicz BA, Bissett DL, Buettner GR. Effect of topically applied tocopherol on ultraviolet radiation-mediated free radical damage in skin. J Invest Dermatol. 1995;104:484–488. doi: 10.1111/1523-1747.ep12605921. [DOI] [PubMed] [Google Scholar]
- 23.Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Niu X, Xiao S, Ma H. Retinoic acid ameliorates photoaged skin through RARmediated pathway in mice. Mol Med Rep. 2017;16:6240–6247. doi: 10.3892/mmr.2017.7336. [DOI] [PubMed] [Google Scholar]
- 25.Bissett DL, Hannon DP, Orr TV. An animal model of solar-aged skin: Histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem Photobiol. 1987;46:367–378. doi: 10.1111/j.1751-1097.1987.tb04783.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Wang L, Ma R, Mu Q, Yu N, Zhang Y, Tang Y, Li Y, Jiang G, Zhao D, et al. JiangTang XiaoKe granule attenuates cathepsin K expression and improves IGF-1 expression in the bone of high fat diet induced KK-Ay diabetic mice. Life Sci. 2016;148:24–30. doi: 10.1016/j.lfs.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 27.Gaspar LR, Maia Campos PM. Rheological behavior and the SPF of sunscreens. Int J Pharm. 2003;250:35–44. doi: 10.1016/s0378-5173(02)00462-3. [DOI] [PubMed] [Google Scholar]
- 28.Ouhtit A, Muller HK, Davis DW, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000;156:201–207. doi: 10.1016/S0002-9440(10)64720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii T, Okuda T, Yasui N, Wakaizumi M, Ikami T, Ikeda K. Effects of amla extract and collagen peptide on UVB-induced photoaging in hairless mice. J Funct Foods. 2013;5:451–459. [Google Scholar]
- 30.Kwon OS, Jung SH, Yang BS. Topical administration of manuka oil prevents UV-B irradiation-induced cutaneous photoaging in mice. Evid Based Complement Alternat Med. 2013;2013(930857) doi: 10.1155/2013/930857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka YT, Tanaka K, Kojima H, Hamada T, Masutani T, Tsuboi M, Akao Y. Cynaropicrin from Cynara scolymus L. suppresses photoaging of skin by inhibiting the transcription activity of nuclear factor-kappa B. Bioorg Med Chem Lett. 2013;23:518–523. doi: 10.1016/j.bmcl.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 32.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 33.Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–161. [Google Scholar]
- 34.Khan A, Bai H, Shu M, Chen M, Khan A, Bai Z. Antioxidative and antiphotoaging activities of neferine upon UV-A irradiation in human dermal fibroblasts. Biosci Rep. 2018;38(BSR20181414) doi: 10.1042/BSR20181414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jo WS, Yang KM, Park HS, Kim GY, Nam BH, Jeong MH, Choi YJ. Effect of microalgal extracts of tetraselmis suecica against UVB-induced photoaging in human skin fibroblasts. Toxicol Res. 2012;28:241–248. doi: 10.5487/TR.2012.28.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sies H, de Groot H. Role of reactive oxygen species in cell toxicity. Toxicol Lett. 1992:64–65. doi: 10.1016/0378-4274(92)90230-h. [DOI] [PubMed] [Google Scholar]
- 37.Khan A, Bai H, Liu E, Chen M, Yu C, Wang R, Khan A, Bai Z. Protective effect of neferine against UV-B-mediated oxidative damage in human epidermal keratinocytes. J Dermatolog Treat. 2018;29:733–741. doi: 10.1080/09546634.2018.1441490. [DOI] [PubMed] [Google Scholar]
- 38.Smith JG Jr, Davidson EA, Sams WM Jr, Clark RD. Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol. 1962;39:347–350. doi: 10.1038/jid.1962.122. [DOI] [PubMed] [Google Scholar]
- 39.Uitto J, Fazio MJ, Olsen DR. Molecular mechanisms of cutaneous aging. Age-associated connective tissue alterations in the dermis. J Am Acad Dermatol. 1989;21:614–622. [PubMed] [Google Scholar]
- 40.Hou H, Li B, Zhao X, Zhuang Y, Ren G, Yan M, Cai Y, Zhang X, Chen L. The effect of pacific cod (Gadus macrocephalus) skin gelatin polypeptides on UV radiation-induced skin photoaging in ICR mice. Food Chem. 2009;115:945–950. [Google Scholar]
- 41.Chiang HM, Lin TJ, Chiu CY, Chang CW, Hsu KC, Fan PC, Wen KC. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem Toxicol. 2011;49:309–318. doi: 10.1016/j.fct.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 42.Fan J, Zhuang Y, Li B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients. 2013;5:223–233. doi: 10.3390/nu5010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.