Significance
Currently, cisplatin, carboplatin, and oxaliplatin are the only platinum agents approved by the US Food and Drug Administration for use in therapeutic regimens. While effective in select cancers, their clinical utility remains limited by intrinsic or acquired resistance. This is attributed, in part, to poor drug tumor localization and uptake. Additionally, one of the more formidable mechanisms of platinum resistance involves dysfunction of the tumor suppressor p53. Lack of wild-type p53 activation can translate to a significant reduction in the 5-y survival rate compared with mutant p53 in some cancers. The inability to overcome p53 dysfunction in concert with the dose-limiting toxicities and poor tumor-specific drug delivery of Pt provides an incentive to develop agents as described in this report.
Keywords: cancer, texaphyrins, drug development, platinum prodrug, drug resistance
Abstract
Described here is the development of gadolinium(III) texaphyrin-platinum(IV) conjugates capable of overcoming platinum resistance by 1) localizing to solid tumors, 2) promoting enhanced cancer cell uptake, and 3) reactivating p53 in platinum-resistant models. Side by side comparative studies of these Pt(IV) conjugates to clinically approved platinum(II) agents and previously reported platinum(II)-texaphyrin conjugates demonstrate that the present Pt(IV) conjugates are more stable against hydrolysis and nucleophilic attack. Moreover, they display high potent antiproliferative activity in vitro against human and mouse cell cancer lines. Relative to the current platinum clinical standard of care (SOC), a lead Gd(III) texaphyrin-Pt(IV) prodrug conjugate emerging from this development effort was found to be more efficacious in subcutaneous (s.c.) mouse models involving both cell-derived xenografts and platinum-resistant patient-derived xenografts. Comparative pathology studies in mice treated with equimolar doses of the lead Gd texaphyrin-Pt(IV) conjugate or the US Food and Drug Administration (FDA)-approved agent oxaliplatin revealed that the conjugate was better tolerated. Specifically, the lead could be dosed at more than three times (i.e., 70 mg/kg per dose) the tolerable dose of oxaliplatin (i.e., 4 to 6 mg/kg per dose depending on the animal model) with little to no observable adverse effects. A combination of tumor localization, redox cycling, and reversible protein binding is invoked to explain the relatively increased tolerability and enhanced anticancer activity seen in vivo. On the basis of the present studies, we conclude that metallotexaphyrin-Pt conjugates may have substantial clinical potential as antitumor agents.
Since Rosenberg et al. (1) first reported the antitumor activity of platinum compounds almost five decades ago, platinum-based drugs have made a major contribution to cancer therapy. Currently, 50% of all chemotherapeutic regimens given to cancer patients include a platinum drug (e.g., cisplatin, carboplatin, and oxaliplatin) (Fig. 1) (2). Unfortunately, despite their success and history, the Food and Drug Administration (FDA)-approved small molecule platinum agents suffer from multiple limitations, including 1) lack of tumor selectivity, 2) high systemic toxicity, 3) multiple mechanisms of resistance (intrinsic and acquired), and 4) a narrow spectrum of activity (3). In certain cancers, such as nonsmall cell lung cancer, mesothelioma, and ovarian cancer, these limitations are manifest in low 5-y survival rates of 5 to 45% in patients treated with cisplatin- and carboplatin-based front-line therapies. Finding ways to overcome the limitations of current platinum drugs and to improve the outcomes for patients suffering from these and other solid tumors represents a broad interdisciplinary challenge (4–7).
Fig. 1.
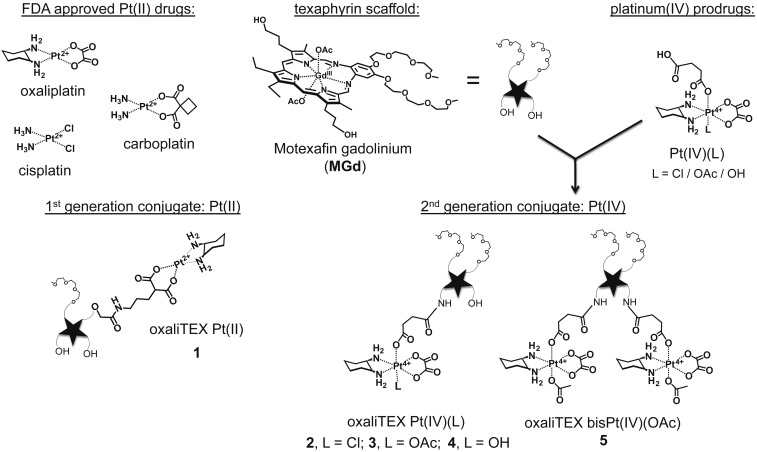
Structures of FDA-approved platinum drugs, the first generation oxaliTEX-Pt(II) conjugate 1, and second generation conjugates 2 to 5. The latter species were synthesized by conjugation of MGd to one or two Pt(IV) prodrugs.
In the specific case of ovarian cancer, the leading cause of gynecological cancer death (8), cisplatin and carboplatin are used as front-line therapies in combination with taxol. This translates to an overall 45% 5-y survival rate (5). However, 60% of the total ovarian cancer population is diagnosed with advanced cancer. Here, the clinical utility of platinum drugs becomes limited by intrinsic or acquired resistance and is reflected in a 5-y survival rate of only 27% (9). Moreover, recent clinical trials focused on addressing platinum-resistant ovarian cancer with chemotherapeutic or biological agents have demonstrated little success (10–13). Here, we report a Pt(IV)-based texaphyrin conjugate that, based on a combination of in vitro and in vivo studies, shows promise in overcoming platinum-resistant ovarian and colon cancers. This conjugate contains a number of key components, including a metallotexaphyrin core, a Pt(IV) center, an oxalate tethered to the texaphyrin through a labile linker, and a diaminocyclohexyl (DACH) ligand around the coordinated platinum center. As discussed below, each of these design elements imparts operational benefits, including 1) reduced toxicity relative to the FDA-approved platinum drugs, 2) ease of formulation, 3) good in vitro and in vivo efficacy, and 4) an ability to overcome platinum drug resistance.
Metallotexaphyrins are a class of expanded porphyrins (e.g., motexafin gadolinium [MGd]) (Fig. 1) that have been shown to accumulate in primary and metastatic tumors both in rodents (14, 15) and humans (16–18). Moreover, metallotexaphyrins have intrinsic anticancer activity through redox activity centered on the macrocylic ligand (19, 20). The relatively large texaphyrin core allows for the formation of stable complexes with lanthanide ions, such as Gd(III) (as in the case of MGd), which then can be used to enhance the contrast of magnetic resonance images (14–18). In recent years, our laboratory has focused on the development of texaphyrin drug conjugates as potential tumor-localizing drug leads (21–25). In 2012, as part of this effort, we reported a Pt(II) gadolinium texaphyrin conjugate that was capable of overcoming key platinum resistance mechanisms in vitro (23). However, difficulties were encountered in terms of formulating this first generation conjugate for use in vivo. This led us to redesign the system.
As a first step in our redesign, we sought to incorporate a Pt(IV) center rather than a Pt(II) species. The potential advantages of Pt(IV) agents compared with traditional Pt(II) drugs (i.e., cisplatin, carboplatin, and oxaliplatin) have been noted by a number of laboratories (22, 26–29). These advantages include relative kinetic inertness and a reduction in off-target toxicity effects. In addition, the presence of two extra ligands in the axial positions relative to typical Pt(II) complexes offers the possibility of tuning various pharmacokinetic parameters, such as the hydrophilicity/lipophilicity ratio, reduction potential, and cancer microenvironment targeting. Although Pt(IV) complexes can interact with DNA in their oxidized forms, adduct formation is relatively slow (30, 31). Thus, Pt(IV) complexes are generally considered to be prodrugs that must undergo reduction to produce the corresponding active Pt(II) form (32). This reduction serves to reduce the number of ligands around the platinum center (typically from six to four) and thus, provides a potential Pt(II) drug release mechanism. Recently, we reported on the ability of MGd to mediate the cancer-specific reduction of Pt(IV) agents (28). We thus sought to incorporate a Pt(IV) center into a conjugate based on a gadolinium texaphyrin core (22, 33). In doing so, we wanted to retain the DACH ligand environment about the platinum center that is present in oxaliplatin.
The DACH ligand is thought to play a key role in producing platinum complexes that are able to overcome p53-based platinum resistance. In fact, one of the most formidable molecular mechanisms of resistance involves loss of function for the tumor suppressor p53, presumably as the result of selection pressures (6). This is particularly problematic in the case of ovarian cancers, where a 96% p53 mutation rate is seen in high-grade serous ovarian cancer and where cisplatin and carboplatin typically produce attenuated responses (4, 34). While one must be cautious in oversimplifying the categorization of p53 status (and patient prognosis), it is noteworthy that DNA damage by cisplatin or carboplatin is mediated, in part, by Chk2 kinases, which are often down-regulated in platinum-resistant cancers and lead to a lack of posttranslational modification of p53 needed for its functional activation. On the other hand, DNA damage by oxaliplatin and other platinum agents containing a DACH carrier ligand is mediated by mitogen-activated protein kinase (MEK)1/2 kinases (35). Thus, these latter species often serve to “reactivate” dormant p53 in resistant tumor cells. We, therefore, reasoned that a texaphyrin conjugate containing a Pt(IV)–DACH complex linked through an axial ligand could be used to 1) deliver an active Pt(II), equivalent to oxaliplatin, in the reducing environments characteristic of many solid tumors and 2) overcome common p53-related cisplatin resistance mechanisms. As detailed below, this and other design expectations have been met in the case of a conjugate (compound 3) (Fig. 1).
Materials and Methods
The chemical synthesis and characterization of all complexes as well as their in vitro and in vivo evaluation are provided in SI Appendix. The pathological evaluation of mice treated with equimolar doses of oxaliplatin and oxaliTEX-Pt(IV) (3) is also provided in Dataset S1.
Synthesis and Stability Studies of Oxaliplatin-Based Pt(IV)-Texaphyrin Conjugates.
In the preparation of a texaphyrin-Pt(IV) conjugate, a Pt(IV) precursor incorporating both a DACH ligand and a succinate linker was used [compare Pt(IV)(L)] (Fig. 1). The choice of a succinate linker reflects an appreciation that oxaliplatin Pt(IV) derivatives with carboxylates as axial ligands are relatively stable to reduction (36). This we viewed as an advantage as it ideally would ensure stability in the bloodstream before reaching the tumor site but then, allow release of the active Pt(II) form after the cancer target was reached.
To allow conjugation of a succinate-functionalized DACH ligand, the MGd core was subject to amination (SI Appendix has the synthesis) (37). This resulted in the formation of monoamine and diamine texaphyrins [MGd(NH2) and MGd(NH2)2] as a statistical mixture (i.e., ∼33% of each species is formed, with the remaining 33% of the reaction mixture being the original MGd). The isolation of both the mono- and bis-amine products enabled the formation of two separate conjugate classes, namely 1) oxaliTEX-Pt(IV) containing a single Pt(IV) unit (e.g., 2 to 4) and 2) oxaliTEX-bis-Pt(IV) (e.g., 5) containing two Pt(IV) units (Fig. 1).
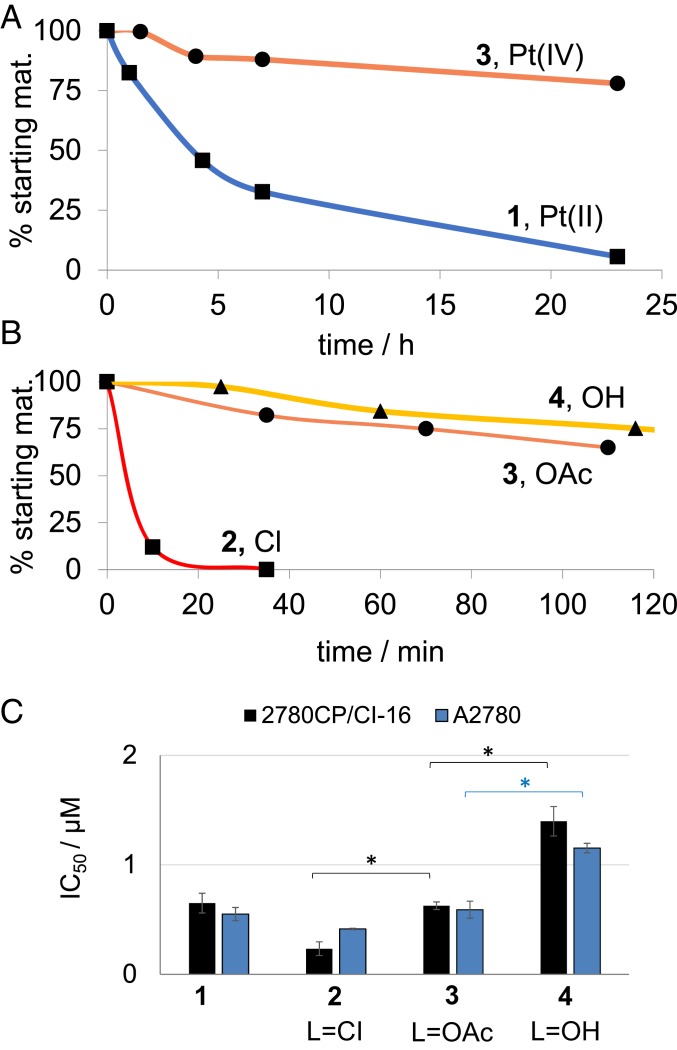
Compared with the first generation Pt(II) texaphyrin conjugate 1, the synthetic route leading to 2 to 5 is considerably shorter (23). Moreover, conjugation with Pt(IV) involves ligation at a different site on the texaphyrin core as compared with 1 (Fig. 1). This has the consequence of keeping intact the two polyethylene glycol chains on the MGd core and enhancing the water solubility of the final conjugates. In fact, the monofunctionalized conjugates 2 to 4 proved highly soluble in water and buffer systems (i.e., more than millimolar concentrations are readily achievable). Appreciable but slightly reduced water solubility was also seen for the bis-Pt(IV) system 5. Moreover, with acetate as the nonconjugated axial ligand on the Pt(IV) center, the stability of these two complexes toward hydrolysis was found to be considerably improved relative to 1 (Fig. 2A). This beneficial feature is ascribed to the inherently higher kinetic stability of Pt(IV) species relative to their Pt(II) counterparts.
Fig. 2.
(A) Hydrolysis kinetic profiles determined in PBS (pH 7) at 37 °C for 1 vs. 3. (B) Reduction kinetic profiles of conjugates 2 to 4 with L = OH (triangles), L = OAc (circles), and L = Cl (squares) at 20 μM in the presence of GSH (66 μM) in PBS (pH 7) at 37 °C. Reverse phase (RP)-HPLC monitoring: detector set at 470 nm. (C) Antiproliferative activities of 1 to 4 against A2780 (blue) and 2780CP/Cl-16 (black) seen following a 5-d incubation time with the indicated platinum species. Error bars represent SD. *P values from t test (two tailed, unpaired) < 0.05.
In an effort to select the best free, nonconjugated axial ligand on the ligated Pt(IV) center, conjugates 2 to 4 were prepared where L = Cl, OAc, or OH. These conjugates are inert and require reduction as the first step toward activation. The rate of this activation can determine antitumor activity, with slow reduction resulting in no meaningful interaction with tumor cells and rapid reduction leading to inactivation through indiscriminate interaction with plasma proteins before the drug reaches the tumor environment. In this regard, the nature of the ligand was found to affect conjugate stability toward reduction by glutathione (GSH) (Fig. 2B). Conjugate reduction studies in the presence of GSH at 37 °C and in the dark yielded the corresponding relative stabilities as 4 > 3 >> 2, where 2 was found to be 90% reduced after 10 min. Cyclic voltammetric analyses of the unconjugated Pt(IV)(L) complexes revealed that the Pt(IV)(Cl) species has a much less negative reduction potential (about −500 mV per Ag/AgCl) than the corresponding Pt(IV)(OAc) (−870 mV) and Pt(IV)(OH) (−950 mV) complexes (SI Appendix, Fig. S18), which rationalizes the complete reduction of the conjugate 2 by GSH. In the event, conjugate 2 containing Cl as an axial ligand (i.e., 2 where L = Cl) was determined to be too unstable for possible use in vitro or in vivo and was discarded from further consideration. Both the L = OAc and L = OH complexes (compounds 3 and 4, respectively) were carried forward for further studies.
We next investigated the stability of 3 in serum. This medium contains potential inactivating/reducing agents, including thiol-containing proteins and small molecules (sodium ascorbate, GSH, etc.) that can sequester Pt(II) species or reduce Pt(IV) prodrugs prematurely (32). Initially, the stability of Pt(IV) conjugate 3 and the previously reported Pt(II) conjugate 1 were evaluated in fetal bovine serum (23). It was found that 1 decayed faster than 3 (t1/2 = 2.5 and 6 h, respectively) (compare with SI Appendix, Fig. S19). The greater stability seen for 3 vs. 1 is initially surprising since Pt(IV) species can become reactive through both reduction and hydrolysis, whereas Pt(II) complexes are only likely to become reactive through hydrolysis (SI Appendix, Fig. S20). However, the greater stability seen for the Pt(IV) conjugate 3 leads us to conclude that the axial ligands in 3 (which are not present in 1) protect against hydrolysis, which only occurs after a reductive step. In this context, it is important to reiterate that the rates of reduction (as well as the associated driving force) for Pt(IV) species can be modulated through the choice of axial ligand. Thus, it is possible that the axial ligands in complex 3 lead to rates of hydrolysis and reductive stability that are fortuitously well balanced.
The controlled release and subsequent DNA platination by 3 were further investigated in the presence and absence of the ascorbate. It was found that treatment of salmon DNA with 3 resulted in minimal DNA platination. In contrast, a corresponding study carried out in the presence of excess ascorbate gave rise to statistically significant DNA platination (SI Appendix, Fig. S21). We ascribe this difference to reductive activation of complex 3 and corresponding release of oxaliplatin.
Antiproliferative Activities and Mechanistic Studies of Pt(IV)-Texaphyrin Conjugates in Cell Culture.
To gain further insights into how the choice of axial ligand might influence the operational features of the present Pt(IV) texaphyrin conjugates, cell proliferation studies were carried out using the A2780 ovarian and 2780CP/Cl-16 platinum-resistant ovarian cell lines (Fig. 2C and SI Appendix, Fig. S21). Each conjugate displayed minimal to no cross-resistance between cell lines, further validating our ability to overcome platinum resistance using rationally designed texaphyrin-oxaliplatin conjugates (23). The potency (defined as 1/IC50 [inhibitory concentration 50]) of 2 to 4 within each cell line was found to be axial ligand dependent in the order Cl > OAc > OH. This finding, considered in concert with the GSH-mediated stability studies discussed above (compare with Fig. 2A), leads us to suggest that 2 is reduced rapidly, resulting in higher potency, which may have associated toxicologic implications. Alternatively, a more stabilizing axial ligand (i.e., 4) would result in delayed reduction-based activation, thus reducing effective potency, with an attendant loss in antitumor benefits. The acetate ligand, as present in conjugate 3, was considered to be optimal. This axial ligand was thus used to create the corresponding bis-Pt(IV) conjugate 5. As expected from a twofold increase in the Pt payload, the IC50 for this latter species proved to be roughly twice as low as that for 3 in both A2780 and 2780CP/Cl-16 cell lines. In other words, the potency of 3 and 5 was essentially equal on a per equivalent of Pt basis (SI Appendix, Table S1).
The activity of 3 and 5 was further evaluated in various cancer cell lines and compared with oxaliplatin, cisplatin, and Pt(IV)(OAc) (SI Appendix, Figs. S22–S24 and Table S1). High anticancer activity across cell lines representing four cancer types was observed for both 3 and 5. In addition, equal anticancer potency was observed in platinum-sensitive A2780 ovarian and platinum-resistant 2780CP/Cl-16 cells when treated with both 3 and 5. In contrast, poor anticancer activity was observed for the unconjugated Pt(IV)(OAc) control. Again, the potency of 5 was roughly equal to that of 3 on a per Pt basis.
Cellular uptake studies of 3 and oxaliplatin were conducted in A549 lung cancer cells as this cell line is well validated for both in vitro and in vivo studies. After a 24-h equimolar drug incubation period, cells were collected and digested in nitric acid. The digests were then analyzed by inductively coupled plasma mass spectrometry (ICP-MS) (SI Appendix, Fig. S25). It was found that 1.7-fold more Pt was detected in cells exposed to 3 relative to oxaliplatin. It is worth mentioning that the Pt(II) conjugate 1 also displayed a higher Pt uptake and lower antiproliferative activity in A2780 cells than oxaliplatin (23). We hypothesize that this surplus of Pt might not be available to bind to DNA due to a modified subcellular localization and/or sequestration. Indeed, texaphyrin is known to accumulate within cytoplasmic compartments (38); as a consequence, Pt within the conjugates would not be able to reach the nucleus and interact with nuclear DNA as long as Pt remains attached to the texaphyrin core.
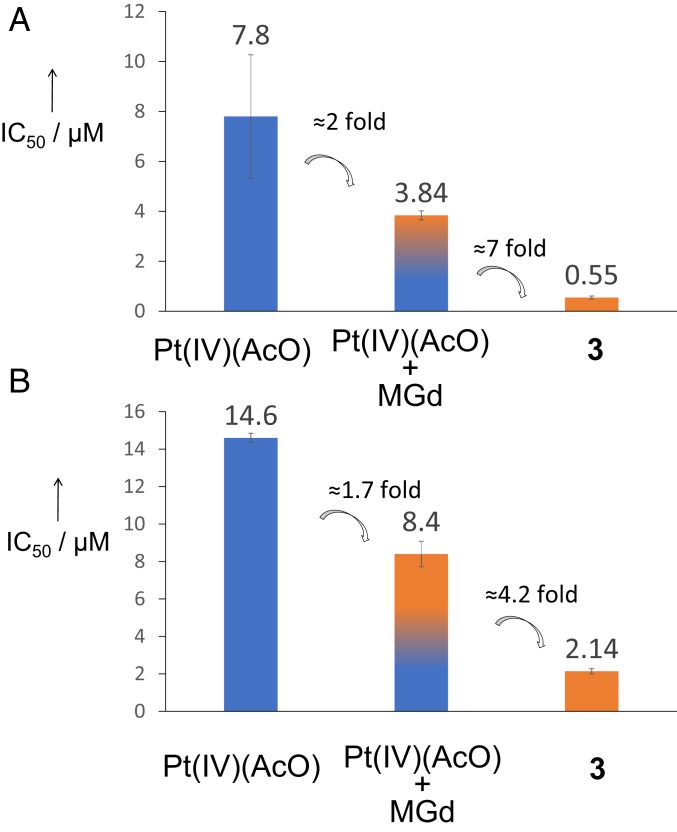
Interestingly, poor anticancer activity was observed for the unconjugated Pt(IV)(OAc) control. We previously noted that MGd is able to mediate the reduction (and hence, activation) of Pt(IV) prodrugs (28). This, in turn, leads to an increase in antiproliferative activity in cell culture relative to the Pt(IV) prodrug alone. This enhancement in activity is further improved (up to sevenfold compared with the combination) when Pt(IV) is covalently attached to MGd in the form of conjugate 3 (see Fig. 4). We believe that this increase reflects both 1) the benefits of intramolecular vs. intermolecular electron transfer between the texaphyrin core and the Pt(IV) center and 2) enforced proximity (essentially a colocalization effect under the conditions of in vitro testing).
Fig. 4.
Antiproliferative activities of Pt(IV)(AcO), of Pt(IV)(AcO) in combination with MGd (1 equivalent), and of conjugate 3 against (A) ovarian A2780 and (B) lung A549 cells; 5-d incubation time. Note that IC50(MGd) >> IC50(Pt[IV][AcO]) in both cell lines. Error bars represent SD.
Upon considering the above results in conjunction with the fact that 3 displays higher water solubility than 5 (making it attractive for administration in vivo), a decision was made to focus primarily on the mono-Pt(IV) conjugate 3. The properties of the latter lead system were thus explored in detail.
Protein Binding Studies with the Lead Pt(IV)-Texaphyrin Conjugate.
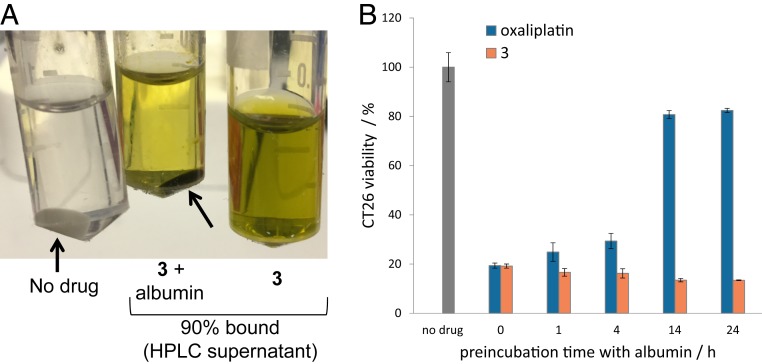
Further efforts were made to test the stability, protein binding, and drug activity of 3 under biologically relevant conditions prior to animal testing. Toward this end, the in vitro antiproliferative activity of 3 and oxaliplatin was compared following preincubation in human serum at 37 °C. At variable time points, preincubated aliquots of either 3 or oxaliplatin were added to CT26 cancer cells at a final concentration reflective of the IC50 value as determined on the basis of the preliminary tests noted above. Cell viability was then assessed using a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (SI Appendix, Fig. S26). We observed that half of the activity of oxaliplatin is lost after only 1 h of incubation in serum. This loss is ascribed to drug activation in the serum and binding to serum components, predominantly proteins. In contrast, a 6-h incubation time resulted in only 50% inactivation of 3. On the basis of these studies, we conclude that oxaliTEX-Pt(IV)(AcO) (conjugate 3) is considerably more stable and hence, more bioavailable than oxaliplatin in serum on a pharmacologically relevant timescale.
Binding to serum proteins can affect the pharmakinetics/pharmacodynamics (PK/PD) profile of a drug (39). Therefore, we decided to investigate the interaction between 3 and albumin (the most abundant protein present in the bloodstream). In fact, a dark green pellet is obtained when albumin is precipitated out from an aqueous solution containing conjugate 3 (0.6 mM) (Fig. 3A). Analysis of the supernatant by reverse-phase high performance liquid chromatography (HPLC) revealed that only 10% of 3 from the reference solution remained in the supernatant, leading to the inference that 90% was albumin bound (Fig. 3A and SI Appendix, Figs. S27 and S28). This binding to albumin by 3 and MGd was further evidenced by isothermal titration calorimetric studies (SI Appendix, Figs. S29 and S30). CT26 cells were then treated with samples of 3 and oxaliplatin that were preincubated in albumin at variable time points. Proliferation analysis revealed that oxaliplatin activity was lost after 14 h on incubation with albumin, whereas the anticancer activity of 3 was maintained over a full 24 h of incubation in the presence of albumin (Fig. 3B). Such a finding is noteworthy in light of suggestions that interactions with albumin can enhance the bloodstream half-lives of drugs (26).
Fig. 3.
(A) Albumin pellets (arrows) obtained in the absence (Left) or presence of 3 (0.6 mM; Center); also shown is a solution of 3 (0.6 mM) in just water (Right). The % bound of 3 to albumin was determined by HPLC analysis. (B) Antiproliferative activities against CT26 cells of oxaliplatin (1.7 μM) and 3 (4 μM) seen on preincubation with albumin (40 mg/mL). Note that cells were exposed to the Pt agent in question for 5 d.
We previously noted that MGd is able to mediate the reduction (and hence, activation) of Pt(IV) prodrugs (28). This, in turn, leads to an increase in antiproliferative activity in cell culture relative to the Pt(IV) prodrug alone. We confirmed that this activation mediated by the texaphyrin ring release of oxaliplatin also occurs with conjugate 3 (SI Appendix, Fig. S31). This enhancement in activity against cancer cells is further improved (up to sevenfold compared with the combination) when Pt(IV) is covalently attached to MGd in the form of conjugate 3 (Fig. 4). We believe that this increase reflects both 1) the benefits of intramolecular vs. intermolecular electron transfer between the texaphyrin core and the Pt(IV) and 2) enforced proximity (essentially a colocalization effect under the conditions of in vitro testing).
Apoptosis and p53 Activation.
To determine whether 3 promotes apoptosis, flow cytometry studies in conjunction with annexin-V staining were carried out. In brief, plated exponential growth-phase A549 cells were exposed to equipotent concentrations of 3 and oxaliplatin (a known inducer of apoptosis). At variable time points, all cells (adhered and floating) were collected, washed, and stained with fluorescein-labeled annexin-V and propidium iodide (PI) and subjected to flow cytometry (SI Appendix, Fig. S32). At early time points, evidence of early-stage apoptosis was seen as inferred from the binding of annexin-V to the still intact and impermeable cell membrane (resulting in fluorescein isothiocyanate [FITC]-only fluorescence). As time progressed, larger percentages of both early- and late-stage apoptosis/necrotic (FITC positive and PI positive from staining of nuclear material) cells became evident. Similar results in both the early- and late-stage apoptotic quadrants were seen for the A549 cells treated with an equipotent concentration of oxaliplatin. On this basis, we conclude that 3 induces controlled cell death via an apoptotic mechanism.
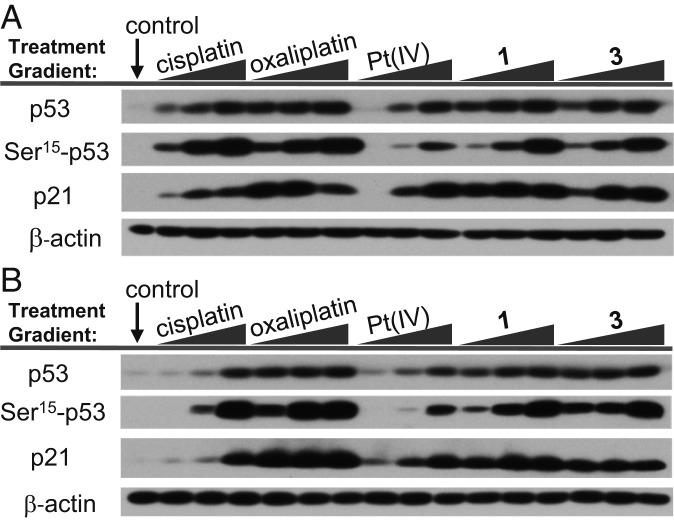
The results presented in Fig. 5 are consistent with the proposal that apoptosis by oxaliplatin and 3 is possible in both a p53-dependent and p53-independent manner. While both A2780 and 2780CP/Cl-16 models have wild-type p53 function, only A2780 cells exhibit facile cisplatin-induced p53 activation through posttranslational modification events, such as Ser-15 phosphorylation. To investigate the effect of p53 induction and Ser-15 phosphorylation by various platinum agents, A2780 and 2780CP/Cl-16 cells were exposed to cisplatin, oxaliplatin, Pt(IV)(AcO), 1, and 3 in a concentration-dependent fashion (1, 5, and 25 µM). At 24 h, cells were collected and washed with cold phosphate buffered saline (PBS), and the cell lysates were prepared for analysis by western blotting. Equal protein loading was confirmed by β-actin immunoblots.
Fig. 5.
p53 pathway activation of (A) A2780 cisplatin-sensitive and (B) 2780CP/Cl-16 cisplatin-resistant ovarian cancer cells treated with variable concentrations of platinum agent.
Induction (or stabilization) of p53 and its functional activation are separate events. p53 function was thus assessed by monitoring the transcriptional activation of p21 as a downstream target of p53. At low concentrations, all tested complexes induced p21 in platinum-sensitive A2780 ovarian cancer cells. In the platinum-resistant 2780CP/Cl-16 ovarian cancer cells, only oxaliplatin, 1, and 3 induced p21 at low (i.e., 1, 5 µM) concentrations at 24 h. This is in contrast to cisplatin and Pt(IV)(AcO), which induced transactivation of p21 only at high concentrations (i.e., 25 µM).
In Vivo Activity.
Tolerability and efficacy.
As a first step in assessing whether the present texaphyrin-oxaliplatin conjugates might be efficacious in animal models, the tolerability of the Pt(II) and Pt(IV) systems 1 and 3 was tested using athymic nude mice. Initial repeat dose studies indicated that both complexes were well tolerated with no significant body weight loss being observed over a 30-d period when mice were dosed via intravenous (i.v.) tail vein injection with 1 (90 mg/kg per dose) or 3 (60 mg/kg per dose) on days 1, 5, 9, and 13 (SI Appendix, Fig. S33). The major difference between these two complexes in this initial study was formulation. Complex 1 proved challenging to formulate, with 10% Trappsol/deionized water being needed to achieve effective solubilization. In contrast, 3 was easily dissolved in 5% dextrose/deionized water.
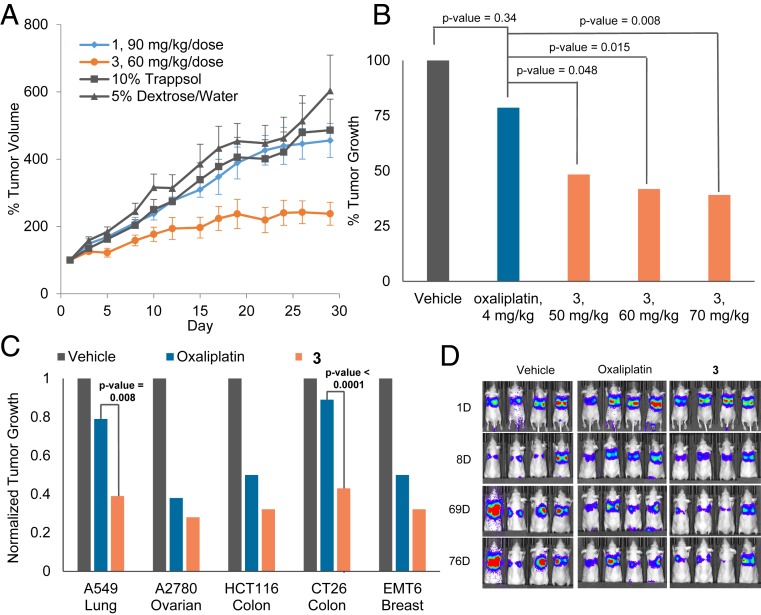
A subsequent efficacy study using these formulations was conducted in athymic mice bearing established subcutaneous (s.c.) A549 xenografts (n = 6 mice per group) (Fig. 6A). Mice were treated on days 1, 5, 9, and 13. It was found that 1 provided minimal tumor growth inhibition (P value > 0.1) relative to the 10% Trappsol control. Alternatively, even at a lower dose of 60 mg/kg per dose, 3 provided a statistically significant tumor growth delay compared with groups treated with 1 or 5% dextrose (vehicle). Taking into account the in vitro stability studies discussed above, it is possible that premature release of Pt from 1 would lead to rapid sequestration of the active species. However, in the case of 3, the controlled release of oxaliplatin would then result in the observed efficacious effect.
Fig. 6.
In vivo efficacy of 3, 1, and oxaliplatin in mice bearing s.c. A549 xenograft tumors (A and B). Error bars represent SD, and P value between 1 and 3 = 0.006. (C) In vivo efficacy of 3 (70 mg/kg per dose on days 1, 5, 9, 13) vs. oxaliplatin (4 mg/kg per dose on days 1, 5, 9, 13) in various s.c. xenograft tumor (A549, A2780, HCT116) and syngeneic tumor (CT26, EMT6) models. The study end point for the A549 model was 30 d. The study end point for all other models was the day at which the vehicle-treated mice reached maximum tumor burden. (D) Bioluminescent monitoring of orthotopic xenografts of A549 lung cancer cells expressing the luciferase gene. Mice were subject to i.v. administrations of 3 (at 70 mg/kg per dose) and oxaliplatin (4 mg/kg per dose) on days 9, 13,17, and 21. Bioluminescent images were acquired 10 min after intraperitoneal injection with luciferin. For A–C, data represent an average for each study arm. SI Appendix has details. This includes a graph showing quantification of the luminescent signal over time corresponding to D (SI Appendix, Fig. S35).
To understand further the significance of the observed tumor growth delay produced by 3, comparative studies were carried out using oxaliplatin. This particular standard of care agent was chosen as a control due to the fact that it contains the same oxalate and DACH carrier ligands present in 3 and indeed, was a starting point in the design of this particular Pt(IV) conjugate. Based on a repeat dosing tolerability study analogous to that used in the case of 3, we found that in our hands mice were able to tolerate up to 4 mg/kg per dose of oxaliplatin before observed body weight loss (SI Appendix, Fig. S34). On a per mole basis, it was found that 3 could be dosed more than four times (i.e., 70 mg/kg per dose) more than a tolerable dose of oxaliplatin (i.e., 4 mg/kg per dose in this model) with little to no observable adverse effects. An efficacy study in athymic nude mice bearing established s.c. A549 human lung cancer xenografts comparable with that conducted with 3 was then carried out (n = 9 mice per group) (Fig. 6B). It was found that, at the study end point (day 28), mice treated with oxaliplatin exhibited a 19% tumor growth inhibition relative to the vehicle group. While this tumor growth delay was evident, it was statistically insignificant (P value = 0.34) relative to the vehicle group. In contrast, xenograft-bearing mice treated i.v. with 3 at 50, 60, and 70 mg/kg per dose resulted in statistically significant (relative to the oxaliplatin group) tumor growth inhibitions of 51, 58, and 61%, respectively.
To assess the potential benefits of 3 in the context of other tumor types, efficacy studies were conducted in mice bearing cell-derived s.c. xenografts (A2780 ovarian and HCT116 colon) and syngeneic tumors (CT26 colon and EMT6 breast) (Fig. 6C). Complex 3 displayed statistically significant anticancer activity against all four tumor types relative to vehicle-treated mice. In addition, the delay in tumor growth was found to be greater in the 3 treatment group relative to the oxaliplatin treatment group. An efficacy study utilizing an orthotopic model of A549 lung cancer was also carried out (Fig. 6D and SI Appendix, Fig. S35). In this study, mice were inoculated with luciferase-expressing A549 cells, and the xenograft growth in the lungs was quantitatively monitored by bioluminescence imaging. As illustrated in Fig. 6D, tumor growth (as measured in bioluminescent output) is significantly delayed as a result of oxaliTEX-Pt(IV) (i.e., 3); however, in this model, no statistically significant benefit relative to oxaliplatin was observed. Cumulatively, these findings provide initial support for the conclusion that 3 demonstrates antitumor activity that extends beyond Pt-resistant ovarian cancer.
To evaluate the ability of 3 to treat tumors that have poor responses to traditional platinum treatment, further efficacy studies in patient-derived xenografts (PDXs) were conducted. PDX models (provided by Champions Oncology) were chosen due to their reported clinical significance (40–42). Initially, a low-powered screening study (three mice per group) was conducted in 10 different in vivo tumor models so as to identify tumor types that would respond to 3 (SI Appendix, Fig. S36). This study identified two platinum-resistant models (0253 ovarian, 0069 colorectal) that responded well to 3. Following this, higher-powered studies (n = 10 per group) in these two PDX models were conducted and compared with the platinum standard of care for that particular tumor type (Fig. 7 and SI Appendix, Fig. S36).
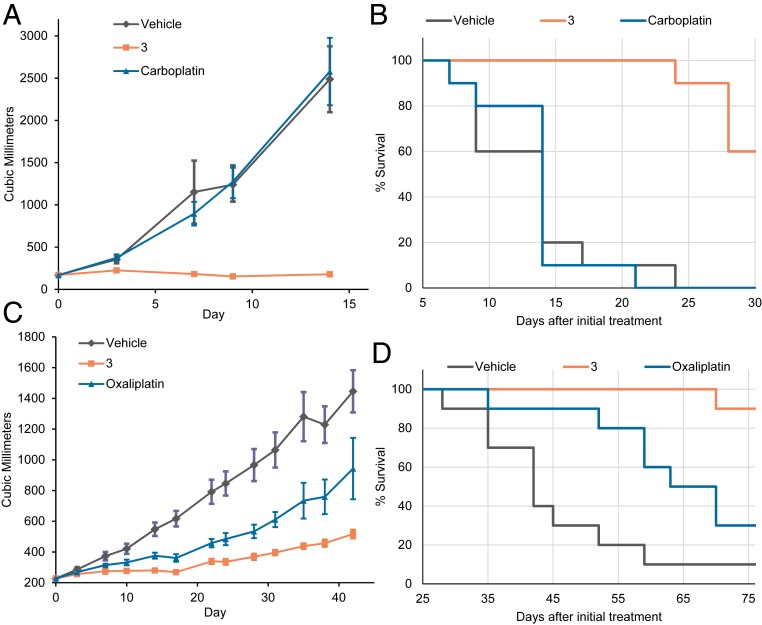
Fig. 7.
(A) Tumor growth delay and (B) Kaplan–Meier curves of mice bearing 0253 ovarian PDX tumors. (C) Tumor growth delay and (D) Kaplan–Meier curves of mice bearing 0069 colon PDX tumors. In both studies, mice were administered 70 mg/kg per dose of 3 i.v. on days 0, 4, 8, and 12 as scheduled. For the 0253 ovarian, mice i.v. received 30 mg/kg per dose of carboplatin on days 0, 4, 8, 12, 16, and 20. For 0069 colon mice, 6 mg/kg per dose of oxaliplatin was administered i.v. on days 0, 4, and 8. For A and C, the data represent an average for each study arm. Error bars represent SD. P value between 3 and carboplatin on day 14 was 0.0002, and P value between 3 and oxaliplatin on day 31 was 0.0001. SI Appendix has details.
Mice bearing 0253 ovarian PDX tumors did not respond to treatment with carboplatin, whereas treatment with 3 resulted in a statistically significant delay in tumor growth inhibition (95% tumor growth inhibition) at the end of study (Fig. 7A and SI Appendix, Fig. S36). This delay also translated to a statistically significant increase in survival (Fig. 7B). Similar results were also observed in 0069 colon PDX models in which treatment with 3 resulted in a 80% tumor growth inhibition, whereas treatment with oxaliplatin resulted in a 50% tumor growth inhibition (Fig. 7 C and D). Taken in concert, these results provide further support for the contention that the lead complex 3 may prove superior to the current standard of care in the case of certain platinum-resistant cancers.
Biodistribution.
It was observed that mice can tolerate higher repeated doses of platinum when it is administered in the form of 3 than as oxaliplatin. Based on literature precedent, we considered it likely that the kinetic inertness associated with the use of a higher oxidation state [Pt(IV) vs. Pt(II)] could account for this difference (43). However, we also suspected that, when attached to texaphyrin, the biodistribution of Pt might be modified in a way that reduces acute systemic toxicity. To test this postulate, side by side biodistribution studies were conducted in both tumor-free mice and mice bearing HCT-116 colon s.c. xenografts. At 24 h postinjection, mice were killed, and various organs were analyzed using atomic absorption (flameless atomic absorption spectroscopy [FAAS]) so as to determine their respective Pt content.
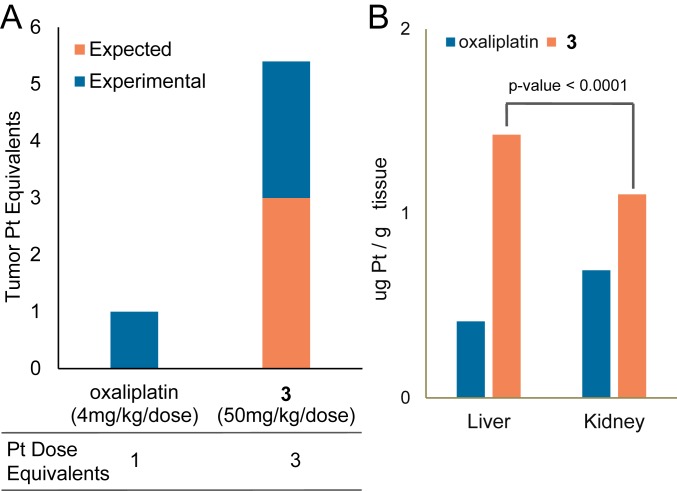
In a first experiment using mice bearing HCT116 colon xenografts, the per mole equivalents of the 3 dose were threefold higher (i.e., 50 mg/kg per dose) than the dose used for oxaliplatin (i.e., 4 mg/kg per dose). If no preferential localizations were occurring, this difference in doses would be expected to translate into a threefold increase in tumor-localized Pt equivalents. However, experimental analyses of the excised tumors revealed that the intratumoral Pt concentration in mice treated with 3 was 5.5-fold higher than in the animals treated with oxaliplatin (Fig. 8A). These findings are consistent with one of the core postulates underlying this project, namely that the gadolinium(III) texaphyrin core in conjugate 3 provides a tumor localization benefit.
Fig. 8.
(A) Intratumoral platinum content (in mice) quantified by FAAS 24 h after injection of oxaliplatin (4 mg/kg) or 3 (50 mg/kg). (B) Platinum levels in liver and kidney determined by FAAS 24 h after equimolar single i.v. injection of oxaliplatin or 3 into nontumor-bearing mice. Data represent an average for each study arm. SI Appendix has details.
Conjugation of a Pt(IV) prodrug to texaphyrin also alters the biodistribution away from the kidney and more toward the liver (Fig. 8B) (44, 45). Since renal toxicity can be dose limiting, particularly for cisplatin (46), this alteration in the biodistribution and presumed clearance pathways may prove beneficial in a clinical setting. We attribute the redistribution seen in the case of 3 to the presence of the texaphyrin core, a moiety in which the known path of metabolism is predominantly hepatic (47).
Pathology.
Studies were conducted to evaluate the tolerability and pathology in CD1 mice treated with 3 and oxaliplatin at equimolar platinum dosages (one dose per day on days 1, 5, 9, 13) (Dataset S1 has more detail). The efficacious and “high dose with acceptable weight loss” (HD-AWL) doses of 3, 70 mg/kg per dose (corresponding on a per mole basis to platinum concentrations of 16.2 mg/kg per dose in oxaliplatin), and the efficacious and approximate maximum tolerated dose (MTD) of oxaliplatin, 6 mg/kg per dose in this animal model (which would correspond to a per mole platinum concentration of 25.9 mg/kg per dose of 3), were tested. Mice treated with 70 mg/kg per dose of 3 experienced recoverable minor weight loss over the treatment period (Table 1) with no notable adverse clinical symptoms being observed. Mice treated with an equimolar platinum dose of oxaliplatin (16.2 mg/kg per dose) experienced significant body weight loss and severe adverse clinical effects (three of the eight mice required early euthanasia).
Table 1.
Dosing and body weight change of CD1 mice treated with 3 and oxaliplatin
| Drug | Dose, mg/kg per dose | Pt per dose, nmol | BW change day 14, % | BW change day 28, % |
| Vehicle | Vehicle | NA | +3.5 | NA |
| 3 | 70 (HD-AWL) | 41 | −7.5 | +1.0 |
| Oxaliplatin | 16.2 | 41 | −16.5* | NA |
| 3 | 25.9 | 15 | +6.4 | +5.8 |
| Oxaliplatin | 6 (MTD) | 15 | +8.9 | +14.8 |
Data represent an average for each study arm. SI Appendix has details. BW, body weight; NA, not applicable.
Note that 40% of mice were not evaluated as they were removed from study due to adverse drug effects.
The primary hematological finding associated with both oxaliplatin and 3 was dose-dependent pancytopenia (leukopenia, anemia, and thrombocytopenia). The pancytopenia was generally more pronounced in oxaliplatin-treated animals compared with those treated with equimolar platinum doses of 3. Experience with cisplatin and carboplatin leads us to suggest that the pancytopenia with 3 would be easily manageable in the clinic (48). Comparisons in hematology parameters were made between these groups (3, 70 mg/kg per dose and oxaliplatin, 6 mg/kg per dose). The leukocyte and erythrocyte values between these groups were generally comparable; however, the recovery of platelet counts in animals treated with 3 were delayed compared with oxaliplatin-treated animals. The primary serum chemistry-related findings were that administration of 3 resulted in several moderate analyte elevations suggestive of muscle degeneration, most of which resolved within 2 wk. The mild decrease in blood urea nitrogen in animals treated with 3 and the mild increase in total bilirubin in the animals treated with this dose of compound 3 could reflect a slight decrease in hepatic function. Given the limited magnitude of the hepatic changes, liver pathology is likely to be of minimal clinical relevance. Test article-related lesions were seen in hematopoietic organs (bone marrow, spleen), kidneys, small intestine, liver, and ovaries in mice treated with both 3 and oxaliplatin. Most lesions related to 3 were considered minor. Bone marrow atrophy was the most clinically significant finding, albeit not at a level that is likely to engender concern. All animal studies are in compliance with each institution’s Institutional Animal Care and Use Committee: University of Texas at Austin (AUP-2019-00114); M.D. Anderson Cancer Center (00001311-RN01); Korean Basic Science Institute (KBSI-AEC1820); and Champions Oncology (2017-TOS-001).
Conclusion
The attachment of a Pt(IV) prodrug (an oxaliplatin-like payload) to a Gd-texaphyrin core (MGd) generates a conjugate, complex 3, that on the basis of in vitro and in vivo studies is found to be more than the sum of its two constituent parts. The known ability of MGd to activate Pt(IV) prodrug species through redox cycling allowed the use a relatively inert Pt(IV) moiety to construct 3, thus precluding premature reduction on contact with bloodstream components (28). This design consideration along with the beneficial biolocalization provided by the texaphyrin core is thought to underlie the experimental finding that relatively high doses of platinum may be administered in vivo by means of 3 without inducing acute toxicity effects. Since antitumor response is dependent on dose, which is, in turn, dependent on tolerability, these design principles [i.e., redox-controlled release of active oxaliplatin from the more inert Pt(IV) species] are considered key design features in the case of 3. The effects of these design principles are culminated in efficacy studies in which conjugate 3 was found to be more effective at retarding/inhibiting tumor growth in xenograft mouse models and more clinically relevant Pt-resistant PDX mouse models than the most closely related current platinum-based standards of care. The present findings, when considered in light of the favorable initial toxicological profile, lead us to suggest that conjugate 3 is an agent with potential clinical significance and one that warrants further development as a drug lead.
Data Availability.
Supporting information for chemical synthesis/biological methods and toxicological evaluation are provided in SI Appendix and Dataset S1, respectively.
Supplementary Material
Acknowledgments
Funding from the Cancer Prevention and Research Institute of Texas (to R.F., Z.H.S., and J.L.S.) and National Cancer Institute Grant R01 CA68682 (to J.L.S.) is acknowledged. Support from Korea Basic Science Institute Grant T39631 (to K.S.H.) is also acknowledged.
Footnotes
Competing interest statement: Since the time of the original submission, the texaphyrin conjugates described in this manuscript were licensed by The University of Texas to the IQ Global Group and planned for further development by a new for-profit company, OncoTEX Inc. J.F.A. is now employed by OncoTEX Inc., and J.L.S. now serves as a nonexecutive board member for OncoTEX Inc. The other authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914911117/-/DCSupplemental.
References
- 1.Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H., Platinum compounds: A new class of potent antitumour agents. Nature 222, 385–386 (1969). [DOI] [PubMed] [Google Scholar]
- 2.Galanski M., Jakupec M. A., Keppler B. K., Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 12, 2075–2094 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Rabik C. A., Dolan M. E., Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 33, 9–23 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozols R. F., Challenges for chemotherapy in ovarian cancer. Ann. Oncol. 17 (suppl. 5), v181–v187 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Siddik Z. H., Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 22, 7265–7279 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Mehta K., Siddik Z. H., “Drug resistance and the tumor supressor p53: The paradox of wild-type genotype in chemorefractory cancers” in Drug Resistance in Cancer Cells, Mehta K., Siddik Z. H., Eds. (Springer Science, New York, NY, 2009), pp. 209–231. [Google Scholar]
- 7.Johnstone T. C., Suntharalingam K., Lippard S. J., The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 116, 3436–3486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel R., Ma J., Zou Z., Jemal A., Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society , Cancer Facts & Figures 2016 (American Cancer Society, 2016), pp. 1–9. [Google Scholar]
- 10.Bozkaya Y., et al. , Effectiveness of low-dose oral etoposide treatment in patients with recurrent and platinum-resistant epithelial ovarian cancer. J. Obstet. Gynaecol. 37, 649–654 (2017) [DOI] [PubMed] [Google Scholar]
- 11.Pujade-Lauraine E., et al. , Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 32, 1302–1308 (2014). [DOI] [PubMed] [Google Scholar]
- 12.McClung E. C., Wenham R. M., Profile of bevacizumab in the treatment of platinum-resistant ovarian cancer: Current perspectives. Int. J. Womens Health 8, 59–75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marth C., et al. , ENGOT-ov-6/TRINOVA-2: Randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. Eur. J. Cancer 70, 111–121 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Miller R. A., et al. , In vivo animal studies with gadolinium (III) texaphyrin as a radiation enhancer. Int. J. Radiat. Oncol. Biol. Phys. 45, 981–989 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Young S. W., et al. , Gadolinium(III) texaphyrin: A tumor selective radiation sensitizer that is detectable by MRI. Proc. Natl. Acad. Sci. U.S.A. 93, 6610–6615 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal D. I., et al. , A phase I single-dose trial of gadolinium texaphyrin (Gd-Tex), a tumor selective radiation sensitizer detectable by magnetic resonance imaging. Clin. Cancer Res. 5, 739–745 (1999). [PubMed] [Google Scholar]
- 17.Carde P., et al. , Multicenter phase Ib/II trial of the radiation enhancer motexafin gadolinium in patients with brain metastases. J. Clin. Oncol. 19, 2074–2083 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Viala J., et al. , Phases IB and II multidose trial of gadolinium texaphyrin, a radiation sensitizer detectable at MR imaging: Preliminary results in brain metastases. Radiology 212, 755–759 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Magda D., et al. , Motexafin gadolinium reacts with ascorbate to produce reactive oxygen species. Chem. Commun. (Camb.) 22, 2730–2731 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Magda D., Miller R. A., Motexafin gadolinium: A novel redox active drug for cancer therapy. Semin. Cancer Biol. 16, 466–476 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Lee M. H., et al. , Liposomal texaphyrin theranostics for metastatic liver cancer. J. Am. Chem. Soc. 138, 16380–16387 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Thiabaud G., Arambula J. F., Siddik Z. H., Sessler J. L., Photoinduced reduction of Pt(IV) within an anti-proliferative Pt(IV)-texaphyrin conjugate. Chemistry 20, 8942–8947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arambula J. F., Sessler J. L., Siddik Z. H., A texaphyrin-oxaliplatin conjugate that overcomes both pharmacologic and molecular mechanisms of cisplatin resistance in cancer cells. MedChemComm 3, 1275–1281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arambula J. F., Sessler J. L., Siddik Z. H., Overcoming biochemical pharmacologic mechanisms of platinum resistance with a texaphyrin-platinum conjugate. Bioorg. Med. Chem. Lett. 21, 1701–1705 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arambula J. F., et al. , Gadolinium texaphyrin (Gd-Tex)-malonato-platinum conjugates: Synthesis and comparison with carboplatin in normal and Pt-resistant cell lines. Dalton Trans. 48, 10834–10840 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y.-R., et al. , Pt(IV) prodrugs designed to bind non-covalently to human serum albumin for drug delivery. J. Am. Chem. Soc. 136, 8790–8798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagopian G. S., Mills G. B., Khokhar A. R., Bast R. C. Jr, Siddik Z. H., Expression of p53 in cisplatin-resistant ovarian cancer cell lines: Modulation with the novel platinum analogue (1R, 2R-diaminocyclohexane)(trans-diacetato)(dichloro)-platinum(IV). Clin. Cancer Res. 5, 655–663 (1999). [PubMed] [Google Scholar]
- 28.Thiabaud G., et al. , Activation of platinum(IV) prodrugs by motexafin gadolinium as a redox mediator. Angew. Chem. Int. Ed. Engl. 55, 12626–12631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He G., et al. , Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 24, 2929–2943 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Pouryasin Z., et al. , Anticancer and DNA binding activities of platinum (IV) complexes; importance of leaving group departure rate. Appl. Biochem. Biotechnol. 172, 2604–2617 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Dolman R. C., Deacon G. B., Hambley T. W., Studies of the binding of a series of platinum(IV) complexes to plasma proteins. J. Inorg. Biochem. 88, 260–267 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Wexselblatt E., Gibson D., What do we know about the reduction of Pt(IV) pro-drugs? J. Inorg. Biochem. 117, 220–229 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Sessler J. L., Arambula J. F., Siddik Z. H., Thiabaud G., “Texaphyrin-Pt(IV) conjugates and compositions for use in overcoming platinum resistance.” US Patent US2017246182 (2015).
- 34.Bast R. C. J. Jr, Hennessy B., Mills G. B., The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 9, 415–428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatt M., Ivan C., Xie X., Siddik Z. H., Drug-dependent functionalization of wild-type and mutant p53 in cisplatin-resistant human ovarian tumor cells. Oncotarget 8, 10905–10918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J. Z., Wexselblatt E., Hambley T. W., Gibson D., Pt(IV) analogs of oxaliplatin that do not follow the expected correlation between electrochemical reduction potential and rate of reduction by ascorbate. Chem. Commun. (Camb.) 48, 847–849 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Wei W.-H., et al. , Gadolinium texaphyrin-methotrexate conjugates. Towards improved cancer chemotherapeutic agents. Org. Biomol. Chem. 3, 3290–3296 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Woodburn K. W., Intracellular localization of the radiation enhancer motexafin gadolinium using interferometric fourier fluorescence microscopy. J. Pharmacol. Exp. Ther. 297, 888–894 (2001). [PubMed] [Google Scholar]
- 39.Yamasaki K., Chuang V. T. G., Maruyama T., Otagiri M., Albumin-drug interaction and its clinical implication. Biochim. Biophys. Acta 1830, 5435–5443 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Tentler J. J., et al. , Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9, 338–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richmond A., Su Y., Mouse xenograft models vs GEM models for human cancer therapeutics. Dis. Model. Mech. 1, 78–82 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerbel R. S., Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: Better than commonly perceived—but they can be improved. Cancer Biol. Ther. 2, 133–138 (2003). [PubMed] [Google Scholar]
- 43.Hall M. D., Hambley T. W., Platinum(IV) antitumour compounds: Their bioinorganic chemistry. Coord. Chem. Rev. 232, 49–67 (2002). [Google Scholar]
- 44.Ford J. M., et al. , Results of the phase I dose-escalating study of motexafin gadolinium with standard radiotherapy in patients with glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 69, 831–838 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Miles D. R., et al. , Validation and use of three complementary analytical methods (LC-FLS, LC-MS/MS and ICP-MS) to evaluate the pharmacokinetics, biodistribution and stability of motexafin gadolinium in plasma and tissues. Anal. Bioanal. Chem. 385, 345–356 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Hartmann J. T., Lipp H.-P., Toxicity of platinum compounds. Expert Opin. Pharmacother. 4, 889–901 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Mani C., Upadhyay S., Lacy S., Boswell G. W., Miles D. R., Reductase-mediated metabolism of motexafin gadolinium (Xcytrin) in rat and human liver subcellular fractions and purified enzyme preparations. J. Pharm. Sci. 94, 559–570 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Siddik Z. H., Boxall F. E., Harrap K. R., Haematological toxicity of carboplatin in rats. Br. J. Cancer 55, 375–379 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting information for chemical synthesis/biological methods and toxicological evaluation are provided in SI Appendix and Dataset S1, respectively.