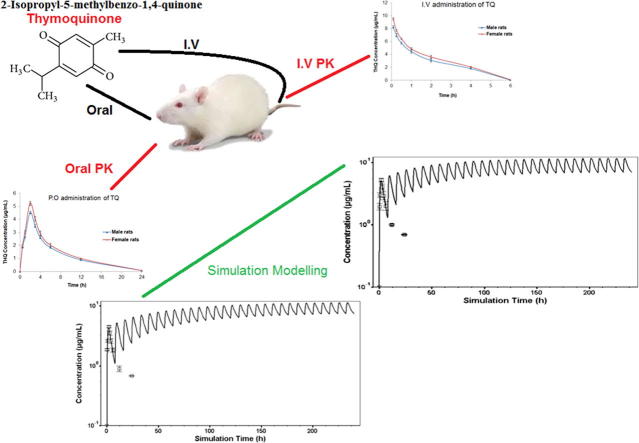
Graphical abstract
Keywords: Thymoquinone, Male and female rats, Plasma, Pharmacokinetics, GastroPlus PK simulations
Abbreviations: THQ, Thymoquinone; I.V., intravenous; P.O., oral; CMC, carboxy methyl cellulose; AUC, area under plasma concentration-time curve; AUMC, area under the first moment curve; MRT, mean residence time; Cl, total clearance; Vss, volume of distribution at steady state; Vz, volume of distribution z; λz, terminal elimination rate constant; T1/2, elimination half-life; Cmax, maximum plasma concentration; Tmax, time to maximum concentration
Abstract
Thymoquinone is the most biologically active constituent of Nigella sativa (black seed). A monoterpene compound chemically known as 2-methyl-5-isopropyl-1, 4-quinone. In this study, the gender-dependent pharmacokinetic behavior of thymoquinone in rats was investigated. Thymoquinone was administered orally (20 mg/kg) and intravenously (5 mg/kg) to male and female rats and blood samples were collected at specific time points. Plasma concentration-time curves were plotted and pharmacokinetic parameters were determined using the non-compartmental analysis. In addition, simulations of steady state concentrations of thymoquinone in male and female rats were performed using GastroPlus PK software. After oral administration, the maximum plasma concentration (Cmax) of thymoquinone was 4.52 ± 0.092 μg/ml in male rats and 5.22 ± 0.154 μg/ml in female rats (p = 0.002). Similarly, after intravenous administration, the Cmax was 8.36 ± 0.132 μg/ml in males and 9.51 ± 0.158 μg/ml in females (p = 0.550). The area under the plasma concentration-time curve (AUC)0-∞ following oral dosing was 47.38 ± 0.821 μg/ml·h in females and 43.63 ± 0.953 μg/ml·h in males (p = 0.014). Pharmacokinetics and plasma concentration vs. time profiles for multiple oral doses of thymoquinone in rats were predicted using a simulation model to compare the simulation results with the experimental plasma pharmacokinetic data. The differences observed in thymoquinone pharmacokinetics between male and female rats after a single dose were not evident for the simulated steady-state parameters. The findings suggest that the gender difference does not seem to play a significant role in thymoquinone disposition at steady state.
1. Introduction
Nigella sativa (N. sativa) is an herb that belongs to the family Ranunculaceae, which has been historically used as medicinal food to treat a wide range of pathological conditions (Ahmad et al., 2013). Recently, black seed (N. sativa seed) has been the subject of extensive medical research to elucidate its biologically active components and to determine its potential therapeutic applications (Ahmad et al., 2013, Hassanien et al., 2015). It has been shown to enhance learning and memory (Sahak et al., 2016) and affect plasma lipids, leading to a lower total cholesterol and triglyceride levels and augmented high-density lipoprotein cholesterol levels (Sahebkar et al., 2016). In addition, N. sativa seed has been shown to have immunomodulatory, anti-inflammatory (Majdalawieh and Fayyad, 2015), cardio-protective, anti-cancer (Shafiq et al., 2014), anti-oxidant (El-Saleh et al., 2004), anti-sepsis (Alkharfy et al., 2015b, Alkharfy et al., 2011), hepatoprotective (Adam et al., 2016, Mabrouk et al., 2016), nephroprotective (Canayakin et al., 2016, Erboga et al., 2016) and anti-diabetic effects (AbuKhader, 2012, Alimohammadi et al., 2016, Kaatabi et al., 2015).
Thymoquinone (THQ; 2-Isopropyl-5-methylbenzo-1,4-quinone) Fig. 1 is the most active chemical constituent of the essential oil of black seed and accounts for the majority of its therapeutic properties. We have previously reported the pharmacokinetics of THQ following intravenous (I.V.) and oral (P.O.) administration in a male rabbit model (Alkharfy et al., 2015a). Interestingly, gender-dependent pharmacokinetic behaviors of various compounds have been demonstrated in several works (Liu et al., 2013, Lyu et al., 2016, Yang et al., 2013, Griffin et al., 1997, Witkamp et al., 1992). This could be due to the gender-related variations in body weight, fat tissue distribution, plasma protein binding, metabolizing enzymes and drug transport activities (Gandhi et al., 2004, Czerniak, 2001). Additionally, gender differences in preclinical studies used in the safety assessments of a new chemical entity may offer a rationale for choosing different preliminary doses based on gender to be used in clinical trials. Gastrointestinal simulations based on the Advanced Compartmental Absorption and Transit (ACAT) model have become a vital in silico tool to forecast the in vivo drug performance during drug development and quality ratification. The essential character of ACAT model which add to its use in biopharmaceutical drug characterization is that it provides association between formulation performance and drug product pharmacokinetics (Okumu et al., 2009, Parrott et al., 2009). In this study, we evaluated the pharmacokinetic parameters of THQ following I.V. and P.O. dosing in male and female rats to assess the gender effect in vivo.
Fig. 1.
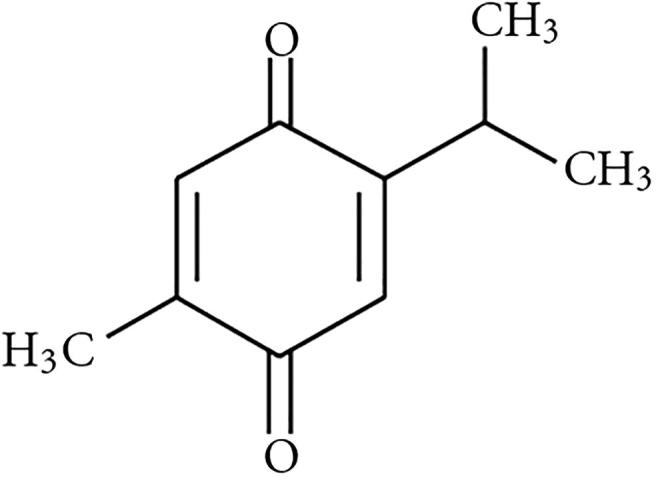
Chemical structure of 2-Isopropyl-5-methylbenzo-1,4-quinone (Thymoquinone, THQ).
2. Materials and methods
2.1. Chemicals
Thymoquinone and internal standard (IS, thymol) were acquired from Sigma-Aldrich (St Louis, USA). Chromatographic grade Methanol was acquired from Panreac Chemicals (Barcelona, Spain) and Potassium dihydrogen phosphate (KH2PO4) was purchased from Winlab Ltd. (Maidenhead, UK). Distilled water (Mili-Q) was produced in our laboratory. All other chemical reagents used were at of analytical grade.
2.2. Study protocol
Twenty-four male and female rats (12 each) weighing 200–220 gm were acquired from the Animal Care and Use Center at College of Pharmacy, King Saud University. The study was approved by the College of Pharmacy Ethical Committee (KSU-SE-19-17). The rats were distributed into four groups (n = 6 animals) for I.V. and P.O. administration. The rats were kept in agreement with the approvals of the “Guide for the Care and Use of Laboratory Animals” of the center. The rats were kept under laboratory conditions of temperature, humidity, 60%; 23 ± 2 °C; and a 12-h/12-h light-dark cycle), with free access to water and food.
Thymoquinone was liquefied in normal saline solution consisting of DMSO (10%). After overnight fasting, THQ was administered in male and female rats through the tail veins (I.V.) at a dose of 5 mg/kg using sterile disposable syringes. For the P.O. dosing, THQ solution was suspended in 10 ml of carboxy methyl cellulose (CMC). This solution was given orally at a dose of 20 mg/kg using a gavage needle. Blood samples (1 ml) were drawn from retro-orbital plexus at the following intervals–immediately before administration and 0.25, 1, 2, 3, 4, 6, 12 and 24 h post P.O. dosing. For the I.V. treatment, the sampling was carried out up to 6 h (i.e., 0.08, 0.25, 0.50, 1, 2, 4 and 6 h). The blood samples were collected in 1 ml anti-coagulant tubes (8 mg of disodium EDTA). Plasma separation was carried out by centrifugation at 5000g (Eppendorf, NY, USA) for 10 min, and stored at −80 °C for the quantitative investigation of THQ.
2.3. Preparation of sample and pharmacokinetics
The concentration of THQ in rat plasma was determined by an HPLC/UV method that has been established and validated in our laboratory (Alkharfy et al., 2015a). Briefly, to 200 µl of plasma comprising of THQ, IS 20 µl (20 µg/ml) was added in an eppendorf tube. The mixture was vortexed and then centrifuged at 2500g for additional 10 min. The supernatant was removed into a new tube. The sample was dried and reconstituted with 200 µl of mobile phase and 10 µl from of the sample was injected into the HPLC column for THQ analysis.
A non-compartmental pharmacokinetic analysis as previously described by Alkharfy et al. was used to check the pharmacokinetic behavior of THQ in plasma (Alkharfy et al., 2015a). The pharmacokinetic analysis was performed using PK Solver software (version 1.0) on the individual plasma concentration-time curves. The calculated parameters were as follows: The elimination rate constant, λz; the apparent half-life, T1/2; time to maximum concentration, Tmax; maximum concentration in plasma, Cmax; area under curve, AUC; area under moment curve, AUMC; mean residence time, MRT; volume of distribution, Vz, total clearance; Cl, volume of distribution at steady state, Vss.
2.4. Prediction of the pharmacokinetic profile and model simulation (ACAT Model)
All simulations and predictions were performed using the commercially accessible software GastroPlus version 9.0 (Simulation Plus Inc., CA, USA) fixed with Advanced Compartmental Absorption Transit (ACAT). The input parameters required to run the simulation were extracted from literature (Anonymous; Salmani et al., 2014) or estimated using GastroPlus and ADMET predictor and are summarized in Table 1. The model (ACAT) was used to simulate and foresee THQ absorption in the small intestine. A rat model of fasting physiology was selected and an optimized log D model was applied for determining the absorption scaling factors for the intestinal compartments. This model was used to fit the simulated PK profiles to the observed PK profiles after administration of 5 mg/kg I.V. and 20 mg/kg P.O. of THQ to male and female rats. Then, the model was used to predict the plasma profile after administration of multiple P.O. doses in both males and females.
Table 1.
GastroPlus simulation inputs model parameters for thymoquinone (THQ).
| Parameter | Value | Methods/Reference |
|---|---|---|
| Molecular weight (g/mol) | 164.2 | (Anonymous) |
| Log P (at pH 7.4) | 2.2 | (Anonymous) |
| pKa | 6.8 | Estimated by ADMET predictor |
| Solubility (mg/ml) | 0.67 | (Salmani et al., 2014) |
| Particle size (μm) | 25 | Default value |
| Effective permeability (10−5 cm/s) | 6.45 | Estimated by GastroPlus® |
| fup | 0.41 | Estimated by GastroPlus® |
| Blood: Plasma ratio | 1.15 | Estimated by GastroPlus® |
(Salmani et al., 2014). Value obtained from Salmani et al. (2014).
(Anonymous). PubChem; https://pubchem.ncbi.nlm.nih.gov/compound/Thymoquinone#section=Top.
2.5. Statistical analysis
The data are expressed as mean with standard error (SEM). The significance of the data was calculated by applying student T-test using Graph Pad Prism version 6 for Windows (Graph Pad Software, CA, USA). A p value of <0.05 was considered significant.
3. Results and discussion
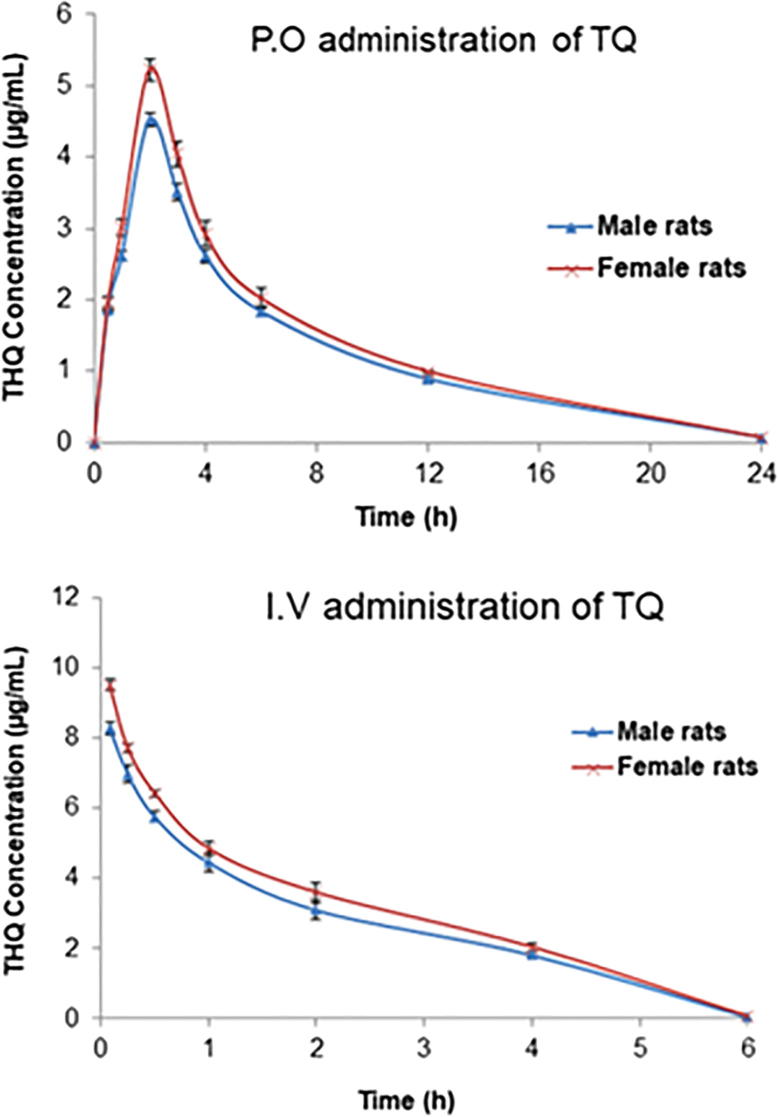
THQ at the I.V. dose of 5 mg/kg and P.O. dose of 20 mg/kg was well tolerated by all animals in the study. The analytical method used was successful in quantifying the plasma concentration of THQ up to 24 h. The plasma concentration–time curves of THQ following P.O. administration of 20 mg/kg to male and female rats as a single dose are shown in Fig. 2. Depicts the plasma concentration–time curves of THQ following I.V. administration (5 mg/kg) to male and female rats as a single dose. The plasma concentration following the P.O. administration increased swiftly, reaching maximum level at 2 h after dosing. The THQ pharmacokinetic parameters calculated using the non-compartmental analysis for both male and female rats are presented in Tables 2 for I.V. and P.O. treatment, respectively. The estimated absolute bioavailability of THQ was 0.58 in males and 0.57 in females. The Cmax following I.V. and P.O. administration was slightly higher (i.e., ∼12–14%) in female than male rats. Upon the P.O. dosing, the Cmax in males was 4.52 ± 0.092 μg/ml, while it was 5.23 ± 0.154 μg/ml in females (p = 0.002). THQ Cmax post I.V. administration in male and female rats was 8.36 ± 0.132 μg/ml and 9.51 ± 0.158 μg/ml, respectively (p = 0.550). Similarly, the AUC0-∞ was ∼ 8% greater in females than in males (43.63 ± 0.953 μg.h/ml vs. 47.38 ± 0.821 μg.h/ml; p = 0.014) after P.O. dosing.
Fig. 2.
Plasma concentration–time curves following P.O. administration (20 mg/kg) and I.V. administration (5 mg/kg) of a single thymoquinone (THQ) dose to male and female rats. Each value is expressed as mean ± SEM (n = 6); data points are connected by a trend line.
Table 2.
Non-compartmental pharmacokinetic parameters of thymoquinone (THQ) after intravenous (I.V.) and P.O. administration in rats (n = 6).
| Parameter Unit | I.V. dose 5 mg/kg |
p-value | P.O. 20 mg/kg |
p-value | ||
|---|---|---|---|---|---|---|
| Male Average ± SEM |
Female Average ± SEM |
Male Average ± SEM |
Female Average ± SEM |
|||
| λz (1/h) | 0.47 ± 0.042 | 0.51 ± 0.045 | 0.551 | 0.07 ± 0.004 | 0.07 ± 0.008 | 0.664 |
| T1/2 (h) | 1.51 ± 0.141 | 1.40 ± 0.133 | 0.610 | 9.52 ± 0.572 | 9.43 ± 1.113 | 0.947 |
| Tmax (h) | 0.10 ± 0.035 | 0.08 ± 0.000 | 0.340 | 2.00 ± 0.000 | 2.00 ± 0.000 | 1.000 |
| Cmax (μg/ml) | 8.36 ± 0.132 | 9.51 ± 0.158 | 0.550 | 4.52 ± 0.092 | 5.23 ± 0.154 | 0.002 |
| AUC0-t (μg.h/ml) | 17.30 ± 0.751 | 19.36 ± 0.772 | 0.086 | 34.27 ± 0.611 | 38.04 ± 1.105 | 0.013 |
| AUC0-∞ (μg.h/ml) | 18.77 ± 0.730 | 20.73 ± 0.763 | 0.092 | 43.63 ± 0.953 | 47.38 ± 0.821 | 0.014 |
| AUMC0-∞ (μg.h2/ml) | 45.27 ± 1.769 | 47.98 ± 1.961 | 0.328 | 631.33 ± 31.861 | 657.66 ± 54.813 | 0.686 |
| MRT0-∞ (h) | 2.41 ± 0.081 | 2.31 ± 0.053 | 0.310 | 14.43 ± 0.486 | 13.85 ± 1.049 | 0.629 |
| Vz (ml/kg) | 584.60 ± 63.11 | 492.19 ± 51.94 | 0.281 | 6279.96 ± 284.62 | 5736.27 ± 639.04 | 0.455 |
| Cl (ml/h/kg) | 268.35 ± 10.47 | 242.80 ± 9.36 | 0.092 | 459.42 ± 9.75 | 422.76 ± 7.44 | 0.014 |
| Vss (ml/kg) | 650.37 ± 38.91 | 562.75 ± 27.77 | 0.100 | – | – | – |
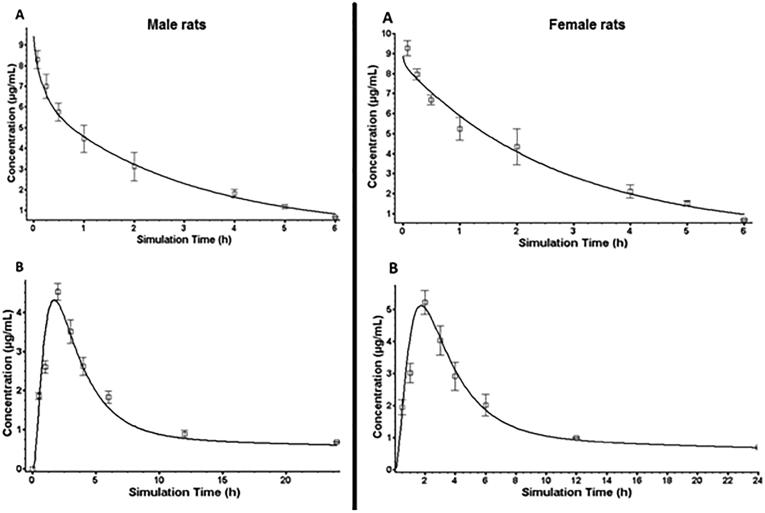
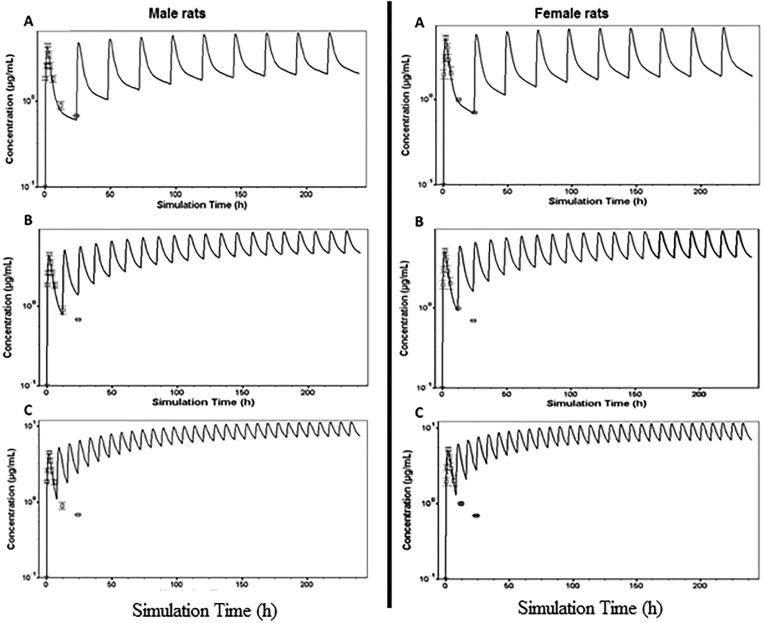
To further assess the effect of gender difference on the pharmacokinetics of THQ, we performed modeling and simulation of P.O. multiple doses to obtain steady-state parameters. The pharmacokinetics and disposition parameters obtained from in vivo studies and other parameters (such as permeability and solubility) predicted by GastroPlus program were used to form the simulation model, and absorption in intestines was imitated by using the ACAT model. The disposition and physicochemical parameters predicted were incorporated to establish the model. Finally, simulation trials of plasma concentration vs. time profiles for THQ using the disposition parameters predicted were performed for 5 mg/kg I.V. and 20 mg/kg P.O dosing. The trials stood effectively able to predict the THQ pharmacokinetic profiles rats. Analogy of the simulation outcomes with the experimental plasma pharmacokinetic profiles in male and female rats is presented in Fig. 3, respectively. The pharmacokinetic profiles predicted were analogous to the profiles observed, and goodness-of-fit was tested by comparing the experimental data with the predicted data at similar points. The correlation coefficient was greater than 0.85 for all doses. This model was also able to generate accurate estimates for the disposition parameters in male and female rats. Table 3 and Fig. 3 show predicted plasma profiles and pharmacokinetic parameters (AUC0-t, AUC0-∞, Cmax, and Tmax), which were analogous to those observed experimentally at different doses of THQ. In addition, Table 4 and Fig. 4 illustrate predicted plasma profile, Cmax, Cmin, Cave, AUCss, and clearance (Cl) at steady state for 20 mg/kg multiple P.O. doses of THQ every 8 h, 12, and 24 h in male and female rats.
Fig. 3.
Simulated data and observed plasma profiles after 5 mg/kg I.V. administration (A) and 20 mg/kg P.O. administration (B) of thymoquinone (THQ) in male and female rats. The symbols represent the observed data and solid line represents the predicted data.
Table 3.
Model predicted and observed parameters of thymoquinone (THQ) after administration of 5 mg/kg I.V. and 20 mg/kg P.O. in male and female rats and model predicted parameters of THQ at steady state for 20 mg/kg P.O. dose administered every 8, 12, and 24 h in male and female rats.
| PK parameter | I.V. administration |
P.O. administration |
||||
|---|---|---|---|---|---|---|
| Predicted | Observed | Pre/Obs | Predicted | Observed | Pre/Obs | |
| Male rats (n = 6) | ||||||
| Cmax (µg/ml) | 9.402 | 8.306 | 0.883 | 4.301 | 4.508 | 0.961 |
| Tmax (h) | 0.081 | 0.082 | 1.000 | 1.763 | 2.000 | 0.882 |
| AUC0-t (µg·h/ml) | 16.802 | 17.308 | 0.971 | 30.801 | 34.204 | 0.906 |
| AUC0-∞ (µg·h/ml) | 19.305 | 18.504 | 1.042 | 75.107 | 64.802 | 1.154 |
| Female rats (n = 6) | ||||||
| Cmax (µg/ml) | 8.803 | 9.204 | 0.964 | 5.105 | 5.202 | 0.982 |
| Tmax (h) | 0.081 | 0.082 | 1.000 | 1.778 | 2.000 | 0.881 |
| AUC0-t (µg·h/ml) | 20.702 | 21.206 | 0.981 | 36.505 | 38.106 | 0.951 |
| AUC0-∞ (µg·h/ml) | 23.305 | 22.102 | 1.053 | 72.702 | 62.106 | 1.178 |
Table 4.
Predicted steady state pharmacokinetic parameters of thymoquinone (THQ) for 20 mg/kg P.O. dose administered every 8, 12, and 24 h in male and female rats.
| PK parameter | Male rats (n = 6) |
Female rats (n = 6) |
||||
|---|---|---|---|---|---|---|
| 8 h | 12 h | 24 h | 8 h | 12 h | 24 h | |
| Cmax (µg/ml) | 11.402 | 8.803 | 6.304 | 11.704 | 9.203 | 6.903 |
| Cmin (µg/ml) | 7.501 | 4.704 | 2.102 | 6.903 | 4.304 | 1.802 |
| Cave (µg/ml) | 8.204 | 5.908 | 3.203 | 8.304 | 5.802 | 3.109 |
| AUCss (µg·h/ml) | 65.302 | 70.502 | 76.101 | 66.506 | 69.901 | 73.202 |
| Cl (ml/h/kg) | 310.01 | 281.00 | 262.09 | 301.05 | 297.14 | 273.26 |
Fig. 4.
Predicted plasma profiles of thymoquinone (THQ) for multiple P.O. administration of 20 mg/kg in male and female rats every 24 h (A), 12 h (B), and 8 h (C).
Previous studies have reported gender-related differences in drug disposition of some compounds. For example, significant gender-dependent differences in veratramine pharmacokinetics following P.O. administration were reported in rats, wherein the absolute bioavailability in males was much higher than that in females (Lyu et al., 2016). Diosbulbin B was also shown to have better absorption in female rats than in male rats after P.O. administration (Yang et al., 2013). Interestingly, females have low levels of CYP3A, which contribute significantly to the first-pass metabolism (Johnson et al., 2000). The results of the present study indicate that THQ clearance slightly differs between male and female rats after a single dose. Irregularity in intestinal expression of P450 enzymes and gut transport of drugs such as p-glycoprotein and MDR-1 could explain the gender differences in plasma drug concentrations (Kim et al., 2001). Hepatic clearance of THQ may also be less in female rats due to variations in cardiac output.
The sex-specific cytochrome P450s CYP2C11, 13 and CYP3A2 are expressed more in males as compared to females, whereas CYP2C12 is found more in females (Czerniak, 2001, Hermann et al., 2003, Kato and Yamazoe, 1992). Each species has a significant gender difference in plasma clearance (Witkamp et al., 1992). The higher CL in the male rats is believed to be caused by the higher metabolism of thymoquinone than in female rats. It is remarkable to note that equally phase I and phase II metabolism can determine gender-based differences (Czerniak, 2001). Female rats had a lower Cl rate than the male rats. Thus, these differences (gender-based) in animals appear in increased Cl; in male rats, Cl decreased when equated to the Cl of female rats. Following the I.V. administration, the drug directly enters the systemic circulation, avoiding the first-pass metabolism in the intestinal wall and in the liver. Therefore, gender-dependent pharmacokinetics of any drug after different routes of administrations (i.e., I.V. vs. P.O.) is considered to be mainly due to variations in the first-pass metabolism. The volume of distribution (Vss) of THQ was slightly larger in males compared with females (∼14%). THQ is a lipophilic compound, which is known to be highly protein bound (Alkharfy et al., 2015a). Therefore, the observed gender-based THQ pharmacokinetic could be explained by variations in drug clearance and/or volume of distribution, which may result in altered measured plasma drug concentrations between the two genders.
Many contributing parameters are compulsory to aid the absorption and disposition predictions of a drug after oral administration. As the physicochemical properties and dosage, the form of a drug, such as diffusivity, lipophilicity and solubility at different pH conditions are vital for predicting the solublization and dissolution of the drug in the gastrointestinal tract and the subsequent diffusion across the intestines (Abuasal et al., 2012). These parameters were successfully used to predict the steady state pharmacokinetic parameters of THQ in male and female rats following 20 mg/kg P.O. dose administered every 8, 12, and 24 h. The findings demonstrated similar PK parameters at steady state in males and females does not seem to play a significant role in thymoquinone disposition.
4. Conclusion
In conclusion, variations in metabolic capacity and volumes of distribution may explain this small gender difference in THQ pharmacokinetics following the administration of a single dose in a male and female rat model. However, simulations of steady state concentrations demonstrated comparable predicted PK parameters of THQ in both gender. Further studies are warranted to verify the gender similarity of THQ pharmacokinetics in humans for a possible future clinical use.
Acknowledgments
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for supporting this work through College of Pharmacy Research Center.
Declaration of Competing Interest
The authors declare no conflict of interests.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abuasal B.S., Bolger M.B., Walker D.K., Kaddoumi A. In silico modeling for the nonlinear absorption kinetics of UK-343,664: a P-gp and CYP3A4 substrate. Mol. Pharm. 2012;9:492–504. doi: 10.1021/mp200275j. [DOI] [PubMed] [Google Scholar]
- AbuKhader M.M. Thymoquinone: a promising antidiabetic agent. Int. J. Diab. Develop. Countries. 2012;32:65–68. [Google Scholar]
- Adam G.O., Rahman M.M., Lee S.J., Kim G.B., Kang H.S., Kim J.S., Kim S.J. Hepatoprotective effects of Nigella sativa seed extract against acetaminophen-induced oxidative stress. Asian Pac. J. Trop. Med. 2016;9:221–227. doi: 10.1016/j.apjtm.2016.01.039. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Damanhouri Z.A., Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac. J. Trop. Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimohammadi S., Hobbenaghi R., Javanbakht J., Kheradmand D., Mortezaee R., Tavakoli M., Khadivar F., Akbari H. Retraction note: protective and antidiabetic effects of extract from Nigella sativa on blood glucose concentrations against streptozotocin (STZ)-induced diabetic in rats: an experimental study with histopathological evaluation. Diagn. Pathol. 2016;11:125. doi: 10.1186/s13000-016-0571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkharfy K.M., Ahmad A., Khan R.M., Al-Shagha W.M. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur. J. Drug. Metab. Pharmacokinet. 2015;40:319–323. doi: 10.1007/s13318-014-0207-8. [DOI] [PubMed] [Google Scholar]
- Alkharfy K.M., Ahmad A., Raish M., Vanhoutte P.M. Thymoquinone modulates nitric oxide production and improves organ dysfunction of sepsis. Life Sci. 2015;143:131–138. doi: 10.1016/j.lfs.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Alkharfy K.M., Al-Daghri N.M., Al-Attas O.S., Alokail M.S. The protective effect of thymoquinone against sepsis syndrome morbidity and mortality in mice. Int. Immunopharmacol. 2011;11:250–254. doi: 10.1016/j.intimp.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Canayakin D., Bayir Y., Kilic Baygutalp N., Sezen Karaoglan E., Atmaca H.T., Kocak Ozgeris F.B., Keles M.S., Halici Z. Paracetamol-induced nephrotoxicity and oxidative stress in rats: the protective role of Nigella sativa. Pharm. Biol. 2016;54:2082–2091. doi: 10.3109/13880209.2016.1145701. [DOI] [PubMed] [Google Scholar]
- Czerniak R. Gender-based differences in pharmacokinetics in laboratory animal models. Int. J. Toxicol. 2001;20:161–163. doi: 10.1080/109158101317097746. [DOI] [PubMed] [Google Scholar]
- El-Saleh S.C., Al-Sagair O.A., Al-Khalaf M.I. Thymoquinone and Nigella sativa oil protection against methionine-induced hyperhomocysteinemia in rats. Int. J. Cardiol. 2004;93:19–23. doi: 10.1016/s0167-5273(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Erboga M., Kanter M., Aktas C., Sener U., Fidanol Erboga Z., Bozdemir Donmez Y., Gurel A. Thymoquinone ameliorates cadmium-induced nephrotoxicity, apoptosis, and oxidative stress in rats is based on its anti-apoptotic and anti-oxidant properties. Biol. Trace Elem. Res. 2016;170:165–172. doi: 10.1007/s12011-015-0453-x. [DOI] [PubMed] [Google Scholar]
- Gandhi M., Aweeka F., Greenblatt R.M., Blaschke T.F. Sex differences in pharmacokinetics and pharmacodynamics. Ann. Rev Pharmacol. Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- Griffin R.J., Godfrey V.B., Kim Y.C., Burka L.T. Sex-dependent differences in the disposition of 2,4-dichlorophenoxyacetic acid in Sprague-Dawley rats, B6C3F1 mice, and Syrian hamsters. Drug Metab. Dispos. 1997;25:1065–1071. [PubMed] [Google Scholar]
- Hassanien M.F., Assiri A.M., Alzohairy A.M., Oraby H.F. Health-promoting value and food applications of black cumin essential oil: an overview. J. Food Sci. Technol. 2015;52:6136–6142. doi: 10.1007/s13197-015-1785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann R., Ferron G.M., Erb K., Knebel N., Ruus P., Paul J., Richards L., Cnota H.P., Troy S. Effects of age and sex on the disposition of retigabine. Clin. Pharmacol. Therap. 2003;73:61–70. doi: 10.1067/mcp.2003.12. [DOI] [PubMed] [Google Scholar]
- Johnson T.N., Tanner M.S., Tucker G.T. A comparison of the ontogeny of enterocytic and hepatic cytochromes P450 3A in the rat. Biochem. Pharmacol. 2000;60:1601–1610. doi: 10.1016/s0006-2952(00)00485-8. [DOI] [PubMed] [Google Scholar]
- Kaatabi H., Bamosa A.O., Badar A., Al-Elq A., Abou-Hozaifa B., Lebda F., Al-Khadra A., Al-Almaie S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015;10 doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R., Yamazoe Y. Sex-specific cytochrome P450 as a cause of sex-and species-related differences in drug toxicity. Toxicol. Lett. 1992;64–65:661–667. doi: 10.1016/0378-4274(92)90245-f. [DOI] [PubMed] [Google Scholar]
- Kim R.B., Leake B.F., Choo E.F., Dresser G.K., Kubba S.V., Schwarz U.I., Taylor A., Xie H.G., McKinsey J., Zhou S., Lan L.B., Schuetz J.D., Schuetz E.G., Wilkinson G.R. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin. Pharmacol. Therap. 2001;70:189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- Liu W., Kulkarni K., Hu M. Gender-dependent differences in uridine 5 '-diphospho-glucuronosyltransferase have implications in metabolism and clearance of xenobiotics. Exp. Opin. Drug Metab. Toxicol. 2013;9:1555–1569. doi: 10.1517/17425255.2013.829040. [DOI] [PubMed] [Google Scholar]
- Lyu C.M., Zhang Y.F., Zhou W.B., Zhang S., Kou F., Wei H., Zhang N., Zuo Z. Gender-dependent pharmacokinetics of veratramine in rats. In vivo and in vitro evidence. Aaps J. 2016;18:432–444. doi: 10.1208/s12248-016-9870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk A., Bel Hadj Salah I., Chaieb W., Ben Cheikh H. Protective effect of thymoquinone against lead-induced hepatic toxicity in rats. Environ. Sci. Pollut. Res. Int. 2016;23:12206–12215. doi: 10.1007/s11356-016-6419-5. [DOI] [PubMed] [Google Scholar]
- Majdalawieh A.F., Fayyad M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int. Immunopharmacol. 2015;28:295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Okumu A., DiMaso M., Lobenberg R. Computer simulations using GastroPlus to justify a biowaiver for etoricoxib solid oral drug products. Eur. J. Pharm. Biopharm. 2009;72:91–98. doi: 10.1016/j.ejpb.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Parrott N., Lukacova V., Fraczkiewicz G., Bolger M.B. Predicting pharmacokinetics of drugs using physiologically based modeling–application to food effects. Aaps J. 2009;11:45–53. doi: 10.1208/s12248-008-9079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahak M.K., Kabir N., Abbas G., Draman S., Hashim N.H., Hasan Adli D.S. The role of Nigella sativa and its active constituents in learning and memory. Evid. Based Complement Alternat. Med. 2016;2016:6075679. doi: 10.1155/2016/6075679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebkar A., Beccuti G., Simental-Mendia L.E., Nobili V., Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol. Res. 2016;106:37–50. doi: 10.1016/j.phrs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Salmani J.M., Asghar S., Lv H., Zhou J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules. 2014;19:5925–5939. doi: 10.3390/molecules19055925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq H., Ahmad A., Masud T., Kaleem M. Cardio-protective and anti-cancer therapeutic potential of Nigella sativa. Iran J. Basic Med. Sci. 2014;17:967–979. [PMC free article] [PubMed] [Google Scholar]
- Witkamp R.F., Yun H.I., Van’t Klooster G.A., van Mosel J.F., van Mosel M., Ensink J.M., Noordhoek J., van Miert A.S. Comparative aspects and sex differentiation of plasma sulfamethazine elimination and metabolite formation in rats, rabbits, dwarf goats, and cattle. Am. J. Vet. Res. 1992;53:1830–1835. [PubMed] [Google Scholar]
- Yang B.H., Wang X.T., Liu W., Zhang Q., Chen K.X., Ma Y.M., Wang C.H., Wang Z.T. Gender-related pharmacokinetics and absolute bioavailability of diosbulbin B in rats determined by ultra-performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2013;149:810–815. doi: 10.1016/j.jep.2013.08.010. [DOI] [PubMed] [Google Scholar]