Keywords: enteroids, intestinal stem cell, organoids, regeneration
Abstract
Intestinal organoid cultures provide an in vitro model system for studying pathways and mechanisms involved in epithelial damage and repair. Derived from either embryonic or induced pluripotent stem cells or adult intestinal stem cells or tissues, these self-organizing, multicellular structures contain polarized mature cells that recapitulate both the physiology and heterogeneity of the intestinal epithelium. These cultures provide a cutting-edge technology for defining regenerative pathways that are induced following radiation or chemical damage, which directly target the cycling intestinal stem cell, or damage resulting from viral, bacterial, or parasitic infection of the epithelium. Novel signaling pathways or biological mechanisms identified from organoid studies that mediate regeneration of the epithelium following damage are likely to be important targets of preventive or therapeutic modalities to mitigate intestinal injury. The evolution of these cultures to include more components of the intestinal wall and the ability to genetically modify them are key components for defining the mechanisms that modulate epithelial regeneration.
INTRODUCTION
The mammalian intestine supports a myriad of functions, including digestion, secretion, absorption, barrier function, movement/elimination of waste, immune responses, and provision of a home for the microbiome. Consisting of a tube that is open at both ends to the outside environment, the lumen is lined by an epithelium that is at the core of the gastrointestinal tract’s many functions. Homeostasis of this epithelium is associated with rapid turnover that results in complete renewal of cells approximately every 3–7 days. Disruptions in homeostasis of the epithelium can occur through a variety of means, including radiation- or chemotherapy-based therapeutic treatments, infection with pathogenic organisms, and inflammation. Repair of the damaged epithelium and restoration of epithelial homeostasis is dependent on the intestinal stem cell (ISC) and its associated environment, referred to as the ISC niche. Although both Drosophila melanogaster and mice have provided valuable insight into epithelial regeneration, they are limited in the ability to fully predict the human ISC response due to differences in intestinal anatomy, physiology, genetics, microbiome, environment, and diet. Transformed human cell lines have provided an in vitro system in which many fundamental biological questions have been addressed. However, their inability to accurately replicate the complex physiology and biology of the normal human epithelium has been an important drawback. Organ cultures of human intestine provide a more biologically relevant model but have limitations due to rapid cell death and the inability to renew cells. In the last 10 years, the development of new in vitro models of the human intestinal epithelium, termed organoids, has significantly advanced understanding of the biology of the human intestine by providing an in vitro model that mirrors the complexity of in vivo epithelium. Recently, these models have come to the forefront as key tools to understand how the epithelium undergoes repair after damage. Here, we review the use of organoid cultures to study intestinal regeneration following injury due to radiation and chemical treatment, as well as damage that results from pathogenic infections.
ORIGIN OF INTESTINAL ORGANOID CULTURES
In vitro models of the intestinal epithelium can be derived from human and animal stem cells (50, 52, 56). These stem cell-derived cultures develop into three-dimensional organoids with a spherical shape that surrounds a lumen (Fig. 1). The organoid cultures are genetically stable and grow indefinitely (52), in contrast to primary intestinal cells or tissue explant models, which only offer short-term culture capabilities. After differentiation by manipulation of various growth factors, the cultures contain polarized differentiated cells of the intestinal epithelium. These self-organizing, multicellular structures recapitulate many properties of the intestine, including the heterogeneity of the cellular composition, appropriate physiology, and region-specific features of the intestine. Additionally, these cultures allow for the study of the human intestinal epithelium in the context of individual genetic variability, disease status, and other demographic factors including age, gender, and ethnicity, and they are expected to become a key component for developing personalized approaches to medical treatments for intestinal diseases.
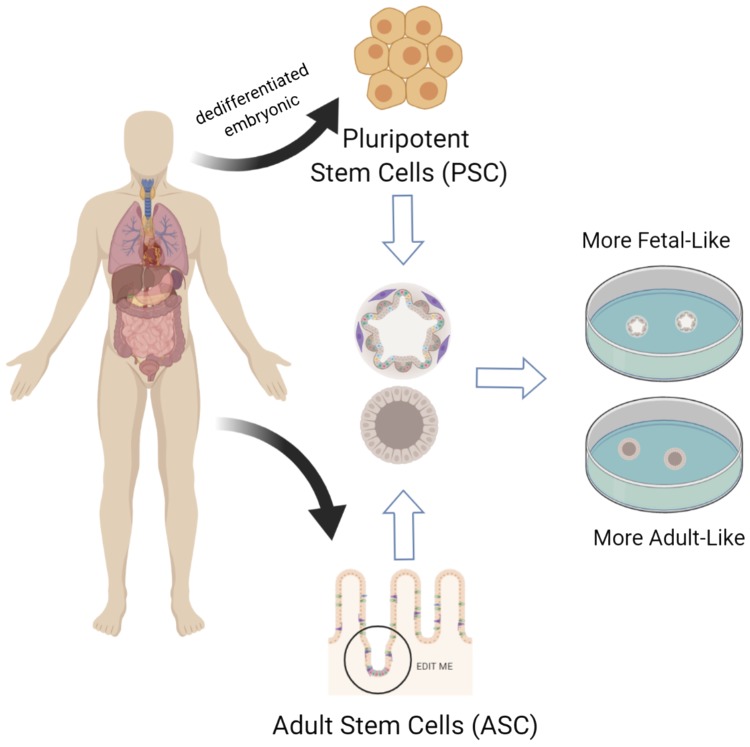
Fig. 1.
The origin of human intestinal organoid cultures. Intestinal organoid cultures can be derived from either human embryonic stem cells or induced human pluripotent stem cells or adult stem cells derived from the intestinal tissue itself. These stem cells are propagated in vitro and form three-dimensional structures grown in matrigel matrix in a tissue culture dish. Figure was created with Biorender.com.
Human Pluripotent Stem Cell-Derived Organoids
Intestinal organoid cultures are derived from either human embryonic or human-induced pluripotent stem cells (PSC) (Fig. 1). Based on gene expression profiling (13), both embryonic and human-induced, PSC-derived organoids contain a more fetal-like epithelium when compared with native adult intestinal epithelium (13). Consisting of an intestinal epithelial layer surrounded by mesenchyme, limited regional specificity (proximal small intestine and colon) can be controlled by growth factor exposure during derivation (35, 60). A more mature phenotype, including formation of crypts and villi as well as underlying stroma and muscle layers, can be induced by transplantation of the PSC organoid into heavily vascularized areas such as below the kidney capsule or in the omentum of immunocompromised mice (7, 66). These organoids have particular advantages in studying epithelial-mesenchymal interactions and intestinal development and can be used to study how these components aid in repair of the epithelium following damage resulting from radiation, chemotherapy, and intestinal infections.
Adult Stem Cell-Derived Organoids
Adult stem cell (ASC)-derived organoids, referred to as enteroids (57), are derived from either the intestinal crypts (50) or isolated leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5)+ ISCs (52, 73) from human or animal intestinal tissue (Fig. 1). These primary cells are cultured with various growth factors necessary for their growth and propagation (23, 52). Removal of these growth factors promotes epithelial differentiation resulting in the presence of mature intestinal epithelial cells (goblet, enteroendocrine, tuft, enterocyte, Paneth) in addition to the ISCs. These differentiated cells are present in the appropriate ratios in the cultures, and their locations within the organoid somewhat replicate the cellular organization in vivo (17, 51). Unlike the PSC-derived organoids, the ASC-derived organoids lack a mesenchymal component but more closely model adult epithelium and retain region-specific functions that are useful for studying mature human epithelial biology. Most importantly, the ASC-organoids contain the fully developed human adult ISC population, which previously could not be generated or propagated in vitro. Thus, they offer a new system in which the biology of stem cells can be more closely studied in the context of epithelial damage following radiation, chemotherapy, and intestinal infections.
USING ORGANOID CULTURES TO STUDY INTESTINAL INJURY AND REGENERATION RESULTING FROM RADIATION AND CHEMOTHERAPY
Radiation and Chemical Damage of the Epithelium
Radiation and chemotherapy, which are used to treat cancer and other conditions, are toxic to proliferating ISCs and dramatically affect the regenerative capacity of the intestinal epithelium. Side effects from these treatments, including nausea, vomiting, dyspepsia, abdominal pain, and ulcerations that occur as a result of decreased stem cell reserves and ability to repair cell damage, cause both short- and long-term complications in the gastrointestinal tract (4, 54). Currently, there is no approved agent to treat radiation- or chemical-induced GI injury or complications. In vivo models of radiation and chemotherapy have been used extensively to interrogate the mechanisms of regeneration of the proliferative ISC (1, 9, 30, 34, 43, 47, 48, 55, 71, 72, 74). Several hypotheses have been proposed to explain how the epithelium recovers from toxic damage. These include crypt fission, in which surviving crypts expand to replace crypts lost due to stem cell depletion (68), the existence of quiescent stem cells that repopulate the cycling ISC (2, 34, 42, 58), and the capacity of differentiated epithelial cells that exhibit cell plasticity and revert into ISCs (1, 5, 22, 59, 62, 71, 72, 74). The signaling pathways that mediate the regeneration of the ISC after damage are important avenues for understanding how ISC and niche homeostasis is regained. Both chemical and radiation damage can be applied to intestinal organoids, and the response of the epithelium in these models will help identify the upstream regulators of the signaling pathways that are modulating epithelial repair.
Intestinal Organoids and Radiation Damage
Currently, organoid cultures are being utilized in two different approaches to examine recovery of the epithelium after radiation damage: 1) irradiation is performed in vivo in the mouse, and subsequently intestinal organoid formation is assessed in vitro (organoid-forming assay (63, 70) and 2) organoids are established from either human or mouse intestine and the organoids themselves are exposed to radiation in vitro (70). The organoid-forming assay has been used primarily to dissect key pathways in regeneration following irradiation of reporter and knockout mice in which specific components of the pathways are deleted or altered. In a recent study, the organoid-forming assay demonstrated that the myeloid translocation gene MTG16 plays a role in negatively regulating regeneration (41). This assay was also used to demonstrate that SRY-box transcription factor 9 (SOX9)+ epithelial cells can survive and expand to form organoids after exposure to radiation in vivo (63). Organoid-forming assays were used to demonstrate that the lack of expression of blood vessel epicardial substance, which negatively regulates WNT, resulted in increased organoid plating efficiency, spheroid formation, and proliferation and expression of stem cell markers following radiation exposure in vivo (45). A radio-resistant cell that expresses kertain 19 (KRT19) has been identified in murine colonic organoid-forming assays that seems to render the LGR5+ ISC dispensable for recovery from radiation (1). Radiation treatment of established organoids has resulted in identification of potential mitigators of radiation-induced gastrointestinal syndrome. Using human colonic organoids, an antineoplastic small molecule, BCN057, was shown to induce regeneration and mitigate toxicity of radiation exposure (3). Regeneration was quantified by increased organoid budding and transcriptional expression of stem cell and proliferative markers, such as LGR5 and KI67, respectively. Wnt-β catenin signaling, which is an important component of the ISC niche (8), was induced by BCN057 and accelerated the repair pathway in the organoids. In similar studies (36), human colonic organoids were used to demonstrate that Auranofin, an anti-inflammatory agent, was protective against radiation-induced damage and improved survival in the cultures via activation of p53/p21 cell cycle arrest that was reversible (36). Addition of CHIR9901, a GSK-3 inhibitor, to human intestinal cultures significantly improved survival and growth after radiation (65) via selective blockade of p53-induced apoptosis in the ISC. These in vitro organoid studies suggest that p53 regulation may be important to radioprotective responses. Using a high-throughput format to identify molecules associated with increased murine small intestinal-organoid recovery following irradiation, a library of 2,534 compounds was screened and 2 compounds were identified that increased recovery from gamma irradiation. Two compounds were identified that improved the viability of the organoid cultures following radiation exposure via mechanisms that had not been previously linked to radiation protection: CGP052608, a synthetic thiosemicarbaszone that acts through retinoic acid receptors, and G023850, which inhibits methionine amnopetidases (29). These organoid studies suggest potential new target pathways that could be modulated to mediate the effects of radiation. Although mouse organoids were used in the screen, this approach seems easily transferable to human organoids. Automation will be a key component in the evolution of using organoids in a high-throughput format to screen for compounds that mitigate radiation injury. An automated organoid morphometry platform has been developed to assess growth survival and regeneration of intestinal organoids following irradiation (46). Platforms such as these are currently facilitating drug screens to identify candidates to counteract the side effects of radiation exposure in the intestine.
Intestinal Organoids and Chemical Damage
Chemotherapeutic agents, similar to radiation, have detrimental side effects on the ISC and cause significant complications. The effect of these agents on epithelial regeneration has begun to be explored using intestinal organoid cultures. Human intestinal organoid cultures were used to understand the dual role of p53 pathways in the damage resulting from treatment with the chemotherapeutic agent, irinotecan (CPT-11), as well as the regenerative response of the epithelium following the damage (27). A small molecule inhibitor of PUMA, a transcriptional target of p53 induction that is important in radiation-induced apoptosis, enhanced preservation of the LGR5+ ISC and promoted organoid growth and epithelial regeneration following CPT-11 damage in both mouse and human colonic organoids. Human organoid cultures have demonstrated the plasticity of Paneth cells to revert to LGR5+ ISC following doxorubicin damage (6). This work provides strong support for a role of the Paneth cell in regeneration when the crypt ISC is damaged by chemical agents. The effect of various inhibitors and growth factors on cell growth and Paneth cell reversion revealed a role for R-spondin, phosphatase and tensin homolog (PTEN), and AKT signaling in the regenerative response. These studies illustrate the capacity of using intestinal organoids to discover new pathways and mechanisms that are potential targets to mitigate chemotherapy-induced intestinal injury.
USING ORGANOID CULTURES TO STUDY REGENERATION FOLLOWING INTESTINAL INFECTIONS
Gastrointestinal Infections and Epithelial Damage and Repair
More than 76 million people in the United States suffer from gastrointestinal infections (viral, bacterial, parasitic) each year (49). In contrast to radiation or chemical damage, which directly target the cycling LGR5+ ISC, pathogenic infections of the intestine predominantly cause damage to the differentiated epithelium that, in many cases, results in villus blunting or atrophy. Because of the clinical importance of these diseases, there is increased interest in understanding the effect of pathogen-induced damage on the ISC response and comparing it to the effects of radiation/chemotherapy. In vitro transformed human cell lines or human explant tissue models have been limited in their use to study regeneration after infection due to the lack of cellular complexity in modeling the intestinal epithelium, the absence of stem cells, and the short-term viability in culture that does not allow infections to be examined. In vivo systems, including studies in mice, better model the complexity of infections and have been used widely; however, these models are not susceptible to many human pathogens or do not exhibit comparable pathogenesis, limiting their use in understanding how the epithelium repairs itself after infections. Despite their limitations, mouse models have revealed that intestinal infections can result in effects on the ISC and its niche. Some pathogenic bacterial products, such as Salmonella AvrA and type III secretion components, appear to increase ISC proliferation through expansion of Paneth cells and upregulation of Wnt signaling in vivo (32, 33). Rotavirus, a common viral infection of the mature enterocytes, stimulates Paneth cell production of WNT and Wnt signaling in the crypt that results in increased proliferation and migration of the epithelial cells in mice (80). In contrast to uninfected tissue where the mesenchyme appears to be the primary modulator of ISC activity (61), epithelial components such as the Paneth cell seem to be a major factor in the regenerative response to infection. Thus, intestinal organoid cultures, which maintain the complexity and physiology of the intestinal epithelium, provide an ideal system to dissect the molecular pathways of the epithelial response and allow for the study of many human intestinal pathogens that previously were unable to be studied outside the human host. Most pathogens initially contact the intestinal epithelium on the apical or luminal side. Modeling this contact interaction using three-dimensional organoids (where the apical side of the epithelium faces the lumen of the organoid) has been somewhat problematic, requiring either labor-intensive microinjection techniques (67) or that the organoids be broken open (53) (Fig. 2). Establishment of culture conditions to grow organoid cultures in a monolayer format that exposes the apical surface (64) makes delivery of infectious organisms much less labor intensive (Fig. 2), and infections using this format have already been demonstrated (12). Pathways identified from these studies will provide a more complete picture of the numerous signals to which the ISC can respond and potentially provide new targets to direct therapeutics to modulate epithelial regeneration.
Fig. 2.
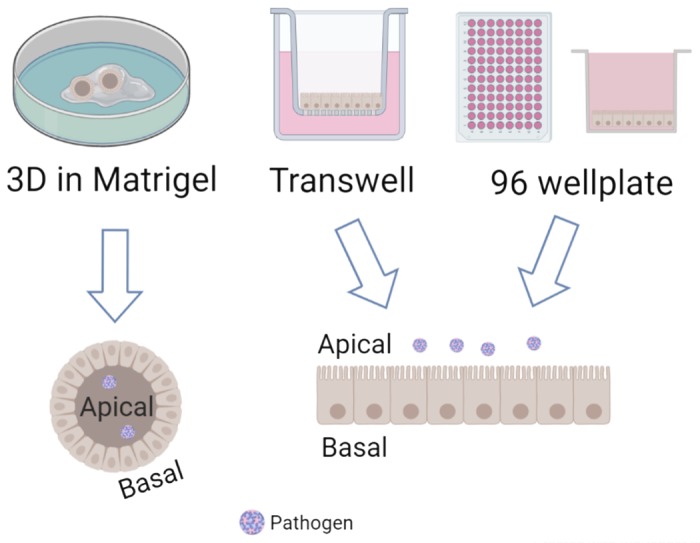
Formats of organoid culturing. Organoids can be cultured as three-dimensional (3D) structures enclosed within a matrigel plug. 3D organoids typically have the apical side of the epithelium facing the inside lumen of the 3D structure, making it difficult to easily access. 3D organoids can be dispersed and grown as a monolayer either in a 96-well plate or on a transwell membrane. In either format, there is easy access to the apical side of the epithelium. Figure was created with Biorender.com.
Organoids and Viral Infections
Despite the role of viral infections as one of the leading causes of acute gastroenteritis worldwide, the effect of viral damage on epithelial regeneration of the human intestinal tract has not been well characterized, in part due to a lack of in vitro models in which the pathogenesis of human viruses recapitulates the in vivo properties. Several human intestinal viruses, including rotavirus, norovirus, enterovirus, adenovirus, and coronavirus, have now been demonstrated to infect human intestinal organoid cultures (10, 12, 14, 19, 53, 79). Human rotavirus infects human organoid cultures much more efficiently than animal rotavirus (53), recapitulating in vivo epidemiology findings. Organoid cultures are able to functionally and physiologically recapitulate some of the damage aspects of in vivo human rotavirus infection, including cytotoxicity and fluid flux characteristic of secretory diarrheas (53). The organoid model has also enabled study of the interactions between human norovirus and the epithelium. Ettayebi et al. (12) demonstrated human noroviruses isolated from stool replicate in human organoid cultures with evidence of cell rounding, destruction of the epithelium, and an increase in dead cells. Additionally, these studies found that the enterocyte is most likely the site for human norovirus replication. In a similar fashion, human adenoviruses were shown to have tropism for goblet cells in organoid cultures, which was not previously known (19). Human enterovirus also appears to replicate in the enterocyte population in organoid cultures and, like rotavirus and norovirus, induces significant cytotoxic effects accompanied by disruption of tight junctions that results in loss of barrier function and cell necrosis (10). Although the organoid cultures are now being widely used to study pathogenesis, studies examining regenerative responses of the epithelium following viral damage are in their infancy. Although spread of avian influenza strains by the fecal oral route is well documented and makes disease hard to prevent and control, little is known about its pathogenesis in the intestine. Human intestinal organoids were used to demonstrate that an avian influenza strain that infects the intestinal tract also impairs ISC proliferation and differentiation (20). This virus appears to damage the Paneth cells in the organoids presumably causing indirect effects on ISCs. The use of organoid cultures to study regeneration of the epithelium following viral damage has the potential to provide insight on epithelial renewal in situations where the differentiated cells are damaged but the crypt remains relatively intact.
Organoids and Bacterial Infections
Intestinal bacterial pathogens also cause a large percentage of acute gastroenteritis worldwide. Organoid cultures have created new models of human bacterial infections in which epithelial regeneration after bacteria damage can be explored. The intestinal organoid culture recapitulates in vivo pathologies of several human pathogenic intestinal bacteria, including Salmonella enterica and typhimurium; Enteropathogenic, Enterotoxigenic, Enterohemorrhagic, and Enteroaggregative Escherichia coli; and Clostridium difficile (11, 15, 21, 24, 28, 44). In particular, some bacterial infections, such as C. difficile (28) and Salmonella (78), recapitulate the tight junction damage that is often associated with reduced barrier function. As with viral infections, regeneration of the intestinal epithelium following bacterial infections is only beginning to be explored via organoid culture models. Recent work using both human and mouse organoids has suggested that the levels of cytokines that are induced by bacterial infections have a significant effect on the ISC niche by modulating proliferation, cell fate, architecture, antimicrobial secretion, and crypt cell hierarchy (31). With the establishment of several models of human bacterial infections in the organoid cultures, scientists are now poised to begin to understand intestinal epithelial regeneration following bacterial injury.
A recent effort is the utilization of organoid cultures to understand the effects of commensal intestinal bacteria on epithelial homeostasis and repair (40). New work has shown that the ISC expresses nucleotide-binding oligomerization domain-containing protein 2 (NOD2), which is stimulated by a peptidoglycan motif that is expressed on most bacterial organisms, suggesting putative pathways that might be conduits for communication between the microbiome and the ISC niche (38). Treatment of organoid cultures with ligands for NOD2 resulted in increased numbers and larger-sized organoids, indicating that these ligands induce epithelial proliferation. These ligands also exhibited cytoprotective effects when organoids were treated with doxorubicin. Support for the concept of a role for bacterial species in the ISC niche comes from studies in mice where a crypt-specific microbiome has been identified that associates with homeostatic proliferation in the colonic crypt. This finding led to organoid studies that demonstrated that modulation of the colonic epithelial balance between proliferation and differentiation occurred through a TLR4-dependent pathway that was triggered by bacterial-derived LPS (37). Other work using the organoid-forming assay has shown that colonic crypts from mice that are devoid of the microbiota lose their regenerative capacity, as assessed by the ability to form organoids (76). The regenerative capacity was recovered by fecal microbiota transplantation that restored the crypt microbial communities (76). Recently, lactate derived from bacteria was shown to mediate small intestinal epithelial proliferation through stimulation of the LGR5+ ISC in murine organoid cultures (26), suggesting there may be specific bacterial-derived factors that interact with the host cells to modulate the ISC response. These initial findings provide strong rationale for exploring the role of the microbiome in homeostasis of the human ISC niche. A role for the microbiome in repair after injury still remains to be defined. The organoid system is just beginning to be used extensively to study whether commensal microbial organisms and their products control crypt homeostasis or contribute to epithelial repair.
Organoids and Parasitic Infections
Although parasites are a significant cause of morbidity and mortality from diarrheal disease, the use of organoids to better understand the pathogenesis of these organisms has lagged behind that of the viral and bacterial fields. Recently, parasitic infections such as Cryptosporidium, Nippostrongylus brasiliensis, and Toxiplasma gondii have been established in organoid cultures (16, 18, 25) and appear to accurately model in vivo infectivity and pathogenesis, surmounting a major barrier in studying these pathogens. Mouse organoids infected with Cryptosporidium demonstrated decreased organoid budding (a marker of proliferation) and LGR5+ ISC marker expression (77), mimicking in vivo findings. Organoid-forming assays performed on crypts isolated from Cryptosporidium-infected mice showed a similar lack of budding compared with uninfected controls (77). The Wnt pathway, a key factor in ISC function, appeared to be inhibited by the pathogen in the organoid culture. Because Cryptosporidium infection is limited to enterocytes, there may be unique signals that arise from the epithelium that modulate the ISC through regulation of the niche. More extensive studies will be necessary to illuminate the mechanisms of epithelial repair following parasitic infection.
SUMMARY
The gap between in vitro human-transformed cell lines and in vivo animal models has now been filled by the establishment of human intestinal organoid culture, in which the human nontransformed epithelium can be cultured and the biology dissected. These cultures allow exquisite control of experimental conditions surrounding epithelial damage and regeneration in vitro. The capability of organoids to be damaged either through irradiation, chemical treatment, or as a result of infection by pathogenic organisms provides an elegant model for defining signals that restore homeostasis after damage occurs. Organoids can also be used to expolore the role of commensal organisms that also may play a role in both controlling epithelial homeostasis and restoring homeostasis following injury. Several pathways are emerging as important in regeneration, including Notch, EGF, bone morphogenetic protein (BMP), phosphatidylinositol 3-kinase (PI3K)/AKT, MAPK, Wnt, and Hippo signaling, which are proposed to induce events such as dedifferentiation of mature epithelial cells, stem cell plasticity, and transduction of inflammatory cues. These pathways can now be explored and validated in the organoid culture. The ability to genetically modify organoids using CRISPR/Cas-9 editing will allow a deeper dissection of these pathways and their individual contributions to epithelial repair. The intestinal organoid cultures are continuing to evolve with improved culture conditions and the incorporation of vasculature, components of the enteric nervous system, immune components, and the incorporation of physical forces that mimic luminal and blood flow as well as peristalsis (39, 69), all of which may contribute to or affect the epithelial repair process. The influence of these components on the molecular mechanisms induced by injury in organoid cultures will be important to fully define the mechanisms that modulate epithelial regeneration.
FUTURE QUESTIONS
Intestinal organoids will make a significant contribution to developing preventive and therapeutic modalities to treat gastrointestinal disorders and will complement the current in vivo models. The cultures hold great potential to provide inroads to understanding the plasticity of intestinal epithelial cell populations and defining under what conditions of damage and by what mechansims they are induced to repopulate the cycling ISC compartment. The molecular pathways that are induced by epithelial damage still remain to be linked to specific responses within the ISC niche and most likely will differ based on type of injury, and upstream regulators that are induced by injury remain poorly defined. Epithelial factors induced by damage can be easily defined using organoids; however, the role of stromal factors cannot be ignored. The evolution of PCS-derived organoids and the incorporation of mesenchymal cells into ASC-derived organoids will be essential to defining the role of the stroma. The interplay of epithelial and mesenchymal factors in repair and regeneration needs to be fully characterized. There has been much discussion about the therapeutic potential of transplanting organoid cultures to repair damaged intestinal epithelium. Initial experiments in mice in which ASC-derived organoids were transplanted to repair chemical damage of the epithelium showed engraftment and proper differentiation of the organoids to the damaged areas (75). More work is necessary to determine if this is a viable therapeutic approach for human gastrointestinal diseases. The organoid models hold the promise of new discoveries that will help promote overall intestinal health and hasten recovery from or prevent intestinal epithelial damage.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.B. prepared figures; S.E.B., O.D.K., M.D., N.S., C.G., and M.K.E. drafted manuscript; S.E.B., M.D., N.S., C.G., and M.K.E. edited and revised manuscript; S.E.B., M.D., N.S., C.G., and M.K.E. approved final version of manuscript.
REFERENCES
- 1.Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, Westphalen CB, von Burstin J, Mastracci TL, Worthley DL, Guha C, Quante M, Rustgi AK, Wang TC, von Burstin J, Mastracci TL, Worthley DL, Guha C, Quante M, Rustgi AK, Wang TC. Krt19+/Lgr5− cells are radioresistant cancer-initiating stem cells in the colon and intestine. Cell Stem Cell 16: 627–638, 2015. doi: 10.1016/j.stem.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J, Chan K, Wrana JL, Gregorieff A. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569: 121–125, 2019. doi: 10.1038/s41586-019-1154-y. [DOI] [PubMed] [Google Scholar]
- 3.Bhanja P, Norris A, Gupta-Saraf P, Hoover A, Saha S. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res Ther 9: 26, 2018. doi: 10.1186/s13287-017-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol 25: 106–118, 2012. [PMC free article] [PubMed] [Google Scholar]
- 5.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69, 2013. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, Lo YH, Butkus J, Zou W, Tseng YJ, Jen HI, Patel S, Groves A, Estes M, Sahin E, Frey M, Dempsey P, Shroyer N. Gfi1-expressing Paneth cells revert to stem cells following intestinal injury (Preprint). bioRxiv 10.1101/364133. [DOI]
- 7.Cortez AR, Poling HM, Brown NE, Singh A, Mahe MM, Helmrath MA. Transplantation of human intestinal organoids into the mouse mesentery: a more physiologic and anatomic engraftment site. Surgery 164: 643–650, 2018. doi: 10.1016/j.surg.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Date S, Sato T. Mini-gut organoids: reconstitution of the stem cell niche. Annu Rev Cell Dev Biol 31: 269–289, 2015. doi: 10.1146/annurev-cellbio-100814-125218. [DOI] [PubMed] [Google Scholar]
- 9.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558: 449–453, 2018. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 10.Drummond CG, Bolock AM, Ma C, Luke CJ, Good M, Coyne CB. Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc Natl Acad Sci USA 114: 1672–1677, 2017. doi: 10.1073/pnas.1617363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engevik MA, Aihara E, Montrose MH, Shull GE, Hassett DJ, Worrell RT. Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. Am J Physiol Gastrointest Liver Physiol 305: G697–G711, 2013. doi: 10.1152/ajpgi.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL, Estes MK. Replication of human noroviruses in stem cell-derived human enteroids. Science 353: 1387–1393, 2016. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkbeiner SR, Hill DR, Altheim CH, Dedhia PH, Taylor MJ, Tsai YH, Chin AM, Mahe MM, Watson CL, Freeman JJ, Nattiv R, Thomson M, Klein OD, Shroyer NF, Helmrath MA, Teitelbaum DH, Dempsey PJ, Spence JR. Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivo. Stem Cell Reports 4: 1140–1155, 2015. doi: 10.1016/j.stemcr.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkbeiner SR, Zeng XL, Utama B, Atmar RL, Shroyer NF, Estes MK. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio 3: e00159-12, 2012. doi: 10.1128/mBio.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbester JL, Goulding D, Vallier L, Hannan N, Hale C, Pickard D, Mukhopadhyay S, Dougan G. Interaction of Salmonella enterica serovar typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect Immun 83: 2926–2934, 2015. doi: 10.1128/IAI.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529: 226–230, 2016. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grün D, Lyubimova A, Kester L, Wiebrands K, Basak O, Sasaki N, Clevers H, van Oudenaarden A. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525: 251–255, 2015. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- 18.Heo I, Dutta D, Schaefer DA, Iakobachvili N, Artegiani B, Sachs N, Boonekamp KE, Bowden G, Hendrickx APA, Willems RJL, Peters PJ, Riggs MW, O’Connor R, Clevers H. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol 3: 814–823, 2018. doi: 10.1038/s41564-018-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holly MK, Smith JG. Adenovirus infection of human enteroids reveals interferon sensitivity and preferential infection of goblet cells. J Virol 92: e00250-18, 2018. doi: 10.1128/JVI.00250-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Hou Q, Ye L, Yang Q, Yu Q. Crosstalk between H9N2 avian influenza virus and crypt-derived intestinal organoids. Vet Res (Faisalabad) 48: 71, 2017. doi: 10.1186/s13567-017-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.In J, Foulke-Abel J, Zachos NC, Hansen AM, Kaper JB, Bernstein HD, Halushka M, Blutt S, Estes MK, Donowitz M, Kovbasnjuk O. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol 2: 48–62.e3, 2016. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadhav U, Saxena M, O’Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, Shivdasani RA. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21: 65–77.e5, 2017. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, Clevers H, Batlle E. Isolation and in vitro expansion of human colonic stem cells. Nat Med 17: 1225–1227, 2011. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 24.Karve SS, Pradhan S, Ward DV, Weiss AA. Intestinal organoids model human responses to infection by commensal and Shiga toxin producing Escherichia coli. PLoS One 12: e0178966, 2017. doi: 10.1371/journal.pone.0178966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klotz C, Aebischer T, Seeber F. Stem cell-derived cell cultures and organoids for protozoan parasite propagation and studying host-parasite interaction. Int J Med Microbiol 302: 203–209, 2012. doi: 10.1016/j.ijmm.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Lee YS, Kim TY, Kim Y, Lee SH, Kim S, Kang SW, Yang JY, Baek IJ, Sung YH, Park YY, Hwang SW, O E, Kim KS, Liu S, Kamada N, Gao N, Kweon MN. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 24: 833–846.e6, 2018. doi: 10.1016/j.chom.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Leibowitz BJ, Yang L, Wei L, Buchanan ME, Rachid M, Parise RA, Beumer JH, Eiseman JL, Schoen RE, Zhang L, Yu J. Targeting p53-dependent stem cell loss for intestinal chemoprotection. Sci Transl Med 10: eaam7610, 2018. doi: 10.1126/scitranslmed.aam7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun 83: 138–145, 2015. doi: 10.1128/IAI.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ley S, Galuba O, Salathe A, Melin N, Aebi A, Pikiolek M, Knehr J, Carbone W, Beibel M, Nigsch F, Roma G, d’Ario G, Kirkland S, Bouchez LC, Gubser Keller C, Bouwmeester T, Parker CN, Ruffner H. Screening of intestinal crypt organoids: a simple readout for complex biology. SLAS Discov 22: 571–582, 2017. doi: 10.1177/2472555216683651. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Nakauka-Ddamba A, Tobias J, Jensen ST, Lengner CJ. Mouse label-retaining cells are molecularly and functionally distinct from reserve intestinal stem cells. Gastroenterology 151: 298–310.e7, 2016. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Moriggl R, Zhang D, Li H, Karns R, Ruan HB, Niu H, Mayhew C, Watson C, Bangar H, Cha SW, Haslam D, Zhang T, Gilbert S, Li N, Helmrath M, Wells J, Denson L, Han X. Constitutive STAT5 activation regulates Paneth and Paneth-like cells to control Clostridium difficile colitis. Life Sci Alliance 2: e201900296, 2019. doi: 10.26508/lsa.201900296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Lu R, Wu S, Sun J. Salmonella regulation of intestinal stem cells through the Wnt/β-catenin pathway. FEBS Lett 584: 911–916, 2010. doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez Rodriguez NR, Eloi MD, Huynh A, Dominguez T, Lam AH, Carcamo-Molina D, Naser Z, Desharnais R, Salzman NH, Porter E. Expansion of Paneth cell population in response to enteric Salmonella enterica serovar Typhimurium infection. Infect Immun 80: 266–275, 2012. doi: 10.1128/IAI.05638-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 108: 179–184, 2011. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Múnera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, Hudson JR, Howell JC, Chatuvedi P, Spence JR, Shannon JM, Zorn AM, Helmrath MA, Wells JM. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21: 51–64.e6, 2017. doi: 10.1016/j.stem.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nag D, Bhanja P, Riha R, Sanchez-Guerrero G, Kimler BF, Tsue TT, Lominska C, Saha S. Auranofin protects intestine against radiation injury by modulating p53/p21 pathway and radio-sensitizes human colon tumor. Clin Cancer Res 25: 4791–4807, 2019. doi: 10.1158/1078-0432.CCR-18-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naito T, Mulet C, De Castro C, Molinaro A, Saffarian A, Nigro G, Bérard M, Clerc M, Pedersen AB, Sansonetti PJ, Pédron T. Lipopolysaccharide from crypt-specific core microbiota modulates the colonic epithelial proliferation-to-differentiation balance. MBio 8: e01680-17, 2017. doi: 10.1128/mBio.01680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nigro G, Rossi R, Commere PH, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15: 792–798, 2014. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7: 45270, 2017. [Erratum in Sci Rep 7: 46790, 2017.] doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peck BC, Shanahan MT, Singh AP, Sethupathy P. Gut microbial influences on the mammalian intestinal stem cell niche. Stem Cells Int 2017: 5604727, 2017. doi: 10.1155/2017/5604727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poindexter SV, Reddy VK, Mittal MK, Williams AM, Washington MK, Harris E, Mah A, Hiebert SW, Singh K, Chaturvedi R, Wilson KT, Lund PK, Williams CS. Transcriptional corepressor MTG16 regulates small intestinal crypt proliferation and crypt regeneration after radiation-induced injury. Am J Physiol Gastrointest Liver Physiol 308: G562–G571, 2015. doi: 10.1152/ajpgi.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet 7: 271–283, 1974. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 43.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158, 2012. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajan A, Vela L, Zeng XL, Yu X, Shroyer N, Blutt SE, Poole NM, Carlin LG, Nataro JP, Estes MK, Okhuysen PC, Maresso AW. Novel segment- and host-specific patterns of enteroaggregative Escherichia coli adherence to human intestinal enteroids. MBio 9: e02419-17, 2018. doi: 10.1128/mBio.02419-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy VK, Short SP, Barrett CW, Mittal MK, Keating CE, Thompson JJ, Harris EI, Revetta F, Bader DM, Brand T, Washington MK, Williams CS. BVES regulates intestinal stem cell programs and intestinal crypt viability after radiation. Stem Cells 34: 1626–1636, 2016. doi: 10.1002/stem.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren J, Niu Z, Li X, Yang J, Gao M, Li X, Zhang T, Fang L, Zhang B, Wang J, Su Y, Wang F. A novel morphometry system automatically assessing the growth and regeneration of intestinal organoids. Biochem Biophys Res Commun 506: 1052–1058, 2018. doi: 10.1016/j.bbrc.2018.10.181. [DOI] [PubMed] [Google Scholar]
- 47.Saha S, Aranda E, Hayakawa Y, Bhanja P, Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, Zhang J, Godwin AK, Tome WA, Wang TC, Guha C, Pollard JW. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun 7: 13096, 2016. doi: 10.1038/ncomms13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One 6: e24072, 2011. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology 122: 1500–1511, 2002. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 50.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 51.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 53.Saxena K, Blutt SE, Ettayebi K, Zeng XL, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR, Graham DY, Qureshi W, Sherman V, Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Donowitz M, Estes MK. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 90: 43–56, 2015. doi: 10.1128/JVI.01930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shadad AK, Sullivan FJ, Martin JD, Egan LJ. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol 19: 185–198, 2013. doi: 10.3748/wjg.v19.i2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Tóth B, Kondo A, Massasa EE, Itzkovitz S, Kaestner KH. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557: 242–246, 2018. [Erratum in Nature 560: E29, 2018.] doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109, 2011. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stelzner M, Helmrath M, Dunn JC, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, Wong MH, Yu J; NIH Intestinal Stem Cell Consortium . A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol 302: G1359–G1363, 2012. doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science 334: 1420–1424, 2011. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18: 203–213, 2016. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Tsai YH, Nattiv R, Dedhia PH, Nagy MS, Chin AM, Thomson M, Klein OD, Spence JR. In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development. Development 144: 1045–1055, 2017. doi: 10.1242/dev.138453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantù C, Aguet M, Basler K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Reports 15: 911–918, 2016. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 62.van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens AC, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104, 2012. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302: G1111–G1132, 2012. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64: 911–920, 2015. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Wei L, Cramer JM, Leibowitz BJ, Judge C, Epperly M, Greenberger J, Wang F, Li L, Stelzner MG, Dunn JCY, Martin MG, Lagasse E, Zhang L, Yu J. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci Rep 5: 8566, 2015. doi: 10.1038/srep08566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM, Helmrath MA. An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20: 1310–1314, 2014. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williamson IA, Arnold JW, Samsa LA, Gaynor L, DiSalvo M, Cocchiaro JL, Carroll I, Azcarate-Peril MA, Rawls JF, Allbritton NL, Magness ST. A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell Mol Gastroenterol Hepatol 6: 301–319, 2018. doi: 10.1016/j.jcmgh.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Withers HR, Brennand JT, Elkind MM. The response of stem cells of intestinal mucosa to irradiation with 14 MeV neutrons. Br J Radiol 43: 796–801, 1970. doi: 10.1259/0007-1285-43-515-796. [DOI] [PubMed] [Google Scholar]
- 69.Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 23: 49–59, 2017. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamauchi M, Otsuka K, Kondo H, Hamada N, Tomita M, Takahashi M, Nakasono S, Iwasaki T, Yoshida K. A novel in vitro survival assay of small intestinal stem cells after exposure to ionizing radiation. J Radiat Res (Tokyo) 55: 381–390, 2014. doi: 10.1093/jrr/rrt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471, 2012. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan KS, Gevaert O, Zheng GX, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, , et al. . Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 21: 78–90.e6, 2017. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11: 106–112, 2014. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu S, Tong K, Zhao Y, Balasubramanian I, Yap GS, Ferraris RP, Bonder EM, Verzi MP, Gao N. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell 23: 46–59.e5, 2018. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18: 618–623, 2012. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 76.Zaborin A, Krezalek M, Hyoju S, Defazio JR, Setia N, Belogortseva N, Bindokas VP, Guo Q, Zaborina O, Alverdy JC. Critical role of microbiota within cecal crypts on the regenerative capacity of the intestinal epithelium following surgical stress. Am J Physiol Gastrointest Liver Physiol 312: G112–G122, 2017. doi: 10.1152/ajpgi.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang XT, Gong AY, Wang Y, Chen X, Lim SS, Dolata CE, Chen XM. Cryptosporidium parvum infection attenuates the ex vivo propagation of murine intestinal enteroids. Physiol Rep 4: e13060, 2016. doi: 10.14814/phy2.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang YG, Wu S, Xia Y, Sun J. Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol Rep 2: e12147, 2014. doi: 10.14814/phy2.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J, Li C, Zhao G, Chu H, Wang D, Yan HH, Poon VK, Wen L, Wong BH, Zhao X, Chiu MC, Yang D, Wang Y, Au-Yeung RK, Chan IH, Sun S, Chan JF, To KK, Memish ZA, Corman VM, Drosten C, Hung IF, Zhou Y, Leung SY, Yuen KY. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv 3: eaao4966, 2017. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zou WY, Blutt SE, Zeng XL, Chen MS, Lo YH, Castillo-Azofeifa D, Klein OD, Shroyer NF, Donowitz M, Estes MK. Epithelial WNT ligands are essential drivers of intestinal stem cell activation. Cell Reports 22: 1003–1015, 2018. doi: 10.1016/j.celrep.2017.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]