Abstract
Purpose
In order to prepare functional Au nanoparticles with low toxicity and high antitumor properties, we have used fruit waste (banana peel) to synthesize a new dendrite-shaped gold nanoparticle and used it for the treatment of tumors.
Methods
Dendrite-shaped gold nanoparticle (Au-dendrite) was synthesized through a facile hydrothermal process. The banana peel was used as both the reducing agent and the protective agent for reducing chloroauric acid to obtain Au-dendrite. The safety assessment of the Au-dendrite was conducted by H&E staining of the mouse’s eyelid skin and CCK-8 assay. The antitumor effects were evaluated through in vitro tumor cytotoxicity experiments and in vivo treatment of animal tumors.
Results
In this work, a new type of gold nanomaterial (Au-dendrite) was synthesized by using a common agricultural waste (banana peel) through a facile hydrothermal process without any extra chemical reducing agent or protective agent. Subsequent experiments showed that, compared with some classical Au nanomaterials, the as-synthesized gold nanocomposites have superior biocompatibility and impressive characteristics of dual inhibition toward tumor growth and migration.
Conclusion
We successfully synthesized a dendrite-shaped gold nanocomposite which was derived from a common agricultural waste (banana peel). A facile and environmentally friendly synthetic process was proposed accordingly without regular chemical additives. The as-prepared Au-dendrite nanocomposites not only had better biocompatibility than some classical gold nanoparticles but also exhibited unique advantages in tumor inhibition.
Keywords: nanomaterials, Au nanoparticles, biocompatibility
Introduction
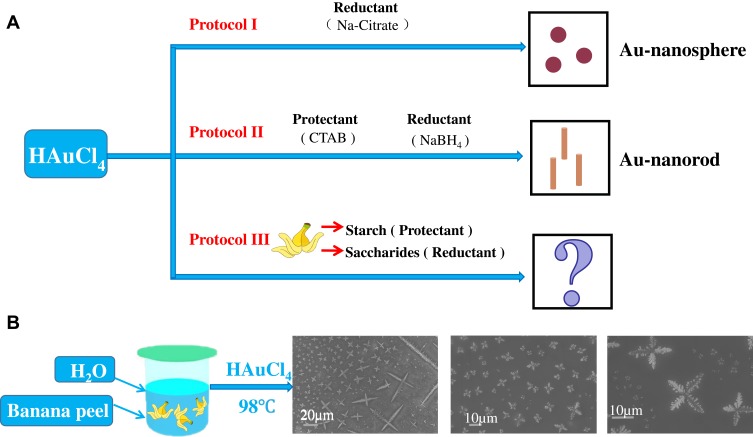
Nanomaterials exhibit optical, magnetic, electronic, and catalytic properties that differ from their macroscopic counterparts due to their high specific surface area, surface defects, and quantum effects. These unique properties made nanomaterials can be widely used in many fields such as biological imaging, nanosensors, biomedicine, photoelectric conversion and so on. Nanoparticles can be divided into organic nanoparticles (carbon-based nanoparticles) and inorganic nanoparticles. Inorganic nanoparticles can be further divided into magnetic nanoparticles (iron), noble metal nanoparticles (silver, gold and platinum), and semiconductor nanoparticles (silica, zinc oxide, and so on).1–6 Gold nanoparticles (Au NPs) have been widely used in biomedical fields due to their diverse morphology, well biocompatibility and remarkable near-infrared response characteristics.7–12 There are many classical methods for the synthesis of Au NPs,13–19 for instance, classical gold nanospheres (Au NSs) can be obtained by directly adding a reducing agent into the chloroauric acid (Figure 1A, Protocol I).20 Furthermore, adding reductants with some protectants to chloroauric acid may lead to other shapes, such as gold nanorods (Au NRs) (Figure 1A, Protocol II).21 These well-known methods have laid a solid research foundation for the synthesis and biomedical applications of various Au NPs. However, the utilization of these chemical protectants or reducing agents may have some negative impacts on the biocompatibility of the final products. Therefore, it is preferred to find some natural substances, in particular, some agricultural wastes (such as corn stalks, peanut shells, fruit peel, etc.) that have the characteristics of both protectants and reductants. By using these natural agricultural wastes, a more environmentally friendly synthesis route of functional Au NPs might be explored. In this case, some waste resources can be effectively utilized and the biocompatibility of the products may also be improved. Based on the above considerations, we conducted a series of experiments and finally discovered a common fruit waste: banana peel, which contains a certain amount of starch (protectant), glucose and fructose (reductants).22,23 What is more, banana peel also contains some anti-cancer properties (phenolic acids, flavonoids and other bioactive substances), which thus have a considerable antioxidant activity and antitumor function.24,25 In this study, we utilized the banana peel as the main natural raw material for the synthesis of new Au NPs (Figure 1A, Protocol III). Through a series of optimization, we have chosen chloroauric acid as the precursor; heated the banana peel, water and acetone at 98°C; and no extra chemical protectant or reductant was added. Finally, Au NPs with four-leaf clover (dendrite) morphology were obtained (Figure 1B), which we named it Au-dendrite.
Figure 1.
Different synthesis strategies to prepare Au nanoparticles.
Notes: (A) Simplified diagram of the synthesis process of Au NSs and Au NRs; (B) the synthesis route of Au-dendrite.
Abbreviations: Au-dendrite, dendrite-shaped gold nanoparticle; Au NSs, Au nanospheres; Au NRs, Au nanorods.
Materials
Chloroauric acid (HAuCl4), silver nitrate (AgNO3) and ascorbic acid were purchased from Sinopharm Chemical Reagent Co. Sodium borohydride (NaBH4), cetyltrimethylammonium bromide (CTAB) were obtained from Sigma-Aldrich. Sodium citrate anhydrous (Na3C6H5O7) was purchased from J&K Scientific Ltd. Acetone (CH3COCH3) was purchased from Xilong Chemical Co., Ltd. Bananas were purchased from Fujian Province, China.
Synthesis of Au-Dendrite
One hundred grams of minced banana peel was added to 100 mL deionized water, heating at 98°C for 30 mins. After filtration, acetone of the same volume was added to the filtrate and then dried at 70°C for 12 hrs after centrifuged. Subsequently, 120 mg of the dried powder was mixed with 1 mL chloroauric acid (0.02 M) and 23 mL deionized water. The mixed liquor was then heated for 3 mins at 98°C.
Cell Migration Test
The cells were prepared to be tested (the cell state was adjusted, plated one day in advance, density 60–70%), and the cell density was adjusted to 1–10×105/mL. And, 100 μL of the cell suspension was taken and added to the transwell chamber. Then, 500–650 μL of complete medium (containing 10% FBS) was added to the lower chamber of the 24-well plate. Finally, the 24-well plate inoculated with cells was placed in a cell culture incubator and cultured for 24–60 hrs. The cells were taken out and staining was performed, and then the medium was discarded. The cells in the upper chamber were gently wiped off with a cotton swab, and 500–700 μL of 0.1% crystal violet was added for staining at room temperature for 15 mins. After the dyeing was finished, it could be repeatedly washed with PBS several times under a decolorizing microscope to select different fields of view for photographing.
Nude Mouse Back LLC Tumor Model
Six-week-old BALA/c nude mice were purchased from Human SJA Experimental Animal Co., Ltd. (Changsha, Hunan). Mice were performed according to protocols approved by the Institutional Animal Care and Use Committee at Institute of Translational Medicine, Nanchang University (no. 2016NC-020-02) and animal handling followed the dictates of the National Animal Welfare Law of China. According to the ethics and related norms of small animals, we collected the cultured LLC tumor cells and planted them in the right back of nude mice according to 1×107 cells of each mouse. After the injection, the mice were returned to the original environment with standard feeding. After 1 week, we injected different materials into the corresponding group of mice by subcutaneous injection and performed near-infrared irradiation in the infrared group. After 3 weeks of experimentation, the mice were euthanized and anatomically photographed for the subsequent evaluation.
Results and Discussion
Au-Dendrite Material Characteristics
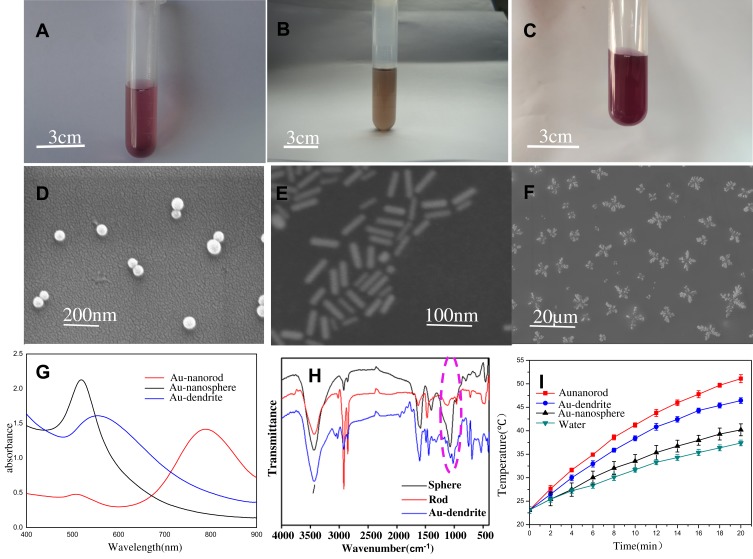
In order to systematically evaluate the material properties of Au-dendrite, we compared Au-dendrite with classic Au NSs and Au NRs. Au-dendrite is significantly different from the other two in terms of color (Figure 2A–C), as well as the location of the maximum absorption peak in the UV-Vis spectrum (Figure 2G), suggesting that their morphologies (Figure 2D–F and functions may be also different. The size of the as-prepared Au-dendrite is approximately 10 microns (Figure 2F). At the same time, Au-dendrite has a special vibration at 1046 nm−1 from the Fourier transform infrared spectroscopy (Figure 2G), indicating that Au-dendrite has some special functional groups compared with the other two Au NPs (which may be C–N bond stretching vibration of fatty amine, alcohol or phenol or C–O–C bond stretching vibration).26 Their energy dispersive x-ray analysis was shown in Figure S1, in which we can see the elemental peaks of gold. These characteristics may make this brand-new Au-dendrite getting a new function in the field of biomedical applications. We also compared the heating response of the three Au materials. As the result, the photothermal heating performance of Au-dendrite is slightly inferior to Au NRs, but it is obviously better than Au NSs, exhibiting a well near-infrared response characteristic of Au-dendrite (Figure 2I).
Figure 2.
Au NSs, Au NRs and Au-dendrite material characteristics.
Notes: (A–C) Photographs of Au NSs, Au NRs and Au-dendrite, respectively; (D–F) the corresponding SEM images; (G–I) their UV-Vis spectrum, Fourier transform infrared and heating curves, respectively.
Abbreviations: Au-dendrite, dendrite-shaped gold nanoparticle; Au NSs, Au nanospheres; Au NRs, Au nanorods.
Material Biosafety
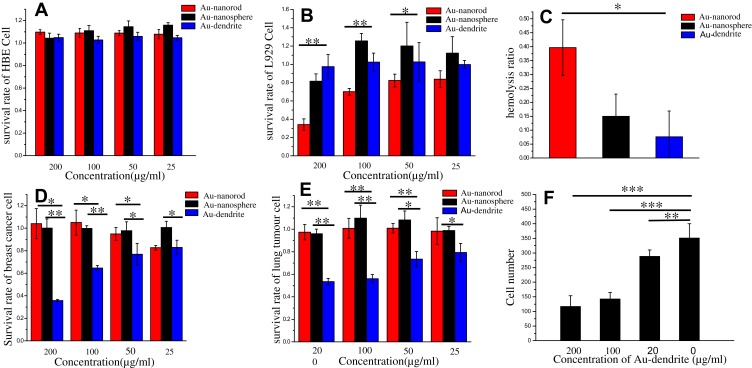
Then, we evaluated the biocompatibility of Au-dendrite. We found that Au-dendrite showed a better biocompatibility than traditional Au NRs or Au NSs on both human bronchial epithelioid cells (HBE cells) (Figure 3A) and mouse fibroblasts (L929 cells) (Figure 3B). For HBE cells, the biocompatibility of the three materials showed little difference. But for L929 cells, Au-dendrite showed better biocompatibility. A similar result was obtained in the hemolysis rate experiment: the hemolysis rate of Au-dendrite was lower than that of Au NRs or Au NSs (Figure 3C). This remarkable good biocompatibility might because that the synthetic raw material of Au-dendrite was purely nature, and no extra chemical reductant or protectant was used. Subsequently, we continued to verify the cytotoxicity of Au-dendrite in breast cancer cells. It was surprisingly found that Au-dendrite had a significant inhibitory effect on breast cancer cells at a certain concentration (200 μg/mL), but the same situation did not appear from Au NRs or Au NSs (Figure 3D). To verify this point, we then repeated cytotoxicity experiments on lung cancer cells and obtained consistent results: Au-dendrite has a significant inhibitory effect on the growth of lung cancer cells, while Au NRs or Au NSs has not (Figure 3E). Therefore, Au-dendrite has an inhibitory effect on tumor cells at the cell level. However, only tumor inhibition is not enough, because 90% of cancer-caused deaths are due to cancer metastasis.27 Therefore, we have done a subsequent experiment on the inhibition of tumor migration by Au-dendrite. The results showed that Au-dendrite exhibits an excellent effect of inhibiting the migration of tumors when the concentration of Au-dendrite is more than 100 μg/mL. (Figure 3F), and these phenomena did not appear in Au NRs or Au NSs (Figure S2, Supporting Information). So, we suspected that this characteristic of Au-dendrite was related to its special functional groups, but the relevant mechanism needs to be verified by future experiments.
Figure 3.
The cellular studies of Au-dendrite.
Notes: (A, B) The cytotoxicity assay histogram for HBE cells, L929 cells after treating with Au-dendrite, Au NRs and Au NSs, respectively; (C) The hemolysis rate of Au-dendrite, Au NRs and Au NSs; (D, E) The cytotoxicity assay histogram for breast cancer cells, lung cancer cells after treating with Au-dendrite, Au NRs and Au NSs, respectively; (F) Tumor cell number analysis for different concentrations of Au-dendrite groups. A probability value (p-value) of <0.05 was considered statistically significant (*p < 0.05, **p < 0.01 and ***p < 0.001).
Abbreviations: Au-dendrite, Dendrite-shaped gold nanoparticle; Au NSs, Au nanospheres; Au NRs, Au nanorods.
Au-Dendrite Animal Experiment to Treat Tumor
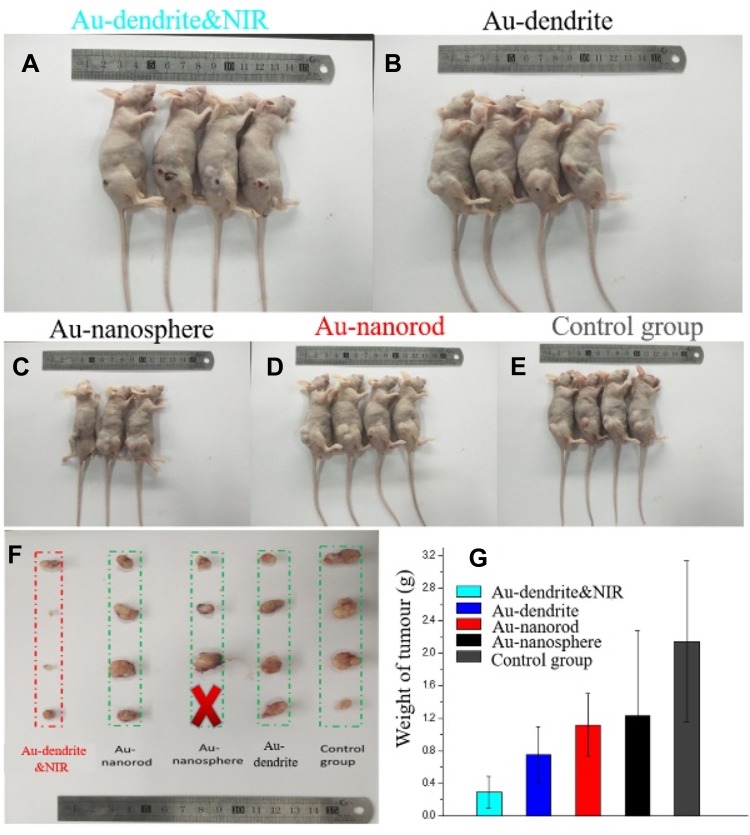
In order to further verify the inhibitory effect of Au-dendrite on tumor, we injected lung cancer cells directly into the back of nude mice to establish a classic subcutaneous lung cancer tumor model. The nude mice were divided into five groups: Au-dendrite & NIR, Au-dendrite, Au NRs, Au NSs and the control (Figure 4A–E). A constant dosage of tumor cells was injected to each group of nude mice along with the corresponding materials (100 μL, 200 μg/mL). In the Au-dendrite-NIR group, near-infrared light was additionally irradiated after the Au-dendrite being injected. Figure 4A–E are photographs of different groups (Au-dendrite & NIR, Au-dendrite, Au NRs, Au NSs and the control group) of mice after animal ethics execution, respectively. The weight of the mice was recorded every 2 days (Figure S3a). As a result, the body weight of each group of mice are basically rising, indicating that Au-dendrite has no negative impact on the health of mice while anti-tumor. In the end of this experiment, tumors in the nude mice were dissected and the volume as well as the weight of each group of tumors were measured. Figure 4F is the tumour anatomy pictures of different groups. From the experimental results, we found that comparing with Au NRs group and Au NSs group, the volume and the weight of the tumors treated with Au-dendrite were the smallest, which demonstrated that Au-dendrite group had the strongest tumor-suppressive effect in material groups (Figures 4F–G and S3b). After combined with near-infrared light, this suppression effect can be further improved (Figure S4).
Figure 4.
Animal experiments.
Notes: (A–E) Photographs of different groups (Au-dendrite & NIR, Au-dendrite, Au NRs, Au NSs and the control group) of mice after animal ethics execution; (F) The tumour anatomy pictures of different groups; (G) The comparison of tumour weights in different groups.
Abbreviations: Au-dendrite, dendrite-shaped gold nanoparticle; Au NSs, Au nanospheres; Au NRs, Au nanorods; NIR, nearinfrared ray.
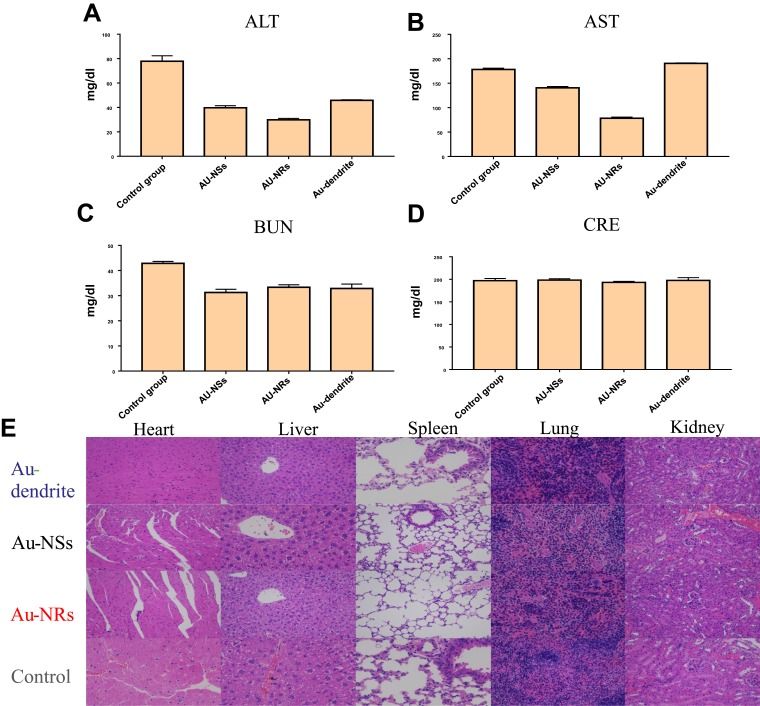
Material in vivo Safety
To further verify the safety of this Au-dendrite-based therapy, we performed the blood analysis of hepatic and renal function, as well as the hematoxylin–eosin staining (H&E staining) of heart, liver, spleen, lung and kidney. In all groups, the amounts of ALT, AST, BUN, and CRE in the blood of mice treated with Au-dendrite were closest to that of the normal group (Figure 5A–D), which showed that the biocompatibility of Au-dendrite was the closest to the health group. As the results of H&E staining (Figure 5E), Au-dendrite does not have a negative effect on vital organs of mice while anti-tumor. These results implied that Au-dendrite is a promising anti-tumor material with high biocompatibility, and which might have great applications in the field of cancer therapy.
Figure 5.
Blood biochemical analysis and H&E staining.
Notes: (A–D) The blood biochemical analysis of Au-dendrite, Au NRs, Au NSs and the control groups; (E) The H&E staining pictures of heart, liver, spleen and lu-ng in different treatment groups.
Abbreviations: H&E, hematoxylin–eosin; Au-dendrite, dendrite-shaped gold nanoparticle; Au NSs, Au nanospheres; Au NRs, Au nanorods.
Conclusion
In this paper, dendrite-shaped gold nanocomposites were derived from a common agricultural waste: banana peel. A facile and environmentally friendly synthetic process was proposed accordingly with the least amount of chemical additives. The subsequent cellular and animal experiments demonstrated that, the as-prepared Au-dendrite nanocomposites not only had better biocompatibility than conventional gold nanoparticles but also exhibited unique advantages in tumor inhibition. One thing should be noted is their impressive tumor migration inhibition capabilities, which is one hot research topic in both functional material and biomedical area. As a proof of concept study, the present work should be evaluated from two perspectives. On the one hand, this work does provide a new and feasible research direction in both the area of agricultural waste recovery and functional nanocomposite development. On the other hand, it also should be realized that, the present study is still in its infancy, and there are many aspects should be investigated in detail. For instance, which is the key functional group on the surface of Au-dendrite? What is the detail mechanism of its tumor migration inhibiting capability? The ongoing research of our group would first focus on two aspects: the optimization of this natural product derived synthesis process, as well as the long-term biosafety of the relative products.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31860263 to Xiaolei Wang; No. 21461015 to Xiaolei Wang; No. 81460393 to Anwen Liu); Science Foundation of Jiangxi Provincial Department of Education (KJLD14010 and 20153BCB23035, 20161ACB21002, 20165BCB19002 to Xiaolei Wang); National Key Basic Research Program of China (2013CB531103 to Hongbo Xin); Nanchang University Seed Grant for Biomedicine.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Suganthy N, Sri Ramkumar V, Pugazhendhi A, Benelli G, Archunan G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ Sci Pollut Res. 2018;25(11):10418–10433. doi: 10.1007/s11356-017-9789-4 [DOI] [PubMed] [Google Scholar]
- 2.Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1):104–111. doi: 10.1016/j.ijpharm.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 3.Murphin Kumar PS, MubarakAli D, Saratale RG, et al. Synthesis of nano-cuboidal gold particles for effective antimicrobial property against clinical human pathogens. Microb Pathog. 2017;113:68–73. doi: 10.1016/j.micpath.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 4.Chellapandian C, Ramkumar B, Puja P, Shanmuganathan R, Pugazhendhi A, Kumar P. Gold nanoparticles using red seaweed gracilaria verrucosa: green synthesis, characterization and biocompatibility studies. Process Biochem. 2019;80:58–63. doi: 10.1016/j.procbio.2019.02.009 [DOI] [Google Scholar]
- 5.Shankar PD, Shobana S, Karuppusamy I, et al. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: formation mechanism and applications. Enzyme Microb Technol. 2016;95:28–44. doi: 10.1016/j.enzmictec.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 6.Vijayan SR, Santhiyagu P, Ramasamy R, et al. Seaweeds: A resource for marine bionanotechnology. Enzyme Microb Technol. 2016;95:45–57. doi: 10.1016/j.enzmictec.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Zhang LM, Zheng WF, et al. Thermo-triggered release of CRISPR-Cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew Chem Int Ed. 2018;57(6):1491–1496. doi: 10.1002/anie.v57.6 [DOI] [PubMed] [Google Scholar]
- 8.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21(23):10644–10654. doi: 10.1021/la0513712 [DOI] [PubMed] [Google Scholar]
- 9.Schulz M, Ma-Hock L, Brill S, et al. Investigation on the genotoxicity of different sizes of gold nanoparticles administered to the lungs of rats. Mutat Res Genet Toxicol Environ Mutagen. 2012;745(1–2):51–57. doi: 10.1016/j.mrgentox.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 10.Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37(9):1896–1908. doi: 10.1039/b712170a [DOI] [PubMed] [Google Scholar]
- 11.Ye D, Zhang X, Yue Y, et al. Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain. J Control Release. 2018;286:145–153. doi: 10.1016/j.jconrel.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Wu M, Dai W, et al. Gold nanoclusters for controlled insulin release and glucose regulation in diabetes. Nanoscale. 2019;11(13):6471–6479. doi: 10.1039/C9NR00668K [DOI] [PubMed] [Google Scholar]
- 13.Narouz MR, Osten KM, Unsworth PJ, et al. N-heterocyclic carbene-functionalized magic-number gold nanoclusters. Nat Chem. 2019;11(5):419–425. doi: 10.1038/s41557-019-0246-5 [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Du Y, Liu J, et al. Aurophilic interactions in the self-assembly of gold nanoclusters into nanoribbons with enhanced luminescence. Angew Chem Int Ed. 2019;58(24):8139–8144. doi: 10.1002/anie.v58.24 [DOI] [PubMed] [Google Scholar]
- 15.Pei Y, Wang P, Ma Z, Xiong L. Growth-rule-guided structural exploration of thiolate-protected gold nanoclusters. Acc Chem Res. 2019;52(1):23–33. doi: 10.1021/acs.accounts.8b00385 [DOI] [PubMed] [Google Scholar]
- 16.Arunachalam KD, Annamalai SK. Chrysopogon zizanioides aqueous extract mediated synthesis, characterization of crystalline silver and gold nanoparticles for biomedical applications. Int J Nanomed. 2013;8:2375–2384. doi: 10.2147/IJN [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JL, Tian B, Li T, et al. Biosynthesis of Au,Ag and Au-Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int J Nanomed. 2018;13:1411–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu JM, Ye ZL, Fan XY, Wang HQ, Wang Z, Chen BY. A highly sensitive biosensor based on Au NPs/rGO-PAMAM-Fc nanomaterials for detection of cholesterol. Int J Nanomed. 2019;14:835–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Wang M, Webster TJ. Growth process and anticancer properties of gold nanorods. J Biomed Mater Res A. 2017;105(9):2616–2621. doi: 10.1002/jbm.v105.9 [DOI] [PubMed] [Google Scholar]
- 20.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o [DOI] [PubMed] [Google Scholar]
- 21.Nikoobakht B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater. 2003;15(10):1957–1962. doi: 10.1021/cm020732l [DOI] [Google Scholar]
- 22.Akhavan O, Ghaderi E, Aghayee S, Fereydooni Y, Talebi A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J Mater Chem. 2012;22(27):13773–13781. doi: 10.1039/c2jm31396k [DOI] [Google Scholar]
- 23.Dong -Y-Y, Li S-M, Ma M-G, Yao K, Sun R-C. Compare study cellulose/Ag hybrids using fructose and glucose as reducing reagents by hydrothermal method. Carbohydr Polym. 2014;106:14–21. doi: 10.1016/j.carbpol.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 24.Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits - A review. Food Chem. 2016;206:1–11. doi: 10.1016/j.foodchem.2016.03.033 [DOI] [PubMed] [Google Scholar]
- 25.Borges CV, de Oliveira Amorim VB, Ramlov F, et al. Characterisation of metabolic profile of banana genotypes, aiming at biofortified Musa spp. cultivars. Food Chem 2014;145:496–504. doi: 10.1016/j.foodchem.2013.08.041 [DOI] [PubMed] [Google Scholar]
- 26.Bankar A, Joshi B, Kumar AR, Zinjarde S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf B Biointerfaces. 2010;80(1):45–50. doi: 10.1016/j.colsurfb.2010.05.029 [DOI] [PubMed] [Google Scholar]
- 27.Gkountela S, Castro-Giner F, Szczerba BM, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176(1–2):98–112. doi: 10.1016/j.cell.2018.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]