Abstract
Newborn piglets develop pulmonary hypertension and have diminished pulmonary vascular nitric oxide (NO) production when exposed to chronic hypoxia. NO is produced by endothelial NO synthase (eNOS) in the pulmonary vascular endothelium using l-arginine as a substrate and producing l-citrulline as a byproduct. l-Citrulline is metabolized to l-arginine by two enzymes that are colocated with eNOS in pulmonary vascular endothelial cells. The purpose of this study was to determine whether oral supplementation with l-citrulline during exposure of newborn piglets to 10 days of chronic hypoxia would prevent the development of pulmonary hypertension and increase pulmonary NO production. A total of 17 hypoxic and 17 normoxic control piglets were studied. Six of the 17 hypoxic piglets were supplemented with oral l-citrulline starting on the first day of hypoxia. l-Citrulline supplementation was provided orally twice a day. After 10 days of hypoxia or normoxia, the animals were anesthetized, hemodynamic measurements were performed, and the lungs were perfused in situ. Pulmonary arterial pressure and pulmonary vascular resistance were significantly lower in hypoxic animals treated with l-citrulline compared with untreated hypoxic animals (P < 0.001). In vivo exhaled NO production (P = 0.03) and nitrite/nitrate accumulation in the perfusate of isolated lungs (P = 0.04) were significantly higher in l-citrulline-treated hypoxic animals compared with untreated hypoxic animals. l-Citrulline supplementation ameliorated the development of pulmonary hypertension and increased NO production in piglets exposed to chronic hypoxia. We speculate that l-citrulline may benefit neonates exposed to prolonged periods of hypoxia from cardiac or pulmonary causes.
Keywords: nitric oxide synthase, nitric oxide, l-arginine recycling
infants with chronic lung disease and cyanotic congenital heart disease frequently suffer from hypoxia. Because of its effects on both existing and developing pulmonary arteries, chronic hypoxia causes progressive changes in both the function and structure of the pulmonary circulation (28, 31). Ultimately, chronic hypoxia results in severe pulmonary hypertension culminating in right-sided heart failure and death. Currently, the therapy for pulmonary hypertension in infants suffering from chronic cardiopulmonary disorders associated with persistent or episodic hypoxia is largely limited to improving the underlying cardiopulmonary disorder and attempts to achieve adequate oxygenation (1, 2, 23, 31). The need for novel therapies to treat infants with chronic progressive neonatal pulmonary hypertension is well acknowledged (1–3, 14, 23).
The piglet is an excellent species for the study of neonatal pulmonary hypertension since adaptation of the pulmonary circulation to extra-uterine life is similar in pigs and humans (14). Changes in pulmonary blood vessels found in piglets exposed to hypoxia approximate those found in human infants with pulmonary hypertension (15). We have previously shown that newborn piglets develop pulmonary hypertension when exposed to chronic hypoxia (9). Moreover, we have shown that the development of pulmonary hypertension in piglets exposed to 10 days of chronic hypoxia is associated with impaired production of the vasodilator nitric oxide (NO) (14).
NO is produced by endothelial NO synthase (eNOS) in the pulmonary vascular endothelium using l-arginine as a substrate and producing l-citrulline as a by-product. In turn, l-arginine can be synthesized from l-citrulline, providing a recycling pathway for the conversion of l-citrulline to NO via l-arginine (30). Plasmalemmal caveolae, the site of the l-citrulline-to-l-arginine recycling pathway, may be the principal source of l-arginine available to eNOS (12, 13, 30). Via this recycling pathway, the availability of l-citrulline may regulate NO production by eNOS in the pulmonary circulation.
The purpose of this study was to determine whether oral supplementation with l-citrulline during exposure of newborn piglets to 10 days of chronic hypoxia would prevent the development of pulmonary hypertension and the concomitant reduction in NO production.
METHODS
Animal care.
All experimental protocols were performed in adherence with the National Institutes of Health guidelines for the use of experimental animals and approved by the Animal Care and Use Committee of Vanderbilt University Medical Center. The animal resource facility is fully accredited by the Association for Assessment and Accredidation of Laboratory Animal Care International. A total of 17 hypoxic and 17 normoxic control piglets were studied. Normoxic control animals were studied on the day of arrival from the farm at 12 days of age. The hypoxic pigs (2 days old) were placed in a normobaric hypoxic chamber for 10–11 days. Normobaric hypoxia was provided using compressed air and nitrogen to create inspired oxygen of 8–11% (Po2 of 60–72 Torr) and CO2 was maintained at 3–6 Torr by absorption with soda lime. The animals were monitored with daily weights and physical exam twice daily. They were fed ad libitum with sow milk replacer from a feeding device in the cage.
l-Citrulline supplementation.
Six of the 17 hypoxic piglets were supplemented with oral l-citrulline starting on the first day of the hypoxic exposure. l-Citrulline supplementation was provided at a dose of 0.13 g/kg body wt twice a day using a syringe to deliver the dose orally. If it appeared to study personnel that the piglet had not ingested the majority of a dose, it was repeated. l-Citrulline was mixed using a preparation (Sigma Pharmaceuticals, 98% purity) at a concentration of 0.13 g/ml of distilled water. When completely dissolved, this solution was passed through a 0.20-μm filter.
In vivo hemodynamics.
In vivo hemodynamics were measured in six of the normoxic control piglets and in all of the hypoxic piglets. For these measurements, the animals were weighed and then preanesthetized with Ketamine (15 mg/kg) and Acepromazine (2 mg/kg) intramuscularly. A tracheostomy, venous and arterial catheters, and thermistor were then placed as previously described using intravenous pentobarbital for sedation (10). Pulmonary artery pressure, left ventricular end diastolic pressure, and cardiac output were measured. Cardiac output was measured by a thermodilution technique (model 9520 thermodilution cardiac output computer, Edwards Laboratory, Irvine, CA) using a thermistor in the aortic arch and the left ventricle catheter as an injection port. Cardiac output was measured at end expiration as the mean of three injections of 3 ml of normal saline (0°C). Exhaled NO was measured as described below. During the in vivo measurements, animals were ventilated with room air using a piston-type ventilator at a tidal volume of 15–20 ml/kg, end-expiratory pressure of 2 Torr, and a respiratory rate of 15–20 breaths/min. Hemodynamic measurements were obtained in all hypoxic animals and six control animals. In our past experience as in this study, it is not always possible to obtain in vivo hemodynamic data on every animal for technical reasons. The most common difficulty encountered is the inability and length of time needed to place and advance a right heart catheter into the pulmonary artery to measure pulmonary artery pressure. Because of this difficulty, we did not attempt to obtain hemodynamic data in all control animals.
Exhaled NO measurement.
For exhaled NO measurement in anesthetized animals, expiratory gas was sampled two to three times for 3-min periods each and passed through a chemiluminescence analyzer (model 270B NOA; Sievers, Boulder, CO) to measure NO concentration as previously described (11). Exhaled NO production (nmol/min) was calculated using minute ventilation and the measured exhaled NO concentration.
Isolated lung perfusions.
All control and hypoxic animals used for hemodynamic measurements and an additional 11 control piglets were used in isolated lung perfusions. The lungs were isolated and perfused in situ with a Krebs Ringer bicarbonate (KRB) solution containing 5% dextran, molecular weight of 70,000, at 37°C and ventilated with a normoxic gas mixture (21% O2 and 5% CO2) as previously described (10). The lungs were perfused for 30–60 min until a stable pulmonary arterial pressure was achieved. Perfusate samples (1 ml) were then removed from the left atrial cannula every 10 min for a 60-min period. The perfusate samples were centrifuged, and the supernatant was stored at −80°C for future analysis of nitrite/nitrate (NOx−) concentrations as described below. At the end of the perfusion, the volume of perfusate remaining in the circuit and reservoir was measured. In some cases, lung tissue was collected immediately following the perfusion, frozen with liquid nitrogen, and then stored at −80°C for later measurement of eNOS and nNOS content as described below. Isolated lung perfusions were attempted in all animals. It is our experience, as in this study, that it is not possible to successfully isolate and perfuse lungs in all animals for technical reasons.
NOx− measurement.
A chemiluminescence analysis described previously was used to determine perfusate NOx− concentration (nmol/ml) at each collection time. (10, 34) Perfusate (20 μl) was injected into the reaction chamber of a chemiluminescence NO analyzer (model 170B NOA, Sievers). The reaction chamber contained vanadium (III) chloride in 1 M HCl heated to 90°C to reduce nitrite and nitrate to NO gas. The NO gas was carried into the analyzer using a constant flow of N2 gas via a gas bubble trap containing 1 M NaOH to remove HCl vapor. A standard curve was generated by adding known amounts of NaNO3 to distilled water and assaying as described for the perfusion samples.
The perfusate NOx− concentration (nmol/ml) was calculated for each collection time by multiplying the perfusate concentration of NOx− at that sample collection time by the volume of the system (perfusion circuit + reservoir) at the sample collection time plus the amount of NOx− removed with all previous samples. The rate of NOx− production was determined from the slope of a linear regression line fit to the amount of NOx− in the perfusate vs. time for the first 60 min of the collection period.
Plasma amino acid measurements.
On the day of hemodynamic measurements and/or lung perfusion study, for normoxic control and both l-citrulline-treated and -untreated chronic hypoxic animals, blood was drawn before the study was started and the plasma frozen at −80°C for later determination of amino acid levels. For the l-citrulline-treated hypoxic animals, a blood sample was obtained 12 h after the last dose of citrulline to measure the trough level of this amino acid. We wanted to verify that l-citrulline levels in treated animals were greater than those in untreated animals. Therefore, in some of the l-citrulline-treated animals (n = 3), after blood sampling for a trough level, a dose of l-citrulline was given via nasogastric tube. Following this dose, blood samples were drawn every 30 min for 90 min (the length of the in vivo studies). All samples were spun, and the plasma was collected and frozen at −80°C for amino acid analysis.
Concentrations of plasma citrulline and arginine were determined by amino-acid analysis on protein-free extracts. Amino acids were separated by cation-exchange chromatography using a Hitachi L8800 amino acid analyzer (Hitachi USA, San Jose, CA). Calibration of the analyzer was performed before piglet samples were tested.
Western blot of eNOS and nNOS in lung tissue.
Using a standard immunoblot technique as previously described, we analyzed samples of whole lung homogenates from normoxic controls (n = 3) and untreated hypoxic (n = 3) and l-citrulline-treated hypoxic (n = 3) animals for eNOS and nNOS. We used 10 and 30 mg of total protein for eNOS and nNOS, respectively, a dilution of primary eNOS or nNOS antibody of 1:500 (BD transduction), and a dilution of secondary anti-mouse antibody conjugated to horseradish peroxidase of 1:5,000 (11).
Calculations and statistics.
Pulmonary vascular resistance was calculated from the in vivo hemodynamic measurements: (pulmonary arterial pressure − left ventricular end diastolic pressure) ÷ (cardiac output/body wt).
Data are presented as means ± SD. The one-way ANOVA with Fisher's protected least significant difference (PLSD) post hoc comparison test was used to compare data between normoxic control and untreated hypoxic and l-citrulline-treated hypoxic animals. A P value of <0.05 was considered significant (21).
RESULTS
In vivo hemodynamic measurements.
Both l-citrulline-treated and -untreated chronic hypoxic animals had lower cardiac output and weights and higher left ventricular end-diastolic pressure measurements on the day of study at 12–13 days of age than comparable age normoxic control piglets (Table 1). We have previously shown that piglets grown under hypoxic conditions have less weight gain than those grown under normoxic conditions (9). Measurements of aortic pressure and arterial Po2 (PaO2) were similar (PaO2 was 74 ± 13 Torr in normoxic control piglets, 74 ± 16 Torr in untreated hypoxic piglets, and 78 ± 16 Torr in l-citrulline-treated hypoxic piglets) among groups. Values for arterial Pco2 (PaCO2) were significantly lower (P = 0.04) in the l-citrulline-treated hypoxic animals (30 ± 3 Torr) compared with both normoxic controls (39 ± 6 Torr) and untreated hypoxic (41 ± 12 Torr) animals. However, since the values of pH did not differ significantly between any of the groups of animals (Table 1), these differences in PaCO2 are unlikely to have had any physiological impact on the hemodynamic measurements.
Table 1.
Data for normoxic control, chronically hypoxic, and l-citrulline-treated chronically hypoxic piglets
| Treatment Group | Weight at 12 Days of Age, kg | Aortic Pressure, cmH2O | LVEDP, cmH2O | Cardiac Output, ml·min−1·kg−1 | Arterial pH |
|---|---|---|---|---|---|
| Controls (n = 6) | 3.94±0.7 | 91±9 | 5.2±1.5 | 414±105 | 7.38±0.12 |
| Chronic hypoxic (n = 11) | 2.76±0.5* | 100±12 | 7.4±1.7* | 244±00* | 7.38±0.04 |
| Citrulline hypoxic (n = 6) | 2.6±0.23* | 97±15 | 7.2±1.1* | 270±71* | 7.36±0.05 |
Values are means ± SD. LVEDP, left ventricular end-diastolic pressure.
Significnat difference vs. normoxic controls (P < 0.05; ANOVA with post hoc comparison test).
Notably, as shown in Fig. 1A, l-citrulline-treated hypoxic animals had significantly lower pulmonary artery pressures than untreated hypoxic animals. In addition, as shown in Fig. 1B, calculated pulmonary vascular resistance in those hypoxic animals treated with l-citrulline were significantly lower than those of untreated hypoxic animals. Furthermore, pulmonary vascular resistances were similar in l-citrulline-treated hypoxic animals and normoxic controls.
Fig. 1.
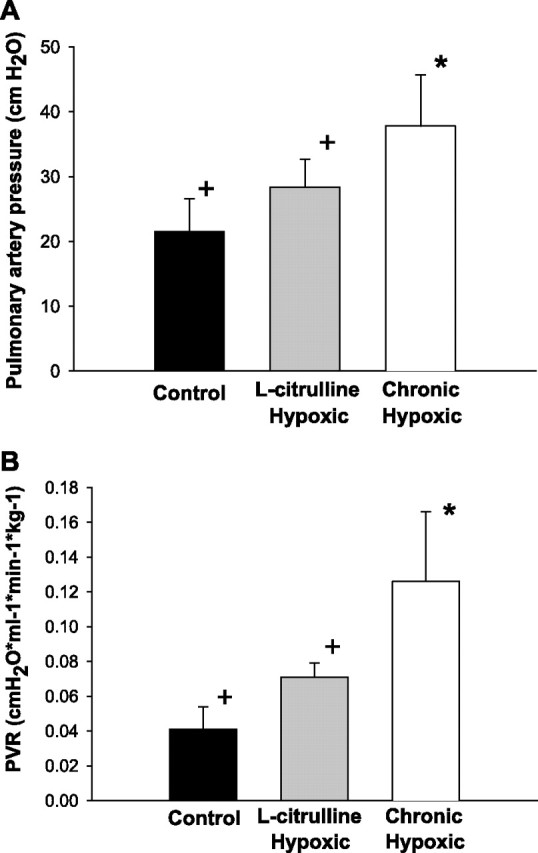
A: mean pulmonary arterial pressure measurements in normoxic control (n = 6), chronically hypoxic (n = 11), and l-citrulline-treated chronically hypoxic (n = 6) piglets. B: calculated pulmonary vascular resistance in normoxic control (n = 6), chronically hypoxic (n = 11), and l-citrulline-treated chronically hypoxic (n = 6) piglets. Values are means ± SD. Significantly different from normoxic control (*) and chronically hypoxic (+) (P < 0.05; ANOVA with post hoc comparison test).
Exhaled NO output and perfusate NOx−.
As shown in Fig. 2A, exhaled NO output in normoxic controls and l-citrulline-treated hypoxic animals were higher than exhaled NO output in untreated hypoxic animals. However, exhaled NO output did not differ between normoxic control and l-citrulline-treated hypoxic animals.
Fig. 2.

A: exhaled nitric oxide in normoxic control (n = 6), chronically hypoxic (n = 11), and l-citrulline-treated chronically hypoxic (n = 5) piglets. B: nitrite/nitrate accumulation in lung perfusate in normoxic control (n = 17), chronically hypoxic (n = 9), and l-citrulline-treated chronically hypoxic (n = 5) piglets. Values are means ± SD. Significantly different from *normoxic control (*) and chronically hypoxic (+) (P < 0.05; ANOVA with post hoc comparison test).
As shown in Fig. 2B, lungs from both the normoxic control and l-citrulline-treated hypoxic animals had significantly higher NOx− accumulation rates than lungs from untreated hypoxic animals. Furthermore, there was no difference in the rate of NOx− accumulation between lungs from l-citrulline-treated hypoxic animals and normoxic controls.
Plasma amino acids.
As shown in Table 2, although not reaching statistical significance, plasma l-citrulline levels in untreated chronic hypoxic piglets were less than trough l-citrulline levels in treated hypoxic piglets. Moreover, when drawn 90 min after a dose, levels of l-citrulline in treated hypoxic animals were almost twice that of the untreated chronic hypoxic animals. Levels of l-citrulline obtained at 30 (135 ± 60 μM) and 60 (156 ± 9 μM) min after a dose did not differ significantly from the 90-min value. Regardless of the time the sample was drawn, plasma arginine levels were not higher in l-citrulline-treated chronic hypoxic animals compared with untreated hypoxic animals.
Table 2.
Plasma amino acid levels for normoxic control, chronically hypoxic, and l-citrulline-treated chronically hypoxic piglets
| Treatment Group | Citrulline, μM | Arginine, μM |
|---|---|---|
| Normoxic controls (n =10) | 71±20 | 112±49 |
| Chronic Hypoxic (n =8) | 111±67 | 51±31* |
| l-Citrulline-treated hypoxic (90 min; n =3) | 219±63*,† | 43±8* |
| l-Citrulline-treated hypoxic (trough; n = 6) | 161±13* | 39±24* |
Values are means ± SD. Trough, plasma level ∼12 h after l-citrulline dose; 90 min, plasma level 90 min after administration of l-citrulline dose.
Significant difference vs. normoxic controls (P < 0.05; ANOVA with post hoc comparison test).
Significant difference vs. untreated chronic hypoxics (P < 0.05; ANOVA with post hoc comparison test).
Western blot for lung eNOS and nNOS protein.
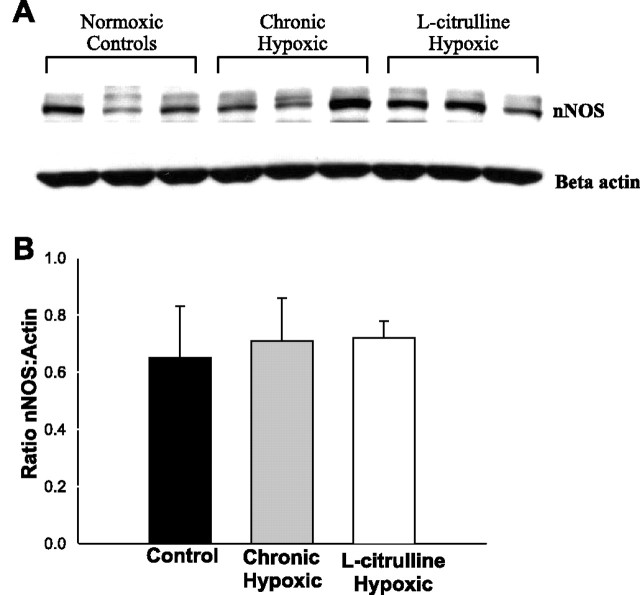
As shown in Fig. 3 and consistent with our previous studies (11), the amount of eNOS protein present in the lung tissue of normoxic control animals was significantly higher than that present in the lungs of untreated hypoxic animals. Furthermore, the amount of eNOS protein present in the lung tissue of l-citrulline-treated hypoxic piglets was not significantly different from that in the untreated hypoxic animals and was significantly lower than eNOS protein levels in normoxic control animals. As shown in Fig. 4, there was no difference in nNOS protein levels among the three groups.
Fig. 3.
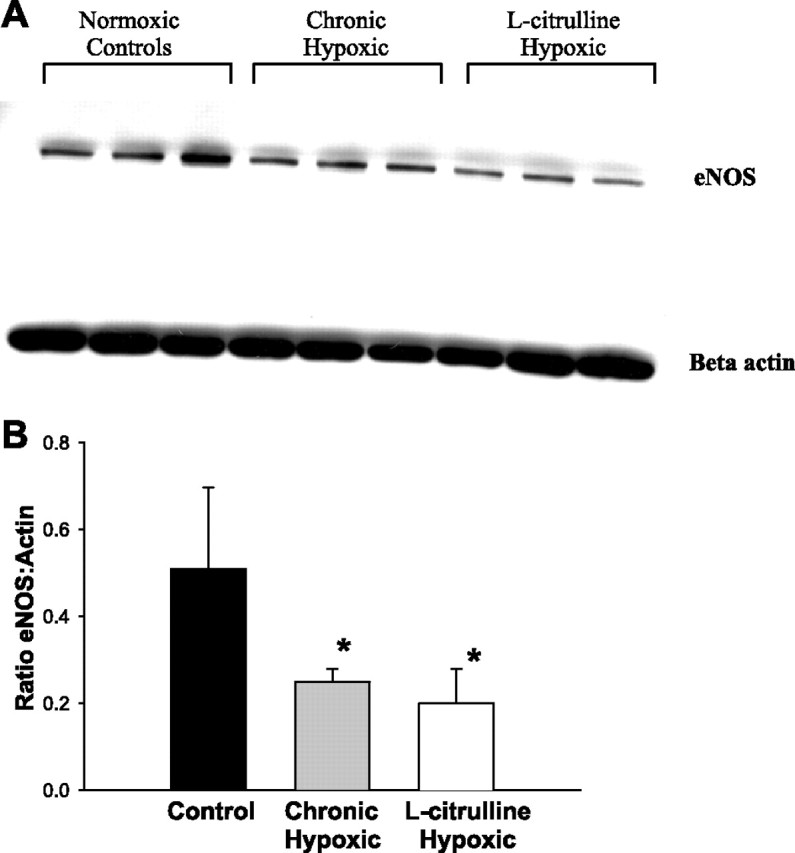
A: immunoblot for eNOS protein reprobed for beta actin for lung tissue from normoxic controls (n = 3), chronic hypoxic (n = 3), and l-citrulline-treated chronic hypoxic (n = 3) piglets. B: densitometry of eNOS normalized to beta actin for lung tissue from normoxic controls (n = 3), chronic hypoxic (n = 3), and l-citrulline-treated chronic hypoxic (n = 3) piglets Values are means ± SD. *Significantly different from normoxic control (P < 0.05; ANOVA with post hoc comparison test).
Fig. 4.
A: immunoblot for nNOS protein reprobed for beta actin for lung tissue from normoxic controls (n = 3), chronic hypoxic (n = 3), and l-citrulline-treated chronic hypoxic (n = 3) piglets. B: densitometry of nNOS normalized to beta actin for lung tissue from normoxic controls (n = 3), chronic hypoxic (n = 3), and l-citrulline-treated chronic hypoxic (n = 3) piglets. Values are means ± SD.
DISCUSSION
In this study, we found that l-citrulline supplementation ameliorates the development of pulmonary hypertension in newborn piglets exposed to 10 days of chronic hypoxia. To our knowledge, this is the first study showing the effectiveness of l-citrulline in preventing the development of pulmonary hypertension in either newborn or more mature animal models of this disease.
Other important findings in this study are that both exhaled NO production and pulmonary vascular NOx− accumulation rates are greater in l-citrulline-treated hypoxic piglets than in untreated hypoxic piglets. Thus our findings clearly show that l-citrulline supplementation significantly increased pulmonary NO production. In addition, our finding that the amounts of eNOS and nNOS protein are unchanged in the l-citrulline-treated hypoxic animals suggests that the mechanism for this increase in pulmonary NO production is not an increase in NOS expression.
Based on the current literature (13, 17, 30, 32), the mechanism by which l-citrulline mediates an increase in NO production could be by improving NOS function. One possible mechanism for improving NOS function is by increasing the amount of l-arginine available as a substrate for eNOS. Assessment of arginine availability for NO synthesis has been a challenge that has been addressed by many investigators. Plasma levels of arginine in the l-citrulline-treated animals in this study were not significantly increased compared with untreated hypoxic animals. However, this finding was not surprising since total cellular levels of l-arginine have not been found to accurately reflect subcellular levels of l-arginine available for NO synthesis. Su and Block (32) attempted to show that decreased NO production in pulmonary endothelial cells exposed to hypoxia was due to a decrease in cellular l-arginine content. They found that, rather than being decreased, cellular l-arginine content was actually increased by degradation of cellular proteins in response to hypoxia and hypothesized that this increased supply of l-arginine was unavailable to eNOS (32). Solomonson et al. in 2003 showed that providing l-arginine to endothelial cells increased NO production only slightly compared with the more dramatic increase in endothelial NO production found with l-citrulline supplementation (30). In addition, l-citrulline supplementation increased total cellular arginine only slightly compared with the significant increase in total cellular arginine after l-arginine supplementation. Thus, similar to Su and Block, these authors concluded that there was no correlation between total cellular arginine and endothelial NO production (30). Based on findings from these and other studies (13, 17), eNOS function seems to be dependent on a pool of arginine that is isolated from the bulk of intracellular arginine and is maintained through an efficient arginine regeneration enzymatic process in close proximity to eNOS.
This discordance between intracellular arginine and NO production, termed the “arginine paradox,” explains the increase in NO production in the face of unchanged plasma arginine levels seen with l-citrulline supplementation in this study. l-Citrulline is a urea cycle intermediate metabolized to arginine by a recycling pathway consisting of two enzymes, argininosuccinate synthase (AS) and argininosuccinate lyase (AL). These two enzymes, AS and AL, have been found colocated with eNOS in pulmonary endothelial cells (7). It is thought that together these enzymes produce a separate subcellular pool of arginine used exclusively for NO synthesis. Tissue and plasma arginine levels cannot accurately measure this subcellular pool.
l-Citrulline may also have improved NO production and eNOS function by additional mechanisms. Recently, it has been suggested that, in the setting of ischemia and reperfusion injury, the enzyme eNOS (a dimer) uncouples and produces superoxide instead of NO (7). There is evidence that this uncoupling of eNOS occurs in the presence of low levels of arginine or BH4, a necessary cofactor for the production of NO (35). Hence, another potential action of l-citrulline in this study is the prevention of the uncoupling of eNOS by maintaining adequate levels of its substrate arginine. We have yet to explore this possibility.
l-Citrulline has been used in several patient populations with some success. In addition to those patients with urea cycle defects, patients with sickle cell disease receiving citrulline have shown improved disease symptoms (36). In children undergoing cardiopulmonary bypass at risk for development of postoperative pulmonary hypertension, Smith et al. recently showed that oral supplementation with l-citrulline increased both plasma citrulline and arginine levels (29). Moreover, postoperative pulmonary hypertension did not develop in those children who had plasma citrulline levels greater than 37 μM/l. Furthermore, intravenous l-citrulline has been shown to be safe and well tolerated in this same patient population of children undergoing bypass by Barr et al. (4).
Notably, l-citrulline therapy has been used in animal models of vascular diseases other than our model of chronic hypoxia-induced pulmonary hypertension. In rabbits fed a high-cholesterol diet, l-citrulline supplementation causes regression of atheromatous lesions (16). In spontaneously hypertensive rats, maternal supplementation with l-citrulline increased renal NO production and ameliorated hypertension in offspring (18). Therefore, it would seem that l-citrulline may be useful for improving NO dysfunction in conditions other than hypoxia-induced pulmonary hypertension.
Although l-citrulline has not been widely studied as a therapy for pulmonary hypertension, l-arginine supplementation has been used frequently with mixed results. For example, treatment with l-arginine has been shown to prevent the development of pulmonary hypertension in two adult rat models of pulmonary hypertension (22, 25). Furthermore, administration of l-arginine was shown to reverse evidence of postoperative pulmonary vascular endothelial dysfunction in children who had undergone cardiopulmonary bypass and to restore impaired pulmonary vasorelaxation in adults with pulmonary hypertension (6, 8, 20, 24, 27). Although these studies provide evidence that l-arginine may help prevent the development of pulmonary hypertension and may be helpful once pulmonary hypertension has developed, serious adverse effects of l-arginine treatment have been suggested, and variable results from l-arginine treatment have been reported (5, 26). Because arginine is involved in other processes in the body and is quickly metabolized by arginases in many cellular compartments, supplementation often requires high doses, i.e., 9 g/day, in adults (26). These massive doses are sometimes poorly tolerated, and patient compliance can be difficult to maintain (33).
There are several limitations of this study that merit comment. First, we have been unable to detect iNOS protein in lung tissue from newborn piglets using those antibodies currently commercially available. Thus, although we have shown that eNOS and nNOS protein levels in lung tissue are unchanged with l-citrulline therapy, we cannot rule out the possibility that an increase in iNOS protein contributes to the increase in NO production and decrease in pulmonary vascular resistance in l-citrulline-treated hypoxic piglets. In addition, eNOS has been shown to be present in respiratory epithelium as well as pulmonary vascular endothelium (34). Therefore, Western blots of whole lung homogenates cannot establish the precise anatomical site of any change in lung eNOS expression.
Another study limitation is that we did not measure AS and AL amounts or activities. It is possible that changes in the amount or activity of these enzymes that are colocated with eNOS could contribute to alterations in NO production. Yet another limitation is that our study findings do not address the possibility that l-citrulline therapy may have effects in normoxic animals. Also, because isolated lung perfusion requires disruption of the right ventricle morphology and can cause edema and distortion of the pulmonary architecture, we were unable to assess the effect of l-citrulline therapy on either right ventricular hypertrophy or pulmonary vascular remodeling. We were unable to assess the changes in pulmonary vasoreactivity since the agonists used to determine reactivity can potentially alter lung NO production. In addition, vessels harvested from isolated perfused lung preparations are no longer viable for use in pressurized, cannulated artery studies. Further studies are required to more extensively evaluate the mechanisms underlying the effect of l-citrulline therapy on NOS function, potential changes in vasoreactivity, and the development of pulmonary hypertension.
In summary, our findings show that l-citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. We also provide evidence that the effectiveness of citrulline is due to increased NO production, which is likely due at least in part to an increase in NOS function since neither eNOS nor nNOS protein levels are changed. It is possible that l-citrulline may be a useful therapy in neonates at risk of developing pulmonary hypertension due to conditions associated with impaired NO function, including chronic or intermittent unresolved hypoxia.
Acknowledgments
This work was supported by an American Heart Association affiliate grant to C. D. Fike.
REFERENCES
- 1.Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed : F15–F18, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen J, subcommittee AoP ATS. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med : 356–396, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Aschner J. New therapies for pulmonary hypertension in neonates and children. Pediatric Pulmonol : S132–S135, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Barr FE, Tirona RG, Taylor MB, Rice G, Arnold J, Cunningham G, Smith HA, Campbell A, Canter JA, Christian KG, Drinkwater DC, Scholl F, Kavanaugh-McHugh A, Summar ML. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery:potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg : 319–326, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Boger RH. l-Arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr and Met Care : 55–61, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Boger RH, Bode-Boger SM, Heinzel D. Differential systemic and pulmonary haemodynamic effects of l-arginine in patients with coronary heart disease and primary pulmonary hypertension. Int Clin Pharmacol Ther : 323–328, 1996. [PubMed] [Google Scholar]
- 7.Dragoni S, Gori Stolfo GD T, Sicuro S, Forconi Parker JD S. Folic acid does not limit endothelial dysfunction induced by ischemia and reperfusion. J Cardiovasc Pharmacol : 494–497, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by l-arginine. Lancet : 1546–1550, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Fike CD, Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol : 2853–2862, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Fike CD, Kaplowitz MR, Rehorst-Paea LA, Nelin LD. l-Arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J Appl Physiol : 1797–1803, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Fike CD, Kaplowitz MR, Thomas CJ, Nelin LD. Chronic hypoxia decreases nitric oxide production and endothelial nitric oxide synthase in newborn pig lungs. Am J Physiol Lung Cell Mol Physiol : L517–L526, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide : 187–197, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin BL, Solomonson LP, Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem : 18353–18360, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Haworth SG, Hislop AA. Adaptation of the pulmonary circulation to extra-uterine life in the pig and its relevance to the human infant. Cardiovasc Res : 108–119, 1981. [DOI] [PubMed] [Google Scholar]
- 15.Haworth SG, Hislop AA. Effect of hypoxia on adaptation of the pulmonary circulation to extra-uterine life in the pig. Cardiovasc Res : 293–303, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Juliet PAR, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. l-Citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc Natl Acad Sci USA : 13681–13686, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of l-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle l-citrulline to l-arginine. Proc Natl Acad Sci USA : 8612–8616, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koeners MP, Van Faassen EE, Wesseling S, Sain-van der Velden M, Koomans HA, Braam B, Joles JA. Maternal supplementation with citrulline increases renal nitric oxide in young spontaneously hypertensive rats and has long-term antihypertensive effects. Hypertension : 1077–1084, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Liu JQ, Zelko IN, Erbynn EM, Sham JSK, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol : L2–L10, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with l-arginine in pulmonary hypertension. Circulation : 1539–1545, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Meier U. A note on the power of Fisher's least significant difference procedure. Pharm Stat : 253–263, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Mitani Y, Maruyama K, Minoru S. Prolonged administration of l-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation : 689–697, 1997. [PubMed] [Google Scholar]
- 23.Mupanemunda RH. Current status of inhaled nitric oxide therapy in the perinatal period. Early Hum Dev : 247–262, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Pernow J, Bohm F, Beltran E, Gonon A. l-Arginine protects from ischemia-reperfusion-induced endothelial dysfunction in humans in vivo. J Appl Physiol : 2218–2222, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki S, Asano M, Ukai T, Nomura N, Maruyama K, Manabe T, Mishima A. Nitric oxide formation and plasma l-arginine levels in pulmonary hypertensive rats. Respir Med : 205–212, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. l-Arginine therapy in acute myocardial infarction: the vascular interaction with age in myocardial infarction (VINTAGE MI) randomized clinical trial. JAMA : 58–64, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Schulze-Neick I, Penny DJ, Rigby ML, Morgan C, Kelleher A, Collins P, Li J,Bush A, Shinebourne EA, Redington AN. l-Arginine and substance P reverse the pulmonary endothelial dysfunction caused by congenital heart surgery. Circulation : 749–755, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Shimoda L, Sham JSK, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res : 549–560, 2000. [PubMed] [Google Scholar]
- 29.Smith HAB, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, Rice GD, Barr FE, Summar ML. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg : 58–65, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol : 2083–2087, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Subhedar NV. Recent advances in diagnosis and management of pulmonary hypertension in chronic lung disease. Acta Paediatr Suppl : 29–32, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Su Y, Block ER. Acute hypoxia increases intracellular l-arginine content in cultured porcine pulmonary artery endothelial cells. J Cell Physiol : 349–353, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Tenenbaum A, Fisman EZ, Motro M. l-Arginine: rediscovery in progress. Cardiology : 153–159, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Turley JE, Nelin LD, Kaplowitz MR, Zhang Y, Fike CD. Exhaled NO is reduced at an early stage of hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol : L489–L500, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Verma S, Maitland A, Weisel RD, Fedak PWM, Pomroy NC, Li SH, Mickle DAG, Li RK, Rao V. Novel cardioprotective effects of tetrahydrobiopterin after anoxia and reoxygenation: identifying cellular targets for pharmacologic manipulation. J Thorac Cardiovasc Surg : 1074–1083, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Waugh WH, Daeschner CWIII, Files BA, McConnell ME, Strandjord SE. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. J Natl Med Assoc : 363–371, 2001. [PMC free article] [PubMed] [Google Scholar]