Significance
Hosts sequester iron as a strategy to limit pathogen acquisition of this essential nutrient in a process termed nutritional immunity. Due to their in vitro antimicrobial activity, iron-binding proteins transferrins are suspected to play a role in iron sequestration. However, little is known about the in vivo role of transferrins. Here, we found that Drosophila melanogaster exhibits infection-induced hypoferremia mediated by Transferrin 1. Due to excessive iron in hemolymph, Transferrin 1 (Tsf1)-deficient flies are hypersusceptible to certain infections. Our study reveals that nutritional immunity is an important, previously unrecognized arm of immune defense in Drosophila. Using fly and bacterial genetics, we show that Tsf1 mediates nutritional immunity by sequestering iron from the pathogens in vivo on the whole-organism level.
Keywords: transferrin, Drosophila, nutritional immunity, iron sequestration, innate immunity
Abstract
Iron sequestration is a recognized innate immune mechanism against invading pathogens mediated by iron-binding proteins called transferrins. Despite many studies on antimicrobial activity of transferrins in vitro, their specific in vivo functions are poorly understood. Here we use Drosophila melanogaster as an in vivo model to investigate the role of transferrins in host defense. We find that systemic infections with a variety of pathogens trigger a hypoferremic response in flies, namely, iron withdrawal from the hemolymph and accumulation in the fat body. Notably, this hypoferremia to infection requires Drosophila nuclear factor κB (NF-κB) immune pathways, Toll and Imd, revealing that these pathways also mediate nutritional immunity in flies. Next, we show that the iron transporter Tsf1 is induced by infections downstream of the Toll and Imd pathways and is necessary for iron relocation from the hemolymph to the fat body. Consistent with elevated iron levels in the hemolymph, Tsf1 mutants exhibited increased susceptibility to Pseudomonas bacteria and Mucorales fungi, which could be rescued by chemical chelation of iron. Furthermore, using siderophore-deficient Pseudomonas aeruginosa, we discover that the siderophore pyoverdine is necessary for pathogenesis in wild-type flies, but it becomes dispensable in Tsf1 mutants due to excessive iron present in the hemolymph of these flies. As such, our study reveals that, similar to mammals, Drosophila uses iron limitation as an immune defense mechanism mediated by conserved iron-transporting proteins transferrins. Our in vivo work, together with accumulating in vitro studies, supports the immune role of insect transferrins against infections via an iron withholding strategy.
Iron plays an indispensable role in numerous physiological processes, such as respiration, the trichloroacetic acid cycle, oxygen transport, gene regulation, and DNA biosynthesis. Owing to its versatile biological utility, iron is an essential element in the biological processes of all living organisms, and is central to metabolic function. As a consequence, iron sequestration by the host is a potent defense against bacterial pathogens, a process termed nutritional immunity (1–7). Early reports dating back to the 1940s documented that intramuscular inoculation of dogs with Staphylococcus aureus leads to a precipitous drop in plasma iron levels, which was named hypoferremia of infection (8). This hypoferremic response is an important facet of the innate immune system aimed at limiting iron availability to invading microbes by withholding iron within the cells and tissues. In line with this, individuals who suffer from iron overload due to mutations affecting iron metabolism have an enhanced risk of infection (9). To sequester iron from pathogens, the host relies on a number of iron-binding proteins, among which members of the transferrin family frequently play a prominent role (1, 10). Transferrins are monomeric glycoproteins that are ubiquitous in metazoans. Mammals have four types of transferrin: serum transferrin, lactoferrin, melanotransferrin, and the inhibitor of carbonic anhydrase (11, 12). Among these, serum transferrin and lactoferrin have been implicated in nutritional immunity via iron sequestration from invading pathogens (5, 12). Serum transferrin is abundant in the blood of mammals and primarily functions as an iron transporter by shuttling the iron from the gut to peripheral sites of storage and use (13). Lactoferrin is found on mucosal surfaces, and in biological fluids including milk and saliva, indicating that it is part of the innate immune response; however, there is no functional in vivo data supporting this role (14–16). Due to their high affinity to iron, transferrins have been shown to inhibit the growth of certain microbes (17). While numerous studies reported the potent antimicrobial activity of purified transferrins in vitro, in vivo studies addressing transferrin function are rather limited (10, 15, 18–24). Although hypotransferrinemic (hpx) mice devoid of serum transferrin exist, how they respond to microbial infection has yet to be examined (25). Hence, the in vivo role of transferrins awaits further investigation.
Due to its genetic tractability, Drosophila melanogaster has been a model of choice to study innate host defense mechanisms (26). The systemic antimicrobial response is probably the best-characterized immune mechanism in Drosophila. It involves the fat body, and, to a lesser extent, hemocytes, producing antimicrobial peptides that are secreted into the hemolymph. This response is regulated at the transcriptional level by two nuclear factor κB (NF-κB) pathways, Toll and Imd, whose inactivation causes a high susceptibility to infection (26–29). However, whether nutritional immunity via iron sequestration constitutes a part of the insect defense response has not been studied. There are three transferrin homologs in Drosophila: Tsf1, Tsf2, and Tsf3. Tsf2 is a component of epithelial septate junctions (30) and is unlikely to play an antimicrobial role, whereas Tsf3 has not been functionally characterized yet but might play a role in circadian rhythms (31). Tsf1 was recently shown to function as an iron transporter in the hemolymph (the insect blood) similar to mammalian serum transferrin (32). Specifically, fat body-derived Tsf1 is secreted into the hemolymph and transports iron from the gut and hemolymph to the fat body. The Tsf1 gene is induced upon infections, pointing to its role in host defense (33–35). Proteomic analysis of hemolymph also revealed Tsf1 up-regulation after infection with the fungus Beauveria bassiana (36). Transferrin genes have been shown to be up-regulated in response to infection in other insect species, including representatives from Diptera, Coleoptera, Hemiptera, Hymenoptera, and Lepidoptera (21, 22, 37–39). Also, the promoter region of Tsf1 genes from several insects is enriched in putative NF-κB binding sites, supporting the immune role of Tsf1 in these animals (37). Indeed, purified iron-free transferrins from Sarcophaga bullata, Bombyx mori, and Manduca sexta were shown to have antibacterial activity in vitro, which was dependent on the transferrin’s ability to sequester iron (21, 22, 40).
However, the in vivo role of insect transferrins in host defense at the organismal level has never been addressed. In this study, we used D. melanogaster as a genetically tractable model to investigate the role of iron and Tsf1 in insect host defense.
Results
D. melanogaster Exhibits Infection-Induced Hypoferremia.
Infections in mammals induce a transient depletion of plasma iron (8), motivating us to investigate whether infection-induced hypoferremia also happens in Drosophila. To this end, we infected flies by pricking with a range of pathogens, including the Gram-positive bacterium Micrococcus luteus, the Gram-negative bacteria Pectobacterium carotovorum (Ecc15) and Pseudomonas entomophila, and the yeast Candida albicans. We measured iron content in the extracted hemolymph from unchallenged and infected flies using inductively coupled plasma optical emission spectrometry (ICP-OES). Compared to uninfected flies, there was a significant decrease in hemolymph iron levels in all tested infections (Fig. 1A). Importantly, pricking with heat-killed bacteria triggered the same drop in hemolymph iron level as infection with live bacteria (Fig. 1B). This suggests that iron withdrawal from hemolymph is a host-mediated process, and does not result from bacterial consumption. To track where hemolymphatic iron might be redistributed to upon infection, we monitored iron levels in other major tissues. The decrease of hemolymph iron after M. luteus infection was correlated with a concomitant increase in iron level in the fat body, while other tissues were not affected (Fig. 1C). This result suggests that iron was relocated from the hemolymph to the fat body after infection.
Fig. 1.
D. melanogaster exhibits infection-induced hypoferremia. (A and B) Iron content of flies’ hemolymph after indicated infections. Asterisks indicate statistical significance relative to unchallenged (UC) (one-way ANOVA) (n = 50 flies per treatment). (C) Iron content in indicated tissues of wild-type (WT) flies 24 h after M. luteus infection compared to uninfected controls (n = 20 organs per group, n = 50 flies for hemolymph). (D and E) Hemolymph iron content (D) in Toll pathway mutants (spzrm7, GNBP1osi, PGRP-SASeml, and ModSP1) 24 h after M. luteus infection and (E) in Imd pathway mutants (PGRP-SDsk1, PGRP-LCE12, and RelishE20) 24 h after heat-killed Ecc15 injection (n = 50 flies per group). For all graphs, iron content in uninfected wild-type flies was set to 100, and all other values were expressed as a percentage of this value. The mean and SD of three independent experiments are shown. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, nonsignificant, P > 0.05.
Infection-Mediated Hemolymph Iron Depletion Requires the Toll and Imd Pathways.
We next explored whether the Toll and Imd immune pathways contribute to the depletion of hemolymphatic iron upon infection. We found that, in contrast to wild-type flies, Toll pathway-deficient mutants, including spzrm7, GNBP1osi, PGRP-SASeml, and ModSP1 flies, were unable to remove iron from the hemolymph after M. luteus infection, a challenge known to predominantly activate the Toll pathway (27, 33). In fact, iron amount after infection stayed at the same level as in uninfected flies (Fig. 1D). Similarly, Imd pathway-deficient mutants PGRP-SDsk1, PGRP-LCE12, and RelishE20 were impaired in iron removal from the hemolymph after pricking with Ecc15 heat-killed bacteria (Fig. 1E), which potently activates Imd pathway but does not kill mutant flies (41). Interestingly, in PGRP-SDsk1 mutant, we observed significant decrease in hemolymph iron, but not as strong as in wild-type flies. This result is explained by the fact that PGRP-SDsk1 mutants have only partial reduction in Imd pathway activity, and therefore they have partial hypoferremic response (41, 42). Thus, the Imd and Toll pathways appear to be required for iron withdrawal from hemolymph after infections that activate these pathways.
Transferrin 1 Is Required for Iron Relocation from Hemolymph to Fat Body after Infection.
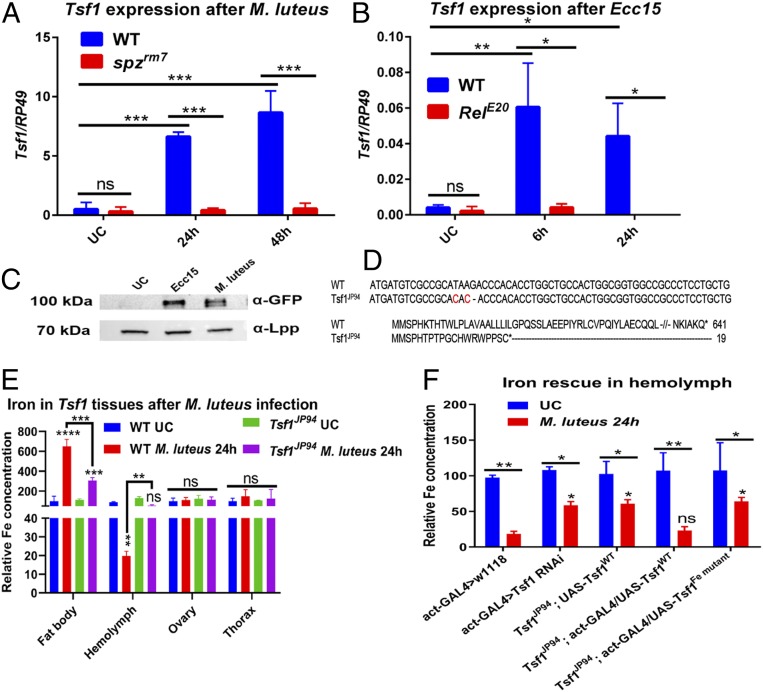
The fact that the Toll and Imd pathways are necessary for iron removal from hemolymph after infection suggests that potential immune effectors downstream of these pathways can transport iron from hemolymph to fat body. A good candidate was the iron transporter transferrin 1 (Tsf1), as transcriptomic studies have shown that this gene is induced upon infection (33, 34). Using RT-qPCR, we showed that this gene is strongly induced by M. luteus in a Toll pathway-dependent manner, and by Ecc15 in an Imd pathway-dependent manner (Fig. 2 A and B). Importantly, Tsf1 up-regulation upon infection was tissue-specific and was restricted to the fat body (SI Appendix, Fig. S1A). Also, Tsf1 is the only infection-responsive transferrin in Drosophila, since none of the other two transferrins was induced by M. luteus or Ecc15 (SI Appendix, Fig. S1 B and C). Using an endogenously GFP-tagged Tsf1 transgenic line, we additionally confirmed that Tsf1 protein abundance is strongly increased in the hemolymph after both M. luteus and Ecc15 infections (Fig. 2C). To further explore the role of Tsf1 in infection-induced iron transport, we generated a Tsf1 mutant (Tsf1JP94) using CRISPR-Cas9. The mutant has two nucleotide substitutions and a single nucleotide deletion, which leads to a frameshift with a premature stop codon at position 19 (Fig. 2D). Using qPCR, we showed that there was no Tsf1 transcript in the Tsf1JP94 mutant in contrast to wild-type flies after M. luteus infection (SI Appendix, Fig. S1D). Tsf1JP94 mutants were viable and did not show any obvious morphological defects under standard laboratory conditions. Also, both Toll and Imd pathways were induced properly in this mutant, as illustrated by the level of Drs and Dpt expression after M. luteus and Ecc15 infections, respectively (SI Appendix, Fig. S1 E and F). Next, we compared iron distribution between wild-type and Tsf1JP94 mutant tissues after M. luteus infection. There was no difference in iron content between uninfected wild-type and Tsf1JP94 mutant in the hemolymph and fly tissues. Strikingly, after M. luteus infection, Tsf1JP94 mutant contained significantly more iron in the hemolymph and significantly less in the fat body compared to wild-type flies (Fig. 2E). Overexpression of a wild-type copy of Tsf1 in the Tsf1JP94 mutant background rescued the phenotype (Fig. 2F). This result suggests that Tsf1 contributes to the iron relocalization from hemolymph to fat body after infection. To confirm this result and identify the source of Tsf1, we performed tissue-specific RNA interference (RNAi)-mediated Tsf1 knockdown. Similar to Tsf1JP94, flies with ubiquitous Tsf1 knockdown retain high iron load in the hemolymph after infection (Fig. 2F). Fat body-specific, but not gut- or hemocyte-specific, Tsf1 knockdown recapitulated this phenotype, indicating that the fat body is the major source of Tsf1 (SI Appendix, Fig. S1G). This result is consistent with a recent study (32) that showed Tsf1 is produced by the fat body and is secreted into the hemolymph (Fig. 2C), where it binds to iron and transports it to the fat body. DmTsf1 is homologous to human plasma Transferrin that has been functionally and structurally well characterized. Structure−function analysis has shown that five amino acid residues of hTsf are required for iron binding (11, 12). Sequence homology analysis showed that three out of these five residues are conserved in Drosophila Tsf1 (SI Appendix, Fig. S2). We substituted these three residues with alanine and generated a fly line that overexpresses this mutated form of Tsf1 (UAS-Tsf1Fe mut) that should not bind iron. Overexpression of this mutated version of Tsf1 did not rescue the Tsf1JP94 mutant (Fig. 2F). This reinforces our conclusion that the ability of Tsf1 to bind iron is necessary to relocate the metal from hemolymph to fat body.
Fig. 2.
Contribution of Tsf1 to infection-induced hypoferremia. (A and B) Tsf1 expression (A) in wild type and spzrm7 mutants after M. luteus infection and (B) in wild type and RelE20 mutants after Ecc15 infection, measured by RT-qPCR (n = 10 flies per group). (C) Western blot of Tsf1-GFP hemolymph extracted 24 h after M. luteus or Ecc15 infection showing Tsf1 induction after these infections. Lipophorin (α-Lpp) was used as a loading control. A representative Western blot out of three independent experiments is shown (n = 30 flies per group). (D) Nucleotide and amino acid sequence alignment of wild-type and Tsf1JP94 transferrin. (E) Iron content in indicated tissues of wild-type and Tsf1JP94 flies 24 h after M. luteus infection compared to uninfected controls (n = 20 organs per group, n = 50 flies for hemolymph). Asterisks above the red bars indicate significance relative to wild-type UC. (F) Hemolymph iron content of indicated fly genotypes 24 h after M. luteus infection compared to uninfected controls (n = 50 flies per group). Asterisks above bars indicate significance relative to act-GAL4 > w1118 M. luteus-infected. Iron content in uninfected wild-type or act-GAL4 > w1118 flies was set to 100, and all other values were expressed as a percentage of this value. The mean and SD of three independent experiments are shown unless otherwise stated. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, nonsignificant, P > 0.05.
Transferrin 1 Mutants Are Susceptible to Pseudomonas and Mucorales Fungal Infections.
Having shown that Tsf1 mediates the transport of iron upon infection, we investigated the relevance of this immune process in host survival to various pathogens. As shown in SI Appendix, Fig. S3, Tsf1JP94 mutants exhibited wild-type levels of survival after systemic infection with Ecc15, Enterobacter cloacae, Listeria monocytogenes, Streptococcus pyogenes, S. aureus, Enterococcus faecalis, C. albicans, and B. bassiana (natural infection). We next explored Tsf1JP94 mutants’ susceptibility to fungi of the order Mucorales and Pseudomonas bacteria, the virulence of which is known to be strongly modulated by iron availability (43–45). Interestingly, we observed an increased susceptibility of Tsf1JP94 mutant and Tsf1 RNAi flies to systemic infection with Cunninghamella bertholletiae, a representative Mucorales that infects humans (Fig. 3 A and B). Notably, Toll pathway activation by C. bertholletiae was not affected in the Tsf1JP94 mutant (Fig. 3C). Overexpression of wild-type Tsf1, but not the iron binding sites mutated form of Tsf1, rescued the increased susceptibility of Tsf1JP94 mutants to this fungus (Fig. 3D). We could almost completely rescue the susceptibility of Tsf1JP94 mutants to C. bertholletiae by injection of the iron chelator bathophenanthrolinedisulfonic acid disodium (BPS) (46), suggesting that excessive iron in the hemolymph of Tsf1JP94 mutants contributes to their increased susceptibility to C. bertholletiae (Fig. 3E). BPS injection also has a protective effect in wild-type flies, although not as significant as in Tsf1JP94 mutants. We obtained similar increased sensitivity of Tsf1JP94 mutants to another Mucorales representative, Rhizopus oryzae (Fig. 3F), suggesting that transferrins are important for the defense against this group of fungi.
Fig. 3.
Tsf1 is required for the defense against Mucorales. (A) Survival rates of wild-type, spzrm7, and Tsf1JP94 flies infected with C. bertholletiae (106 spores per ml). (B) Survival rates of flies with ubiquitous knockdown of Tsf1 is significantly reduced compared to wild-type flies after infection with C. bertholletiae. (C) Drs expression in wild-type, spzrm7, and Tsf1JP94 flies after C. bertholletiae infection measured by RT-qPCR (n = 10 flies per group). The mean and SD of three independent experiments are shown. (D) Increased susceptibility of Tsf1JP94 mutant flies to C. bertholletiae infection is rescued by the ubiquitous overexpression of the wild-type (UAS-Tsf1WT) but not mutated form of Tsf1 (UAS-Tsf1Fe mutant). (E) Survival rates of wild-type and Tsf1JP94 flies preinjected with 13.4 nL of H2O (control) or with 13.4 nL of 200 µM iron chelator BPS prior to infection with C. bertholletiae. (F) Survival rates of wild-type, spzrm7, and Tsf1JP94 flies infected with R. oryzae (106 spores per mL). Survival graphs show one representative experiment out of three independent experiments with similar results with two or three cohorts of 20 male flies per treatment. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, nonsignificant, P > 0.05.
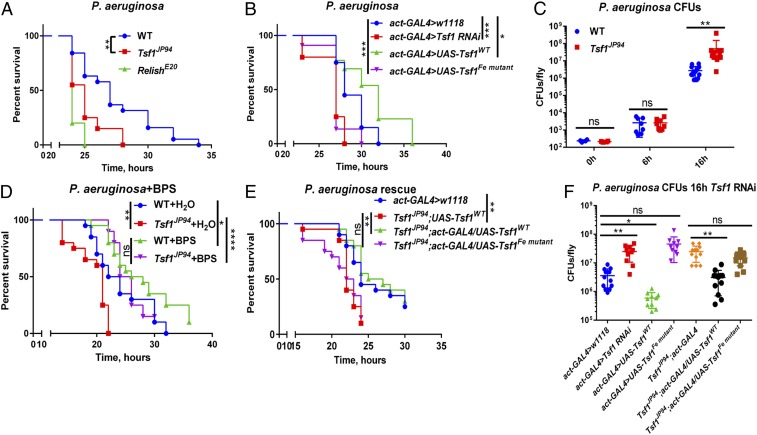
We also observed an increased susceptibility of Tsf1JP94 and Tsf1 RNAi flies to systemic infections with two Pseudomonas species, Pseudomonas aeruginosa (Fig. 4 A and B) and P. entomophila (SI Appendix, Fig. S4 A and B). Consistent with the impaired resistance of Tsf1JP94 flies, P. aeruginosa (Fig. 4C) and P. entomophila (SI Appendix, Fig. S4C) reached significantly higher loads in Tsf1JP94 mutants. As Tsf1JP94 mutants showed wild-type levels of Imd pathway activation after P. entomophila and P. aeruginosa infections (SI Appendix, Fig. S4D), the increased susceptibility of these mutants was not due a general immune deficiency but rather due to their inability to sequester iron away from the hemolymph. Consistent with this, injection of the iron chelator BPS into the hemolymph significantly improves survival of Tsf1JP94 mutants upon P. aeruginosa infection (Fig. 4D). Ubiquitous overexpression of wild-type but not the mutated Tsf1 form was sufficient to rescue the enhanced susceptibility of Tsf1JP94 mutants to P. aeruginosa (Fig. 4E) and P. entomophila (SI Appendix, Fig. S4E). Similarly, P. aeruginosa elevated load in Tsf1JP94 mutants was not observed when wild type but not the mutated Tsf1 form was ubiquitously overexpressed (Fig. 4F). Interestingly, overexpression of wild type but not the mutated Tsf1 form in wild-type background led to a significant reduction in P. aeruginosa load (Fig. 4F), which correlated with improved survival of the flies (Fig. 4B). This protective effect of Tsf1 overexpression is comparable to the effect of BPS injection (Fig. 4D), indicating that Tsf1 may function as endogenous iron chelator.
Fig. 4.
Tsf1-mediated iron sequestration protects against P. aeruginosa infection. (A) Survival rates of wild-type, RelE20, and Tsf1JP94 flies infected with P. aeruginosa. (B) Survival rates of flies with ubiquitous knockdown of Tsf1, and overexpression of either wild-type (UAS-Tsf1WT) or mutated Tsf1 after P. aeruginosa infection. (C) Measurement of P. aeruginosa burden at different time points after infection of wild type and Tsf1JP94 mutant. (D) Survival rates of wild-type and Tsf1JP94 flies preinjected with 13.4 nL of H2O (control) or with 13.4 nL of 200 µM iron chelator BPS prior to infection with P. aeruginosa. (E) Increased susceptibility of Tsf1JP94 mutant flies to P. aeruginosa infection is rescued upon ubiquitous overexpression of wild-type (UAS-Tsf1WT) but not mutated form of Tsf1 (UAS-Tsf1Fe mutant). (F) Pseudomonas aeruginosa load 16 h after infection of flies with indicated genotypes. For cfu counts, each dot represents cfus from a pool of five animals, calculated per fly. The mean and SD are shown. Survival graphs show one representative experiment out of three independent experiments with similar results with two or three cohorts of 20 male flies per treatment. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, nonsignificant, P > 0.05.
Pyoverdine-Mediated Iron Acquisition Is Essential for P. aeruginosa Infection in Drosophila.
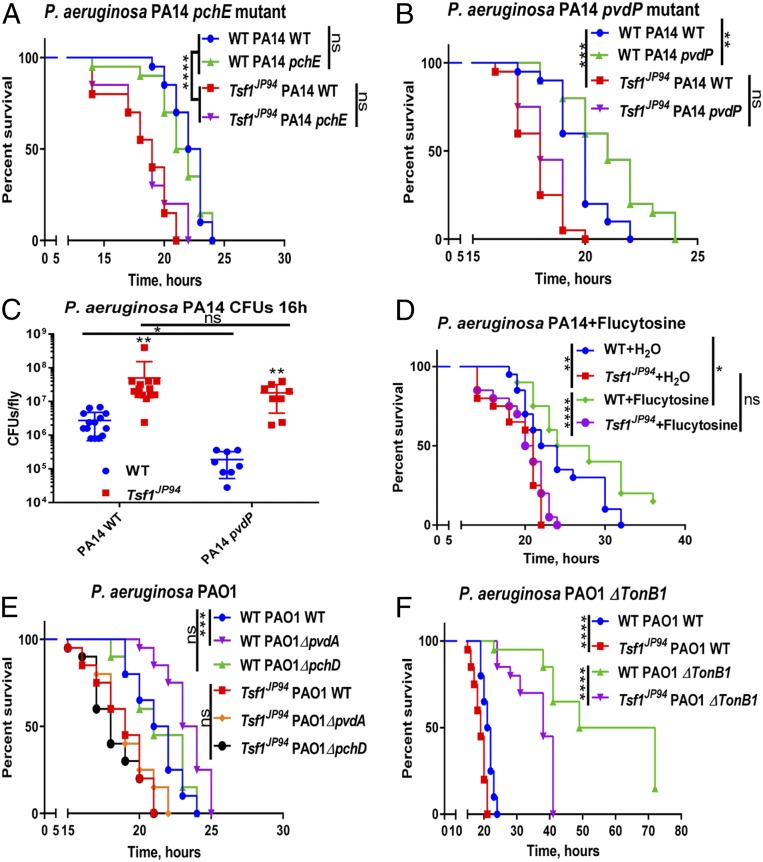
Siderophore production by pathogens is a key mechanism for scavenging iron from a variety of host iron sources. Siderophores are small ferric iron chelators capable of binding iron with high affinity, and can therefore effectively outcompete host transferrin (1). P. aeruginosa produces large amounts of pyoverdine and pyochelin siderophores that scavenge iron and deliver it to the bacteria (47). We next compared the susceptibility of wild-type and Tsf1JP94 mutant flies to wild-type P. aeruginosa PA14 and to its transposon insertion derivatives pchE and pvdP that lack the siderophores pyochelin and pyoverdine, respectively (48). As shown in Fig. 5A, the pchE mutant was as efficient as wild-type P. aeruginosa at killing both wild-type and Tsf1JP94 mutant flies, indicating that pyochelin is not required for P. aeruginosa virulence in Drosophila. Interestingly, the virulence of pvdP mutant was attenuated compared to wild-type P. aeruginosa in wild-type flies, indicating that pyoverdine contributes to Pseudomonas pathogenicity. In contrast, the pathogenicity of pvdP was similar to that of wild-type P. aeruginosa when assayed in Tsf1JP94 mutant background (Fig. 4B). In line with this, pvdP colony-forming unit (cfus) were the same as for wild-type P. aeruginosa in Tsf1JP94 mutant but were significantly lower in wild-type flies (Fig. 5C). To further reinforce the role of pyoverdine, we assessed the survival of flies preinjected prior to infection with flucytosine, a known repressor of pyoverdine (49). We observed that flucytosine was protective in wild-type flies but had no effect in Tsf1JP94 mutant (Fig. 5D), which is similar to what we found with the genetic disruption of pyoverdine (Fig. 5B). Using another P. aeruginosa strain (PAO1) and its derived pyochelin and pyoverdine mutants (50), we confirmed that pyoverdine is essential for virulence in wild-type flies but not in Tsf1JP94 mutant (Fig. 5E). This result suggests that 1) pyoverdine is necessary for P. aeruginosa to acquire iron from wild-type Drosophila, and 2) pyoverdine becomes dispensable in Tsf1JP94 mutant due to excessive iron present in the hemolymph. Additionally, we assessed the virulence of P. aeruginosa PAO1 tonB1 mutants that are defective for siderophore-mediated iron uptake (51). These mutants were severely attenuated in both wild-type and Tsf1JP94 mutant flies. Nevertheless, they still killed Tsf1JP94 mutants faster than wild-type flies, likely due to high iron levels in the hemolymph of Tsf1JP94 mutants (Fig. 5F). Thus, using bacterial and fly genetics, we could show that Tsf1 is required for the Drosophila defense against certain pathogens, by sequestering iron from hemolymph and limiting pathogen access to this essential element.
Fig. 5.
Pyoverdine is required for P. aeruginosa virulence against wild-type but not Tsf1JP94 flies. (A and B) Survival rates of wild-type and Tsf1JP94 mutant flies infected with (A) wild-type, pyochelin-deficient pchE or (B) pyoverdine-deficient pvdP P. aeruginosa PA14. (C) Measurement of wild-type and pvdP PA14 load 16 h after infection of wild-type and Tsf1JP94 flies. Each dot represents cfus from a pool of five animals, calculated per fly. The mean and SD are shown. (D) Survival rates of wild-type and Tsf1JP94 flies preinjected with 13.4 nL of H2O (control) or with 13.4 nL of 100 µM repressor of pyoverdine flucytosine prior to infection with P. aeruginosa. (E and F) Survival rates of wild-type and Tsf1JP94 flies infected with wild-type, (E) ΔpchD, ΔpvdA, and (F) ΔTonB1 P. aeruginosa PAO1. Survival graphs show one representative experiment out of three independent experiments with similar results with two or three cohorts of 20 male flies per treatment. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, nonsignificant, P > 0.05.
Transferrin 1 Plays a Role in Intestinal Immunity.
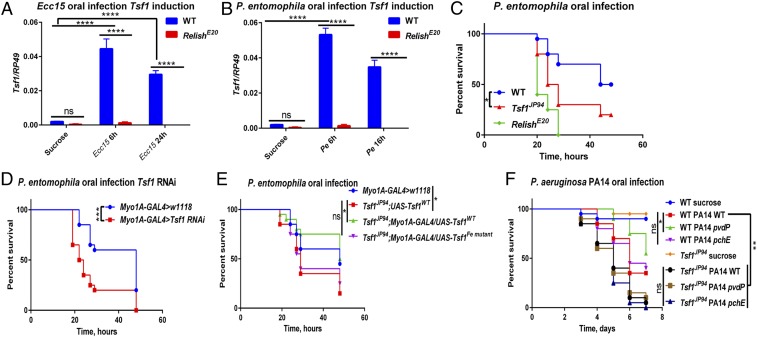
Considering that Tsf1 is induced by Ecc15 and P. entomophila oral infections in the gut in an Imd pathway-dependent manner (Fig. 6 A and B) (52), we explored whether this iron transporter is also implicated in intestinal immunity. As shown in Fig. 6C, Tsf1JP94 mutants succumbed faster to P. entomophila oral infection compared to wild-type flies. Enterocyte-specific Tsf1 knockdown by RNAi also resulted in increased sensitivity to P. entomophila oral infection (Fig. 6D). This increased susceptibility was not due to an impaired Imd pathway activity (SI Appendix, Fig. S5A) or compromised gut repair, as PH3 staining revealed that Tsf1JP94 mutants had the same number of proliferating stem cells after Ecc15 infection as wild-type flies (SI Appendix, Fig. S5B). In line with this, upd3, a ligand of the JAK-STAT pathway playing a crucial role in epithelial renewal (52), was expressed in Tsf1JP94 mutants at wild-type levels after both Ecc15 and P. entomophila infections (SI Appendix, Fig. S5C). Importantly, we found that the susceptibility of Tsf1JP94 mutants to P. entomophila oral infection could be rescued by gut-specific overexpression of wild-type but not iron binding-defective Tsf1 (Fig. 6E), indicating that the ability of Tsf1 to bind iron is necessary for defense against P. entomophila intestinal infection, similar to its effect in systemic infection. Therefore, Tsf1 plays a similar role in the gut as in the hemolymph. To further reinforce this conclusion, we performed oral infection with P. aeruginosa PA14 and observed that Tsf1JP94 mutants were also more susceptible to this pathogen (Fig. 6F). Consistent with systemic infections, pyoverdine-deficient pvdP mutant virulence was attenuated in wild-type but not in Tsf1JP94 mutant flies, while pyochelin-deficient mutant virulence was comparable to wild-type P. aeruginosa (Fig. 6F). The fact that pyoverdine is unnecessary for virulence in Tsf1JP94 mutant suggests that there is enough available iron in the guts of these flies. Indeed, we could detect significantly more iron in Tsf1JP94 mutant guts compared to wild-type unchallenged guts (SI Appendix, Fig. S5D). We conclude that iron sequestration by transferrin is also a potent mechanism contributing to intestinal immunity.
Fig. 6.
Tsf1 contributes to intestinal immunity. (A and B) Tsf1 expression in wild-type and RelE20 guts after (A) Ecc15 and (B) Pe infection, measured by RT-qPCR (n = 20 guts per group). The mean and SD of three independent experiments are shown. (C) Survival rates of wild-type, RelE20, and Tsf1JP94 flies orally infected with P. entomophila. (D) Survival rates of flies with gut-specific (Myo1A) knockdown of Tsf1 are significantly reduced compared to wild-type flies after Pe oral infection. (E) Increased susceptibility of Tsf1JP94 mutant flies to Pe oral infection is rescued by gut-specific overexpression of the wild-type (UAS-Tsf1WT) but not mutated form of Tsf1 (UAS-Tsf1Fe mutant). (F) Survival rates of wild-type and Tsf1JP94 flies orally infected with wild-type, pchE, and pvdP P. aeruginosa PA14. Survival graphs show one representative experiment out of three independent experiments with similar results with two to three cohorts of 20 flies per treatment. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, nonsignificant, P > 0.05.
Discussion
Despite the well-established role of iron and iron-binding proteins in mammalian immunity, their role in insect immunity remains understudied. Using D. melanogaster as a model, we found that 1) flies trigger a hypoferremic response after infection to limit iron availability to invading microbes, and 2) the iron transporter Tsf1 mediates nutritional immunity by sequestering iron from invading pathogens (see model in SI Appendix, Fig. S6).
In mammals, hypoferremia of infection has been known since the 1940s and is characterized by iron withdrawal from the serum and accumulation in storage organs, like the liver (8). Consistent with mammalian studies, we discovered that flies trigger a hypoferremic response upon challenge with a variety of pathogens. During this response, iron was relocated from the hemolymph to the fat body, which is the equivalent of mammalian liver. Given that the same response was also triggered by heat-killed bacteria, depleting hemolymph iron appears to be a host-mediated process. Notably, this mechanism requires the Drosophila Toll and Imd pathways, since mutants for these pathways were not able to induce a hypoferremic response to infection. Thus, beyond regulating antimicrobial effector-mediated immunity, the Toll and Imd pathways also mediate nutritional immunity in flies.
We hypothesized that Tsf1 might play a major role in Drosophila nutritional immunity downstream of Toll and Imd pathways. Out of the three Drosophila transferrins, Tsf1 is consistently induced by a variety of immune challenges (33, 34, 36). Using a Transferrin null mutant, we indeed found that Tsf1 is required for iron trafficking from the hemolymph to the fat body after infection. Therefore, our study agrees with a recently published work that Tsf1 is indeed an iron transporter (32). However, in contrast to Xiao et al. (32), who used a Tsf1 RNAi, we did not observe any lethality or developmental defects in Tsf1JP94 mutants and Tsf1 RNAi. This discrepancy could be due to the fact that we used different RNAi lines targeting different parts of the transcript or because, in contrast to Xiao et al., we used conditional knockdown specifically during adult stage.
Our study raises an intriguing question regarding how Tsf1 relocates iron specifically to the fat body and not to other tissues. One possibility could be that fat body expresses transferrin receptor that directs iron transport by Tsf1. To date, no transferrin receptor homolog has been identified in Drosophila (and other insects). Finding this receptor and mechanism of iron uptake by the fat body during infection would be an interesting future research avenue.
Despite the elevated level of iron in the hemolymph, Tsf1JP94 mutants did not show any increased susceptibility to the majority of pathogens that we tested, including several Gram-positive and Gram-negative bacteria, fungi, and yeast. However, we observed increased susceptibility of Tsf1JP94 mutants to Mucorales fungi and to Pseudomonas bacteria. This increased susceptibility was linked to the ability of Tsf1 to bind iron, as we could not rescue Tsf1JP94 mutants’ susceptibility with a form of Tsf1 mutated in iron binding sites. Given that Mucorales virulence is known to be enhanced by increased iron supply (43), it is reasonable to assume that Tsf1JP94 flies are more susceptible to these infections because of elevated hemolymph iron levels. P. aeruginosa virulence is also known to be strongly regulated by iron. The importance of iron to P. aeruginosa is exemplified by the fact that 6% of its transcribed genes are iron responsive (44, 45). Not surprisingly, these bacteria evolved a diversity of mechanisms to scavenge iron from a variety of host iron sources. Siderophore production is one such mechanism. Pyoverdine and pyochelin are two major siderophores produced by P. aeruginosa (47), and pyoverdine is essential for P. aeruginosa pathogenesis in various mammalian and invertebrate host models (48, 53–55). A recent study showed that P. aeruginosa mutants for the algR regulator are deficient for pyoverdine production, and virulence is attenuated in the algR mutant in a Drosophila oral infection model (56). In line with this, we showed that the P. aeruginosa pyoverdine mutant is less pathogenic compared to its wild-type counterpart during both systemic and oral infections. Importantly, Tsf1JP94 mutant flies were killed by the P. aeruginosa pyoverdine mutant as efficiently as by wild-type bacteria. This suggests that 1) pyoverdine is necessary for iron acquisition by P. aeruginosa during Drosophila infection and, 2) in the absence of transferrin, pyoverdine becomes unessential, as there is an excess of free iron. The extreme dependence of P. aeruginosa on iron makes these bacteria vulnerable to iron chelation therapy by transferrin, which has been proposed as a novel antimicrobial therapy (24). Efficacy of such therapy is also supported by our results showing that Tsf1 overexpression is sufficient to increase the survival of flies to Pseudomonas infections.
Why Tsf1 flies are not sensitive to the majority of pathogens is an intriguing question that our work raises. A likely explanation for this result is that, beyond iron sequestration, the host relies on other arms of defense, like phagocytosis or production of antimicrobial peptides, to combat pathogens. Those additional arms of defense might be sufficient to eliminate most pathogens at the infectious doses we used, even if iron sequestration is impaired. There is accumulating evidence that some elements of the immune system are specifically required against certain pathogens. For instance, from Drosophila studies, it is known that melanization is important to survive S. aureus infection (57), phagocytosis [S. aureus and Salmonella typhimurium (58, 59)], the antimicrobial peptide Diptericin [Providencia rettgeri (60, 61)], and Drosocin [E. cloacae (61)]. Our study suggests that iron sequestration is an important defense mechanism against Mucorales and Pseudomonas, while, in the case of other pathogens, other arms of defense might play a more prominent role. It will be an interesting avenue for future research to explore functional redundancy between different arms of the host defense against specific pathogens.
Taken together, our results reveal that nutritional immunity is an important arm of innate immune defense in Drosophila. Using fly and bacterial genetics, we showed that the iron transporter Tsf1 mediates nutritional immunity by sequestering iron from the pathogens in vivo on the whole-organism level. So far, two studies have identified immune-related phenotypes resulting from RNAi-mediated knockdown of transferrin: increased prevalence of trypanosome infections in Glossina morsitans, and increased mortality of Bacillus thuringiensis-infected Plutella xylostella (62, 63). Those in vivo and accumulating in vitro studies support the immune role of insect transferrins against infections via an iron withholding strategy. Considering the multifactorial function of iron beyond immunity, our work opens avenues for future research addressing the role of transferrins in the host physiology.
Materials and Methods
Pathogen Strains and Survival Experiments.
The bacterial strains used and their respective optical densities (OD) at 600 nm were, unless otherwise stated, the Gram-negative bacteria P. carotovorum (Ecc15, OD 200), E. cloacae β12 (OD 200), P. entomophila (OD 1), P. aeruginosa PA14 (OD 1), P. aeruginosa PA14 pvdP (OD 1), P. aeruginosa PA14 pchE (OD 1), P. aeruginosa PAO1 (OD 1), P. aeruginosa PAO1 ΔpvdA (OD 1), P. aeruginosa PAO1 ΔpchD (OD 1), and P. aeruginosa PAO1 ΔtonB1 (OD 1); the DAP-type peptidoglycan-containing Gram-positive bacteria L. monocytogenes BUG2377 (, OD 40); the Lys-type peptidoglycan containing Gram-positive bacteria M. luteus (OD 200), S. aureus (OD 0.5), S. pyogenes ATCC19615 (OD 200), and E. faecalis OG1RF (OD 15); and the yeast C. albicans (OD 200). Microbes were cultured in Brain-Heart Infusion Broth (L. monocytogenes and E. faecalis), Yeast extract-Peptone-Glucose Broth (C. albicans), or Luria Broth (all others) at 29 °C (E. carotovora, M. luteus, C. albicans, and P. entomophila) or 37 °C (all others). To compare the virulence of P. aeruginosa wild type and siderophore mutants, bacteria were grown in M9 minimal media at 37 °C to stimulate siderophore production. P. aeruginosa PAO1 ΔtonB1 mutant was grown in media supplemented with 100 µM FeSO4. The pvdP and pchE P. aeruginosa PA14 mutants were grown in the presence of 15 µg/mL gentamicin. Spores of the entomopathogenic fungus B. bassiana 802 and Mucorales C. bertholletiae 506313 and R. oryzae 557969 were grown on malt agar plates at 29 °C for ∼3 wk until sporulation. Natural infections were performed by shaking anesthetized flies in a Petri dish containing a sporulating culture of B. bassiana. Systemic infections (septic injury) were performed by pricking adult flies (2 d to 5 d old) in the thorax with a thin needle previously dipped into a concentrated pellet of a bacterial culture or in a suspension of fungal (C. bertholletiae and R. oryzae) spores. Infected flies were subsequently maintained at 29 °C (most of the infections) or at 25 °C (E. faecalis, S. aureus, S. pyogenes, and P. aeruginosa). In some experiments, flies were injected prior to infection with 13.4 nL of 200 µM BPS (iron chelator) or with 13.4 nL of 100 µM Flucytosine using a Nanoject apparatus (Drummond). Oral infections were performed as described previously (42, 52, 64). At least two vials of 20 flies were used for survival experiments, and survivals were repeated at least three times.
Iron Measurement Using ICP-OES.
Flies were infected with different pathogens as described above. Right before hemolymph collection, 50 flies were pricked in the thorax to breach the cuticle and increase hemolymph yield. These flies were placed on a 10-µm filter of an empty mobicol spin column (MoBiTec), covered with glass beads, and centrifuged for 5 min at 4 °C, 5,000 rpm. Then 5 µL of hemolymph per each sample were digested with 0.5 mL of 32% ultrapure hydrochloric acid (VWR Chemicals) under heating conditions (60 °C) for 2 h; 9.5 mL of nitric acid was added to each sample, and the total iron concentration was measured using ICP-OES (Perkin-Elmer Optima 8300 ICP-OES). To measure iron content of tissues, tissues of interest were dissected in phosphate-buffered saline and digested in 0.5 mL of 32% ultrapure hydrochloric acid at 60 °C for 2 h. The samples were filtered to remove impurities and any undigested material. Protein concentration in digested samples was determined using the Pierce BCA protein assay kit. Iron concentration in each sample was normalized to the total protein amount to standardize sample size differences.
RT-qPCR.
For quantification of messenger RNA, whole flies (n = 10) or dissected tissues (n = 20) were collected at indicated time points. Total RNA was isolated using TRIzol reagent and dissolved in RNase-free water. Five hundred nanograms of total RNA was then reverse-transcribed in 10-µL reactions using PrimeScript RT (Takara) and random hexamer primers. The qPCR was performed on a LightCycler 480 (Roche) in 96-well plates using the LightCycler 480 SYBR Green I Master Mix. RP49 was used as a housekeeping gene for normalization.
All data are available in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We appreciate the gifts of P. aeruginosa PA14 pyoverdine mutants from Natalia Kirienko (Rice University) and Mucorales isolates from Dimitrios P. Kontoyiannis (University of Texas MD Anderson Cancer Center). We are extremely grateful to Prof. Paolo Visca and Daniela Visaggio for kindly and timely providing P. aeruginosa PAO1 mutant strains. We thank the Bloomington Stock Centre and the Vienna Drosophila Resource Center for fly stocks. We are grateful to Laetitia Monbaron (University of Lausanne) for the ICP-OES analysis of the samples, and to Mark Hanson for critical reading of the manuscript. This work was partially funded by Swiss National Science Foundation Sinergia Grant CRS II5_186397.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914830117/-/DCSupplemental.
References
- 1.Cassat J. E., Skaar E. P., Iron in infection and immunity. Cell Host Microbe 13, 509–519 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood M. I., Skaar E. P., Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson E. E., Wessling-Resnick M., Iron metabolism and the innate immune response to infection. Microbes Infect. 14, 207–216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez C. A., Skaar E. P., The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23, 737–748 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong S. T., Ho J. Z., Ho B., Ding J. L., Iron-withholding strategy in innate immunity. Immunobiology 211, 295–314 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Schaible U. E., Kaufmann S. H. E., Iron and microbial infection. Nat. Rev. Microbiol. 2, 946–953 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Soares M. P., Weiss G., The iron age of host-microbe interactions. EMBO Rep. 16, 1482–1500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartwright G. E., et al. , The anemia associated with chronic infection. Science 103, 72 (1946). [PubMed] [Google Scholar]
- 9.Khan F. A., Fisher M. A., Khakoo R. A., Association of hemochromatosis with infectious diseases: Expanding spectrum. Int. J. Infect. Dis. 11, 482–487 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Bezkorovainy A., “Antimicrobial properties of iron-binding proteins” in Diet and Resistance to Disease, Phillips M., Baetz A., Eds. (Springer, 1981), pp. 139–154. [DOI] [PubMed] [Google Scholar]
- 11.Lambert L. A., Perri H., Halbrooks P. J., Mason A. B., Evolution of the transferrin family: Conservation of residues associated with iron and anion binding. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 142, 129–141 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Lambert L. A., Molecular evolution of the transferrin family and associated receptors. Biochim. Biophys. Acta 1820, 244–255 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Gkouvatsos K., Papanikolaou G., Pantopoulos K., Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 1820, 188–202 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Farnaud S., Evans R. W., Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 40, 395–405 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Jenssen H., Hancock R. E. W., Antimicrobial properties of lactoferrin. Biochimie 91, 19–29 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Ward P. P., Mendoza-Meneses M., Park P. W., Conneely O. M., Stimulus-dependent impairment of the neutrophil oxidative burst response in lactoferrin-deficient mice. Am. J. Pathol. 172, 1019–1029 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schade A. L., Caroline L., An iron-binding component in human blood plasma. Science 104, 340–341 (1946). [DOI] [PubMed] [Google Scholar]
- 18.Arnold R. R., Brewer M., Gauthier J. J., Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infect. Immun. 28, 893–898 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rooijakkers S. H. M., et al. , Human transferrin confers serum resistance against Bacillus anthracis. J. Biol. Chem. 285, 27609–27613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward P. P., Conneely O. M., Lactoferrin: Role in iron homeostasis and host defense against microbial infection. Biometals 17, 203–208 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Yun E.-Y., et al. , Bombyx mori transferrin: Genomic structure, expression and antimicrobial activity of recombinant protein. Dev. Comp. Immunol. 33, 1064–1069 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Brummett L. M., Kanost M. R., Gorman M. J., The immune properties of Manduca sexta transferrin. Insect Biochem. Mol. Biol. 81, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruhn K. W., Spellberg B., Transferrin-mediated iron sequestration as a novel therapy for bacterial and fungal infections. Curr. Opin. Microbiol. 27, 57–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L., et al. , Transferrin iron starvation therapy for lethal bacterial and fungal infections. J. Infect. Dis. 210, 254–264 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bu J. T., Bartnikas T. B., The use of hypotransferrinemic mice in studies of iron biology. Biometals 28, 473–480 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaitre B., Hoffmann J., The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Buchon N., Silverman N., Cherry S., Immunity in Drosophila melanogaster—From microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 14, 796–810 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrandon D., Imler J.-L., Hetru C., Hoffmann J. A., The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Tanji T., Ip Y. T., Regulators of the toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 26, 193–198 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Tiklová K., Senti K.-A., Wang S., Gräslund A., Samakovlis C., Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila. Nat. Cell Biol. 12, 1071–1077 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Mandilaras K., Missirlis F., Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics 4, 928–936 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Xiao G., Liu Z.-H., Zhao M., Wang H.-L., Zhou B., Transferrin 1 functions in iron trafficking and genetically interacts with ferritin in Drosophila melanogaster. Cell Rep. 26, 748–758.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 33.De Gregorio E., Spellman P. T., Rubin G. M., Lemaitre B., Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl. Acad. Sci. U.S.A. 98, 12590–12595 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troha K., Im J. H., Revah J., Lazzaro B. P., Buchon N., Comparative transcriptomics reveals CrebA as a novel regulator of infection tolerance in D. melanogaster. PLoS Pathog. 14, e1006847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshiga T., et al. , Drosophila melanogaster transferrin. Cloning, deduced protein sequence, expression during the life cycle, gene localization and up-regulation on bacterial infection. Eur. J. Biochem. 260, 414–420 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Levy F., Bulet P., Ehret-Sabatier L., Proteomic analysis of the systemic immune response of Drosophila. Mol. Cell. Proteomics 3, 156–166 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Geiser D. L., Winzerling J. J., Insect transferrins: Multifunctional proteins. Biochim. Biophys. Acta 1820, 437–451 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Yoshiga T., Hernandez V. P., Fallon A. M., Law J. H., Mosquito transferrin, an acute-phase protein that is up-regulated upon infection. Proc. Natl. Acad. Sci. U.S.A. 94, 12337–12342 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandilaras K., Pathmanathan T., Missirlis F., Iron absorption in Drosophila melanogaster. Nutrients 5, 1622–1647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciencialová A., et al. , Mapping the peptide and protein immune response in the larvae of the fleshfly Sarcophaga bullata. J. Pept. Sci. 14, 670–682 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Iatsenko I., Kondo S., Mengin-Lecreulx D., Lemaitre B., PGRP-SD, an extracellular pattern-recognition receptor, enhances peptidoglycan-mediated activation of the Drosophila Imd pathway. Immunity 45, 1013–1023 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Iatsenko I., Boquete J.-P., Lemaitre B., Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase nox and shortens Drosophila lifespan. Immunity 49, 929–942.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Chamilos G., et al. , Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc. Natl. Acad. Sci. U.S.A. 105, 9367–9372 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinhart A. A., Oglesby-Sherrouse A. G., Regulation of Pseudomonas aeruginosa virulence by distinct iron sources. Genes (Basel) 7, E126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasil M. L., Ochsner U. A., The response of Pseudomonas aeruginosa to iron: Genetics, biochemistry and virulence. Mol. Microbiol. 34, 399–413 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Cowart R. E., Singleton F. L., Hind J. S., A comparison of bathophenanthrolinedisulfonic acid and ferrozine as chelators of iron(II) in reduction reactions. Anal. Biochem. 211, 151–155 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Visca P., Imperi F., Lamont I. L., Pyoverdine siderophores: From biogenesis to biosignificance. Trends Microbiol. 15, 22–30 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Kirienko N. V., et al. , Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host Microbe 13, 406–416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imperi F., et al. , Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc. Natl. Acad. Sci. U.S.A. 110, 7458–7463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minandri F., et al. , Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect. Immun. 84, 2324–2335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takase H., Nitanai H., Hoshino K., Otani T., Requirement of the Pseudomonas aeruginosa tonB gene for high-affinity iron acquisition and infection. Infect. Immun. 68, 4498–4504 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchon N., Broderick N. A., Poidevin M., Pradervand S., Lemaitre B., Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Meyer J. M., Neely A., Stintzi A., Georges C., Holder I. A., Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64, 518–523 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirienko N. V., Ausubel F. M., Ruvkun G., Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 112, 1821–1826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaborin A., et al. , Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc. Natl. Acad. Sci. U.S.A. 106, 6327–6332 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Little A. S., et al. , Pseudomonas aeruginosa AlgR phosphorylation status differentially regulates pyocyanin and pyoverdine production. MBio 9, e02318-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudzic J. P., Hanson M. A., Iatsenko I., Kondo S., Lemaitre B., More than black or white: Melanization and toll share regulatory serine proteases in Drosophila. Cell Rep. 27, 1050–1061.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Defaye A., et al. , Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J. Innate Immun. 1, 322–334 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Charroux B., Royet J., Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc. Natl. Acad. Sci. U.S.A. 106, 9797–9802 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unckless R. L., Rottschaefer S. M., Lazzaro B. P., The complex contributions of genetics and nutrition to immunity in Drosophila melanogaster. PLoS Genet. 11, e1005030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanson M. A., et al. , Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. eLife 8, e44341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J., Kim Y., A viral histone H4 suppresses expression of a transferrin that plays a role in the immune response of the diamondback moth, Plutella xylostella. Insect Mol. Biol. 19, 567–574 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Lehane M. J., Gibson W., Lehane S. M., Differential expression of fat body genes in Glossina morsitans morsitans following infection with Trypanosoma brucei brucei. Int. J. Parasitol. 38, 93–101 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Vodovar N., et al. , Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. U.S.A. 102, 11414–11419 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.