Abstract
Aims
Atrial fibrillation (AF) has been associated with reduced brain volume, cognitive impairment, and reduced cerebral blood flow. The causes of reduced cerebral blood flow in AF are unknown, but no reduction was seen in individuals without the arrhythmia in a previous study. The aim of this study was to test the hypothesis that brain perfusion, measured with magnetic resonance imaging (MRI), improves after cardioversion of AF to sinus rhythm (SR).
Methods and results
All patients undergoing elective cardioversion at our institution were invited to participate. A total of 44 individuals were included. Magnetic resonance imaging studies were done before and after cardioversion with both brain perfusion and cerebral blood flow measurements. However, 17 did not complete the second MRI as they had a recurrence of AF during the observation period (recurrent AF group), leaving 17 in the SR group and 10 in the AF group to complete both measurements. Brain perfusion increased after cardioversion to SR by 4.9 mL/100 g/min in the whole brain (P < 0.001) and by 5.6 mL/100 g/min in grey matter (P < 0.001). Cerebral blood flow increased by 58.6 mL/min (P < 0.05). Both brain perfusion and cerebral blood flow remained unchanged when cardioversion was unsuccessful.
Conclusion
In this study of individuals undergoing elective cardioversion for AF, restoration, and maintenance of SR for at least 10 weeks after was associated with an improvement of brain perfusion and cerebral blood flow measured by both arterial spin labelling and phase contrast MRI. In those individuals where cardioversion was unsuccessful, there was no change in perfusion or blood flow.
Keywords: Atrial fibrillation, Cardioversion, Brain volume, Cognitive impairment, Cerebral blood flow, Brain perfusion
What’s new?
Cardioversion of atrial fibrillation (AF) to sinus rhythm resulted in improvement in cerebral blood flow and brain perfusion measured by phase contrast and arterial spin labelling magnetic resonance imaging of the brain.
In those where cardioversion was unsuccessful no improvement in these parameters was seen.
Decreased cerebral blood flow and brain perfusion could be a key mechanism in causing reduced brain volumes and cognitive decline seen with AF.
These results might have important clinical implications but need to be further evaluated in large-scale clinical trials.
Introduction
Atrial fibrillation (AF) has become a serious public health problem and its prevalence is expected to triple in the next four decades.1 Atrial fibrillation is a significant risk factor for cardioembolic stroke. Atrial fibrillation has furthermore been linked to cognitive decline and vascular dementia, independent of stroke.2,3 The mechanisms behind the association of AF and a detriment in cognitive function are not clear despite multiple shared risk factors including hypertension, diabetes, heart failure, chronic kidney disease, and alcohol abuse.4 Atrial fibrillation has been associated with decreased brain volume as well as cognitive impairment suggesting a link between these.5 There are many possible factors linking AF to decreased brain volume and dementia including altered neurohumoral regulation of the blood flow to the brain secondary to the arrhythmia, multiple microemboli causing small cerebral infarcts and decreased cerebral blood flow, in part due to beat-to-beat variation in stroke volume and lack of atrial contraction.
While cerebral blood flow in AF is not a particularly well-studied subject, there is nevertheless some recent clinical data that supports brain hypoperfusion and diminished cerebral blood flow in AF. In a large cohort of elderly individuals persistent AF was associated with both lower total cerebral blood flow and lower estimated brain perfusion measured by phase contrast magnetic resonance imaging (MRI) of the brain compared to those in sinus rhythm (SR).6 While these results showing decreased cerebral blood flow and brain perfusion in AF with MRI were intriguing, there were some important limitations including the cross-sectional design of the study and indirect measurement of brain perfusion. The results also raise the important question of whether restoration of SR in those with AF could lead to improvement in or even restoration of normal cerebral blood flow.
In a recent large population study, lower cerebral perfusion was associated with brain volume loss, accelerated cognitive decline and a higher risk of dementia during a 7-year follow-up.7 This same mechanism could contribute to the detriment of cognitive function seen in individuals with AF.5,8
The goal of this study was to test the hypothesis that blood flow to the brain is lowered in AF, resulting in decreased brain perfusion and cerebral blood flow, and that successful cardioversion to SR leads to improvement in these parameters. If this would be the case it would strengthen our previous observation as a kind of ‘proof of concept’.
Methods
Individuals aged 18–75 years who were referred to the Landspitali—The National University Hospital in Reykjavik for elective direct-current cardioversion for AF were invited to participate in the study. Those with a pacemaker, an implantable cardioverter-defibrillator, other contraindications for MRI or severe claustrophobia were excluded. A study coordinator went through a safety questionnaire regarding MRI compatibility and collected baseline information. Informed written consent was obtained and the participant was advised not to use tobacco or caffeine at least 4 h before the MRI examination. When the date of the cardioversion was set the coordinator made an appointment for the first MRI study to be done prior to the cardioversion, preferably on the same day or the day before. On the day of the MRI, the patient underwent an electrocardiogram (ECG) to confirm that the patient was still in AF and a blood test including measurement of N-terminal pro brain natriuretic peptide (NT-proBNP). After the cardioversion, an appointment was made for the second MRI examination at least 10 weeks later. This timeframe was chosen to allow for the return of mechanical atrial systole, which takes a few weeks after restoration of electrical SR.
Approximately a week before the second MRI, the patient underwent a new ECG and was asked about symptoms of recurrent AF. If a patient who previously had been cardioverted to SR had a recurrence of AF on the ECG, they were excluded from further participation in the study as the time from cardioversion to recurrence was uncertain and the second MRI was cancelled. No 24-h Holter monitoring was performed. Those that achieved continued SR after a successful cardioversion, or continued in AF after unsuccessful cardioversion, that is maintained unchanged rhythm at the second MRI; went for the imaging procedure as planned. Those that went back into AF during the observation period were excluded from the study as it was not known when they had reverted to AF again, this could have happened anytime from a few hours after the cardioversion to some weeks later. The lack of knowledge on this would have made the results on this group very difficult to interpret.
Baseline data collected included information on age, sex, height and weight of the participant, as well as blood pressure and heart rate. Body mass index (BMI) was calculated from measured height and weight. Information on the individual’s past medical history and cardiovascular risk factors was also collected, including information on the nature of AF (paroxysmal or persistent), length of AF episode, and history of previous cardioversion. Information on medication use included use of beta-blockers, anti-hypertensive medication, warfarin or novel oral anticoagulants (NOACs), aspirin or platelet inhibitors, diabetes medication, lipid-lowering medication, and digoxin was registered. Information was also obtained from medical records and results of echocardiography studies noted if done within 6 months prior to entering the study.
The study was approved by the Icelandic National Bioethics Committee, which acts as the Institutional Review Board for the Icelandic Heart Association and The National University Hospital (VSN-13-043) and by The Icelandic Data Protection Authority.
All participants without a contraindication for MRI underwent a brain scan on a 1.5-T Signa Excite Twinspeed system (General Electric Medical Systems, Waukesha, WI, USA). The scan protocol included acquisition of pseudo-continuous arterial spin labelling (ASL) perfusion sequences for calculating brain perfusion with post-labelling delay of 1525 ms. Additionally, a phase contrast sequence for flow measurements in the cervical arteries was performed as well as anatomical imaging sequences of the whole brain for calculations of brain tissue volumes, including cerebrospinal fluid, grey matter, white matter, and white matter hyperintensities that were computed with a validated automatic image post-processing pipeline.9,10 Total brain volume (TBV) was computed in millilitres (mL) as the sum of grey matter volume, white matter volume, and white matter hyperintensities volume. The intracranial volume was computed as the sum of TBV and cerebrospinal fluid volume. Brain volumes, in this study, were normalized to intracranial volume and presented as percentages of intracranial volume (TBV/ICV * 100).
Arterial spin labelling is a completely non-invasive method of measuring brain perfusion by using magnetically labelled arterial blood water as an endogenous tracer for quantification of cerebral blood flow in the capillary bed. Hence, this technique does not require an exogenous gadolinium-based tracer as in dynamic susceptibility contrast perfusion, as the blood itself acts as the freely diffusible tracer and the perfusion is measured directly. Inflowing hydrogen nuclei in the arterial blood water are magnetized and then imaged by first labelling the water molecules just proximal to the region of interest. After a certain time, transit time, the paramagnetic tracer flows into the plane to be imagined and interacts with tissue water. The inflow of inverted spins alters total tissue magnetization and consequently the tissue magnetization and image intensity, creating the tag image. The final product, the perfusion image, will reflect the amount of arterial blood delivered to each voxel within the slice within the transit time, determining the delivery rate of oxygen and nutrients to the capillary bed.10
Total cerebral blood flow was measured for comparison with ASL using phase contrast MRI for flow measurement at the level of the skull base in both the internal carotid arteries and the basilar artery. The method of studying the blood flow by phase-contrast MRI is a reliable and highly reproducible method and by measuring the total cerebral blood flow in the carotids and the basilar artery the sum of blood flowing to the brain through the cervical arteries is directly measured.11 More details on the acquisition and analysis of the images as well as calculation of perfusion and flow can be found elsewhere, previously described in detail by Sigurdsson et al.10 The operators of the MRI system and the MR image analysts were blinded to all clinical information on the study participants.
Statistical analysis
Data were presented as mean (standard deviation) for continuous variables and as percentage for categorical variables. A P-value <0.05 was considered statistically significant. Characteristics between groups were compared using t-tests and χ2 tests. The assumption of a normal distribution of the two continuous blood flow measures was verified by inspecting qq-plots of residuals from the regression models. All analyses were performed using R version 3.5.3. Analysis of change in total cerebral blood flow by and between groups was performed using linear mixed models, using the lme4 package in R, with age and sex adjustment, as well as adjustment for brain volume. Analysis of brain perfusion was done similarly with adjustments but adjustment for brain volume was not necessary since the outcome is independent of brain volume due to its direct nature. Adjusted marginal means at each time-point and change between time-points was estimated using the emmeans package in R.
Analytical sample
In total, 142 individuals were initially contacted either with a letter or by telephone. Of those, 60 persons never replied or were not reached in time before their planned cardioversion. Fourteen individuals declined participation in the study, which left 68 individuals that accepted to take part in the study. Of those, 11 were excluded because of spontaneous return to SR before the cardioversion, 6 individuals were further excluded because of a pacemaker or an implanted cardioverter-defibrillator, 5 were excluded because of atrial flutter being the primary arrhythmia and one patient was excluded due to equipment malfunction (MRI machine malfunction at the day of the first examination). During the study period, one individual was further excluded because of a reclassification of the primary arrhythmia as atrial flutter rather than AF discovered while reviewing ECGs. That left 44 eligible and willing individuals to participate in the study.
Results
Of the 44 patients enrolled in the study, 35 (80%) were men and 9 women, mean age 64.4 years (Table 1). A total of 27 individuals (21 men) completed the study with two MRI examinations, 17 that were cardioverted to SR and remained in SR at the time of the second MRI examination (SR group) and 10 who continued to be in AF despite the cardioversion attempt (AF group). The remaining 17 participants had a recurrence of AF during the period from successful cardioversion to the second MRI examination and hence were excluded from further evaluation with a second MRI examination according to the study protocol (recurrent AF group). One of the 27 participants had missing blood flow measurement values due to a technical failure on the day of the second examination and therefore cerebral blood flow values measured with phase contrast MRI only exist for 9 individuals but perfusion values measured with ASL were available for all 10 individuals in the AF group.
Table 1.
Patient characteristics
| Male (n = 35) | Female (n = 9) | P-value | SR group (n = 17) | AF group (n = 10) | Recurrent AF group 3 (n = 17) | P-value all groups | |
|---|---|---|---|---|---|---|---|
| Age (years) (at entry into study) | 64.1 (8.3) | 65.8 (8.5) | 0.59 | 62.6 (9.8) | 65.9 (6.3) | 65.3 (7.6) | 0.53 |
| Height (cm) | 181.2 (6.4) | 165.0 (5.5) | <0.001 | 177.2 (10.2) | 175.9 (7.9) | 179.8 (8.6) | 0.53 |
| Weight (kg) | 95.4 (14.7) | 87.0 (16.2) | 0.14 | 94.4 (19.5) | 95.3 (10.6) | 92.1 (13.2) | 0.86 |
| Body mass index (kg/m2) | 29.0 (3.4) | 31.8 (5.1) | 0.05 | 29.8 (4.6) | 30.8 (2.3) | 28.5 (3.8) | 0.33 |
| Systolic blood pressure (mmHg) | 134.2 (17.5) | 128.8 (16.5) | 0.41 | 132.3 (16.8) | 134.0 (15.7) | 133.3 (19.3) | 0.97 |
| Diastolic blood pressure (mmHg) | 86.7 (12.7) | 83.3 (12.8) | 0.48 | 82.9 (11.9) | 85.6 (7.0) | 89.2 (15.3) | 0.37 |
| Heart rate (at baseline) (b.p.m.) | 77.3 (12.0) | 83.9 (11.9) | 0.15 | 77.6 (14.5) | 80.2 (12.4) | 78.8 (9.8) | 0.87 |
| Ever smoker, former, or current | 25 (71.4) | 7 (77.8) | 1.0 | 10 (58.8) | 10 (100.0) | 12 (70.6) | 0.07 |
| Smoking (never) | 10 (28.6) | 2 (22.2) | 0.69 | 7 (41.2) | 0 (0.0) | 5 (29.4) | <0.01 |
| Hypertension (reported, history of) | 21 (60.0) | 7 (77.8) | 0.55 | 13 (76.5) | 6 (60.0) | 9 (52.9) | 0.35 |
| Lipid disorder (reported, history of) | 11 (31.4) | 2 (22.2) | 0.90 | 3 (17.6) | 6 (60.0) | 4 (23.5) | 0.052 |
| Coronary heart disease (reported) | 10 (28.6) | 1 (11.1) | 0.52 | 2 (11.8) | 5 (50.5) | 4 (23.5) | 0.09 |
| PCI with or without stent insertion, CABG (reported, history of) | 4 (11.4) | 1 (11.1) | 1.0 | 1 (5.9) | 3 (30.0) | 1 (5.9) | 0.11 |
| Valve disease, history of or reported at echo or other cardiac disease | 14 (40.0) | 5 (55.59 | 0.75 | 8 (47.1) | 4 (40.0) | 7 (41.2) | 0.78 |
| Other cardiac surgery or intervention | 1 (2.9) | 1 (11.1) | 0.87 | 1 (5.9) | 0 (0.0) | 1 (5.9) | 0.74 |
| Reduced cardiac contractility, history of or reported at echo (EF < 55%), clinical HF | 12 (34.3) | 6 (66.7) | 0.17 | 4 (23.5) | 5 (50.0) | 9 (52.9) | 0.18 |
| Other cardiac disease, history of | 2 (5.7) | 0 (0.0) | 1.0 | 0 (0.0) | 0 (0.0) | 2 (11.8) | 0.19 |
| Stroke (cerebral infarct or embolus) | 1 (2.9) | 1 (11.1) | 0.87 | 2 (11.8) | 0 (0.0) | 0 (0.0) | 0.19 |
| Anti-hypertensive medication (including beta-blockers) | 28 (80.0) | 9 (100.0) | 0.34 | 14 (82.4) | 9 (90.0) | 14 (82.4) | 0.85 |
| Beta-blocker use | 22 (62.9) | 9 (100.0) | 0.08 | 13 (76.5) | 8 (80.0) | 10 (58.8) | 0.40 |
| Warfarin use | 8 (22.9) | 4 (44.4) | 0.38 | 5 (29.4) | 4 (40.0) | 3 (17.6) | 0.44 |
| Thrombin/Xa inhibitor use | 25 (71.4) | 5 (55.6) | 0.61 | 12 (70.6) | 5 (50.5) | 13 (76.5) | 0.35 |
| Aspirin and anti-platelet medication use | 6 (17.2) | 0 (0.0) | 0.41 | 0 (0.0) | 4 (40.0) | 2 (11.8) | <0.05 |
| Lipid lowering medication | 10 (28.6) | 1 (11.1) | 0.52 | 1 (5.9) | 7 (70.0) | 3 (17.6) | <0.01 |
| Diabetes medication | 1 (2.9) | 1 (11.1) | 0.87 | 1 (5.9) | 0 (0.0) | 1 (5.9) | 0.74 |
| Digoxin | 4 (11.4) | 3 (33.3) | 0.28 | 4 (23.5) | 1 (10.0) | 2 (11.8) | 0.54 |
| Class III anti-arrhythmic use | 3 (8.6) | 4 (44.4) | <0.05 | 4 (23.5) | 0 (0.0) | 3 (17.6) | 0.26 |
Data are shown as mean (standard deviation) for continuous variables and % for categorical variables.
AF, atrial fibrillation; AF group: those that were not converted into sinus rhythm; CABG, coronary artery bypass graft; EF, ejection fraction; HF, heart failure; PCI, percutaneous coronary intervention; recurrent AF: those that were successfully converted into sinus rhythm but had recurrent atrial fibrillation at follow-up; SR, sinus rhythm; SR group: those successfully converted to sinus rhythm.
With regards to baseline clinical characteristics, there was no significant difference between the three groups; SR group, AF group, and recurrent AF group in age, BMI, blood pressure, or heart rate at baseline (Table 1). Likewise, there was no significant difference between the groups with regards to history of coronary artery disease, valvular heart disease, heart failure, or hypertension. Pre-cardioversion heart rate on ECG was 80.5 ± 15.0 b.p.m. for the SR group, 83.1 ± 10.2 for the AF group, and 85.1 ± 14.8 for the recurrent AF (P = 0.64). There was no difference in pre-cardioversion and post-cardioversion heart rate on ECG for the AF group (83.1 ± 10.2 vs. 89.6 ± 13.7, P = 0.13) but a significant lowering was seen in the SR group (80.5 ± 15.0 vs. 58.1 ± 7.4, P < 0.001). Left ventricular ejection fraction measured by echocardiography as well as NT-proBNP values measured immediately before the cardioversion were also in the same ranges (Table 2). All patients were taking oral anticoagulants and the proportion of those taking NOACs was similar in all three groups (Table 1).
Table 2.
Brain perfusion and cerebral blood flow at baseline
| Baseline value | SR group (n = 17) | AF group (n = 10) | Recurrent AF group (n = 17) | P-value |
|---|---|---|---|---|
| ASL whole brain | 36.6 (8.2) | 34.0 (7.9) | 36.1 (8.6) | 0.73 |
| ASL grey matter | 40.2 (9.6) | 37.2 (9.4) | 39.8 (10.3) | 0.72 |
| Total CBFa | 557.4 (109.9) | 588.8 (144.2) | 528.2 (110.5) | 0.45 |
| Relative BV | 71.4 (4.6) | 69.9 (2.3) | 69.1 (3.7) | 0.32 |
| EF | 57.6 (8.1) | 57.6 (9.1) | 55.5 (8.6) | 0.83 |
| BNP | 1172.6 (950.1) | 437.3 (236.6) | 927.3 (584.2) | 0.06 |
Data are age and sex adjusted and shown as mean (standard deviation). For ASL whole brain and grey matter the units were mL/min but for total CBF mL. BNP units were pg/mL. Relative BV and EF are expressed as %.
AF, atrial fibrillation; AF group, those that were not converted into sinus rhythm; ASL, arterial spin labelling; BNP, brain natriuretic peptide; BP, brain perfusion; BV, brain volume; CBF, cerebral blood flow; EF, ejection fraction; recurrent AF, those that were successfully converted into sinus rhythm but had recurrent atrial fibrillation at follow-up; SR, sinus rhythm; SR group, those successfully converted to sinus rhythm.
Adjusted for brain volume.
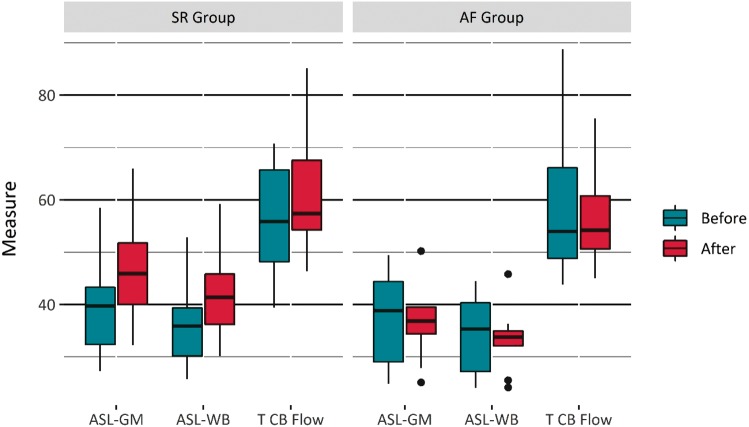
Baseline brain perfusion measured with ASL, both in the whole brain as well as in the grey matter of the brain and total cerebral blood flow measured with phase contrast MRI during the first MRI examination prior to the cardioversion in all 44 individuals enrolled into the study was not significantly different between the three outcome groups (Table 2). Furthermore, there was no difference in relative brain volume at baseline. On the other hand, brain perfusion measured by ASL was significantly higher after cardioversion in the SR group when compared with the AF group, both with regards to whole brain perfusion as well as grey matter perfusion (P < 0.05) (Table 3, Figure 1). The change in ASL perfusion in the SR group was significant for both whole brain, 4.9 mL/min (P < 0.001), and grey matter perfusion, 5.6 mL/min (P < 0.001), as opposed to the AF group, were no significant change was detected, −1.6 mL/min (P = 0.36) and −1.9 mL/min (P = 0.34), respectively (Table 3, Figures 2and3).
Table 3.
Perfusion and cerebral blood flow values at MRI visit 1 and visit 2
| Perfusion and cerebral blood flow | First visit | Second visit | Change | P-valuea | |
|---|---|---|---|---|---|
| ASL whole brain | SR group (n = 17) | 36.3 (1.9) | 41.2 (1.9) | 4.9 (1.3) | <0.001 |
| AF group (n = 10) | 34.4 (2.6) | 32.8 (2.6) | −1.6 (1.7) | 0.36 | |
| ASL grey matter | SR group (n = 17) | 39.9 (2.3) | 45.5 (2.3) | 5.6 (1.4) | <0.001 |
| AF group (n = 10) | 37.8 (2.9) | 35.9 (3.0) | −1.9 (2.0) | 0.34 | |
| Total cerebral blood flowb | SR group (n = 17) | 555.2 (29.2) | 613.8 (29.5)c | 58.6 (24.1) | <0.05 |
| AF group (n = 10) | 586.8 (38.2) | 566.0 (39.9)c | −20.8 (32.0) | 0.52 |
Data are age and sex adjusted and shown as estimated marginal means (standard errors) using Kenward–Roger Degrees of Freedom Approximation. For ASL whole brain and grey matter the units were mL/min but for total cerebral blood flow mL.
AF, atrial fibrillation; AF group, those that were not converted into sinus rhythm; ASL, arterial spin labelling; MRI, magnetic resonance imaging; recurrent AF, those that were successfully converted into sinus rhythm but had atrial fibrillation at follow-up; SR, sinus rhythm; SR group, those successfully converted to sinus rhythm.
Change between visits.
Adjusted for brain volume.
N = 9.
Figure 1.
Box whisker plot of brain perfusion by ASL MRI in the grey matter of the brain and the whole brain and by phase contrast MRI of the total cerebral blood flow in the SR group and the AF group before and after cardioversion. The right column for each parameter in both groups shown the increment in perfusion and blood flow after a successful cardioversion or no change after an unsuccessful cardoversion. Total cerebral blood flow measurements were scaled by a factor of 10. AF, atrial fibrillation; ASL, arterial spin labelling; MRI, magnetic resonance imaging; SR, sinus rhythm; T CB Flow, total cerebral blood flow.
Figure 2.
Box whisker plot showing the change in brain perfusion by ASL MRI in the grey matter of the brain and the whole brain and by phase contrast MRI of the total cerebral blood flow in the SR group and the AF group after cardioversion. The upper columns show a positive change in perfusion and cerebral blood flow in the SR group after a successful cardioversion, whereas the lower columns show no change in the AF group after an unsuccessful cardioversion. Total cerebral blood flow measurements were scaled by a factor of 10. AF, atrial fibrillation; ASL, arterial spin labelling; MRI, magnetic resonance imaging; SR, sinus rhythm.
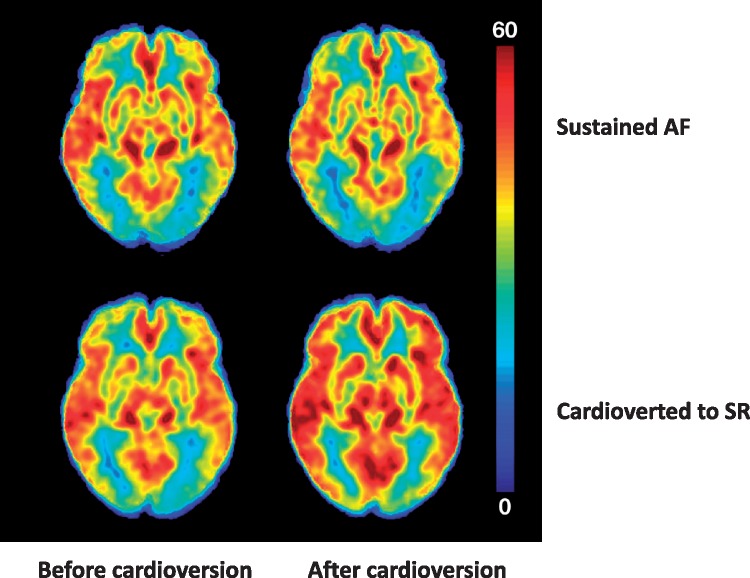
Figure 3.
Average brain perfusion of the whole brain before and after cardioversion measured by ASL MRI. Upper panel shows average baseline brain perfusion of the AF group (left) and no detectable change (right) after an unsuccessful cardioversion. Lower panel shows baseline brain perfusion in the SR group (right) and a visible improvement of perfusion with restoration of sinus rhythm after a successful cardioversion (left). The perfusion is most prominent in the cortex of the brain where perfusion is naturally more apparent. The scale on the right shows colour coded perfusion, with the lowest value (0 mL/100 g/min) being blue and the highest (60 mL/100 g/min) being red. AF, atrial fibrillation; ASL, arterial spin labelling; MRI, magnetic resonance imaging; SR, sinus rhythm.
There was also no significant difference between the groups in total cerebral blood flow measured with phase contrast MRI during the first examination (Table 2). Total cerebral blood flow furthermore increased between the pre- and post-cardioversion MRI examinations in the SR group by 58.6 mL (P < 0.05) but remained unchanged in the AF group, −20.8 mL (P = 0.52) (Table 2, Figures 1and2).
There was no significant difference in interval from the first to the second MRI examination between the SR group and the AF group (20.7 weeks and 24.5 weeks, respectively, P = 0.52).
Discussion
In this prospective study of individuals undergoing elective cardioversion for AF, restoration, and maintenance of SR for at least 10 weeks after was associated with an improvement of brain perfusion and cerebral blood flow measured by both ASL and phase contrast MRI. In those individuals where cardioversion was unsuccessful, there was no change in perfusion or blood flow.
Brain perfusion in AF has not been particularly well-studied. Some studies have indicated that cerebral blood flow is lowered during AF and that brain oxygenation and cerebral blood flow may be improved after restoration of SR.12–16 Those studies used indirect methods for measurement of cerebral blood flow and some investigated regional perfusion only. Additionally, not all studies compared perfusion in AF to perfusion in SR or included patients undergoing cardioversion of AF.
In our study, we used the relatively novel method of ASL for measuring brain perfusion. ASL is an accurate, non-invasive method for directly measuring perfusion in the capillary bed of the whole brain in a reliable and safe manner. At the same time, ASL makes it possible to extract information on both regional and tissue level during the same session.10,17 In a previous cohort study of ours we have shown that the presence of AF was associated with lower cerebral blood flow and estimated brain perfusion than in those in SR.6 Decreased brain perfusion and cerebral blood flow could be contributing factors to reduced brain volume and subsequent cognitive impairment, although the pathophysiologic mechanisms linking AF to cognitive impairment are most likely complex and multifactorial. Cognitive function has been recently been shown to improve after AF ablation.18 The results of our investigation on cerebral blood flow in AF however show that reduced brain perfusion does occur with this arrhythmia and that it may be reversible with return to SR, suggesting that hypoperfusion could play a role. Other possible mechanisms that might link AF with decreased brain volume include cerebral microemboli from the heart, systemic inflammation, cerebral microbleeds, and possibly even genetic factors.3,19,20
Wolters et al.7 showed in a recent population-based study that lower cerebral perfusion measured by phase contrast MRI was linked to accelerated cognitive decline and higher risk of developing dementia, supporting this association, but interestingly this rather large study of almost 5000 individuals did not report whether individuals had AF or not. The mechanism of decreased perfusion in AF could be a combination of reduced stroke volume due to variability in R-R intervals, resultant changes in atrial filling and ventricular loading and simultaneous loss of atrial contractility. It is also conceivable that AF with a rapid ventricular response might additionally result in even lower brain perfusion. In this study, the rate control both before and after cardioversion was adequate and therefore unlikely that heart rate played a major role in the changes seen in brain perfusion.
Cardiovascular risk factors were similar between those in the SR group and the AF group, suggesting that they did not play a significant role in influencing the difference in measured perfusion of the brain and cerebral blood flow. Atherosclerosis may, along with age and autoregulation, affect the threshold for perfusion, but there was no difference either in age or presence of coronary artery disease between the groups in the present study. Whether older brains are more susceptible to the effect of reduced blood flow and perfusion is not known, but Bunch et al.8,21 actually found more reduction of regional cerebral blood flow in younger age groups with AF compared to normal subjects and higher risk of Alzheimer disease in the younger age groups. However, it stands to reason that superimposed atherosclerosis would further compromise the cerebral circulation, possibly showing a consequent decline with advancing age.
In this study, restoration of SR resulted in improvement of perfusion as opposed to individuals remaining in AF after unsuccessful cardioversion. These results may have important clinical implications. The difference in both perfusion and blood flow after successful cardioversion was almost 15% measured by ASL and phase contrast MRI in the whole brain and grey matter. No changes in brain volumes were detected pre- and post-cardioversion, although it should be emphasized that the study cohort was younger than in previous studies showing an effect of AF on brain volume.5,6,9,22 Also, the time between the pre- and post-cardioversion MRI examination was only a few weeks. Therefore, we did not include cognitive testing nor did we actually expect this to be affected in such a short observation period. The main focus was on changes in blood flow with SR in comparison to AF.
Whether all patients demonstrate these changes in perfusion is not clear and larger studies would be needed to further investigate this important effect of AF on brain perfusion and cerebral blood flow, preferably with brain volume and cognition considered in addition to perfusion as endpoints. Whether maintenance of SR can then delay onset of brain atrophy or cognitive decline also needs to be explored further although the results of this study are clearly intriguing.
Limitations
The sample in this study was rather small and hence yields low power in statistical calculations. This was especially apparent in calculations for total cerebral blood flow, due to high variability in measured values, even though there is high correlation with ASL.10 The initial goal was to include a higher number of patients in this study but as indicated in the Methods section 17 patients dropped out as they went back in to AF during the period between the cardioversion and second MRI. Given the results of our much larger recent study (n = 2291) showing that patients in AF have lower total cerebral blood flow and brain perfusion than those in SR, we were very interested to evaluate if this phenomenon in AF patients could be reversed by cardioversion to SR. Despite the small sample, the results do suggest that cerebral blood flow and brain perfusion improve after restoration of SR which in our opinion is an important observation. An important strength of this study is the use of ASL for measurement of brain perfusion and the study design with individual observations both before and after cardioversion.
It can be difficult to ascertain whether individuals might have a brief recurrence of AF or not after cardioversion or even pulmonary vein ablation. Perhaps a Holter monitor for 24 h would have been of help but nevertheless an AF free Holter monitor would not have excluded a potential paroxysmal recurrence at another point in time during the observation period. Ideally, an implantable loop recorder might have been best for this purpose. In this study, however, clinical follow-up with an ECG and brief interval history was chosen.
Conclusion
Restoration of SR in individuals with AF resulted in improvement of cerebral blood flow. It has previously been suggested that subjects with AF may have decreased cerebral blood flow, diminished brain volume, and impaired cognitive function. These results imply that decreased cerebral blood flow might be one of the mechanisms of cognitive decline in individuals with AF. The fact that this is reversed with cardioversion to SR offers a proof of concept in kind and may have important clinical implications, but requires further investigation, preferably in a larger cohort and with longer follow-up.
Acknowledgements
The authors would like to express their sincere gratitude to the participants of the study.
Conflict of interest: none declared.
Funding
This work was supported by the Science Fund of Landspitali—The National University Hospital of Iceland and The Helga Jonsdottir and Sigurlidi Kristjansson Memorial Fund. Landspitali—National University Hospital Science Fund and The Helga Jonsdottir and Sigurlidi Kristjansson Memorial Fund have also funded the AGES Reykjavik Study, in addition to National Institutes of Health contract N01-AG-1-2100, the National Institute of Aging Intramural Research Program, the Icelandic Heart Association, and Althingi, the Icelandic Parliament.
References
- 1. Lane DA, Skjøth F, Lip GYH, Larsen TB, Kotecha D.. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017;6 pii: e005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 3. Dietzel J, Haeusler KG, Endres M.. Does atrial fibrillation cause cognitive decline and dementia? Europace 2018;20:408–19. [DOI] [PubMed] [Google Scholar]
- 4. Kalantarian S, Ruskin JN.. Atrial fibrillation and cognitive decline: phenomenon or epiphenomenon? Cardiol Clin 2016;34:279–85. [DOI] [PubMed] [Google Scholar]
- 5. Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H. et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke 2013;44:1020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardarsdottir M, Sigurdsson S, Aspelund T, Rokita H, Launer LJ, Gudnason V. et al. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. Europace 2018;20:1252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW. et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017;136:719–28. [DOI] [PubMed] [Google Scholar]
- 8. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS. et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm 2010;7:433–7. [DOI] [PubMed] [Google Scholar]
- 9. Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B. et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage 2012;59:3862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sigurdsson S, Forsberg L, Aspelund T, van der Geest RJ, van Buchem MA, Launer LJ. et al. Feasibility of using pseudo-continuous arterial spin labeling perfusion in a geriatric population at 1.5 tesla. PLoS One 2015;10:e0144743.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonekamp D, Degaonkar M, Barker PB.. Quantitative cerebral blood flow in dynamic susceptibility contrast MRI using total cerebral flow from phase contrast magnetic resonance angiography. Magn Reson Med 2011;66:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petersen P, Kastrup J, Videbæk R, Boysen G.. Cerebral blood flow before and after cardioversion of atrial fibrillation. J Cereb Blood Flow Metab 1989;9:422–5. [DOI] [PubMed] [Google Scholar]
- 13. Efimova I, Efimova N, Chernov V, Popov S, Lishmanov Y.. Ablation and pacing: improving brain perfusion and cognitive function in patients with atrial fibrillation and uncontrolled ventricular rates. Pacing Clin Electrophysiol 2012;35:320–6. [DOI] [PubMed] [Google Scholar]
- 14. Porebska A, Nowacki P, Safranow K, Drechsler H.. Nonembolic, hemodynamic blood flow disturbances in the middle cerebral arteries in patients with paroxysmal atrial fibrillation without significant carotid stenosis. Clin Neurol Neurosurg 2007;109:753–7. [DOI] [PubMed] [Google Scholar]
- 15. Wutzler A, Nee J, Boldt L-H, Kuhnle Y, Graser S, Schroder T. et al. Improvement of cerebral oxygen saturation after successful electrical cardioversion of atrial fibrillation. Europace 2014;16:189–94. [DOI] [PubMed] [Google Scholar]
- 16. Elbers PW, Prins WB, Plokker HW, van Dongen EP, van Iterson M, Ince C.. Electrical cardioversion for atrial fibrillation improves microvascular flow independent of blood pressure changes. J Cardiothorac Vasc Anesth 2012;26:799–803. [DOI] [PubMed] [Google Scholar]
- 17. Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X.. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 2016;281:337–56. [DOI] [PubMed] [Google Scholar]
- 18. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A. et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivard L, Khairy P.. Mechanisms, clinical significance, and prevention of cognitive impairment in patients with atrial fibrillation. Can J Cardiol 2017;33:1556–64. [DOI] [PubMed] [Google Scholar]
- 20. Smith EE, Schneider JA, Wardlaw JM, Greenberg SM.. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012;11:272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS. et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:839–45. [DOI] [PubMed] [Google Scholar]
- 22. Ikram MA, Vrooman HA, Vernooij MW, den Heijer T, Hofman A, Niessen WJ. et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging 2010;31:378–86. [DOI] [PubMed] [Google Scholar]