Abstract
The protective surfaces of bacteria are comprised of polysaccharides and are involved in host invasion and colonization, host immune system evasion, as well as antibacterial resistance. A major barrier to our fundamental understanding of these complex surface polysaccharides lies in the tremendous diversity in glycan composition among bacterial species. The polyisoprenoid bactoprenyl phosphate (or undecaprenyl phosphate) is an essential lipid carrier necessary for early stages of glycopolymer assembly. Because of the ubiquity of bactoprenyl phosphate in these critical processes, molecular probes appended to this lipid carrier simplify identification of enzymatic roles during polysaccharide bioassembly. A limited number of these probes exist in the literature or have been assessed with such pathways, and the limits of their use are not currently known. Herein, we devise an efficient method for producing fluorescently modified bactoprenyl probes. We further expand our previous efforts utilizing 2-nitrileaniline, and additionally prepare nitrobenzoxadizol tagged bactoprenyl phosphate for the first time. We then assess enzyme promiscuity of these two probes utilizing four well characterized initiating phosphoglycosyltransferases: CPS2E (Streptococcus pneumoniae), WbaP (Salmonella enterica), WecA (Escherichia coli) and WecP (Aeromonas hydrophilia). Both probes serve as substrates for these enzymes and could be readily used to investigate a wide range of bacterial glycoassembly pathways. Interestingly, we have also identified unique solubility requirements for the nitrobenzoxadizol moiety for efficient enzymatic utilization that was not observed for the 2-nitrileaniline.
Keywords: Bactoprenyl phosphate, undecaprenyl phosphate, phosphoglycosyltransferases, polyisoprenoid, undecaprenyl diphosphate synthase
Graphical Abstract
Introduction
The unique composition of the bacterial cell surface accounts for a large degree of diversity observed among microbial communities. One species of bacteria, such as Escherichia coli, may have numerous sub-types that possess a unique composition in O-antigen, lipopolysaccharide, or capsule.1 A common lipid carrier, bactoprenyl phosphate (BP) (also referred to as undecaprenyl phosphate), is utilized for the bioassembly of cell wall glycans, or modifications of these materials. The addition of the first sugar residue to BP marks a critical step in establishing the ultimate glycan end product. Thus, BP expenditure is considered to sequester this common anchor from a finite pool, and ultimately influences cell physiology, cell shape, as well as adaptations for antibiotic resistance.2–4 Because of its ubiquity among surface polysaccharide bioassembly, BP is essential to recapitulate surface glycan synthesis in vitro but is difficult to detect.
Our group has previously demonstrated that fluorescently tagged BP (fl-BP) can be utilized in vitro for two polysaccharide assembly pathways: E. coli colanic acid, and Bacteroides fragilis capsular polysaccharide A.5, 6 Tagged glycans, including fluorescent and radiolabeled moieties, have been extensively employed in the literature to serve a similar purpose.7, 8 Bacteria utilize a plethora of glycans, generating the considerable diversity among bacterial serotypes. Commercial sources of all known glycans are limited, and a smaller fraction are available labeled. Efforts to prepare these synthetically have been successfully reported, and require a different synthetic strategy for each individual glycan. The primary advantage of fl-BP is that this anchor is unbiqutious throughout bacterial surface glycan assembly, and preserves the native glycan structure. However, the consequences associated with the isoprenoid tag structure and their influence on enzymatic activity have yet to be broadly assessed.
As the glycan oligomer is built on fl-BP, the terminal tag is increasingly distanced from the catalytic site. Therefore, we reason that the initial glycosylation step to fl-BP could be a limiting factor for broader use of these probes. Initiating phosphoglycosyltransferases (PGTs) are integral membrane proteins that catalyze the transfer of hexose-1-phosphates or N-acetylhexosamine-1-phosphates to BP. This first step is then followed by subsequent glycosylation by highly specific pathway-dependent enzymes. A few well studied examples of initiating PGTs include: CPS2E from Streptococcus pneumoniae, which aids in capsule formation; WbaP from Salmonella enterica, WecAfrom Escherichia coli, and WecP from Aeromonas hydrophilia, the latter of which aids in formation of O-antigen.9–12
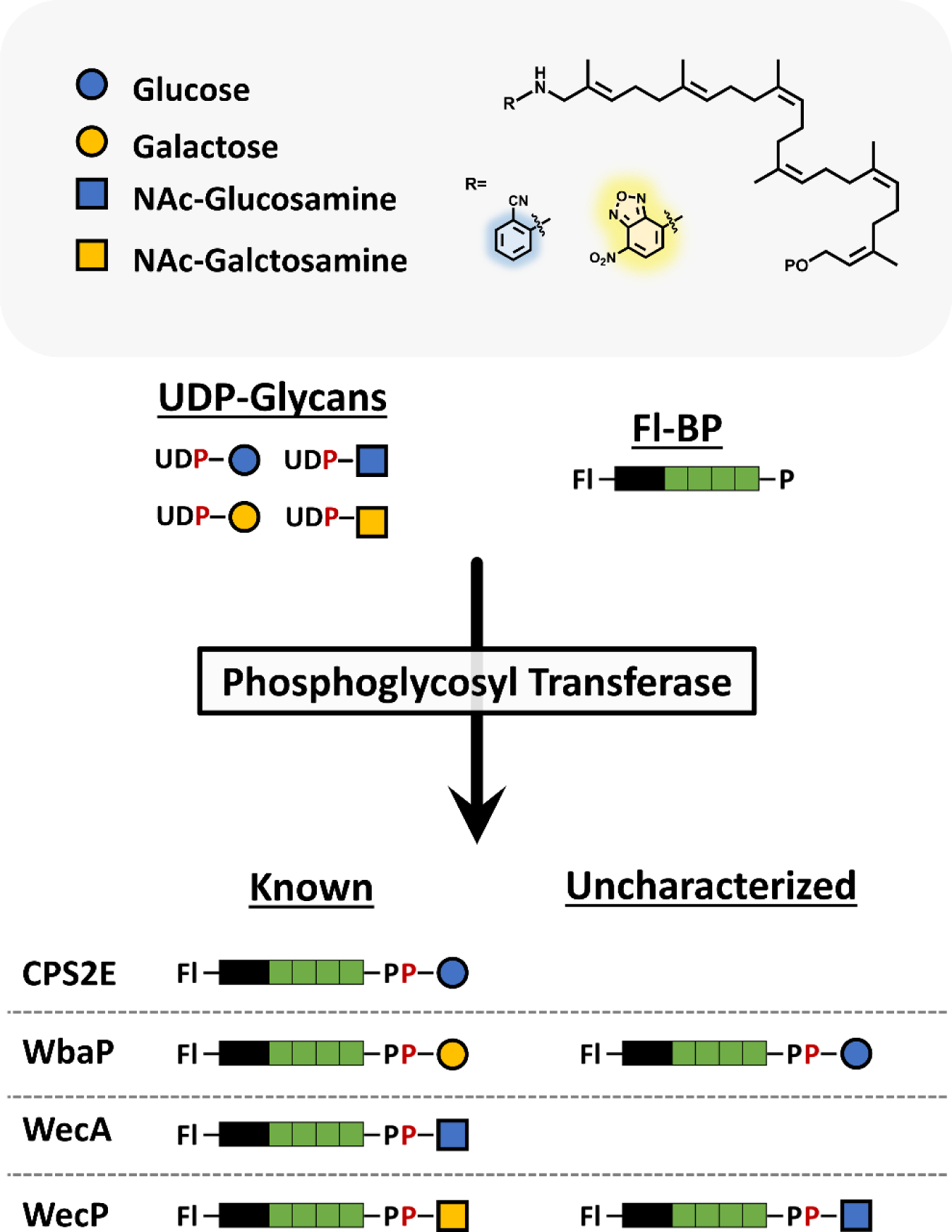
The above well characterized PGTs are herein used to assess fl-BP tag influence on enzymatic activity (Figure 1). Broadly, the fl-BPs are promising tools to probe PGT activity and glycan specificity with their native, unlabeled nucleotide-linked sugar substrates. The fluorescent moiety used in previous works, 2-nitrileaniline (2CN), is prepared by chemical and enzymatic synthesis to produce variable-length isoprenoids, which are length tunable by surfactant choice.13 While the 2CN analogue has been very useful in these applications, the primary shortcomings of this probe include poor detection limit (relative to larger fluorophores or radiolabels) and high UV-background noise. We sought to address these issues by selecting to install a nitrobenzoxadizol (NBD) fluorophore to both increase the detection limit and reduce background noise since the excitation and emission lie in the visible range. Farnesyl labeled NBD probes have been developed for mammalian protein prenylation, but this moiety has not been applied to elongated isoprenoids necessary for bacterial glycobiology.14 Since BP is common to many surface polysaccharide assembly systems, highly fluorescent BP poses an attractive method for following these fundamental processes that is comparable with current methods in glycan labeling.7, 15
Figure 1.
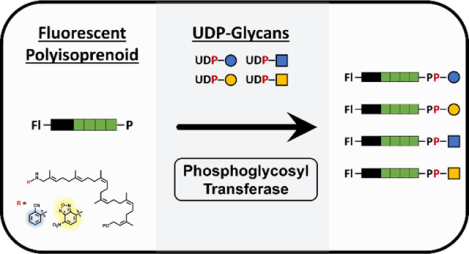
Model PGTs and their transferase activity towards two fluorescently modified substrates. Shortened polyisoprenoids have been chemoenzymatically prepared with one of two fluorescent tags (fl-BP). These alternative substrates were then assessed for transferase activity among four model PGTs with known functions. Both fl-BPs were suitable substrates for all PGT’s in the presence of their preferred UDP-linked glycan substrates. Additional specificity analysis revealed previously uncharacterized transferase activity in two of the model PGTs.
We report herein the development of a new fl-BP that is based on the well characterized NBD fluorophore. Moreover, we have scaled fl-BP production 100 fold relative to our previous efforts by exploiting a highly efficient chemoenzymatic procedure to afford 10 μmol of fl-BPP. We have found that 2CN and NBD are near equivalent substrates for the model PGT enzymes used here. We have also identified previously uncharacterized alternative glycan substrate specificities for two of these PGTs, WbaP and WecP (Figure 1). This work also highlights the conditional dependence of fl-BP enzymatic substrate utilization in the presence of n-propanol, suggesting new insights into the molecular arrangements of these polyisoprenoids in substrate utilization by PGTs. The use of fl-BPs is promising for future applications as effective probes for solubilized enzyme kinetics, background cell envelope fraction activity, and specificity of common bacterial PGTs.
Materials and Methods
General.
Isoprenoids were synthesized as described in the SI methods section with minor modifications of previous reports.14, 16–18 Sequences in the SI were used to generate recombinant proteins in the vector backbone of pET24a (Genscript). Overexpression of proteins was conducted in E. coli strains C41 (UppS) or C43 (WbaP, WecA, and WecP). UppS and WecP were used as soluble proteins, where as WecA and WecP were used as cef. CPS2E, also prepared as cef from BL21-AI, was provided by the Yother lab and has been previously reported in the literature.9 Induction times and temperatures, as well as detailed purification conditions can be found in the SI. All HPLC analysis was performed using a reverse phase Agilent Eclipse XDB-C18, 3.5 μM 4.6 × 50 mm column on an Agilent 1100 HPLC system, unless noted otherwise. All LCMS analysis was performed using a Waters Xbridge Peptide BEH C18 3.5 uM, 4.6 × 50 mm column on an Agilent 1260 Single Quadrupole with a TEE connector splitting the column eluant between the MSD and FLD. All LC solvent conditions are as specified below.
Two-step fl-BP Preparation with Potato Acid Phosphatase.
Preparation of fl-BPP was carried out in a total volume of 10 mL in a conical tube. Reaction mixtures contained: 10 mM fl-GPP (2CN or NBD), 60 mM IPP, 50 mM bicine (pH 8.5), 5 mM KCl, 0.5 mM MgCh, 2.4% octyl thioglucoside (OTG), and 100 μM UppS. Using this quantity of enzyme was critical to the success of this preparatory scale reaction. After incubating at 37 °C for 1 h, the reactions were evaluated by HPLC. More IPP was added to encourage a desired product length (4–6 Z additions), such that an additional 10 and 40 mM of IPP was added to the 2CN and NBD reactions, respectively. Reactions were quenched with the addition of 1 mL n-butanol with vortexing, followed by a brief centrifugation at 2,500 relative centrifugal force (RCF) for 2 min. The upper layer containing n-butanol and fl-BPP (approx. 100 mM) was then separated and either stored at −20 °C for several weeks, used immediately, or lyophilized. To prepare large scale fl-BPs, mixed fl-BPPs in n-butanol (5% n-butanol carry over) were added to 20% of n-propanol, 0.1% Triton-X100, and 50 mM sodium acetate buffer (pH 4.5) and then were generously vortexed and sonicated to promote complete solubilization. Finally, 20 pg/mL of potato acid phosphatase was added and the reaction was allowed to incubate at 37 °C overnight. Suspensions of 2 mg/mL potato acid phosphatase (3.2 M ammonium sulfate pH 5.0 in 20% glycerol) were used here and could be stored at −80 °C for several weeks. HPLC analysis was used to ensure complete hydrolysis, since downstream purification would result in fl-BP/BPP mixtures.
Purification of fl-BP was performed on an Eclipse XDB-C18 (9.4 × 250 mm, 5 μm) column with a stepping gradient. For 2CN (4–7 Z) the column was first equilibrated to 55:45 n-propanol:100 mM ammonium bicarbonate, and then n-propanol was incrementally stepped up after the elution of each size fl-BP (55% 0–15 min, 60% 15–25 min, 75% 25–35 min). For NBD (3–7 Z) the column was first equilibrated to 40:60 n-propanol:100 mM ammonium bicarbonate and then n-propanol was incrementally stepped up after the elution of each size fl-BP (40% 0–20 min, 45% 20–30 min, 50% 30–40 min, 65% 40–50 min). A 10 min rinse at 95% n-propanol was used in both methods to elute any remaining fl-bactoprenol formed using acid phosphatase (typically <5% of the product). Fractions were lyophilized, and the purified fl-BP’s were then resuspended in a single phase mixture of Hexanes:Acetone:DMSO (10:10:0.5) which permitted rapid and efficient solubilization of fl-BPs from glass tubes. This solution of fl-BPs was transferred to a clear non-binding tube (2CN) or amber tubes (NBD) and the volatile solvents were removed under a gentle stream of air resulting in DMSO solubilized fl-BP. The concentrations were determined spectrophotometrically (2CN εDMSO = 2,400 M−1cm−1 and NBD εDMSO = 20,885 M−1cm−1) and could be stored at −20 °C for several months. Both HPLC and LCMS were used to characterize the length of purified fl-BP material.
Plate Reader Assay.
Kinetic reads were performed on a Molecular Devices M5 plate reader with SoftMaxPro software. Representative enzyme reactions occurred in 96-well opaque plate, with two fluorescent reads (excitation/emission 340/390 and 475/525) occurring simultaneously. Fluorescence was taken in 30 s intervals over 60 min. Pre-mixed reactions contained 25 mM bicine (pH 8.5), 0.1% DDM, 5 mM KCl, 0.5 mM MgCl, 10 μM fl-FPP, and 100 nM UppS from either S. aureus or B. fragilis. This reaction premix was allowed to incubate at 37 °C for 5 min to equilibrate the temperature. In a separate, but adjacent, well IPP was also allowed to prewarm. After the 5 min incubation period, a multichannel pipettor was used to transfer the IPP (or water for the control) to the premixed reactions so that the final concentration of IPP was 1 mM.
WecP Kinetic Assays.
WecP reactions mixtures were prepared in 200 mM Bicine pH = 8.5, 20 mM MgCh, 4 mM KCl, 0.019% n-dodecyl-β-D-maltoside and 10–30 nM WecP. When held constant fl-BP concentration was 2.5 μM and UDP-GalNAc was 225 μM. When UDP-GlcNAc was used as the sugar donor 30–90 nM WecP was used. Each reaction was sampled by HPLC through multiple injections over a period of two hours, where product was still being produced linearly with respect to time. Reaction yield at each time point was determined by peak integrals of product and substrate. The fluorescence integral of the substrate and product did account for 100% of signal with no appreciable changes after transfer of the phosphor-sugar. Rates were calculated based on the input fl-BP substrate and product formed over time. Rates were fit to the Michaelis-Menten equation v/Et=kcatapp[S]/(Kmapp+[S]) where v/Et was the reaction rate per total enzyme and S was the concentration of the varied substrate.
PGT Glycan Specificity Reactions.
Standard reactions were carried out in a total volume of 40 μL in the presence of 1 μM fl-BP, 100 μM UDP-sugar, and 0.10 mg/mL total protein cef. For CPS2E, WbaP, WecA, and WecP reactions buffers consisted of 200 mM bicine pH 8.5, 5 mM MgCh, 100 mM KCl, and 15 mM cholate. Alternatively, 5% n-propanol was used in place of cholate as a component of the fl-BP solvent. All reactions were allowed to sit at 37 °C for 2 h and then were quenched with an equal volume of n-propanol.
Results and Discussion
Preparation of Highly Fluorescent Bactoprenyl Diphosphate Analogues.
Chemical preparation of farnesyl diphosphate analogues of 2CN and NBD have been previously reported in the literature.14, 19 Efficient enzymatic preparation of fluorescently modified 2CN bactoprenyl diphosphate (fl-BPP) has been achieved with recombinant undecaprenyl diphosphate synthase (UppS) from Bacteroides fragilis (UppSBf).19 The 2CN analogue was shown to be effective for monitoring enzyme activity with an increase in fluorescence intensity observed upon isoprenoid chain elongation, which was attributed to an increasingly hydrophobic environment around the fluorophore. Similar to 2CN, the NBD fluorophore is known to be highly sensitive to its molecular environment. To rapidly assess if the NBD-GPP compound was a substrate for UppSBf and to determine if a similar increase in fluorescence could be observed with the NBD analogue upon elongation, UppSBf reactions were prepared in 96-well plate format and chain elongation of 5 μM 2CN-GPP or NBD-GPP was monitored by fluorescence increase (Figure 2). We observed a minimum increase in fluorescence in the NBD-GPP reactions over 50 min (1.1 fold), while 2CN-GPP fluorescence increased 2.5 fold. HPLC analysis of products demonstrated that very little substrate had been consumed with the NBD-GPP analogue, while nearly all of the 2CN-GPP had been consumed under identical conditions (data not shown). We were then curious to examine if UppS from different bacterial species would be more effective at utilizing tagged substrates. We found that the enzyme encoded by Staphylococcus aureus (UppSsa) was highly effective for the elongation of both 2CN- and NBD-GPP. A 4.8 fold increase in fluorescence with NBD-GPP was observed relative to a 3.5 fold increase with 2CN-GPP under identical conditions (Figure 2) with the S. aureus protein.
Figure 2.
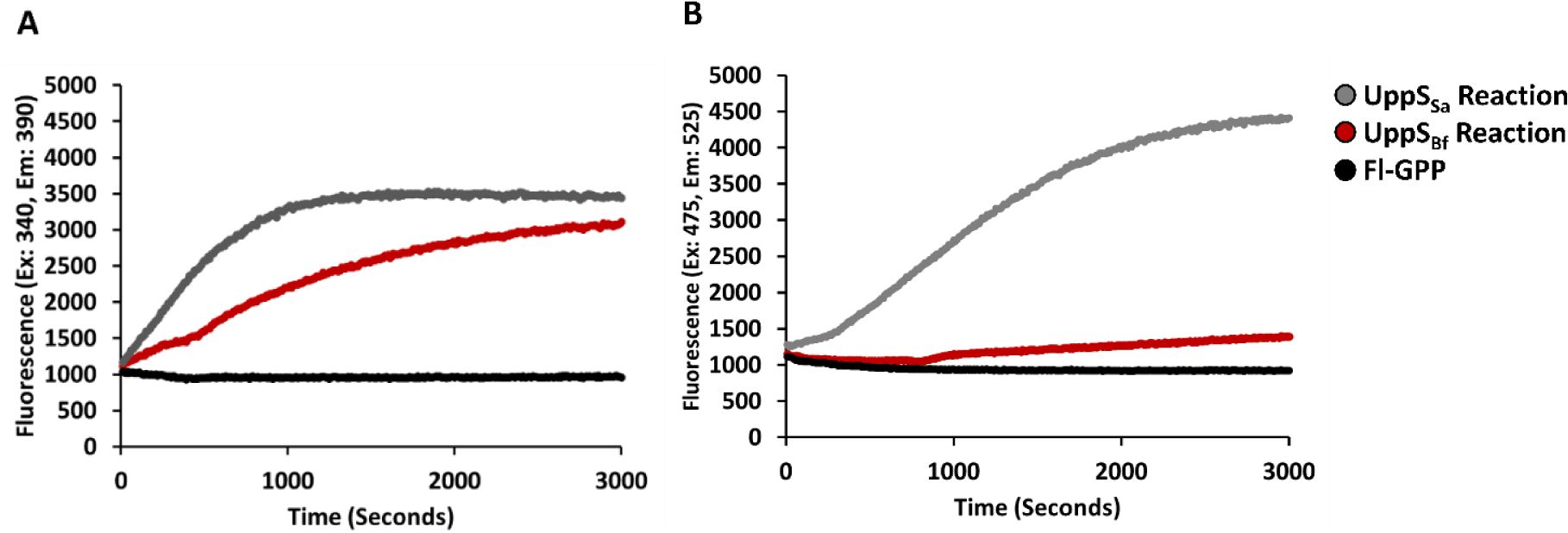
UppS reactions with 2CN- (A) or NBD-GPP (B) monitored. Black lines show the GPP control, in the absence of IPP or UppS. Red lines show reaction using UppS from B. fragilis, while gray lines show reactions using UppS from S. aureus.
From these data it was clear that UppSSa was able to accept the NBD-labeled analogue more readily than the UppSBf, suggesting that subtle differences in the structures of these proteins could lead to more promiscuous activity with alternative substrate structures. A trifluoromethyl (TFM) coumarin based fluorescent analogue was previously shown to be an ineffective substrate with UppS from E. coli (UPPSEc), where the analogue was capable of only accepting a single isoprene unit before being released from the enzyme.20 Analysis of the X-ray crystal structure of UppSEc suggested that the terminal isoprene of FPP moves through a narrow hydrophobic tunnel beneath the active site of the enzyme as isoprene units are added.21 It was proposed that this analogue was a poor substrate for UppSEc because the bulky TFM-coumarin fluorophore could not fit through this hydrophobic tunnel and was therefore released after just a single isoprene unit addition. The NBD and TFM-coumarin moieties have a similar estimated size relative to an isoprene unit or the smaller 2-nitrileaniline. NBD-GPP was also found to be a poor substrate for UppSEc (data not shown). The ineffectiveness of NBD-GPP with B. fragilis and E. coli UppS may have been similarly related to factors that made the TFM-coumarin analogue an ineffective substrate for UppSEc. It is interesting to consider if the more promiscuous S. aureus protein would utilize the TFM-coumarin analogue more readily, and if this protein could be generally used to prepare BPP analogues with bulkier fluorophores.
Two step formation of fl-BP.
In previous work our focus has been on relatively small (100 nmol) preparations of fl-BPP.19 We sought to exploit the robust nature of UppSSa by scaling up BPP production to 10 μmols in the same 1 mL volume used previously, or 100 μmols by scaling the volume ten-fold. Typical soluble protein expression yields from a 1 L culture of UppSSa were around 30–40 mg of purified protein, which could be stored at −80 °C for several weeks. Variable length isoprenoid production was expected, and is tunable based on surfactant choice – herein designated nZ where n is the number of isoprene additions in Z configuration.13 Additionally, we found that a narrow distribution of isoprenoid additions could be modulated by sequential addition of isopentenyl diphosphate, in the presence of relatively high concentrations of UppSSa (Figure S1). This approach enabled us to achieve a range of fl-BPPs of 2CN and NBD with 4–6Z additions (Figure 3). Generally, for practical purposes, shorter isoprenoids (4–6Z) were preferred for their solubility and ease of handling.
Figure 3.
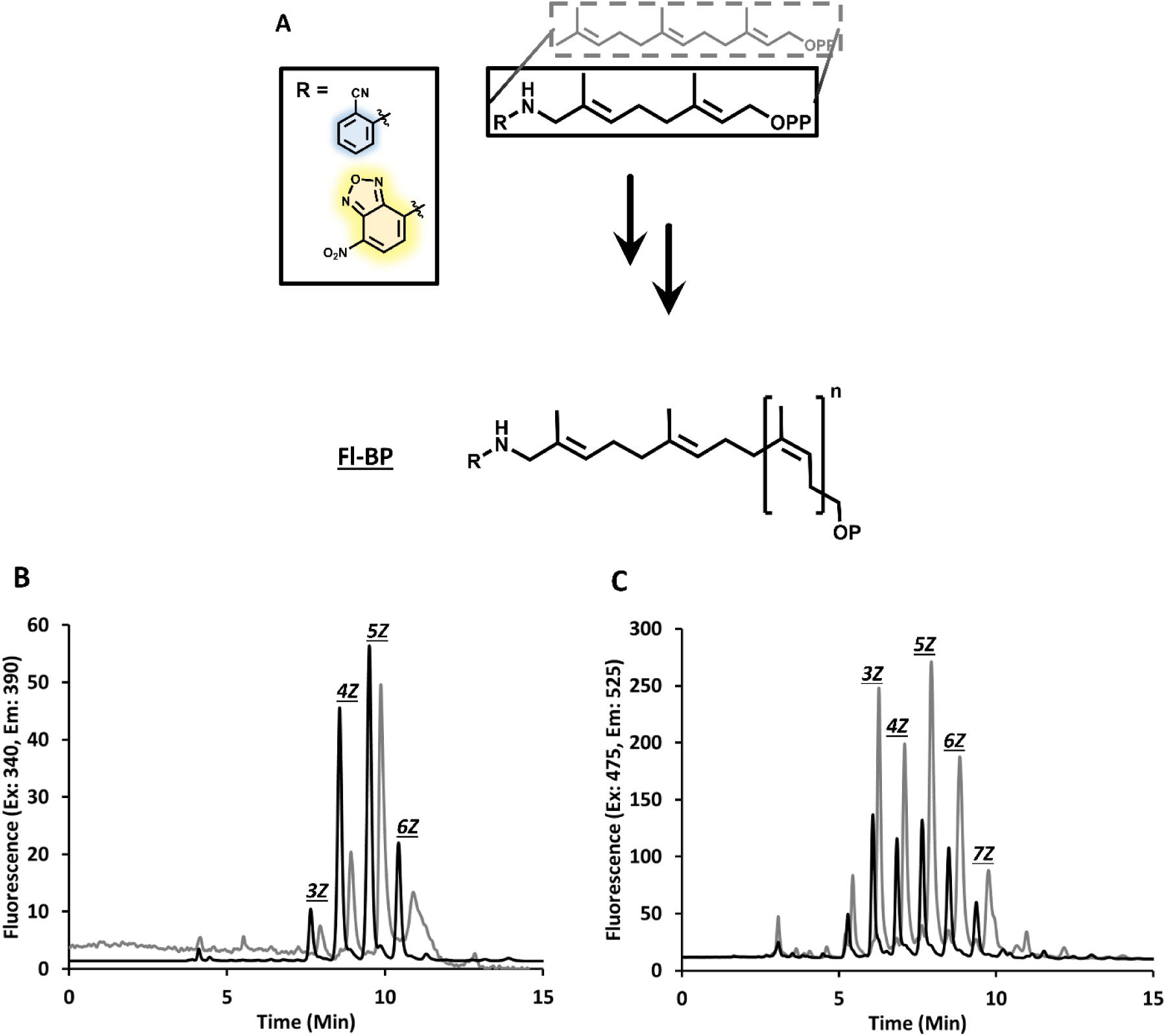
Two step formation of multi-milligram scale fl-BP. A general scheme for two-step fl-BP formation (A) with representative HPLC of 2CN (B) and NBD (C). The black line shows fl-BPP formation from UppSSa reaction, while the gray line shows fl-BP formation upon potato acid phosphatase treatment in the presence of 20% n-propanol.
An essential step for BP production is phosphoanhydride hydrolysis, which provides a polyisoprenyl monophosphate substrate for bacterial PGTs. Previous work from our group utilized an alkaline phosphatase to convert the diphosphate to monophosphate after separation and purification of UppS products. However, we found that there is considerable batch-to-batch variation with alkaline phosphatase activity prompting us to look for an alternative method. Additionally, separation of BPP prior to dephosphorylation was time consuming and decreased overall yield for this process. To address this problem, we chose instead to use commercially available potato acid phosphatase.20, 22 Since acidic conditions were required for the acid phosphatase, a two-step sequential reaction was employed. To do this, we first chose to extract UppSSa reaction products with n-butanol, and then treat the dried extract with acid phosphatase in an acidic buffer. Reactions containing 5 mM of each fl-BPP were readily converted to monophosphate with 20 pg/mL acid phosphatase (Sigma 0.5–3 units/mg) in the presence of 20% n-propanol (Figure 3). Alternatively, acid phosphatase reactions could be carried out directly from n-butanol extracts (with up to 5% n-butanol carryover) in addition to all other reaction components. Monophosphate product formation was confirmed by LCMS (Figure S2), and these were isolated by semi-preparatory HPLC according to isoprenoid size. Dried fractions were optimally resuspended in a single phase mixture of Acetone:Hexanes:DMSO (6:5:0.5 v/v/v) and concentrated as described in methods and materials. Purity and identity of the isolated fl-BP products were confirmed by LCMS and then quantitated by the extinction coefficients associated with the fluorophores. We also tested whether the acid phosphatase reaction could be monitored in a plate reader format, but detected no significant change in fluorescence upon diphosphate hydrolysis (data not shown). Fl-BP with four Z-isoprene additions were further utilized to test the HPLC limit of detection for the isoprenoids. Using three standard deviations of peak-to-peak chromatogram noise, the approximate limit of detection was found to be 5 and 1 pmol for 2CN and NBD, respectively, demonstrating that we had indeed increased the limit of detection for labeled bactoprenyl from our previous attempts by five-fold (Figure S3).
The hydrophobic nature of phospho-polyisoprenoids has led to a number of challenges associated with their limited solubility at high concentrations. Fluorescently tagged materials have somewhat increased solubility, albeit still relatively difficult to solubilize in aqueous amenable solvents alone. We employed a number of solutions to ensure efficient recovery of fl-BPs. Dried fl-BP elutions were resuspended in a single phase solution of Acetone:Hexanes:DMSO, which could then be readily transferred to microcentrifuge tubes. The volatiles were then removed under a ReactiVap thus reducing the total number of transfer steps and ultimately yielding DMSO solubilized fl-BPs. Given the scalability of the chemoenzymatic preparation and robustness of UppSSa, the fundamental bottleneck for purification is column loading capacity during HPLC purification.
NBD-BP and 2CN-BP are nearly equivalent substrates for the PGT WecP.
We were next interested in whether these fluorescent moieties altered the substrate effectiveness for isoprenoid utilizing enzymes. The PGT WecP, which transfers N-acetyl-galactosamine phosphate (GalNAc-P) to BP, is a transmembrane protein; however, previous work has demonstrated that the functional form of the full length protein can be solubilized away from membranes and purified.12 We chose to use this protein as a representative because, unlike many other PGTs, it was easily purified away from cell envelope fraction (cef) and therefore would not have complicating factors in its analysis. The wecP gene was commercially synthesized then incorporated into a pET-24a vector for overexpression and subsequent purification. As expected the protein initially fractionated with the cef, and we were able to extract and purify it to homogeneity (Figure S4). We tested WecP activity with UDP-GlcNAc and -GalNAc, with the expectation that it would only be capable of transferring GalNAc-P to our fl-BP. GalNAc-P was transferred by the protein to both NBD-B(4Z)P and 2CN-B(4Z)P analogues. Interestingly, while GalNAc-P was clearly a better substrate for the enzyme, the protein was also able to catalyze the transfer of GlcNAc-P to a limited extent (Figure 4). Neither UDP-glucose (Glc) nor UDP-galactose (Gal) was a substrate for the enzyme under these conditions. LCMS analysis confirmed that the new fluorescent peaks in these reactions were indeed NBD- and 2CN- B(4Z)PP-linked GlcNAc and GalNAc (Figures S5 and S6).
Figure 4.
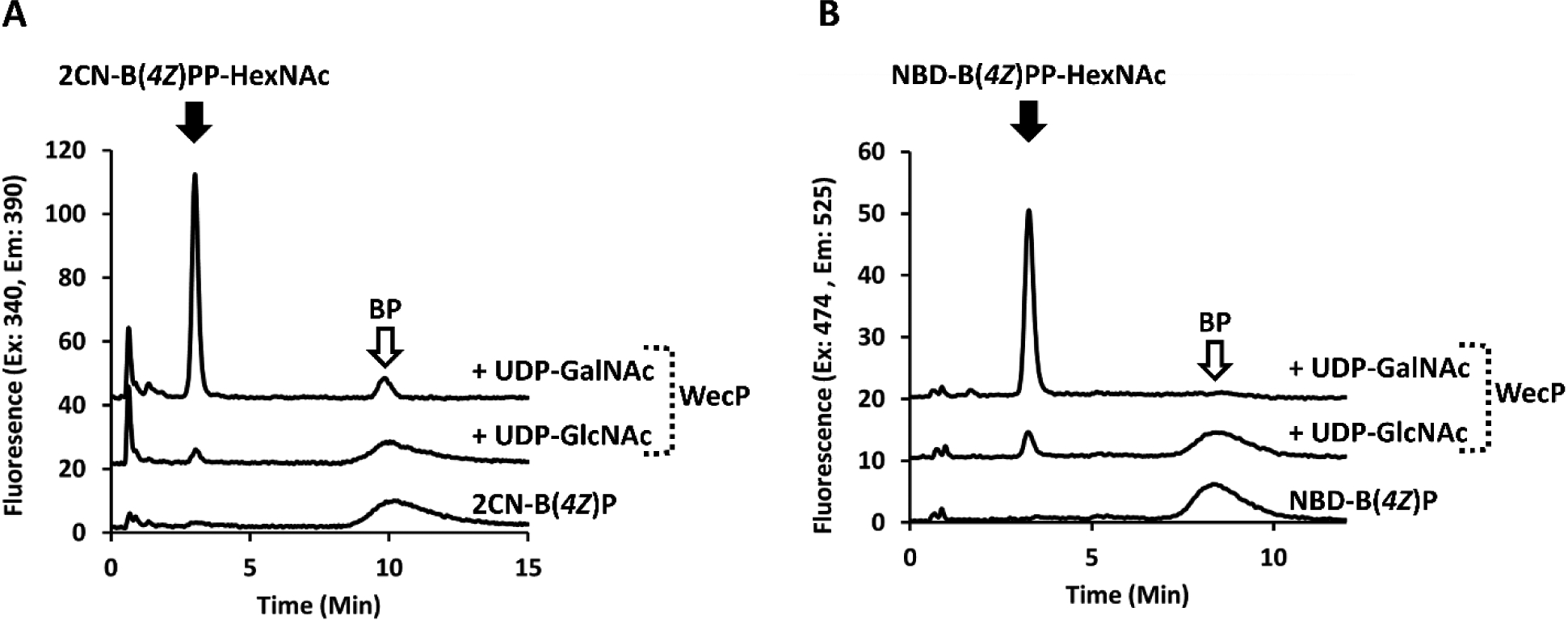
WecP activity with 2CN and NBD-B(4Z)P, A and B respectively. Transferase activity is observed with UDP-GalNAc and UDP-GlcNAc with both fl-BPs. A co-eluting contaminate is present in the 2CN-4Z-BP standard, which appears at 10 min only after 2CN-BP consumption.
Chain length and tag identity have little influence on isoprenoid utilization by WecP.
To determine how well fl-BPs serve as substrates, we chose to perform kinetic analysis on WecP with analogues of varying chain length and tags. Our fl-BP provides a unique opportunity to readily measure kinetics, and are considerably more facile to detect than unlabeled isoprenoids. Optimization of WecP activity was first carried out to identify an optimal concentration of divalent cations and surfactant (Figures S7 and S8). Reactions were next prepared with 2CN-B(4Z)P and 2CN-B(7Z)P at variable concentration with 225 μM UDP-GalNAc (Table 1). Under these conditions the apparent kcat, Kmisoprenoid and kcat/Kmisoprenoid were nearly identical suggesting that the length of the isoprenoid within this range had no influence on enzyme recognition with the 2CN analogue. When the isoprenoid was changed to the NBD-B(7Z)P the analogue was utilized slightly less efficiently (1.5 fold) than the 2CN-B(7Z)P (appkcat/Kmisoprenoid) due to a slightly lower appkcat. When the smaller NBD-B(5Z)P was utilized, the efficiency (appkcat/Kmisoprenoid) was indistinguishable from the other compounds, although the turnover rate (appkcat) was 2.7 fold lower, as the appKmisoprenoid proportionally decreased relative to appkcat. Overall, there was little influence on the WecP isoprenoid utilization by altering the tag or changing the length of the isoprenoid substrate.
Table 1.
WecP Kinetics with UDP-GalNAc.
| fl-BP | UDP-GalNAc | |||||
|---|---|---|---|---|---|---|
| Isoprenoid | kcat (s−1·10−2) | Km (μM) | kcat/Km (M−1·s−1·103) | kcat (s−1·10−2) | Km (μM) | kcat/Km (M−1·s−1) |
| 2CN-B(4Z)P | 1.49 ± 0.23 | 3.0 ± 1.2 | 4.9 ± 2.0 | 1.13 ± 0.05 | 520 ± 89 | 21.7 ± 3.8 |
| 2CN-B(7Z)P | 1.77 ± 0.24 | 3.5 ± 1.3 | 5.1 ± 2.1 | 1.00 ± 0.09 | 166 ± 54 | 60.4 ± 20.4 |
| NBD-B(5Z)P | 0.55 ± 0.08 | 1.3 ± 0.5 | 4.2 ± 1.7 | 1.03 ± 0.07 | 293 ± 60 | 35.2 ± 7.6 |
| NBD-B(7Z)P | 1.00 ± 0.18 | 2.9 ± 1.1 | 3.5 ± 1.5 | 0.97 ± 0.05 | 152 ± 31 | 63.7 ± 13.5 |
Reaction mixtures were prepared with 200 mM Bicine pH = 8.5, 20 mM MgCl2, 4 mM KCl, 0.019% n-dodecyl-β-D-maltoside (DDM 2.4 × CMC) and 10–30 nM WecP. When held constant BP concentration was 2.5 μM and UDP-GalNAc was 225 μM. All parameters are apparent steady-state kinetic values from a non-linear fit to the Michaelis-Menten equation with a minimum of three repeats of six concentrations.
Chain length, but not tag identity influences UDP-GalNAc utilization by WecP.
Since there was little change in the utilization of isoprenoids by WecP, we next tested whether UDP-GalNAc activity was altered by changing the acceptor isoprenoid substrate. To do this, the isoprenoid concentration was held at 2.5 μM and UDP-GalNAc concentration was varied. The appkcat of these reactions was unaffected by the isoprenoid chain length or fluorophore. However, the isoprenoid structure did have a major impact on the UDP-GalNAc appKmGalNAc. With both the 2CN-B(7Z)P and NBD-B(7Z)P, the appKmGalNAc was similar at 166 and 152 μM, respectively. However, when the 2CN-BP was changed to the four Z-configuration isoprene the appKmGalNAc increased over three-fold. When the NBD-BP isoprenoid was changed to the five Z-configuration isoprene analogue the appKmGalNAc increased nearly two-fold. This data suggests that the length of the isoprene has an important influence on the interaction of the UDP-GalNAc with the enzyme.
UDP-GlcNAc is nearly 50-fold less effective as a substrate with WecP.
Our data suggested that UDP-GlcNAc could also be a substrate for WecP although the product formed in our assay was considerably lower than with UDP-GalNAc. We next quantified the effectiveness of UDP-GlcNAc as a substrate by testing 2.5 μM 2CN-B(7Z)P with varying concentrations of the sugar donor (Table 2). Surprisingly, the appKmGlcNAc was only two-fold higher than that of UDP-GalNAc. However, the appkcat was 24-fold lower with UDP-GlcNAc as the donor substrate. These data suggested that the lack of activity with UDP-GlcNAc was more influenced by defective catalysis rather than simply a decrease in the ability of the nucleotide-linked sugar to interact with the protein.
Table 2.
WecP Kinetics with UDP-GlcNAc.
| kcat (s−1·10−2) | Km (μM) | kcat/Km (M−1·s−1) |
|---|---|---|
| 0.041 ± 0.004 | 330 ± 140 | 1.25 ± 0.54 |
Reaction mixtures were prepared and kinetics analyzed as in Table 1 with 30–90 nM WecP, and 2.5 μM 2CN-B(7Z)P with varying UDP-GlcNAc.
WecP likely does not follow a double displacement substrate interaction mechanism.
Recent work on an important monotopic PGT from Campylobacter jejuni has demonstrated that the enzyme follows a double displacement type of reaction mechanism.23 To determine if this was the case for WecP, another kinetic analysis was performed with the 2CN-B(7Z)P analogue where both the UDP-GalNAc and isoprenoid concentrations were varied. For double displacement mechanisms, we would expect to see similar values for kcat/Km at each concentration of UDP-GalNAc while varying the concentration of isoprenoid. The data clearly demonstrated that this was not the case whether UDP-GalNAc was held constant and isoprenoid was varied, or isoprenoid was held constant and UDP-GalNAc was varied (Figure S9). Interestingly, in this analysis the apparent appkcat/Km and appkcat differed at each UDP-GalNAc concentration, but the appKm of UDP-GalNAc remained largely the same with different isoprenoid concentrations, indicating that isoprenoid concentration had very little influence on nucleotide-linked sugar binding under these conditions. Overall these results suggest either an ordered or random sequential binding of substrates, and are inconsistent with double displacement kinetic mechanisms.
Endogenous E. coli transferases are active with fl-BPs.
Because both the NBD and 2CN BP analogues were accepted as substrates by WecP, we next chose to test whether or not these analogues could serve as substrates for other important model PGTs. WecA is a well-established membrane localized protein known to transfer GlcNAc-P to BP. This protein originating from E. coli has not previously been purified to homogeneity, and all attempts herein did not yield purified protein.24, 25 Because WecA is native to E. coli, we first tested whether native cef from C43 E. coli strains, commonly used for protein overexpression, could serve as a functional source of the protein. When cef was incubated with 1 mM UDP-GlcNAc and either 2CN- or NBD-B(4Z)P, partial consumption of the fl-BPs was observed at a retention time and mass consistent with fl-BPP-GlcNAc formation (Figure S10). Alternatively, a pET-24a construct with the wecA gene inserted (pwecA) was commercially prepared and transformed into C43 protein expression cells. The cef of these cells containing overexpressed recombinant WecA protein (Figure S4) was also able to convert fl-BPs with far lower concentrations of UDP-GlcNAc (Figure 5).
Figure 5.
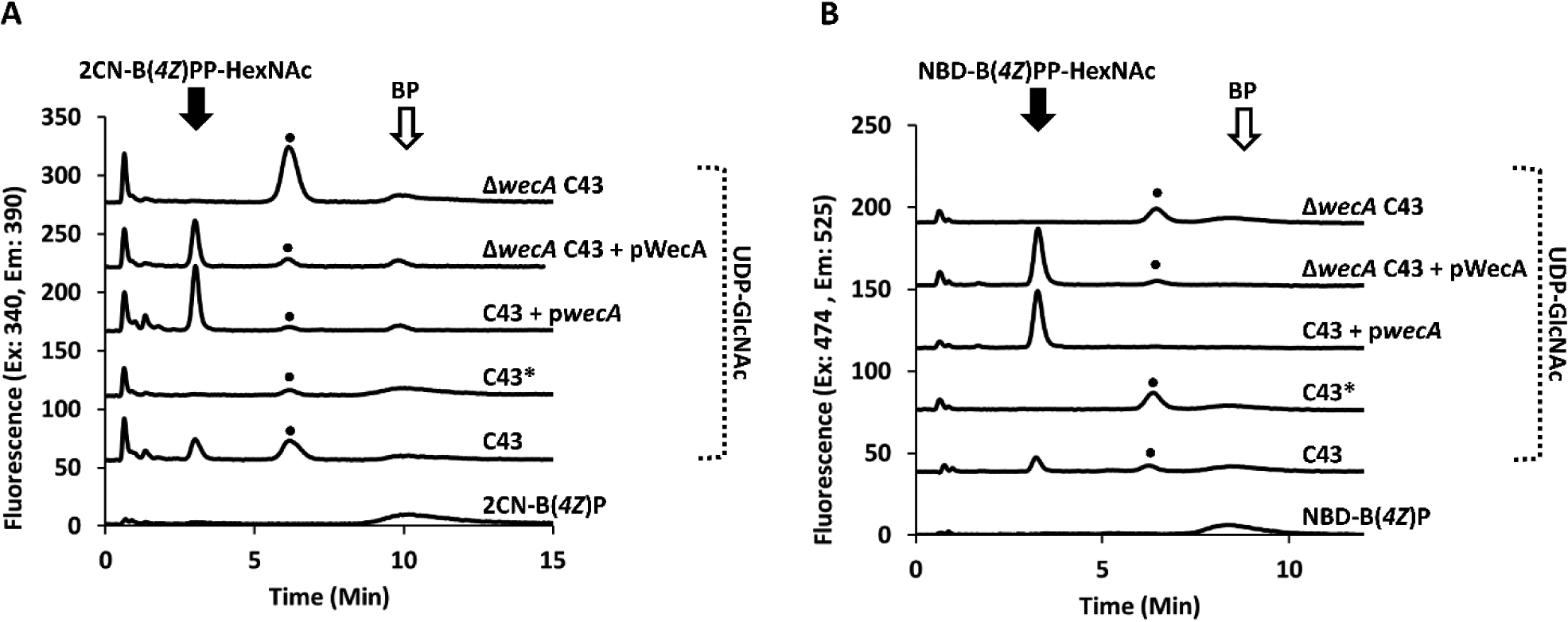
WecA UDP-GlcNAc transferase activity with fl-BPs. Cef from indicated strains was incubated with either 2CN (A) or NBD-B(4Z)P (B) and UDP-GlcNAc as the donor sugar. The cef C43 forms fl-BPP-HexNAc at a retention time of 3.0 and 3.4 for 2CN and NBD, respectively, when co-incubated with UDP-GlcNAc. Adding 1 μg/mL of tunicamycin, designated by (*), eliminates transferase activity. An additional fluorescence peak (•) is observed at 6.2 and 6.4 min for 2CN and NBD respectively. A co-eluting contaminate is present in the 2CN-B(4Z)P standard, which appears at 10 min only after BP consumption.
We next wanted to ensure that endogenous WecA was indeed responsible for the transfer of GlcNAc-P to the fl-BPs and also avoid this background activity in subsequent cef preparations. We attempted two routes to inhibit endogenous WecA transferase activity and to identify it as the protein responsible for fl-BPP-GlcNAc formation. First, we prepared a ΔwecA mutant in the C43 background strain. Neither the 2CN nor NBD BP analogues led to the formation of the presumed fl-BPP-GlcNAc when the wecA gene was removed. However, transferase activity was restored through complementation with pwecA overexpressing the wecA gene (Figure 5). The second route we chose to verify endogenous WecA transferase activity was to supplement reactions with an inhibitory concentration of tunicamycin, the well-known inhibitor of WecA.26 Background WecA activity was abolished under these conditions. Reactions containing cef led to the formation of a new, unanticipated fluorescent material (•) at a retention time of 6.2 and 6.4 min for 2CN and NBD BP, respectively, and the peaks were most prominent when WecA activity was ablated. This unidentified product formed with cef controls, and would form even when sugar was not added to the reaction mixture.
Phosphohexosetransferases utilize fl-BP substrates.
The above data clearly demonstrated that the phospho-HexNAc transferases WecA and WecP could utilize both the 2CN and NBD fl-BP analogues as substrates. We were next interested in testing whether or not two model phosphohexosetransferases could also utilize fluorescently tagged BP probes. Previous work from our group has shown that the S. pneumoniae protein CPS2E will catalyze the transfer of a Glc-P from UDP-Glc to a 2CN fl-BP analogue.6 WbaP from S. enterica is known to transfer Gal-P to BP, but has not yet been assessed with a fl-BP.11 Both phosphohexosetransferases were prepared as cefs from plasmid constructs similar to those for WecP and WecA, and their presence was verified by SDS-PAGE and western blot (Figure S4). We next tested these cef preparations with the two fl-BP probes. The fl-BPP-Hexoses eluted at a similar retention time as fl-BPP-HexNAc under our HPLC conditions. 2CN-B(4Z)P was an effective substrate for both phosphohexosetransferases and their respective glycan donors (Figure 6). Surprisingly, NBD was only an effective substrate for CPS2E, and not WbaP. Previous work with WbaP suggested that isoprenoid chain length may influence the ability of the protein to utilize shorter substrates.27 However, utilizing a longer NBD-B(7Z)P also did not produce a corresponding product (Figure S11). This data suggests that the NBD fluorophore itself, and not chain length, influences catalytic activity for WbaP. The fact that NBD-BPs were ineffective substrates for WbaP was unexpected considering their effectiveness with all other PGTs tested in this work.
Figure 6.
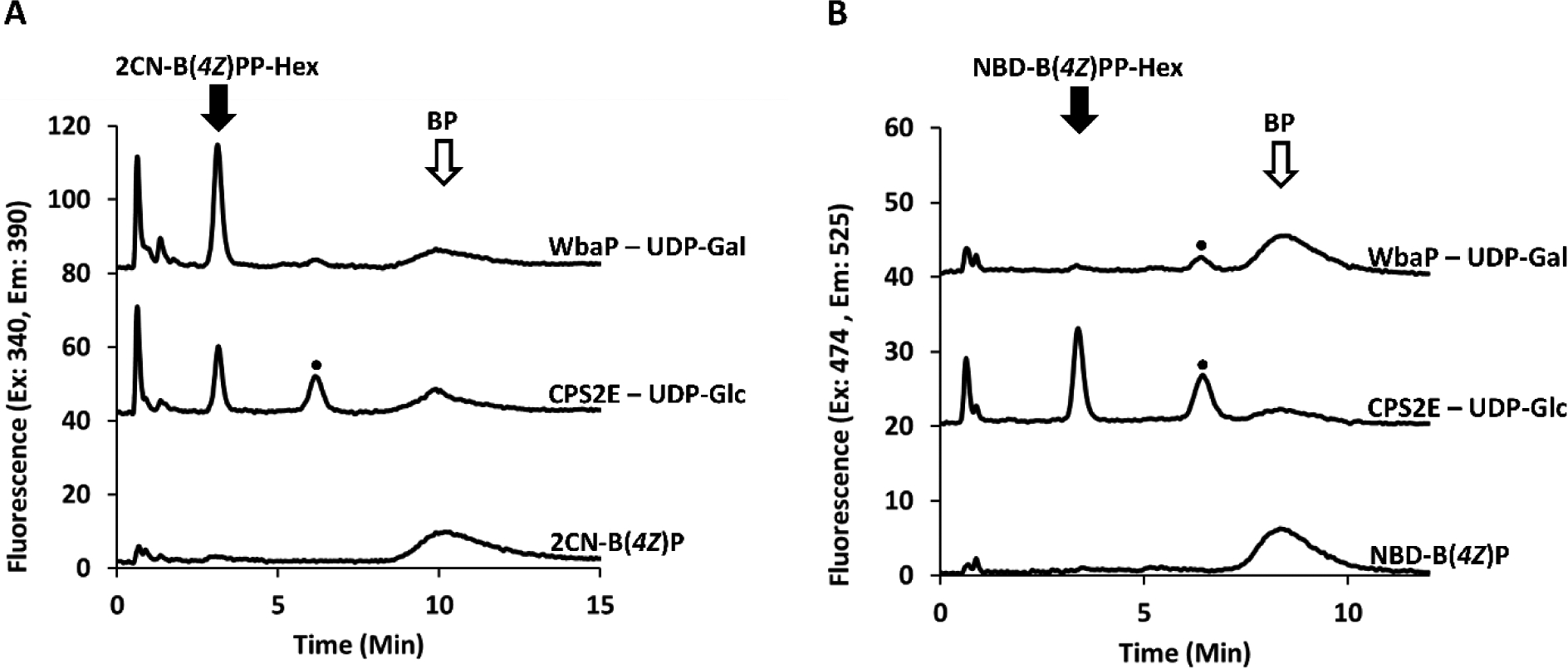
Phosphohexosetransferase activity with fl-BPs. Formation of fl-BPP-Hexose appears at a retention time of 3.0 and 3.4 for 2CN (A) and NBD (B), respectively, when co-incubated with their respective UPD-linked sugar donor. NBD-B(4Z)P does not appear to be a substrate for WbaP. An additional fluorescence peak (•) is observed at 6.2 and 6.4 min for 2CN and NBD respectively.
WbaP activity with NBD-BP is recovered when surfactant is replaced with n-propanol.
While surfactants are commonly employed in PGT reactions, previous work has shown that organic alcohols are needed in addition to prevent aggregation of isoprenoids.28 We found that the surfactant could be replaced entirely with the addition of fl-BPs solubilized in n-propanol, amounting to 5% n-propanol in the total final reaction volume (Figure 7). Notably, WbaP activity was recovered with NBD-B(4Z)P under these conditions, demonstrating that all model PGTs were in fact functionally compatible with both fl-BPs. In the presence of n-propanol WecA activity was completely suppressed, as was the formation of the unidentified material. For all PGTs, there was a limit to how much n-propanol could be added (≥15%) before enzymatic activity was quenched, presumably due to protein denaturation. When reactions were supplemented with 5% w-propanol after the fl-BP had already been added in DMSO, the turnover was markedly decreased (Figure S12). This suggests that the effect organic alcohol has is limited to the initial solution of BP. Perhaps, the way BPs are introduced to solvent conditions changes their availability to enzymes in reaction conditions.
Figure 7.
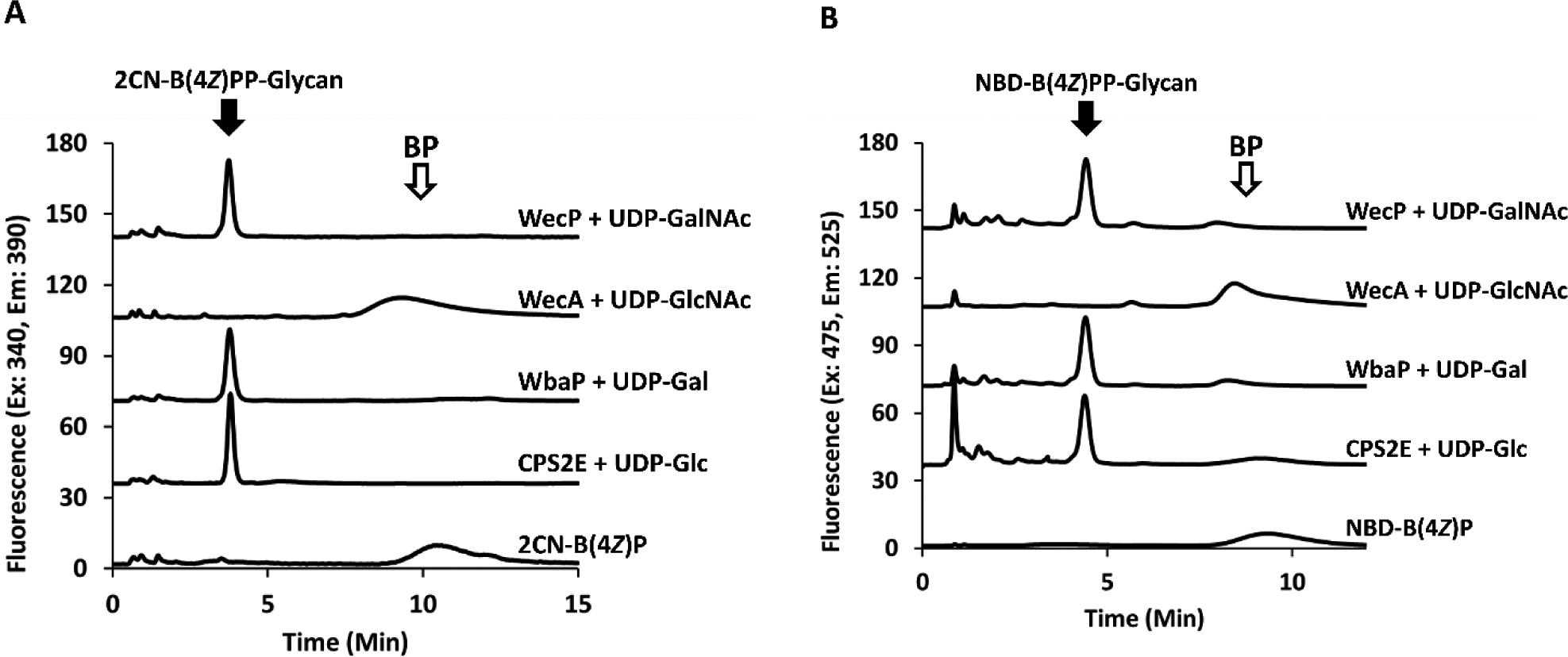
PGT activity with fl-BPs in the presence of 5% n-propanol. Formation of fl-BPP-glycans appears at a retention time of 3.8 and 4.7 for 2CN (A) and NBD (B), respectively, when co-incubated with their respective UDP-linked sugar donor. WecA transferase activity is, however, not observed under these conditions. NBD-B(4Z)P is a substrate for WbaP only when cholate is replaced with 5% n-propanol.
Polyisoprenoids have also been proposed to take on organized micellar structures in vitro22 The role of organic alcohol addition to the isoprenoid reactions to prevent aggregation is corroborated by previous work describing acid phosphatase activity on polyisoprenoids.22 Likewise, the supplementation of either 15% methanol and/or surfactants have been essential for enzymatic turnover for full length BP.26–29 Notably, when crude endogenous BP is used instead, in vitro reactions do not necessitate the use of surfactants or organic alcohols.11, 12, 25, 30 It has also been recently suggested that the unique geometry of BP may aid in binding to PGTs and may also facilitate interactions at the membrane interface.31 It is possible that lipophilic polyisoprenoids, when added with surfactants or organic alcohols, do not necessarily share these same properties as an artifact of exogenous supplementation.
An interesting consideration arises in the fact that the NBD fluorophore is more hydrophilic, and presumably more amenable to aqueous conditions, than the 2CN tag or the native isoprene. Thus solubility alone cannot explain WbaP inactivity with surfactants. Phospholipids bearing an NBD tag on the acyl chain are thought to “loop-back” to the polar headgroup.32, 33 The unique hydrophilicity of NBD could lead to changes in the ability of the PGT enzymes to interact with the isoprenoid, particularly if a severe alteration in the micelle structure is induced by the potentially bolaamphiphilic isoprenoid. Perhaps it is the addition of n-propanol that changes the orientation of NBD-B(4Z)P in solution, which then makes the phosphate more available for catalysis. This is supported by the fact that co-solubilization of the fl-BP and n-propanol simultaneously is required for efficient catalysis.
WecA fails to exhibit activity with any quantity of organic alcohol (Figure 7). One trivial explanation for this difference is that WecA may exhibit poor tolerance of organic solvents, likely due to protein denaturation. In addition, all other PGTs used here exhibit distinctly similar topology whether predicted or experimentally determined, except for WecA which is predicted to have 11 transmembrane domains. It is not yet clear based on these results if a change in BP orientation or WecA conformation has a larger influence on the lack of activity. These findings highlight the trade-off between the positive influence of solubilizing fl-BP and the protein denaturation effect of n-propanol. Likewise, we naturally questioned whether these alcohols could replace the need for a surfactant in UppS reactions, but no observable activity was detected at any concentration in these organic alcohols (data not shown).
Alternative glycan donor specificity with phosphoglycosyltransferases.
Because our fluorescent probes provide a unique opportunity to assess transferase promiscuity, we next performed glycan specificity analysis with the three PGTs (CPS2E, WbaP, WecA) with all four donor substrates (UDP-Glc, -Gal, -GalNAc, or -GlcNAc) in the presence of surfactant (Figures S13 and S14). Additional transferase promiscuity was observed only for WbaP with UDP-Glc as the donor sugar (Figure 8). Similarly, when cholate was replaced with n-propanol, WbaP NBD-B(4Z)P was also active with UDP-Glc (Figure 8). Predictive topology suggests a similar structure of WbaP to WcaJ, a Glc-P transferase.34 However, complementation with wbaP in a ΔwcaJ mutant was unable to restore the mucoid phenotype expected to result from production of the exopolysaccharide colanic acid. This may be indicative of poor substrate utilization of UDP-Glc by WbaP in vivo.
Figure 8.
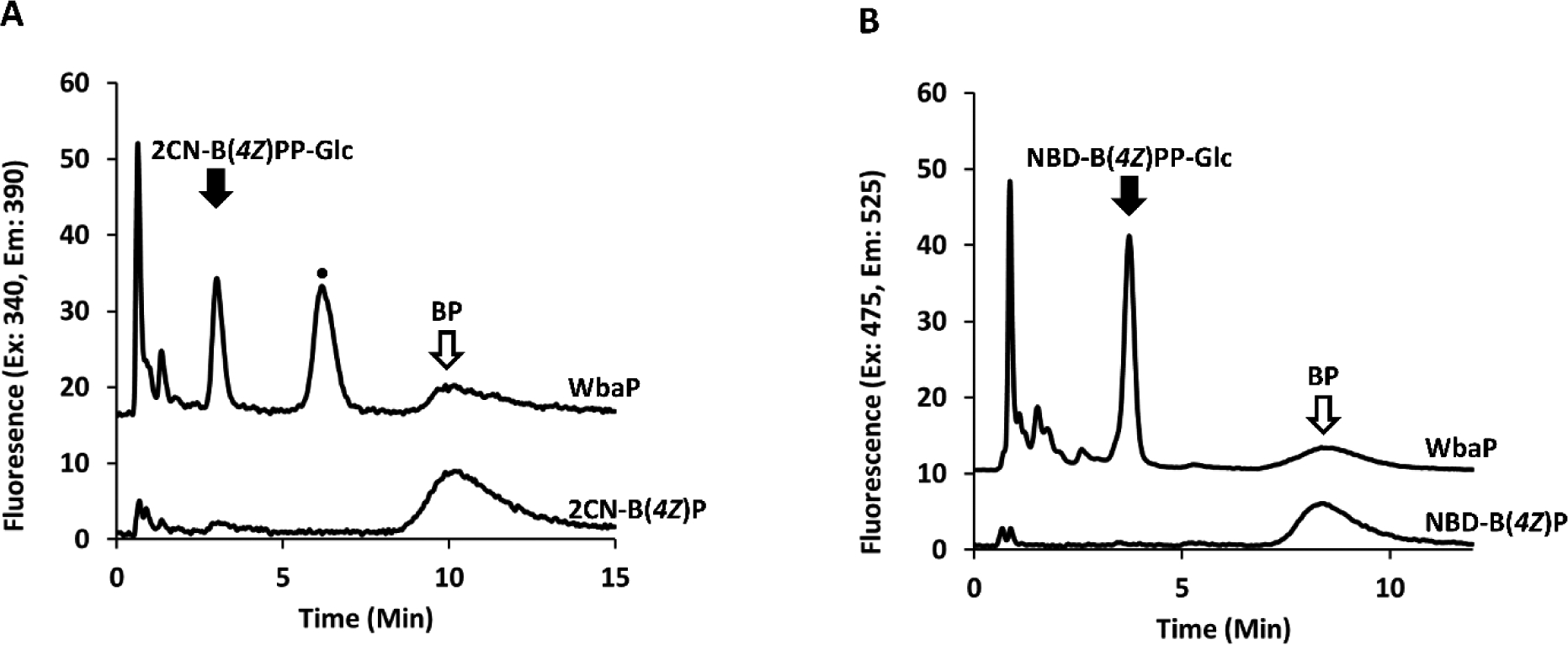
WbaP catalyzes the transfer of UDP-Glc to fl-BPs. Promiscuous transferase activity is demonstrated with cholate and 2CN (A) or 5% n-propanol supplementation and NBD (B). Fl-BPP-Glc is observed at 3.0 and 3.7 min for 2CN and NBD, respectively. A prominent unanticipated fluorescence peak (•) is observed at 6.3 min for 2CN only.
All cef displayed UDP-GlcNAc transferase activity was inhibited by tunicamycin suggesting that this activity was due to native WecA. To ensure that background WecA activity (and not enzymatic promiscuity for UDP-GlcNAc) was responsible for this, PGTs were incubated in the same concentration of tunicamycin with their respective sugar. Expectedly, enzyme function was still observed for all PGTs, except for WecA (Figure 9). Despite also demonstrating additional activity towards UDP-GlcNAc, WecP was not inhibited by tunicamycin. The topology of WecP is reported to be more similar to WbaP than with the HexNAc transferase WecA. In fact, WecA topology is strikingly different from CPS2E, WbaP, or WecP. Members of the HexNAc-phosphate transferase family that utilize UDP-GlcNAc as a donor sugar are expected to demonstrate potent inhibition by tunicamycin, a transition state analogue containing GlcNAc and isoprenoid character. Under reaction conditions used here, WecP was still able to catalyze fl-BPP-GlcNAc transfer, suggesting that efficient inhibition is affected by the stereochemistry of the inhibitor sugar moiety.
Figure 9.
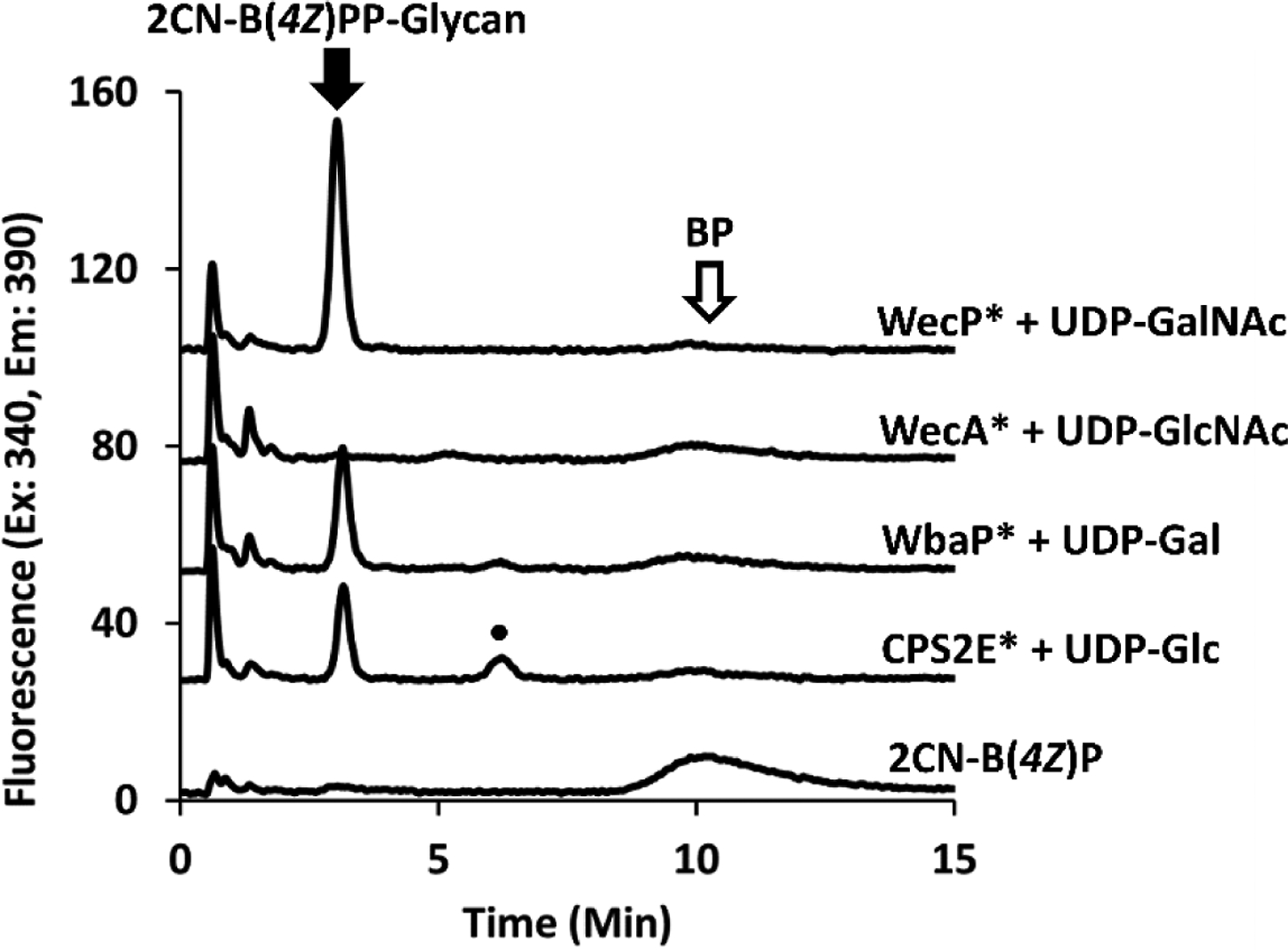
PGT transferase activity in the presence of tunicamycin. 2CN-B(4Z)P is used here in the presence of cholate only, as WecA activity is not observed with n-propanol. Fl-BPP-glycan products are seen at a retention time of 3.0.
Conclusions
In vitro characterization of bacterial glycopolymers depends on the presence of an isoprenoid carrier. The full length, C55, lipid carrier with correct stereochemistry is sparse on the commercial market. Other polyisoprenoids that are more accessible are often derived from plant sources, and may not represent the isomer configuration most tolerable to individual bacterial enzymes.28 Recently it was demonstrated that isoprenoid geometry was critical for binding of PglC, a PGT from Campylobacter jejuni, to BP.31 Alternative to commercial sources, BP has been obtained either through crude isolation from bacterial cell membranes, by chemical synthesis of full or partial length polyisoprenoids, or through shortened isoprenoids which are commercially available but are not necessarily the preferred double bond configuration.11, 30, 35–37 Owing to the importance of BP as a carrier lipid, and the biological importance of glycosyltransferases, it is reasonable for native transferases to be present in the cef background of protein expression cell lines. Endogenous WecA activity appears to be one such transferase exerting activity in the background of our specificity analysis. Therefore, it is reasonable to hypothesize that other such endogenous transferases native to E. coli could explain the unanticipated peak present in our cef reactions. Further, this peak is completely absent in the case of our standards and solubilized WecP reactions. This unanticipated material formed with a great deal of variability only when cef was added to the reaction, and was most prominent in the absence of fl-BPP-GlcNAc formation by WecA. The new peak was not dependent on exogenous UDP-Glc, -Gal, -GalNAc, or -GlcNAc suggesting that none of these sugar donors were substrates for proteins co-isolated in the cef (SI Figures S13 and S14). This suggests that the transferase substrate (possibly a sugar) is also native to the membrane. We are curious to explore such types of proteins, and where they might obtain their sugar source in the absence of exogenous substrate. The identity of this new product is the subject of upcoming work from our laboratory.
Supplementary Material
ACKNOWLEDGMENT
We thank Janet Yother (University of Alabama at Birmingham) for providing bacterial strains with an expression vector of cps2E. We thank Miguel Valvano for supplying a soluble construct of WbaP (Queen’s University Belfast) used for preliminary data associated with this work.
Funding Sources
Funding supporting this work was received through grants from the National Institutes of Health General Medical Sciences grant numbers: R01GM123251 and F31GM130065.
ABBREVIATIONS
- BP
Bactoprenyl phosphate
- Und-P
Undecaprenyl phosphate
- BPP
Bactoprenyl diphosphate
- Fl-BP
Fluorescently tagged BP
- 2CN
2-nitrileaniline
- NBD
Nitrobenzoxadizol
- PGTs
Initiating phosphoglycosyltransferases
- FPP
Farnesyl diphosphate
- UppS
Undecaprenyl diphosphate synthase
- HPLC
High performance liquid chromatography
- LCMS
Liquid chromotography-Mass spectroscopy
- DMSO
Dimethylsulfoxide
- cef
Cell envelope fraction
- UDP
Uridine diphosphate
- Glc
Glucose
- Gal
Galactose
- GlcNAc
N-Acetylglucoseamine
- GalNAc
N-Acetylgalactoseamine
- SDS-PAGE
Sodium dodecyl sulfate Polyacrylamide Gel Electrophoresis
- RCF
Relative Centrifugal Force
Footnotes
REFERENCES
- 1.Whitfield C (2006) Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75, 39–68. [DOI] [PubMed] [Google Scholar]
- 2.Ranjit DK; Young KD (2016) Colanic Acid Intermediates Prevent De Novo Shape Recovery of Escherichia coli Spheroplasts, Calling into Question Biological Roles Previously Attributed to Colanic Acid. J Bacteriol 198, 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cain BD; Norton PJ; Eubanks W; Nick HS; Allen CM (1993) Amplification of the Baca Gene Confers Bacitracin Resistance to Escherichia-Coli. J Bacteriol 175, 3784–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorgenson MA; Young KD (2016) Interrupting Biosynthesis of O Antigen or the Lipopolysaccharide Core Produces Morphological Defects in Escherichia coli by Sequestering Undecaprenyl Phosphate. J Bacteriol 198, 3070–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S; Erickson KM; Troutman JM (2017) Complete Tetrasaccharide Repeat Unit Biosynthesis of the Immunomodulatory Bacteroides fragilis Capsular Polysaccharide A. ACS Chem Biol 12, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott PM; Erickson KM; Troutman JM (2019) Identification of the Functional Roles of Six Key Proteins in the Biosynthesis of Enterobacteriaceae Colanic Acid. Biochemistry-Us 58, 1818–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark EL; Emmadi M; Krupp KL; Podilapu AR; Helble JD; Kulkarni SS; Dube DH (2016) Development of Rare Bacterial Monosaccharide Analogs for Metabolic Glycan Labeling in Pathogenic Bacteria. ACS Chem Biol 11, 3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertozzi CR; Kiessling LL (2001) Chemical glycobiology. Science 291, 2357–2364. [DOI] [PubMed] [Google Scholar]
- 9.Cartee RT; Forsee WT; Bender MH; Ambrose KD; Yother J (2005) CpsE from type 2 Streptococcus pneumoniae catalyzes the reversible addition of glucose-1-phosphate to a polyprenyl phosphate acceptor, initiating type 2 capsule repeat unit formation. J Bacteriol 187, 7425–7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehrer J; Vigeant KA; Tatar LD; Valvano MA (2007) Functional characterization and membrane topology of Escherichia coli WecA, a sugar-phosphate transferase initiating the biosynthesis of enterobacterial common antigen and O-antigen lipopolysaccharide. J Bacteriol 189, 2618–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saldias MS; Patel K; Marolda CL; Bittner M; Contreras I; Valvano MA (2008) Distinct functional domains of the Salmonella enterica WbaP transferase that is involved in the initiation reaction for synthesis of the O antigen subunit. Microbiology-Sgm 154, 440–453. [DOI] [PubMed] [Google Scholar]
- 12.Merino S; Jimenez N; Molero R; Bouamama L; Regue M; Tomas JM (2011) A UDP-HexNAc:Polyprenol-P GalNAc-1-P Transferase (WecP) Representing a New Subgroup of the Enzyme Family. J Bacteriol 193, 1943–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troutman JM; Erickson KM; Scott PM; Hazel JM; Martinez CD; Dodbele S (2015) Tuning the production of variable length, fluorescent polyisoprenoids using surfactant-controlled enzymatic synthesis. Biochemistry-Us 54, 2817–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YW; Alexandrov K; Brunsveld L (2007) Synthesis of a fluorescent analogue of geranylgeranyl pyrophosphate and its use in a high-throughput fluorometric assay for Rab geranylgeranyltransferase. Nat Protoc 2, 2704–2711. [DOI] [PubMed] [Google Scholar]
- 15.Kiessling LL; Splain RA (2010) Chemical approaches to glycobiology. Annu Rev Biochem 79, 619–653. [DOI] [PubMed] [Google Scholar]
- 16.Turek TC; Gaon I; Gamache D; Distefano MD (1997) Synthesis and evaluation of benzophenone-based photoaffinity labeling analogs of prenyl pyrophosphates containing stable amide linkages. BioorgMed Chem Lett 7, 2125–2130. [Google Scholar]
- 17.Das D; Tnimov Z; Nguyen UTT; Thimmaiah G; Lo H; Abankwa D; Wu YW; Goody RS; Waldmann H; Alexandrov K (2012) Flexible and General Synthesis of Functionalized Phosphoisoprenoids for the Study of Prenylation in vivo and in vitro. Chembiochem 13, 674–683. [DOI] [PubMed] [Google Scholar]
- 18.Davisson VJ; Woodside AB; Neal TR; Stremler KE; Muehlbacher M; Poulter CD (1986) Phosphorylation of Isoprenoid Alcohols. J Org Chem 51, 4768–4779. [Google Scholar]
- 19.Dodbele S; Martinez CD; Troutman JM (2014) Species differences in alternative substrate utilization by the antibacterial target undecaprenyl pyrophosphate synthase. Biochemistry-Us 53, 5042–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AP; Chen YH; Liu HP; Li YC; Chen CT; Liang PH (2002) Synthesis and application of a fluorescent substrate analogue to study ligand interactions for undecaprenyl pyrophosphate synthase.JAm Chem Soc 124, 15217–15224. [DOI] [PubMed] [Google Scholar]
- 21.Ko TP; Chen YK; Robinson H; Tsai PC; Gao YG; Chen AP; Wang AH; Liang PH (2001) Mechanism of product chain length determination and the role of a flexible loop in Escherichia coli undecaprenyl-pyrophosphate synthase catalysis. J Biol Chem 276, 47474–47482. [DOI] [PubMed] [Google Scholar]
- 22.Fujii H; Koyama T; Ogura K (1982) Efficient enzymatic hydrolysis of polyprenyl pyrophosphates. Biochim Biophys Acta 712, 716–718. [PubMed] [Google Scholar]
- 23.Das D; Kuzmic P; Imperiali B (2017) Analysis of a dual domain phosphoglycosyl transferase reveals a ping-pong mechanism with a covalent enzyme intermediate. Proc Natl Acad Sci USA 114, 7019–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Dabbagh B; Mengin-Lecreulx D; Bouhss A (2008) Purification and characterization of the bacterial UDP-GlcNAc:undecaprenyl-phosphate GlcNAc-1-phosphate transferase WecA. J Bacteriol 190, 7141–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehrer J; Vigeant KA; Tatar LD; Valvano MA (2007) Functional characterization and membrane topology of Escherichia coli WecA, a sugar-phosphate transferase initiating the biosynthesis of enterobacterial common antigen and O-antigen lipopolysaccharide. J Bacteriol 189, 2618–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Dabbagh B; Mengin-Lecreulx D; Bouhss A (2008) Purification and Characterization of the Bacterial UDP-GlcNAc: Undecaprenyl-Phosphate GlcNAc-1-Phosphate Transferase WecA. J Bacteriol 190, 7141–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel KB; Ciepichal E; Swiezewska E; Valvano MA (2012) The C-terminal domain of the Salmonella enterica WbaP (UDP-galactose:Und-P galactose-1-phosphate transferase) is sufficient for catalytic activity and specificity for undecaprenyl monophosphate. Glycobiology 22, 116–122. [DOI] [PubMed] [Google Scholar]
- 28.Chen L; Men H; Ha S; Ye XY; Brunner L; Hu Y; Walker S (2002) Intrinsic lipid preferences and kinetic mechanism of Escherichia coli MurG. Biochemistry-Us 41, 6824–6833. [DOI] [PubMed] [Google Scholar]
- 29.Al-Dabbagh B; Olatunji S; Crouvoisier M; El Ghachi M; Blanot D; Mengin-Lecreulx D; Bouhss A (2016) Catalytic mechanism of MraY and WecA, two paralogues of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily. Biochimie 127, 249–257. [DOI] [PubMed] [Google Scholar]
- 30.Patel KB; Furlong SE; Valvano MA (2010) Functional analysis of the C-terminal domain of the WbaP protein that mediates initiation of O antigen synthesis in Salmonella enterica. Glycobiology 20, 1389–1401. [DOI] [PubMed] [Google Scholar]
- 31.Entova S; Guan Z; Imperiali B (2019) Investigation of the conserved reentrant membrane helix in the monotopic phosphoglycosyl transferase superfamily supports key molecular interactions with polyprenol phosphate substrates. Arch Biochem Biophys 108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chattopadhyay A; London E (1987) Parallax Method for Direct Measurement of Membrane Penetration Depth Utilizing Fluorescence Quenching by Spin-Labeled Phospholipids. Biochemistry-Us 26, 39–45. [DOI] [PubMed] [Google Scholar]
- 33.Huster D; Muller P; Arnold K; Herrmann A (2001) Dynamics of membrane penetration of the fluorescent 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) group attached to an acyl chain of phosphatidylcholine.Biophys J 80, 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furlong SE; Ford A; Albarnez-Rodriguez L; Valvano MA (2015) Topological analysis of the Escherichia coli WcaJ protein reveals a new conserved configuration for the polyisoprenyl-phosphate hexose-1-phosphate transferase family. Sci Rep 5, 9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu BL; Woodward R; Wen LQ; Wang X; Zhao GH; Wang PG (2013) Synthesis of a Comprehensive Polyprenol Library for the Evaluation of Bacterial Enzyme Lipid Substrate Specificity. Eur JOrg Chem 2013, 8162–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye XY; Lo MC; Brunner L; Walker D; Kahne D; Walker S (2001) Better substrates for bacterial transglycosylases. J Am Chem Soc 123, 3155–3156. [DOI] [PubMed] [Google Scholar]
- 37.Li L; Woodward RL; Han WQ; Qu JY; Song J; Ma C; Wang PG (2016) Chemoenzymatic synthesis of the bacterial polysaccharide repeating unit undecaprenyl pyrophosphate and its analogs. Nat Protoc 11, 1280–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


