Abstract
The androgen receptor (AR) has been linked to bladder cancer (BCa) progression, but if this involves circular RNAs (circRNAs) remains unclear. Here, we find that AR alters the levels of circRNA‐FNTA (circFNTA) to increase BCa cell invasion and chemo‐resistance. Mechanistically, AR represses the RNA editing gene ADAR2 via direct binding to its 5′ promoter region to increase circFNTA levels, which then sponges the microRNA miR‐370‐3p to increase the expression of its host gene FNTA. This AR‐mediated ADAR2/circFNTA/miR‐370‐3p/FNTA pathway then activates KRAS signaling to alter BCa cell invasion and chemo‐sensitivity to cisplatin. A clinical BCa sample survey shows that circFNTA expression is elevated in BCa tissues, and results from a BCa mouse model indicate that depletion of circFNTA leads to the suppression of BCa metastases and increased cisplatin chemo‐sensitivity. Together, based on our results using multiple BCa cell lines and an in vivo mouse model we suggest that targeting this newly identified AR/ADAR2/circFNTA/miR‐370‐3p/FNTA/KRAS axis may lead to the development of therapies to suppress BCa metastasis and to increase its chemo‐sensitivity.
Keywords: bladder cancer, chemo‐sensitivity, circular RNA
Subject Categories: Cancer, RNA Biology, Signal Transduction
The androgen receptor‐regulated circFNTA alters bladder cancer invasion and chemo‐resistance via modulating the miR‐370‐3p/FNTA/KRAS axis. Targeting this signaling pathway might establish novel therapies to suppress bladder cancer progression.
Introduction
Bladder cancer (BCa) is one of the most common malignant genitourinary tumors, with high morbidity and mortality rates 1. BCa ranks as the 9th most common malignancy in men worldwide 2 and ranks as the 4th leading cancer type, with an estimated 81,190 new cases and 17,240 deaths in the United States in 2018 3. At initial diagnosis, approximately 75% of cases are diagnosed as non‐muscle‐invasive BCa (NMIBC) and 25% as muscle‐invasive BCa (MIBC) 4. NIMBC patients have a life‐long risk of recurrence, with 10–20% progression to MIBC following transurethral surgery, while those with MIBC are at a high risk for metastasis development following radical cystectomy 5. Although the cisplatin/gemcitabine (GC) regimen remains as the first‐line chemotherapy for advanced BCa, the median time to progression is only 6 months and it has limited improvement for the overall survival in high‐risk patients 6. Therefore, metastasis and chemo‐resistance remain the two major obstacles for the effective treatment of BCa.
Early studies also indicated that BCa has a gender difference with a male‐to‐female ratio of 3–4 to 1 7. It remains a preferential disease in men even after adjusting for lifestyle or environmental factors, including smoking/tobacco and exposure to industrial chemicals 7. The etiology of this gender difference has been linked to the sex hormones and their receptors, including the androgen receptor (AR) 8, and recent studies also indicated that AR could promote BCa development and progression 9. Furthermore, AR might also be able to alter the cisplatin sensitivity to suppress BCa cell growth 10 and targeting AR with the antiandrogen hydroxyflutamide (HF) or AR degradation enhancer ASC‐J9® could increase bacillus Calmette–Guerin (BCG) therapy efficacy to better suppress BCa progression 11. The significance of AR in BCa therapy is being tested in several ongoing clinical trials using either the recently FDA‐approved antiandrogen enzalutamide on BCa recurrence in NMIBC patients (NCT02605863), or abiraterone in advanced BCa (NCT02788201), or the combination of enzalutamide with gemcitabine and cisplatin in urothelial cancer (NCT02300610) 12.
Circular RNAs (circRNAs) were first found in RNA viruses in the 1970s 13 and are defined as a class of endogenous non‐coding RNAs formed by a covalently closed loop 14. Next‐generation sequencing and bioinformatics analyses provided emerging evidence that circRNAs are abundant, diverse, stable, and conserved and thus may play an indispensable role in the RNA interaction network and in regulating gene expression 15. Recently, several functional circRNAs have been identified in BCa, such as circTCF25, circMYLK, circHIPK3, and circ‐ITCH 16, 17, 18, 19. It remains to be examined, though, if AR has roles to alter circRNA signaling, and if this might impact BCa progression.
Here, we found that the AR‐regulated circular RNA circFNTA competes with the microRNA miR‐370‐3p to increase the expression of its host gene FNTA, which then activates KRAS signaling to promote BCa cell invasion and resistance to cisplatin.
Results
AR alters circFNTA expression to increase BCa cell invasion and cisplatin chemo‐resistance
While results from recent studies indicated that AR can promote BCa cell invasion and cisplatin chemo‐resistance 9, 10, its linkage to circRNA expression for altering BCa progression remains unclear. We first altered AR expression and used Western blot analyses to show that AR levels are increased after adding AR‐cDNA (oeAR) to J82 cells with low AR expression (Fig 1A). In contrast, AR levels are decreased after adding AR‐shRNA (shAR) to UMUC3 cells with high AR expression (Fig 1A).
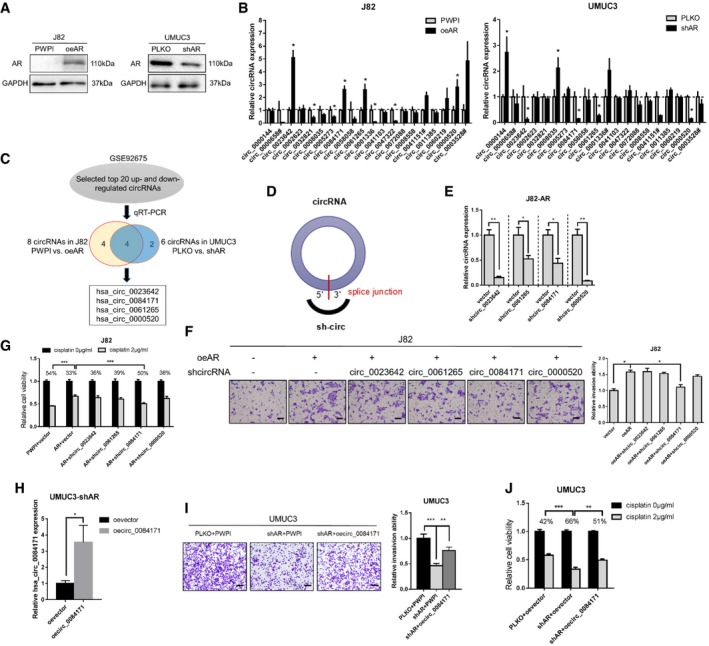
Figure 1. Regulation of circRNA‐FNTA expression and function in response to androgen receptor (AR) in bladder cancer (BCa) cells.
-
AThe expression of AR was determined using Western blots (overexpressing AR in J82 cells and knocking down AR in UMUC3 cells).
-
BRelative expression of the top 20 up‐regulated circRNAs from the GEO dataset GSE92675 (BCa tumor tissues compared to para‐tumor tissues) in response to AR modulation in J82 and UMUC3 cells. The # indicates an RNA with a relatively low expression (qRT–PCR cycle number > 35); thus, the circRNA was excluded for further analysis.
-
CSchematic illustration showing the 4 circRNAs identified that could be regulated by AR.
-
DSchematic illustration showing the position of the targeting shRNA to knock down the circRNAs (sh‐circ) by targeting the specific 5′–3′ splice junction.
-
EReal‐time quantitative PCR (qRT–PCR) results show the knockdown efficiency for these 4 circRNAs in J82 oe‐AR (J82‐AR) cells.
-
FTranswell invasion assays showed that knockdown of hsa_circ_0084171 (circ_0084171), but not the other circRNAs, can partly reverse oeAR‐increased invasion in J82 cells. Scale bar, 100 μm.
-
GMTT assays showed that shcirc_0084171 can partly reverse oeAR‐increased cisplatin chemo‐resistance (2 μg/ml) in J82 cells. To calculate the mean, the value of 0 μg/ml cisplatin groups was set as 1.
-
HThe qRT–PCR results showed the overexpression (oe) efficacy for hsa_circ_0084171 (oecirc_0084171) in UMUC3‐shAR cells.
-
ITranswell invasion assays showed that oecirc_0084171 can partly rescue shAR‐induced invasion reduction in UMUC3 cells. Scale bar, 100 μm.
-
JMTT assays showed that oecirc_0084171 can partly overcome shAR effect for cisplatin chemo‐resistance (2 μg/ml) in UMUC3 cells.
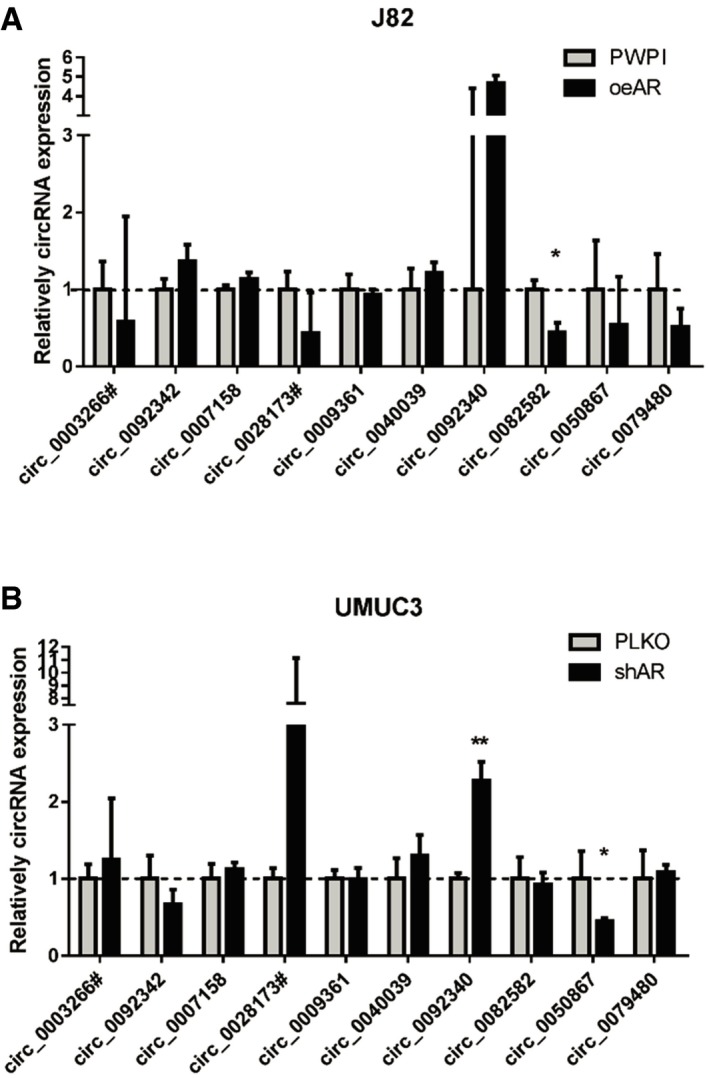
We then utilized the GEO dataset GSE92675 and found several differentially expressed circRNAs in four pairs of BCa tumor tissues vs. para‐tumor bladder tissues [16, Data ref: 20]. We further performed qRT–PCR assays to examine the impact of AR on the expression of 30 circRNAs (20 top up‐regulated and 10 top down‐regulated circRNAs, P < 0.05 and FDR < 0.05), and the results revealed that there were four circRNAs (hsa_circ_0023642, hsa_circ_0084171, hsa_circ_0061265, and hsa_circ_0000520) whose expression were consistently altered by increasing or decreasing AR levels (Figs 1B and C, and EV1A and B).
Figure EV1. Relative circRNA expression changes by manipulating AR in BCa cells.
-
ARelative expression of the 10 top down‐regulated circRNAs in J82 cells (PWPI vs. oeAR).
-
BRelative expression of the 10 top down‐regulated circRNAs in UMUC3 cells (PLKO vs. shAR).
To further examine whether AR functions by altering these four circRNAs to increase BCa cell invasion and to modulate chemo‐sensitivity to cisplatin, we constructed shRNAs for these four circRNAs (sh‐circRNA) by targeting specific 5′–3′ splice junctions (Fig 1D) and used qRT–PCR to confirm the knockdown efficacy for these four circRNAs in J82‐AR cells (Fig 1E).
We then depleted these four circRNAs to reverse/block AR‐dependent BCa cell invasion. The results from the transwell assays with matrigel‐coated filters revealed that knocking down hsa_circ_0084171 (circFNTA), but not the other circRNAs, can partly reverse/block oeAR‐increased BCa J82 cell invasion (Fig 1F and Appendix Fig S1). Results from the MTT cell viability assays also showed that knocking down circFNTA can partly reverse/block cisplatin treatment (2 μg/ml) in AR overexpressing J82 cells (Fig 1G). As expected, adding oecircFNTA to UMUC3‐shAR cells (Fig 1H) partly rescued the shAR's effect in transwell invasion assays and MTT cell viability assays (Fig 1I and J).
Together, the results from Figs 1A–J and EV1 suggest that AR functions by altering circFNTA expression to increase BCa cell invasion and cisplatin chemo‐resistance.
circFNTA promotes cell invasion and cisplatin chemo‐resistance in BCa cells
To further study the impact of AR‐altered circFNTA on BCa cell invasion, we examined potential circFNTA pathological functions using the CircBase database annotation, and the results indicated that circFNTA is derived from the expressed FNTA gene on chr8, 42914234‐42932507, and has a length of 582 nt (Fig 2A). Results from qRT–PCR assays revealed that its expression was elevated in multiple BCa cell lines (T24, J82, UMUC3, and 5673) as compared to normal bladder epithelial SVHUC cells (Fig 2B).
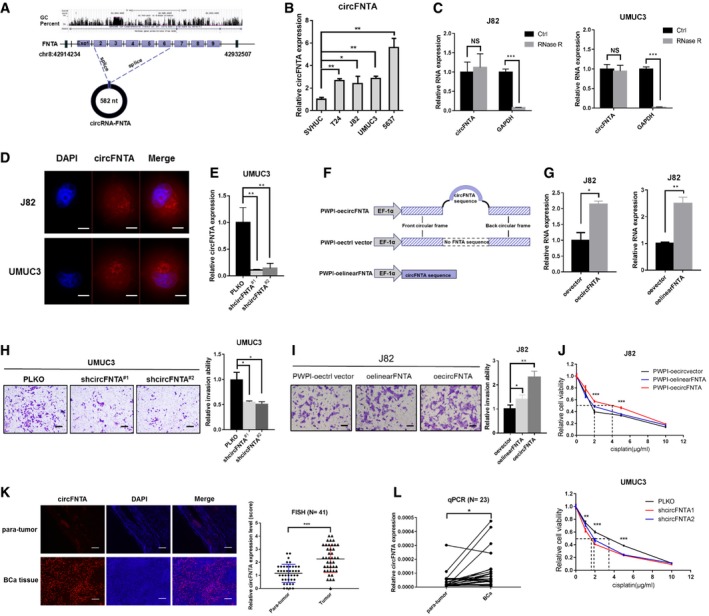
Figure 2. circFNTA expression is higher in BCa and promotes invasion and cisplatin chemo‐resistance in BCa cells.
-
AThe schematic diagram shows the genomic location and splicing patterns of circFNTA (hsa_circ_0084171).
-
BThe relative expression of circFNTA was elevated in multiple BCa cell lines (T24, J82, UMUC3, and 5637) compared to normal bladder epithelial cell line SVHUC as shown by qRT–PCR.
-
CThe expression of circFNTA and GAPDH mRNA in J82 and UMUC3 cells treated with or without RNase R was detected by qRT–PCR.
-
DRNA fluorescence in situ hybridization (FISH) demonstrated that circFNTA was predominantly localized to the cytoplasm of J82 and UMUC3 cells. DAPI was used to indicate the nucleus. Scale bar, 20 μm.
-
ETwo shcircFNTA plasmids using PLKO.1 vector were transfected into UMUC3 cells. The knockdown efficacy was measured by qRT–PCR.
-
FThe structure of PWPI‐oecircFNTA, PWPI‐control‐vector and PWPI‐oelinearFNTA overexpression constructs.
-
GThe qRT–PCR results show oecircFNTA efficacy in J82 cells compared with PWPI vector (left panel) and oecircFNTA or oelinearFNTA in J82 cells (right panel).
-
H, ITranswell invasion assays showed that shcircFNTA can decrease UMUC3 cell invasion (H) and oecircFNTA can increase J82 cells invasion (I) more profoundly than oelinearFNTA as compared with the oevector group. Scale bar, 100 μm.
-
JMTT assays showed that oecircFNTA enhances J82 cells chemo‐resistance to cisplatin (0, 1, 2, 5, and 10 μg/ml were tested). This effect was more obvious in the oecircFNTA group than in the oelinearFNTA group (upper panel). MTT assays showed that shcircFNTA increases UMUC3 cells chemo‐sensitivity to cisplatin (lower panel).
-
KFISH analyses revealed that circFNTA was significantly up‐regulated in BCa tissues as compared with the adjacent para‐tumor tissues (N = 41 patients) and representative images are shown. Scale bar, 250 μm.
-
LThe qRT–PCR assays also demonstrated that circFNTA is significantly elevated in fresh BCa tissues compared to para‐tumor tissues (N = 23 patients).
Furthermore, qRT–PCR assays confirmed that circFNTA was in a circular form as it was resistant to RNase R, while GAPDH mRNA was significantly reduced after RNase R treatment (Fig 2C). In addition, fluorescent in situ hybridization (FISH) assays demonstrated that circFNTA was predominantly localized in the cytoplasm in J82 and UMUC3 cells (Fig 2D and Appendix Fig S2). We then constructed two shcircFNTA plasmids using the PLKO vector (Fig 2E) as well as overexpressed circFNTA, linear‐formed FNTA, and control vector using the PWPI plasmid (Fig 2F), and validated their efficacy in J82 cells (Fig 2G). Results from transwell assays confirmed that suppressing circFNTA with shcircFNTA decreased the UMUC3 cell invasion (Fig 2H), while increasing circFNTA expression increased J82 cell invasion more profoundly than expression of the linearFNTA sequence compared with the vector control group (Fig 2I).
In addition, MTT cell viability assays using multiple cisplatin concentrations (0, 1, 2, 5, 10 μg/ml) also confirmed that oecircFNTA can increase cisplatin chemo‐resistance of J82 cells and shcircFNTA can increase the chemo‐sensitivity of UMUC3 cells against cisplatin (Fig 2J).
Together, the results from Fig 2A–J suggest that circFNTA increases BCa cell invasion and cisplatin chemo‐resistance.
Human clinical sample survey for circFNTA expression comparing BCa and para‐tumor tissues
To further strengthen the above in vitro cell line data, we also studied human clinical BCa samples. The results from FISH analysis revealed that circFNTA was significantly increased in BCa tissues compared with adjacent para‐tumor tissues from 41 patients (Fig 2K). In addition, qRT–PCR assays also indicated that circFNTA was significantly elevated in fresh BCa tissues compared to para‐tumor tissues from 23 patients (Fig 2L), which was in agreement with in vitro data showing that circFNTA was increased in multiple BCa cells compared to SVHUC cells.
AR alters circFNTA levels by repressing the RNA editing gene ADAR2
To dissect the molecular mechanism of how AR can increase circFNTA expression, we first tested the expression of the host gene FNTA in UMUC3 cells at protein and mRNA levels. Results revealed that decreased AR expression after adding shAR led to a decrease in FNTA protein expression while increased AR expression in J82 cells after adding oeAR led to an increase in FNTA protein expression (Fig 3A). However, altering AR expression had little effect on FNTA mRNA expression (Fig 3B), suggesting that AR does not alter circFNTA expression via direct transcriptional regulation.
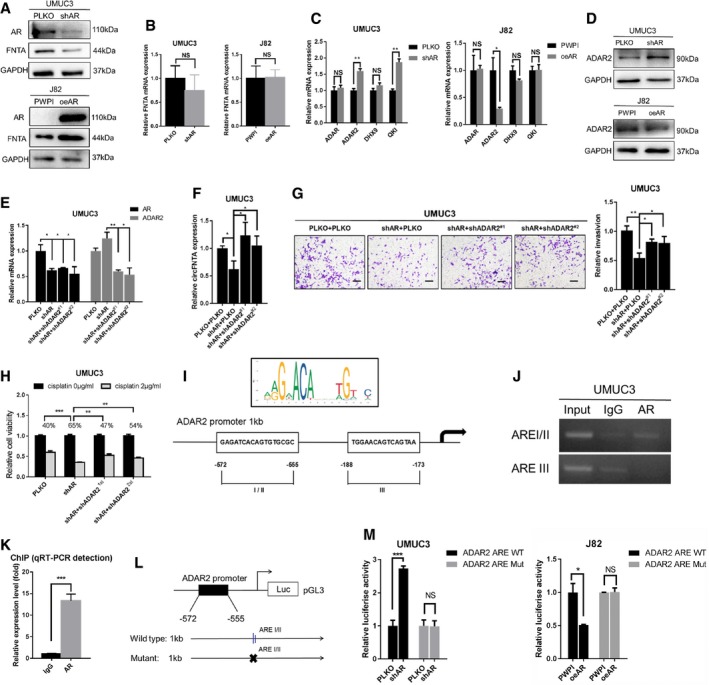
Figure 3. AR regulates circFNTA via the RNA editing gene ADAR2.
-
AThe protein expression of AR and FNTA (circFNTA host gene) was determined using Western blot and showed that shAR can decrease FNTA protein expression in UMUC3 cells and oeAR can increase FNTA protein expression in J82 cells.
-
BqRT–PCR results showed that mRNA levels of FNTA are not changed dramatically in UMUC3 cells with shAR and J82 cells with oeAR.
-
CThe mRNA levels of 4 genes that can regulate circRNA formation (ADAR, ADAR2, DHX9, and QKI) were tested with manipulated AR (UMUC3 cells in left panel and J82 cells in right panel). Results showed ADAR2 mRNA is negatively correlated with AR expression.
-
DWestern blot analysis also confirmed that ADAR2 can be negatively regulated by AR at the protein level.
-
EKnockdown efficiency of AR and ADAR2 (shADAR2#1 and shADAR#2) in UMUC3 cells, as determined by qRT–PCR.
-
FshADAR2#1 and shADAR#2 could both reverse shAR‐dependent circFNTA reduction in UMUC3 cells.
-
GTranswell invasion assays confirmed that both shADAR2 constructs can partly reverse shAR‐dependent UMUC3 cells invasion reduction. Scale bar, 100 μm.
-
HMTT assays confirmed that both shADAR2 constructs can partly overcome shAR‐dependent effects on cisplatin chemo‐resistance (2 μg/ml) in UMUC3 cells.
-
IThe sketch shows the position of the ARE binding sites in the ADAR2 promoter region.
-
JChIP assays confirmed that AR can directly bind to ADAR2 AREI/II (−572nt to −555nt).
-
KChIP pull‐down DNA products were amplified by qRT–PCR reaction. The IgG antibody was used as a negative control.
-
LCloning of the 1 kb ADAR2 promoter into the pGL3 luciferase reporter. Site‐directed mutagenesis was used to generate a mutant ARE.
-
MCo‐transfection of ARE wild type (WT) or mutant (Mut) ADAR2 promoter pGL3‐Luciferase constructs into UMUC3 cells with/without shAR (left panel), and J82 cells with/without oeAR (right panel). The luciferase assay was used to detect promoter activity.
We then focused our attention on several reported RNA editing genes (ADAR, ADAR2, DHX9, and QKI) that could influence circRNA expression 21, 22, 23. Results from qRT–PCR (Fig 3C) and Western blots (Fig 3D) revealed that oeAR in UMUC3 cells decreases ADAR2 expression and shAR in J82 cells increases ADAR2 expression.
To confirm that AR functions by regulating ADAR2 expression to alter circFNTA levels, we used two shRNA constructs for ADAR2 (shADAR2#1 and shADAR2#2), and results revealed that depletion of ADAR2 in UMUC3 cells reversed shAR‐decreased circFNTA expression (Fig 3E and F, and Appendix Fig S3). Importantly, results from functional invasion and cell viability assays also demonstrated that suppressing ADAR2 in UMUC3 cells can partly reverse/block shAR‐decreased cell invasion (Fig 3G) and cisplatin chemo‐resistance (Fig 3H).
Together, the results from Fig 3A–H suggest that AR functions by decreasing ADAR2 expression to alter circFNTA levels in BCa cells.
AR regulates ADAR2 expression by direct binding to the ADAR2 promoter
To dissect the mechanism how AR can regulate ADAR2 expression at the transcriptional level, we first utilized the Ensembl and PROMO 3.0 website to search for potential androgen response elements (AREs) in the ADAR2 1 kb promoter region and found three potential AREs (I/II −572nt to −555nt and III −188nt to −173nt) (Fig 3I). We then performed chromatin immunoprecipitation (ChIP) in UMUC3 cells and found that AR can specifically bind to ADAR2 ARE I/II (Fig 3J and K, and Appendix Fig S4). We then mutated the key sequences of ARE I/II from −572nt to −555nt and conducted luciferase reporter assays in UMUC3 and J82 cells (Fig 3L). Results revealed that shAR increased luciferase activity in UMUC3 cells with the wild type (WT) reporter, but not in the cells with the reporter containing the mutant (Mut) ARE (Fig 3M). Conversely, adding AR significantly decreased luciferase activity in J82 cells transfected with WT reporter, but not in the cells with the Mut reporter (Fig 3M).
Together, the results from Fig 3I–M suggest that AR can alter circFNTA expression by regulating the RNA editing gene ADAR2 by direct binding to the ARE I/II (−572nt to −555nt) in the ADAR2 promoter.
AR/ADAR2/circFNTA signaling increases BCa invasion and chemo‐resistance by competing with miR‐370‐3p to modulate its host gene FNTA
Next, to investigate how AR/ADAR2/circFNTA signaling can increase BCa cell invasion and cisplatin chemo‐resistance, we focused on its host gene FNTA that encodes farnesyltransferase, an enzyme that can regulate cancer cell invasion and chemo‐sensitivity 24, 25. Results from Western blot assays revealed that decreasing circFNTA by adding shcircFNTA to UMUC3 cells led to decreased FNTA protein expression (Fig 4A). In contrast, increasing circFNTA by adding oecircFNTA to J82 cells led to increased FNTA protein expression (more profoundly than with oelinearFNTA) (Fig 4A).
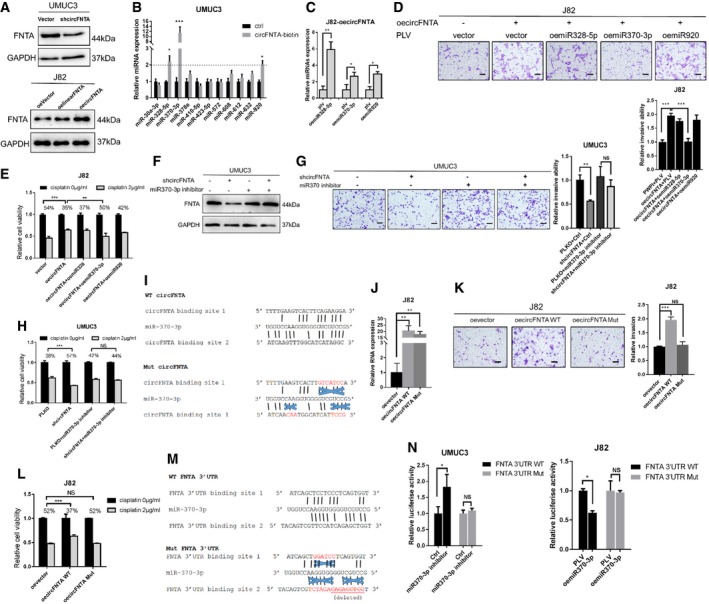
Figure 4. circFNTA regulates invasion and cisplatin chemo‐resistance by competing with miR‐370‐3p to modulate its host gene FNTA .
-
AWestern blot analysis showed that shcircFNTA can decrease FNTA expression in UMUC3 cells (upper panel) and oecircFNTA can increase FNTA expression, and that this was more profound than using oelinearFNTA in J82 cells (lower panel).
-
BA RNA pull‐down assay was used to test the binding of circFNTA to 11 miRNAs (predicted by online database) in UMUC3 cells. The results showed that miR‐328‐5p, miR‐370‐3p, and miR‐920 can be pulled down by circFNTA.
-
CRelative miRNA expressions were quantified by qRT–PCR to test miRNA overexpression efficacy.
-
D, ETranswell invasion assay (D) confirmed that only oemiR‐370‐3p can reverse oecircFNTA induced invasion in J82 cells, and MTT assays (E) confirmed that oemiR‐370‐3p can reverse oecircFNTA induced cisplatin chemo‐resistance (2 μg/ml) in J82 cells. Scale bar, 100 μm.
-
F, GTransducing the miR‐370‐3p inhibitor into UMUC3 cells can reverse FNTA protein reduction by shcircFNTA shown by Western blot (F) and transwell assay (G). Scale bar, 100 μm.
-
HMTT assays also confirmed that transducing with a miR‐370‐3p inhibitor in UMUC3 cells can partly reverse cisplatin chemo‐resistance (2 μg/ml) reduction by shcircFNTA.
-
IThe structure of the circFNTA (WT and Mut) binding sites of miR‐370‐3p. Mut and WT circFNTA's were constructed based on the miRNA target sites.
-
JThe transfection efficacy of oecircFNTA WT and oecircFNTA Mut was tested by qRT–PCR.
-
KTransfection with oecircFNTA WT could increase J82 cells invasion, while oecircFNTA Mut cannot. Scale bar, 100 μm.
-
LTransfection with oecircFNTA WT could increase J82 cells cisplatin chemo‐resistance (2 μg/ml), while oecircFNTA Mut did not have this effect.
-
MThe structures of target sites of miR370‐3p at the FNTA 3′UTR.
-
NLuciferase reporter activity after transfection of WT and Mut FNTA 3′UTR reporter constructs into UMUC3 cells (left panel), comparing transduced miR‐370‐3p inhibitor vs. Ctrl and J82 cells (right panel), and comparing oemiR‐370‐3p vs. control PLV.
To further dissect the mechanism how circFNTA modulates the expression of its host gene FNTA, we focused on miRNAs, since circRNAs may compete for miRNAs to regulate their target genes. LinearFNTA had a similar, but less pronounced impact than circFNTA (See Fig 2I and J), which suggests that circFNTA likely sponges miRNAs rather than acting through other known functions of circRNAs to regulate cell invasion and cisplatin chemo‐resistance.
Using the bioinformatic prediction databases CircNet and StarBase v2.0, we identified 11 potential miRNAs (miR‐30a‐3p, miR‐328‐5p, miR‐370‐3p, miR‐378e, miR‐410‐5p, miR‐423‐5p, miR‐572, miR‐608, miR‐612, miR‐632, and miR‐920), which could bind to both circFNTA and the 3′UTR of FNTA. We then conducted RNA pull‐down assays to test whether circFNTA could interact with one of the above miRNA candidates using biotinylated oligonucleotides (5′‐Biotin‐TGCTCTGTCCTGGACTTCTC‐3′) that bind to the circular junction of circFNTA in UMUC3 cells. The results revealed three miRNAs (miR‐328‐5p, miR‐370‐3p, and miR‐920) enriched in the pull‐down that can directly interact with circFNTA (Fig 4B and Appendix Fig S5).
To further confirm which of these miRNAs is sponged by circFNTA to impact FNTA expression, we constructed and transfected these three miRNAs into J82‐oecircFNTA cells (Fig 4C), and results from transwell invasion assays (Fig 4D) and MTT cell viability assays both confirmed that only oemiR‐370‐3p can partly reverse the effects of oecircFNTA (Fig 4E).
Importantly, transducing a miR‐370‐3p inhibitor into UMUC3 cells can then rescue shcircFNTA‐decreased FNTA expression using Western blots (Fig 4F), transwell invasion assays (Fig 4G), and MTT cell viability assays (Fig 4H). Thus, adding miR‐370‐3p inhibitor to UMUC3 cells can reverse shcircFNTA‐suppressed BCa cell invasion and cisplatin chemo‐resistance. Furthermore, circFNTA mutant (Mut) was constructed and transfected into J82 cells based on the miRNA‐370‐3p target sites (Fig 4I and J). Transwell invasion assays (Fig 4K) and MTT cell viability assays (Fig 4L) revealed that only oecircFNTA WT, not oecircFNTA Mut, can increase J82 cell invasion and cisplatin chemo‐resistance.
Together, the results from Fig 4A–L suggest that AR/ADAR2/circFNTA signaling can regulate circFNTA expression to sponge miR‐370‐3p to modulate its host gene FNTA to increase BCa invasion and cisplatin chemo‐resistance.
miR‐370‐3p modulates FNTA expression by direct binding to the FNTA mRNA 3′UTR
To dissect the mechanism of how miR‐370‐3p modulates FNTA expression, we first utilized online database to identify structures of target sites of miR‐370‐3p in the 3′UTR of FNTA. We then prepared Mut and WT constructs of these two miRNA target sites on the reporter construct of the 3′UTR of FNTA mRNA for luciferase assays (Fig 4M). Results revealed that miR‐370‐3p inhibitor and oemiR‐370‐3p can alter luciferase reporter activity of WT 3′UTR of FNTA, while having little effect on the Mut 3′UTR of FNTA in UMUC3 and J82 cells (Fig 4N).
Together, the results from Fig 4M and 4N suggest that miR‐370‐3p can alter the FNTA expression via direct binding to the FNTA mRNA 3′UTR binding site.
AR/ADAR2/circFNTA/miR‐370‐3p/FNTA signaling increases BCa cell invasion and chemo‐resistance by altering KRAS activity
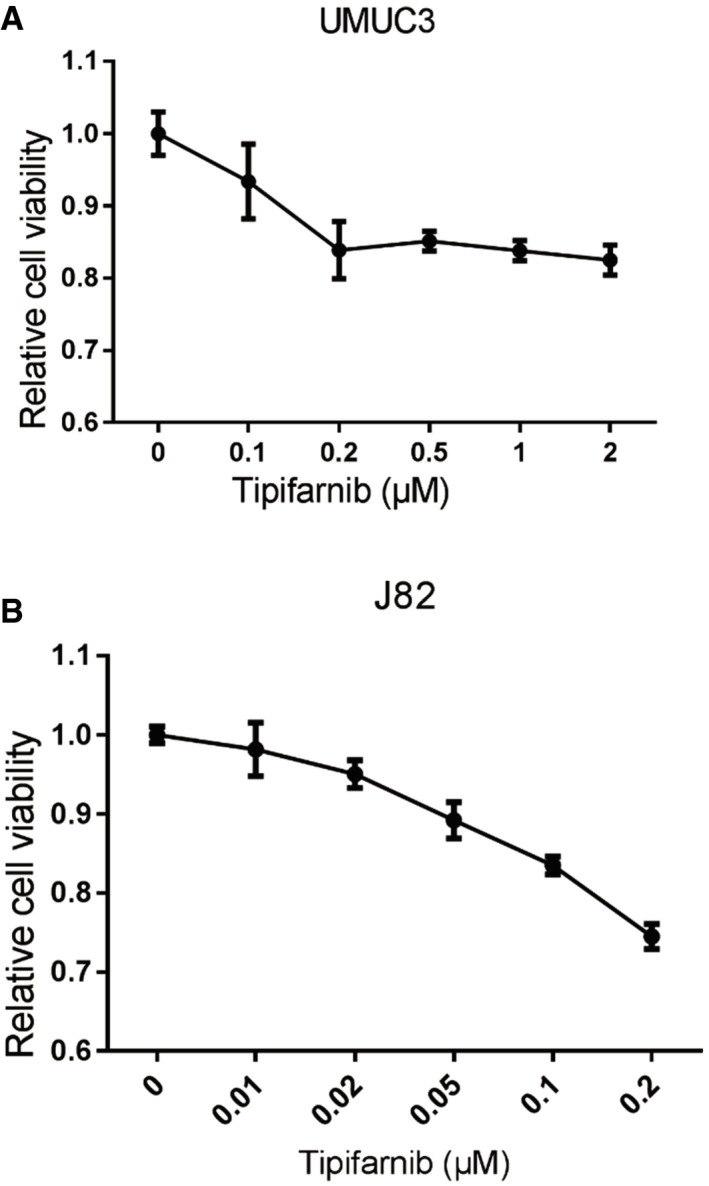
We confirmed that decreasing FNTA by adding shFNTA to both J82 and UMUC3 cells (Fig 5A) can block effects of oecircFNTA on the transwell invasion assay (Fig 5B) and MTT cell viability assay (Fig 5C). We also treated the cells with Tipifarnib (a CAAX inhibitor of farnesyltransferase) at a relatively low concentration (0.05 μM in J82 and 0.1 μM in UMUC3 cells) (Fig EV2A and B), and results of transwell invasion assays (Fig 5D) and MTT cell viability assays (Fig 5E) revealed that Tipifarnib can partly rescue oecircFNTA‐dependent effects in both J82 and UMUC3 cells.
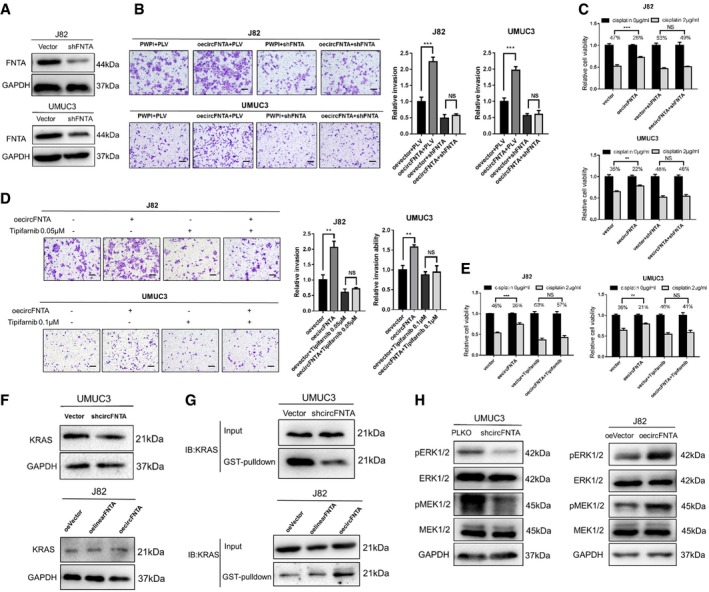
Figure 5. circFNTA modulates FNTA to promote BCa invasion and chemo‐resistance by activating KRAS .
-
AWestern blots show the knockdown efficiency of shFNTA in J82 and UMUC3 cells.
-
BTranswell assays indicated that shFNTA can partly reverse oecircFNTA‐increased invasion in J82 and UMUC3 cells. Quantifications are shown on the right. Scale bar, 100 μm.
-
CMTT assays also confirmed that shFNTA can reverse oecircFNTA‐increased chemo‐resistance to cisplatin (2 μg/ml) in J82 and UMUC3 cells.
-
D, ETranswell assays (D, with quantitations at the right) and MTT assays (E) indicated that treatment with Tipifarnib (farnesyltransferase inhibitor) can partly reverse oecircFNTA‐increased invasion in J82 and UMUC3 cells. Scale bar, 100 μm.
-
FWestern blots showed that shcircFNTA in UMUC3 or oecircFNTA in J82 cells did not change KRAS protein level significantly.
-
GRaf‐1 RBD GST pull‐down assays confirmed that shcircFNTA can decease KRAS activity in UMUC3 cells (upper panel), and oecircFNTA can increase KRAS activity in J82 cells (lower panel).
-
HWestern blots showed that shcircFNTA can decrease ERK1/2 and MEK1/2 phosphorylation in UMUC3 cells (left panel), and oecircFNTA can increase their phosphorylation in J82 cells (right panel).
Figure EV2. Tipifarnib (farnesyltransferase inhibitor) concentration test for BCa cell viability.
-
AThe MTT assay shows relative cell viability using UMUC3 cells treated with Tipifarnib (0, 0.1, 0.2, 0.5, 1, and 2 μM).
-
BThe MTT assay shows relative cell viability using J82 cells treated with Tipifarnib (0, 0.01, 0.02, 0.05, 0.1, and 0.2 μM).
As earlier studies indicated that farnesyltransferase regulates cancer cell progression by altering KRAS activity 26, we examined the potential impact of circFNTA on regulating KRAS activity. Western blots revealed that total KRAS protein expression was not affected by adding shcircFNTA and/or oe‐circFNTA (or oelinearFNTA) to UMUC3 and J82 cells, respectively (Fig 5F). In contrast, results from Raf‐1 RBD GST pull‐down assays confirmed that adding shcircFNTA deceases KRAS activity in UMUC3 cells and adding oecircFNTA increases KRAS activity (more profoundly than adding oelinearFNTA) in J82 cells (Fig 5G). Also, KRAS downstream targets (e.g., ERK1/2 and MEK1/2 phosphorylation status) were altered in response to shcircFNTA or oecircFNTA (Fig 5H).
Together, the results from Fig 5A–H suggest that AR/ADAR2/circFNTA/miR‐370‐3p/FNTA signaling increases BCa cell invasion and chemo‐resistance by altering the KRAS activity.
Depletion of circFNTA suppresses BCa cell invasion and increases cisplatin chemo‐sensitivity
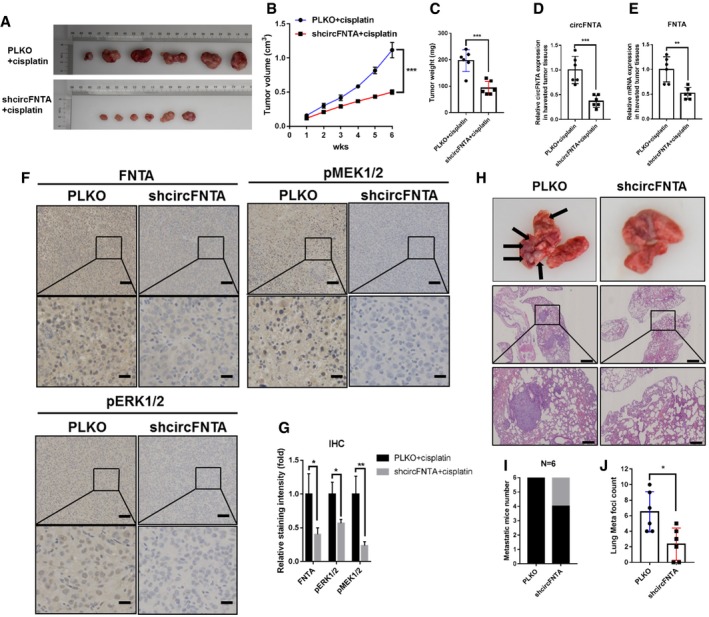
To further confirm all the above in vitro data, we conducted a preclinical study using the xenograft BALB/c nude mice model. UMUC3 cells stably transfected with a shcircFNTA construct or control vector (PLKO) were subcutaneously injected into nude mice followed by treatment with intraperitoneal injection of 1 mg/kg cisplatin every 3 days. Decreased xenograft growth rates and tumor weights were found in the shcircFNTA xenografted mice compared with vector xenografted mice (Fig 6A–C). qRT–PCR using mice tumor tissues following sacrifice confirmed efficient knockdown of circFNTA and that the expression of its downstream host gene FNTA correspondingly decreased (Fig 6D and E). Furthermore, IHC tissues staining revealed that the expressions of FNTA, p‐ERK1/2, and p‐MEK1/2 were decreased by targeting circFNTA (Fig 6F and G). In addition, UMUC3‐PLKO or UMUC3‐shcircFNTA cells were injected into two other sets of mice via the tail vein to establish an experimental metastasis model. The results showed that knocking down circFNTA led to a decrease of tumor metastases and total lung metastatic foci compared to the vector group (Fig 6H–J).
Figure 6. Preclinical study using in vivo mouse model to confirm the role of circFNTA in bladder cancer cell progression and cisplatin chemo‐resistance.
-
AUMUC3‐PLKO and UMUC3‐shcircFNTA cells were used to establish a subcutaneous nude mouse xenograft model, and cisplatin (1 mg/kg) was intraperitoneally administered. Images of tumors are shown after mice were sacrificed (N = 6).
-
BCompared with the vector (PLKO) group, the tumor growth rate was significantly inhibited in shcircFNTA nude mice.
-
CTumor weights also decreased in the shcircFNTA group.
-
D, EThe qRT–PCR results using mouse tumor tissues revealed that circFNTA expression was significantly reduced (D), and the expression of its host gene FNTA was correspondently decreased in the shcircFNTA group as compared to the PLKO group (E).
-
FRepresentative immunohistochemistry (IHC) images detecting FNTA, p‐ERK1/2, and p‐MEK1/2 in PLKO tumors as compared to shcircFNTA xenografted tumors. Scale bars: 100 μm (upper panels) and 25 μm (lower panels).
-
GQuantification of relative IHC staining intensity revealed that targeting circFNTA led to decreased expression of FNTA, p‐ERK1/2, and p‐MEK1/2.
-
HThe tumor tail vein injection model was established to observe lung metastasis (N = 6). Representative images of lung metastasis foci in gross and microscopic HE examination. Scale bars: 400 μm (upper panels) and 100 μm (lower panels).
-
IKnocking down circFNTA significantly decreased metastatic foci numbers as compared to the PLKO group.
-
JTargeting circFNTA significantly reduced lung metastatic foci as compared to the PLKO group.
Together, the results shown in Fig 6A–J demonstrate that targeting AR/ADAR2/circFNTA/miR‐370‐3p/FNTA/KRAS signaling with shcircFNTA suppresses BCa cell invasion and increases cisplatin chemo‐sensitivity also in vivo.
Discussion
Continued efforts have explored the expression profiles of circRNAs in multiple cell types or patient tissue samples, aiming to clarify their potential functions, regulatory networks, and relationships with disease initiation and development 27, 28. CircRNAs have been shown to be involved in many biological processes, such as miRNA sponging, gene splicing, transcription regulation, and parental gene expression modification 29, 30. For cancer studies, circRNAs may act as oncogenic molecules or tumor suppressors to influence cancer initiation and/or progression. Therefore, their expression is another layer of cellular regulatory mechanisms that likely represent promising candidates for clinical cancer diagnosis and treatment 31, 32. For the role of circRNAs in BCa development, several reports of differential expression of circRNAs and their associated pathways broadly implicate the role of circRNAs in BCa development through regulation of the cell cycle and angiogenesis, as well as cell migration and invasion. Whether these circRNAs are coordinately expressed to regulate BCa progression as well as their upstream regulators remains to be studied 16, 17, 18, 19.
On the other hand, we examined the role of circRNAs in BCa phenotypes that are closely tied to a significant player of BCa initiation and development, namely the AR, as well as to establish a chain of regulatory steps to identify a circRNA, circFNTA. This circFNTA is directly connected to one of the most significant oncogenes in BCa, KRAS 33, through regulating the expression of its host gene FNTA, the alpha subunit of farnesyltransferase, which was shown to be a key factor for various cancer metastases and chemo‐resistance 34. Indeed, several inhibitors for this enzyme have been developed for cancer prevention and treatment 35, 36. Not surprisingly, this association makes this circRNA potent enough to impact diverse tumor behaviors including cell invasion and sensitivity to chemotherapeutic drugs. As targeting KRAS for suppressing cancer development remains an unmet and ongoing goal of cancer therapy, our efforts in characterizing a novel regulatory mechanism through circFNTA for regulating KRAS activity represent an innovative approach for identifying potential therapeutic targets for BCa therapy.
The miRNA sponge effect is one of the most classical mechanisms for non‐coding RNAs (ncRNAs), including circRNAs 37, 38. We examined 11 miRNAs from online database prediction and subsequently validated them by RNA pull‐down assay. Results showed three miRNAs might be pulled down and directly interact with circFNTA. While previous HITS‐CLIP data failed to find a connection between miR‐370‐3p and FNTA mRNA in their experimental settings, our functional assays revealed that miR‐370‐3p could partly reverse circFNTA effects in BCa cells. These results are consistent with those that indicated miR‐370‐3p could directly repress Wnt7a expression and thereby suppress BCa cell invasion 39, as well as the role of miR‐370‐3p in regulating glioblastoma chemo‐sensitivity to temozolomide by influencing MGMT expression 40. These results also indicate that it is possible that the circFNTA may be capable of regulating biological processes that were not tested in our current studies and may function through the other miRNAs to regulate BCa development in a particular stage or tumor microenvironment.
In summary, AR‐mediated circFNTA can promote BCa invasion and cisplatin chemo‐resistance via miR‐370‐3p/FNTA/KRAS signals (Fig 7), and targeting this newly identified pathway may help to develop new therapies to better suppress BCa progression.
Figure 7. Illustration of AR/ADAR2/circFNTA/miR‐370‐3p/FNTA/KRAS signaling in BCa invasion and cisplatin chemo‐sensitivity.
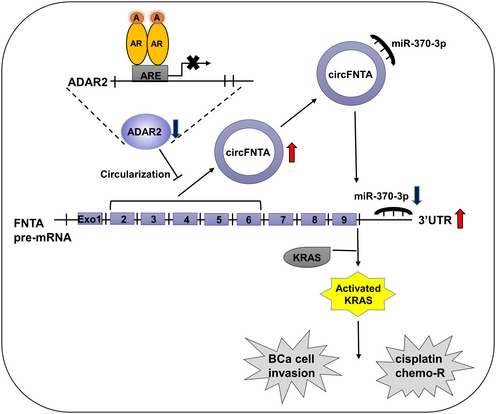
Upon ligand binding (androgen, A), the androgen receptor (AR) interacts with an androgen response element (ARE) in the promoter region of the RNA editing gene ADAR2, promoting its repression. This in turn increases circFNTA levels, which leads to elevated sponging of the microRNA miR‐370‐3p and increased expression of FNTA. High levels of FNTA activate KRAS to promote cell invasion and chemo‐resistance (chemo‐R).
Materials and Methods
Cell lines
Normal human urothelial epithelial cell line SVHUC, BCa cell lines T24, J82, 5637, and UMUC3 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). SVHUC cells were cultured in Kaighn's Modification of Ham's F‐12 Media. T24, J82 and UMUC3 cells were maintained in DMEM, and 5637 cells were maintained in RPMI‐1640. The culture media were supplemented with 10% fetal bovine serum (FBS), antibiotics (100 units/ml penicillin, 100 mg/ml streptomycin), and 2 mM glutamine (Invitrogen, Grand Island, NY, USA) in a humidified 5% CO2 environment at 37°C.
Reagents and materials
AR (N‐20), GAPDH (6c5), p‐MEK‐1/2 (7E10), and MEK‐1/2 (9G3) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The p44/42 MAPK (Erk1/2, 137F5) and phospho‐p44/42 MAPK (p‐Erk1/2, Thr202/Tyr204, 197G2) were purchased from Cell Signaling (Danvers, MA, USA). ADAR2 antibody (GTX114237) was purchased from GeneTex (Irvine, CA, USA), and FNTA antibody (A304‐267A) was purchased from Bethyl Laboratories, Inc (Montgomery, TX, USA). Tipifarnib (CAS192185‐72‐1) was purchased from Cayman Chemical (Ann Arbor, MI, USA), and anti‐mouse/rabbit secondary antibody for Western blots was from Invitrogen.
Lentivirus packaging and infection
The standard CaCl2 transfection method was performed. The psAX2 packaging plasmid and pMD2G envelope plasmid, with the gene of interest knockdown/overexpression plasmids (PLKO‐shAR, PWPI‐oeAR, PLKO‐shcircFNTA (has_circ_0084171, has_circ_0023642, has_circ_0061265, has_circ_0000920), PWPI‐circFNTA, PLVTHM‐miR‐370‐3p/miR‐328‐5p/miR‐920, or PLVTHM‐shFNTA), were transfected into HEK293T cells for 48 h to generate the lentivirus supernatant, and oligo sequences were listed in Appendix Table S1. The lentivirus supernatants were harvested through a 0.45‐μm nitrocellulose filter and used immediately or frozen at −80°C for later use. The method of generating circRNA overexpression plasmids was according to our previous paper 40. To clone and PCR circRNA from the pBSK_circFNTA, the forward primer was as follows: 5′‐GTGAGGAATTTCGACATTTAAATTTAAAAGTGCTGAGATTACAGGCG‐3′, and the reverse primer was as follows: 5′‐TCCTGCAGCCCGTAGTTTTGCTGGGATTACAGGTGTGA‐3′.
Transwell invasion assay
Cell invasion capacity was assessed by a matrigel invasion assay using matrigel (BD Biosciences, Sparks, MD) coated 8.0 μm filter membranes (Corning, NY, USA). Cells (1 × 105) in 150 μl of serum‐free media were plated in upper chambers, with 750 μl of 10% serum‐containing media placed in the lower chambers. After 24 h, cells remaining on the upper surface of the filters were removed with cotton swabs and invaded cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet, and positively stained cells were counted. The cell numbers were obtained by counting six random fields. Quantitation indicates mean ± SD of triplicate repeats.
MTT assay
Cells (3 × 103 per well) seeded in 96‐well tissue culture plates were incubated with/without cisplatin. After 48 h of treatment, 10 ml methylthiazolyldiphenyl‐tetrazolium bromide (MTT) stock solution (5 mg/ml; Sigma‐Aldrich) was added to each well with 100 ml media for 4 h at 37°C. The media were replaced with 100 μl dimethyl sulfoxide (DMSO), followed by incubation for 5 min at room temperature. The absorbance at a wavelength of 570 nm was then measured.
RNA analysis by quantitative RT–PCR
Total RNAs from transfected cells were isolated in two steps using Trizol reagent (Invitrogen). RNA at 2 μg was reverse transcribed using Superscript III transcriptase (Invitrogen). Quantitative real‐time PCR (qRT–PCR) was conducted using a Bio‐Rad CFX96 system. SYBR green was used to determine the mRNA expression level of a gene of interest. Primers were listed in Appendix Table S2. Expression levels were normalized to the mRNA expression of GAPDH. RNase R treatment was performed for 15 min at 37°C using 2 U/mg RNase R (Epicenter, Madison, WI).
Western blot analysis
Cells were lysed in RIPA buffer, and 30 mg total proteins was separated on 10% SDS–PAGE gel and then transferred onto PVDF membranes (Millipore, Billerica, MA). After blocking membranes with 5% non‐fat milk TBST solution, they were incubated with appropriate dilutions of specific primary antibodies. The blots were then incubated with HRP‐conjugated secondary antibodies and then visualized using the ECL system (Thermo Fisher Scientific, Waltham, MA).
RNA fluorescence in situ hybridization
FISH was performed to detect the presence of circFNTA using a Dig‐labeled probe (5′‐TGCTCTGTCCTGGACTTCTC‐3′). The signals of the probes were detected by FISH Kit (K2191050, BioChain, Newark, CA, USA) according to the manufacturer's instructions. The FISH intensity of tissues stainings was blindly scored and termed as expression level by the intensity score (0 = negative, 1 = low, 2 = medium, 3 = high) and the percentage score (0 = none stained, 1 = 1–49% stained, 2 = 50–100% stained) in five separate fields. Total score was intensity score × percentage score.
Chromatin immunoprecipitation
Lysates were pre‐incubated with protein A‐agarose‐conjugated normal rabbit IgG (sc‐2027, Santa Cruz), and 2 μg anti‐AR antibody (N‐20, Santa Cruz) was then added to the cell lysates overnight at 4°C. IgG was used as the negative control. Specific primer sets were designed to amplify a target sequence within ADAR2 gene's promoter. PCR products were analyzed by agarose gel electrophoresis and qRT–PCR.
The circRNA and miRNA pull‐down assay
The specific steps of pull‐down assay were according to our previous paper 41 for the use of the biotin‐labeled anti‐sense oligos against circRNA‐FNTA (5′‐TGCTCTGTCCTGGACTTCTC‐3′). Total RNA was extracted by Trizol (Invitrogen) according to the manufacturer's protocol, reverse transcribed, and subjected to qRT–PCR analysis to detect the circFNTA pulled‐down miRNAs.
Activated KRAS affinity precipitation assay
Activated KRAS affinity precipitation assay was performed as follows: 5 μg of Raf‐1 RBD agarose beads was incubated with cell lysates for 30 min at 4°C, and then, the agarose beads were extensive washed with washing buffer three times. The activated KRAS was bound to Raf‐1 Ras binding domain (RBD) agarose beads and the bead pellet resuspended in 40 μl of 2× reducing SDS–PAGE sample buffer. The amount of activated KRAS was determined by immunoblotting with a KRAS antibody.
Luciferase assay
The human 5′‐promoter region of the ADAR2 gene was constructed into pGL3‐basic luciferase reporter vector. Site‐directed mutagenesis of the AR binding site in the ADAR2 5′‐promoter was achieved with the Quick Change mutagenesis. The 3′UTR of FNTA with WT or Mut miRNA‐responsive elements was constructed into psiCheck2. Cells were plated in 24‐well plates, and the cDNA was transfected using Lipofectamine 3000 (Invitrogen). Luciferase activity was measured 36–48 h after transfection by Dual‐Luciferase Assay (Promega, Madison, WI) according to the manufacturer's manual.
In vivo studies
UMUC3 cells were transfected with PLKO vector or PLKO‐shcircFNTA. The positive stable clones were selected and expanded in culture. For in vivo tumor growth studies, male, athymic BALB/c, nude mice were randomly divided into two groups (n = 6 per group). UMUC3 cells were harvested, washed, resuspended in serum‐free media and mixed 1:1 with matrigel, and then injected subcutaneously into upper back of the nude mice (1 × 107 cells/mouse). One week after inoculation, all of the mice were intraperitoneally treated with 1 mg/kg cisplatin every 3 days. Six weeks later, mice were sacrificed by cervical dislocation and subjected to tumor weight and immunohistochemistry (IHC) studies. For tumor metastasis studies, UMUC3‐PLKO vector and UMUC3‐shcircFNTA cells were injected into other sets of nude mice via the tail vein (2 × 106 cells/mouse). Eight weeks later, mice were sacrificed and lung metastatic foci were examined. Protocols for animal care and experimentation were approved by the Animal Care Committee of Xiangya Hospital, Central South University.
Immunohistochemistry staining
IHC was performed on the samples from mouse xenografted tumors and metastases. Briefly, the samples were fixed in 4% neutral buffered paraformaldehyde, embedded in paraffin, and cut into 5 μm slices. After deparaffinization, hydration, and antigen retrieval, these sliced sections were incubated with corresponding primary antibodies, incubated with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, USA), and then visualized by VECTASTAIN ABC peroxidase system and 3,3′‐diaminobenzidine (DAB) kit (Vector Laboratories, Burlingame, CA, USA). The slides were reviewed and scored blindly by two experienced pathologists. The degree of positivity was determined according to the percentage of positive tumor cells.
Statistics
Experiments were repeated at least three times with triplicate data points. Results are expressed as mean ± SD. Student's t‐test was used to determine statistical significance. A P‐value of less than 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was approved by the Xiangya Hospital, Central South University Ethics Committee. Signed informed consents were obtained from all the patients. And the animal experiments were conducted strictly in accordance with the Animal Care Committee of Xiangya Hospital, Central South University.
Author contributions
JBC, YS, SYY, XBZ, and CSC designed the experimental protocols. JBC, YS, ZYO, YCT, and T‐JS performed the studies. JBC, YS, and SYY analyzed the data. JBC, YS, ZYO, SYY, C‐PH, BY, YCT, T‐JS, XBZ, and CSC wrote the manuscript with contributions from all of the other authors.
Conflict of interest
ASC‐J9® was patented by the University of Rochester, the University of North Carolina, and AndroScience Corp., and then licensed to AndroScience Corp. Both the University of Rochester and CSC own royalties and equity in AndroScience Corp. The other authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Acknowledgements
We thank Karen Wolf for help preparing the manuscript. This work was supported by NIH grant (CA155477), in part by the National Natural Science Foundation of China (81572523, 81873626, 81902592), the Hunan Province Funds for Distinguished Young Scientists of China (2016JJ1026), Hunan Province Key R&D Program (2019SK2202), and Xiangya Hospital Youth Fund (2018Q09).
EMBO Reports (2020) 21: e48467
Contributor Information
Xiongbing Zu, Email: whzuxb@163.com.
Chawnshang Chang, Email: chang@urmc.rochester.edu.
Data availability
All the datasets and materials generated and/or analyzed during the current study are available to the public.
References
- 1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F (2017) Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 71: 96–108 [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87–108 [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68: 7–30 [DOI] [PubMed] [Google Scholar]
- 4. Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, Guo CC, Lotan Y, Kassouf W (2016) Bladder cancer. Lancet 388: 2796–2810 [DOI] [PubMed] [Google Scholar]
- 5. Tan WS, Rodney S, Lamb B, Feneley M, Kelly J (2016) Management of non‐muscle invasive bladder cancer: a comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat Rev 47: 22–31 [DOI] [PubMed] [Google Scholar]
- 6. Milowsky MI, Rumble RB, Booth CM, Gilligan T, Eapen LJ, Hauke RJ, Boumansour P, Lee CT (2016) Guideline on muscle‐invasive and metastatic bladder cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol 34: 1945–1952 [DOI] [PubMed] [Google Scholar]
- 7. Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA (2016) Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol 69: 300–310 [DOI] [PubMed] [Google Scholar]
- 8. Chang C, Lee SO, Yeh S, Chang TM (2014) Androgen receptor (AR) differential roles in hormone‐related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 33: 3225–3234 [DOI] [PubMed] [Google Scholar]
- 9. Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY et al (2007) Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst 99: 558–568 [DOI] [PubMed] [Google Scholar]
- 10. Kashiwagi E, Ide H, Inoue S, Kawahara T, Zheng Y, Reis LO, Baras AS, Miyamoto H (2016) Androgen receptor activity modulates responses to cisplatin treatment in bladder cancer. Oncotarget 7: 49169–49179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shang Z, Li Y, Zhang M, Tian J, Han R, Shyr CR, Messing E, Yeh S, Niu Y, Chang C (2015) Antiandrogen therapy with hydroxyflutamide or androgen receptor degradation enhancer ASC‐J9 enhances BCG efficacy to better suppress bladder cancer progression. Mol Cancer Ther 14: 2586–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li P, Chen J, Miyamoto H (2017) Androgen receptor signaling in bladder cancer. Cancers (Basel) 9: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK (1976) Viroids are single‐stranded covalently closed circular RNA molecules existing as highly base‐paired rod‐like structures. Proc Natl Acad Sci USA 73: 3852–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495: 333–338 [DOI] [PubMed] [Google Scholar]
- 15. Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19: 141–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhong Z, Lv M, Chen J (2016) Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25‐miR‐103a‐3p/miR‐107‐CDK6 pathway in bladder carcinoma. Sci Rep 6: 30919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong Z, Huang M, Lv M, He Y, Duan C, Zhang L, Chen J (2017) Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett 403: 305–317 [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang C, Liu D, Wang M, Wang L, Zeng F et al (2017) CircHIPK3 sponges miR‐558 to suppress heparanase expression in bladder cancer cells. EMBO Rep 18: 1646–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang C, Yuan W, Yang X, Li P, Wang J, Han J, Tao J, Li P, Yang H, Lv Q et al (2018) Circular RNA circ‐ITCH inhibits bladder cancer progression by sponging miR‐17/miR‐224 and regulating p21, PTEN expression. Mol Cancer 17: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhong Z, Lv M, Chen J (2016) Gene Expression Omnibus GSE92675 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE92675). [DATASET]
- 21. Tan MH, Li Q, Shanmugam R, Piskol R, Kohler J, Young AN, Liu KI, Zhang R, Ramaswami G, Ariyoshi K et al (2017) Dynamic landscape and regulation of RNA editing in mammals. Nature 550: 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aktas T, Avsar Ilik I, Maticzka D, Bhardwaj V, Pessoa Rodrigues C, Mittler G, Manke T, Backofen R, Akhtar A (2017) DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature 544: 115–119 [DOI] [PubMed] [Google Scholar]
- 23. Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ (2015) The RNA binding protein quaking regulates formation of circRNAs. Cell 160: 1125–1134 [DOI] [PubMed] [Google Scholar]
- 24. Ferguson D, Rodriguez LE, Palma JP, Refici M, Jarvis K, O'Connor J, Sullivan GM, Frost D, Marsh K, Bauch J et al (2005) Antitumor activity of orally bioavailable farnesyltransferase inhibitor, ABT‐100, is mediated by antiproliferative, proapoptotic, and antiangiogenic effects in xenograft models. Clin Cancer Res 11: 3045–3054 [DOI] [PubMed] [Google Scholar]
- 25. Smalley KS, Eisen TG (2003) Farnesyl transferase inhibitor SCH66336 is cytostatic, pro‐apoptotic and enhances chemosensitivity to cisplatin in melanoma cells. Int J Cancer 105: 165–175 [DOI] [PubMed] [Google Scholar]
- 26. Liu M, Sjogren AK, Karlsson C, Ibrahim MX, Andersson KM, Olofsson FJ, Wahlstrom AM, Dalin M, Yu H, Chen Z et al (2010) Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K‐RAS‐induced lung cancer. Proc Natl Acad Sci USA 107: 6471–6476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Liu T, Wang X, He A (2017) Circles reshaping the RNA world: from waste to treasure. Mol Cancer 16: 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Mo Y, Gong Z, Yang X, Yang M, Zhang S, Xiong F, Xiang B, Zhou M, Liao Q et al (2017) Circular RNAs in human cancer. Mol Cancer 16: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H (2015) Circular RNA: a new star of noncoding RNAs. Cancer Lett 365: 141–148 [DOI] [PubMed] [Google Scholar]
- 30. Zhou R, Wu Y, Wang W, Su W, Liu Y, Wang Y, Fan C, Li X, Li G, Li Y et al (2018) Circular RNAs (circRNAs) in cancer. Cancer Lett 425: 134–142 [DOI] [PubMed] [Google Scholar]
- 31. Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, Ren D, Ye X, Li C, Wang Y et al (2018) Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer 17: 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M (2017) CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer 16: 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCormick F (2015) KRAS as a therapeutic target. Clin Cancer Res 21: 1797–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma Y, Cheng Z, Liu J, Torre‐Healy L, Lathia JD, Nakano I, Guo Y, Thompson RC, Freeman ML, Wang J (2017) Inhibition of farnesyltransferase potentiates NOTCH‐targeted therapy against glioblastoma stem cells. Stem Cell Reports 9: 1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding N, Cui XX, Gao Z, Huang H, Wei X, Du Z, Lin Y, Shih WJ, Rabson AB, Conney AH et al (2014) A triple combination of atorvastatin, celecoxib and tipifarnib strongly inhibits pancreatic cancer cells and xenograft pancreatic tumors. Int J Oncol 44: 2139–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Untch BR, Dos Anjos V, Garcia‐Rendueles MER, Knauf JA, Krishnamoorthy GP, Saqcena M, Bhanot UK, Socci ND, Ho AL, Ghossein R et al (2018) Tipifarnib inhibits HRAS‐driven dedifferentiated thyroid cancers. Cancer Res 78: 4642–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen L, Zhang S, Wu J, Cui J, Zhong L, Zeng L, Ge S (2017) circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR‐29 family. Oncogene 36: 4551–4561 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. He R, Liu P, Xie X, Zhou Y, Liao Q, Xiong W, Li X, Li G, Zeng Z, Tang H (2017) circGFRA1 and GFRA1 act as ceRNAs in triple negative breast cancer by regulating miR‐34a. J Exp Clin Cancer Res 36: 145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang X, Zhu H, Gao Z, Li J, Zhuang J, Dong Y, Shen B, Li M, Zhou H, Guo H et al (2018) Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR‐370‐3p. J Biol Chem 293: 6693–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gao YT, Chen XB, Liu HL (2016) Up‐regulation of miR‐370‐3p restores glioblastoma multiforme sensitivity to temozolomide by influencing MGMT expression. Sci Rep 6: 32972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han Z, Zhang Y, Sun Y, Chen J, Chang C, Wang X, Yeh S (2018) ERbeta‐mediated alteration of circATP2B1 and miR‐204‐3p signaling promotes invasion of clear cell renal cell carcinoma. Cancer Res 78: 2550–2563 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zhong Z, Lv M, Chen J (2016) Gene Expression Omnibus GSE92675 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE92675). [DATASET]
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Data Availability Statement
All the datasets and materials generated and/or analyzed during the current study are available to the public.


