Abstract
We present here the results of a first-in-human, first-in-child trial for patients with relapsed/refractory solid tumors using Celyvir, an advanced therapy medicine that combines autologous mesenchymal stem cells (MSCs) carrying an oncolytic adenovirus. Celyvir was manufactured from a bone marrow aspirate and then given intravenously. Patients received weekly infusions for 6 weeks at a dose of 2 × 106 cells/kg (children) or 0.5–1 × 106 cells/kg (adults), 2 × 104 viral particles per cell. Fifteen pediatric and 19 adult patients were recruited, but 18 were screen failures, mainly because rapid disease progression before Celyvir was available. No grade 2–5 toxicities were reported. Adenoviral replication detected by PCR was found in all but 2 pediatric patient and in none of the adult ones. Absolute numbers of circulating leukocytes suffered minor changes along therapy, but some subsets showed differences comparing the pediatric versus the adult cohorts. Two patients with neuroblastoma showed disease stabilization, and one of them continued on treatment for up to 6 additional weeks. Celyvir, the combination of MSCs and oncolytic adenovirus, is safe and warrants further evaluation in a phase 2 setting. The use of MSCs may be a strategy to increase the amount of oncolytic virus administered to patients, minimizing toxicities and avoiding direct tumor injections.
Keywords: oncolytic virotherapy, mesenchymal stem cells, clinical trial, pediatric tumor
Ramírez and colleagues report here the first clinical trial of oncolytic virotherapy systemically delivered in autologous mesenchymal cells in pediatric and adult patients with advanced tumors. The results show that the infusion of repeated doses is a safe strategy and warrants further evaluation in a phase 2 setting.
Introduction
Oncolytic viruses offer great promise in the field of immuno-oncology, with some products reaching late-phase clinical development for several adult cancers1 and one product (Imlygic/Talimogen laherparepvec/T-Vec) already approved for clinical use in melanoma by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA).2 A known limitation of their use is the need for direct administration into the tumor, which poses significant challenges for central nervous system (CNS), thoracic, and abdominal cancers and limits its use to easily injectable tumors, such as melanoma,3 sarcomas,4 or head and neck cancers.5 As an example, intratumoral injection of DNX-2401 in adult patients with recurrent glioblastoma led to prolonged, overall survival beyond 3 years in a significant proportion of patients as a result of a direct oncolytic effect, followed by elicitation of an immune-mediated antiglioma response.6 Whereas this approach may be suitable for patients with localized, recurrent disease, most of the patients in the advanced setting will present with disseminated disease in need of rather a systemic approach.
Oncolytic viruses administered intravenously (i.v.) in patients with metastatic tumors encounter nevertheless many physiological barriers before reaching cancer lesions. Repeated doses are equally threatened by the recognition and attack of the immune system. A “Trojan horse” strategy using carrier cells has been proposed to overcome the abovementioned limitations. We have worked with autologous, bone marrow-derived mesenchymal stem cells (MSCs) as carriers of Icovir-5, an oncolytic adenovirus,7,8 resulting in a final product called Celyvir.9 Our working hypothesis is that MSCs hide the virus from the recognition and attack of the innate and adaptive immune system before Celyvir delivers their load at the metastasis, favoring better conditions for the in situ oncolytic effect. Once the MSCs have eventually disappeared, due to completion of the adenoviral cycle, the increased oncolysis that would ensue should increment the chances for the initiation or reactivation of an antitumor immune response.
Preclinical and clinical data suggest that MSC tumor homing is a very inefficient process, and the vast majority of infused MSCs is entrapped in the lung, working indirectly via effects on systemic inflammation or as a result of phagocytic uptake of MSC debris by monocytes.10, 11, 12 The possibility that oncolytic particles might be associated with exosomes13 or even carried by them14 has also been reported in preclinical models. We have conducted several preclinical studies testing sources of MSCs, cell doses, toxicities, in vivo tumor-targeting capacity, antiadenoviral immune responses, and antitumor effects using the Celyvir strategy in different animal models15, 16, 17 and a veterinary trial in canine patients with spontaneous cancer.18 No significant adverse effects were found in any of the models. Celyvir produced with either syngeneic or allogeneic MSCs achieved similar tumor-growth reduction and immune activation. We found higher accumulation of the virus in the tumor masses and lungs compared to weekly infusions of naked viruses, with no differences in spleens, livers, and kidneys. The spleens and the lungs were the organs in which the highest amount of virus was detected, likely reflecting the entrapping of MSCs in those organs.17 Tumor lesions were highly infiltrated by immune cells in the core of the tumor.16 The tumor microenvironment showed a more proinflammatory profile after systemic Celyvir therapy.17 The response rate of a veterinary trial with Celyvir was high (74%), even with complete responses in animals with metastatic disease (14.8%).18 The presence of viruses and intratumoral immune changes was detected 4 weeks after the end of treatment. A systemic, humoral antiadenoviral response did not hamper the clinical benefit of this therapy. All of our preclinical data support the Celyvir strategy as an effective cancer immunotherapy. We propose a double mechanism of action for Celyvir. First, selective replication of the oncolytic viruses will produce the death of the tumor cells while amplifying the number of viral particles (vp) locally; circulating viral particles from secondary replication should not be efficacious as antitumor medicines, since they encounter the same limitations as i.v.-infused, naked viruses. Second, the presence of viral antigens, together with the release of molecules associated with tissue damage and the presence of pathogens (damage-associated molecular patterns [DAMPs] and pathogen-associated molecular patterns [PAMPs]), will cause an activation of the immune system.
Children with recurrent or refractory tumors have a dismal prognosis, and therefore, there is an urgent need to identify new therapies. Immunotherapy is changing the paradigm of treatment of several adult and pediatric cancers, mainly by means of immune-checkpoint inhibitors or adoptive T cell therapy, whereas oncolytic virus therapy has scarcely been explored. Initial results of a compassionate-use program for children with metastatic solid tumors were previously reported.19 Here, we present the results of the first-in-man, first-in-child trial (ClinicalTrials.gov: NCT01844661) of Celyvir in adult and pediatric patients with relapsed or refractory solid tumors.
Results
Patient Characteristics
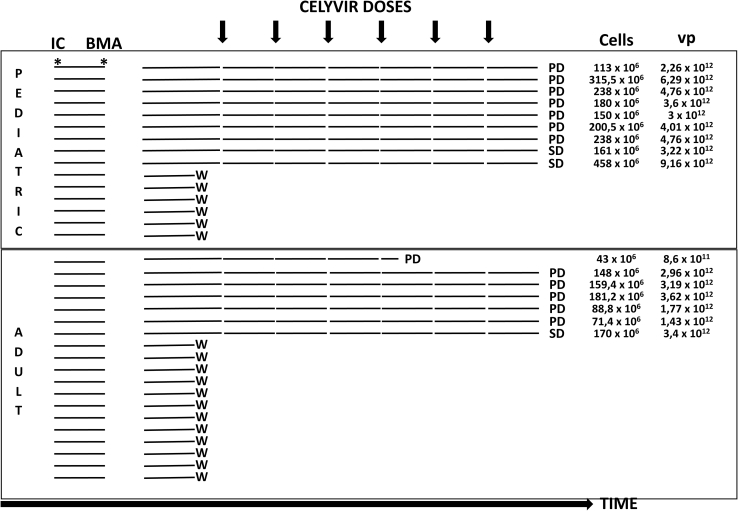
Fifteen pediatric and nineteen adult patients (n = 34) signed informed consent for the study between January 2013 and May 2015. Nine pediatric and seven adult patients (n = 16) received treatment with Celyvir. Eighteen patients (53%) were screen failures: two patients moved to receive therapy at another center, one patient withdrew consent before starting Celyvir, and the other 15 patients experienced rapid disease progression before Celyvir was available, requiring the initiation of chemotherapy (n = 11), or rapidly died (n = 4) (Figure 1).
Figure 1.
Patients Recruited and Procedures during the Trial
IC, informed consent signed; BMA, bone marrow aspirate; vp, viral particles; W, withdrawn; PD, progressive disease; SD, stable disease.
The median age was 7.5 years (range: 3.5–17.3 years) for pediatric patients and 38.6 years (range: 25–66) for adults. Patient characteristics are provided in Table 1. All but one patient enrolled completed the six planned doses of Celyvir. One adult patient received four doses and experienced rapid, progressive disease, dying shortly after stopping Celyvir. In total, 40 and 54 doses were administered to adult and pediatric patients, respectively.
Table 1.
Demographic Characteristics of Patients Treated with Celyvir
| Pediatric Patients (n = 9) | Adult Patients (n = 7) | |
|---|---|---|
| Evaluable for toxicity | 9 | 7 |
| Evaluable for response | 9 | 6 |
| Age, years: median (range) | 7.5 (3.5–17.3) | 38.6 (25–66) |
| Performance Status (Lansky or Karnofsky for Children/ECOG for Adults) | ||
| 90–100/0–1 | 5 | 6 |
| 70–80/1–2 | 4 | 1 |
| Tumor Types | ||
| CNS tumors | 1 | 3 |
| Medulloblastoma | 1 | |
| Astrocytoma | 3 | |
| Non-CNS pediatric/adolescent tumors | 8 | 1 |
| Neuroblastoma | 4 | |
| Non-rhabdo soft-tissue sarcoma | 1 | |
| Ewing sarcoma | 1 | 1 |
| Rhabdomyosarcoma | 1 | |
| Osteosarcoma | 1 | |
| Non-CNS adult tumors | 4 | |
| Colorectal carcinoma | 1 | |
| Lung carcinoma | 1 | |
| Thyroid carcinoma | 1 | |
| Thymoma | 1 | |
| Prior Therapies | ||
| Chemotherapy | 8 | 7 |
| Surgery | 6 | 6 |
| Radiotherapy | 5 | 6 |
| Other (biological, immunological) | 0 | 0 |
Toxicities
All 16 patients that received Celyvir were evaluable for safety. No grade 2–5 toxicities related to Celyvir were reported. In pediatric patients, grade 1 fever was reported in three patients (33%) and grade 1 headache in one (11%). In adult patients, grade 1 fever was reported in two patients (28%) and asthenia in one (14%). Table S1 shows adverse effects recorded during the trial, including related and unrelated with the administration of Celyvir.
Antitumor Activity
All nine pediatric patients completed 6 doses and were evaluated for a response. Two patients (both diagnosed with metastatic neuroblastoma) had disease stabilization, and seven had progressive disease. Two patients (PCPT7 and PCPT8) with stable disease were offered access to a compassionate-use program, and PCPT7 received Celyvir for 6 additional cycles before progression.
Six out of 7 adult patients were evaluated for a response. Five patients (83%) had progressive disease at completion of therapy. One patient with anaplastic astrocytoma had stable disease.
Adenoviral Replication
Table 2 shows information on circulating adenovirus detected by PCR in the pediatric cohort. Adenovirus was absent in all patients before starting therapy. Viral replication detected by PCR was found in all pediatric patients except PCPT2 and PCPT8. Of note, one of these 2 cases only had the PCR test done the first 2 time points (PCPT8). The median number of Celyvir doses to become PCR positive was 2 (range: 1–4). Only 2 patients that had a positive PCR in some moment of the study became permanently negative in the following time points (PCPT5 and PCPT6). On the other hand, we did not detect adenovirus in any of the samples drawn from patients of the adult cohort.
Table 2.
Detection of Adenoviral Particles in Peripheral Blood
| PCR Adenovirus |
|||||||
|---|---|---|---|---|---|---|---|
| Pre-dose 1 | Pre-dose 2 | Pre-dose 3 | Pre-dose 4 | Pre-dose 5 | Pre-dose 6 | Post | |
| PCPT1 | neg | neg | pos | pos | pos | pos | pos |
| PCPT2 | neg | neg | neg | neg | neg | neg | neg |
| PCPT3 | neg | pos | pos | pos | pos | pos | pos |
| PCPT4 | neg | neg | neg | pos | pos | pos | pos |
| PCPT5 | neg | pos | pos | neg | neg | neg | neg |
| PCPT6 | neg | pos | neg | neg | neg | neg | neg |
| PCPT7 | neg | neg | neg | neg | pos | pos | pos |
| PCPT8 | neg | neg | nd | nd | nd | nd | nd |
| PCPT9 | neg | neg | neg | pos | pos | pos | pos |
| ACPT1 | neg | neg | neg | neg | neg | neg | neg |
| ACPT2 | neg | neg | neg | neg | neg | neg | neg |
| ACPT3 | neg | neg | neg | neg | neg | neg | neg |
| ACPT4 | neg | neg | neg | neg | neg | neg | neg |
| ACPT5 | neg | neg | neg | neg | neg | neg | neg |
| ACPT6 | neg | neg | neg | neg | neg | neg | neg |
| ACPT7 | neg | neg | neg | neg | neg | neg | neg |
| ACPT8 | neg | neg | neg | neg | neg | neg | neg |
PCPT#, pediatric cohort patient #; ACPT#, adult cohort patient #; neg, negative; pos, positive; nd, not done.
Analysis of Leukocyte Subpopulations
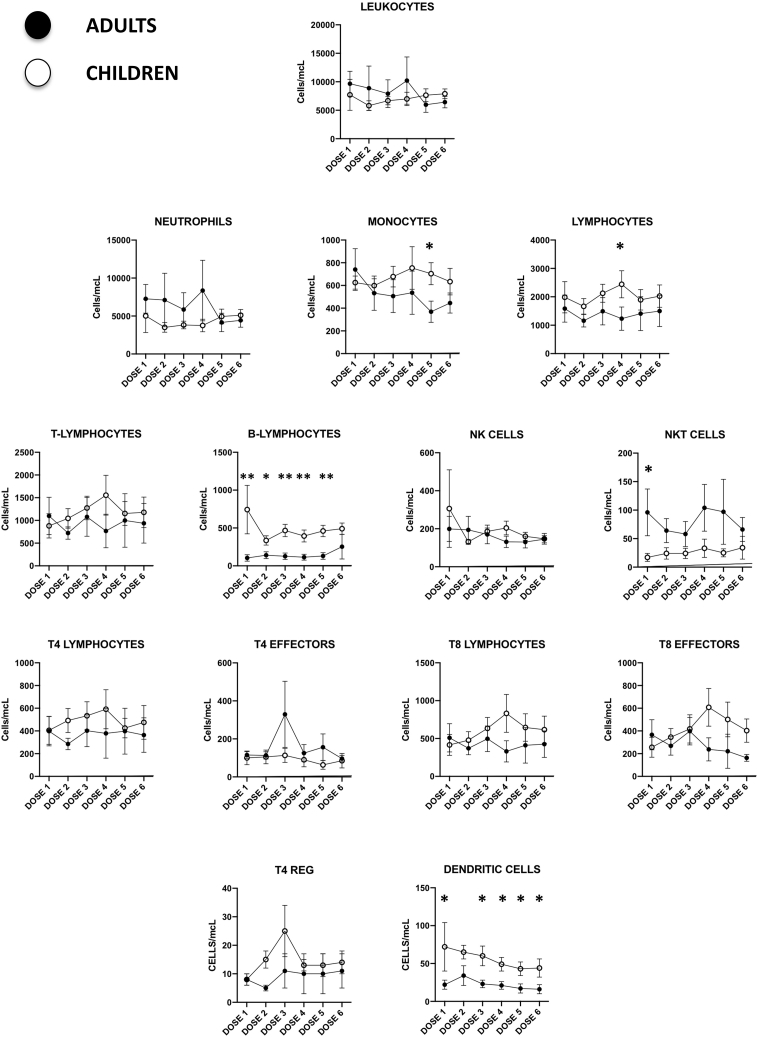
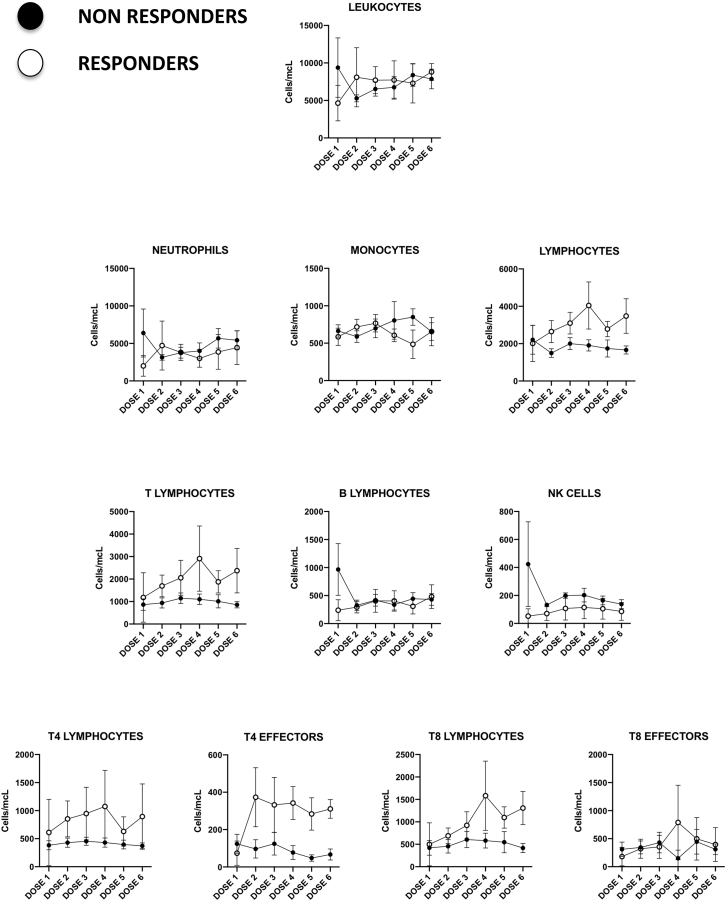
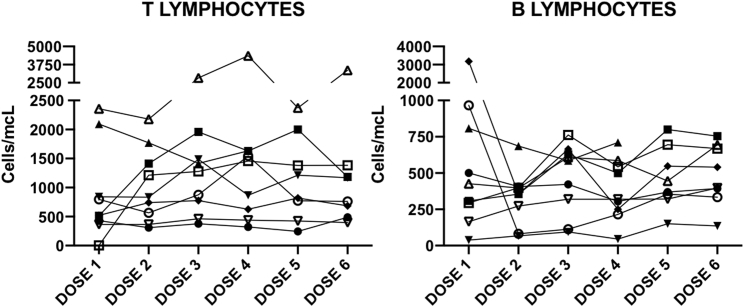
Several leukocyte subpopulations were analyzed during therapy by flow cytometry of peripheral blood samples drawn previous to each Celyvir infusion, as previously described by our group.20 Results are shown in Figure 2. Absolute numbers of circulating leukocytes suffered minor changes along therapy globally; no significant variation of any immune cell subset compared to baseline was found. Some subsets showed differences, comparing the pediatric versus the adult cohorts. For instance, a number of circulating B-lymphocytes (CD45+CD19+) and dendritic cells (lineagenegativehuman leukocyte antigen–DR isotype [HLADRhigh]) were significantly higher among pediatric patients. Numbers of CD4 (CD45+CD3+CD4+) and CD8 (CD45+CD3+CD8+) T lymphocytes were also higher among children at most time points studied, although these differences were not statistically significant. On the other hand, a number of circulating NKT lymphocytes (CD45+CD3+CD56+) were initially higher among adult patients. We compared the kinetics of leukocyte subpopulations among pediatric patients with no response (n = 7) versus those who showed disease stabilization (n = 2) and found that responder patients showed increasingly higher numbers of circulating lymphocytes (B and T) compared to nonresponders. In fact, the numbers of B and T lymphocytes among nonresponder pediatric patients did not change during the trial. This scenario was also seen when analyzing CD4 and CD8 T lymphocyte subpopulations. The major differences are shown in Figure 3. Examples of the kinetics of B and T lymphocytes for the pediatric cohort are shown in Figure 4, as examples of changes at individual patients.
Figure 2.
Changes in Circulating Leukocyte Subpopulation Counts in Patients Treated with Celyvir
Absolute peripheral blood cell counts of different leukocyte subpopulations from pediatric (white) and adult (black) patients. Figures represent the mean ± standard mean error. Dose 1 corresponds to baseline, since blood samples were always drawn immediately before the administration of Celyvir. *p < 0.05; **p < 0.01.
Figure 3.
Changes in Circulating Leukocyte Subpopulation Counts in Pediatric Patients Treated with Celyvir
Absolute peripheral blood cell counts of different leukocyte subpopulations from nonresponder (filled) and responder (empty) pediatric patients. Figures represent the mean ± standard mean error. Dose 1 corresponds to baseline, since blood samples were always drawn immediately before the administration of Celyvir.
Figure 4.
Changes in Circulating Lymphocyte Counts in Pediatric Patients Treated with Celyvir
Changes in circulating T (left) and B (right) lymphocyte subpopulation counts in pediatric patients treated with Celyvir. Individual patients are shown. Dose 1 corresponds to baseline, since blood samples were always drawn immediately before the administration of Celyvir.
Discussion
We present here the results of a first-in-human, first-in-child trial of Celyvir, a new strategy that allows repeated intravenous administration and delivery of oncolytic adenoviruses carried by autologous MSCs. Previous trials with oncolytic virotherapy in pediatric patients have explored the safety of systemic administration of naked viruses in children with metastatic tumors, either alone or in combination with chemotherapy.21,22 Repeated i.v. administration of Seneca Valley Virus (SVV) or reovirus was feasible in these type of patients, with mild to moderate adverse effects. Viral particles were transiently detected in peripheral blood within the first 10 days after viral infusion in the reovirus trial, with seroconversion in all tested patients. Virus clearance occurred rapidly after infusion in the SVV trial, in parallel with increasing titers of neutralizing antibodies. Other groups have tested oncolytic vaccinia4 or herpes virus23,24 virotherapy, injected intratumorally in children and adolescents with intra- and extracranial solid tumors. Mainly, grade 1–2 adverse effects were reported, as well as seroconversion in some participants. The same group has reported results from the first trial testing a single intravenous administration of an oncolytic herpes virus in patients 11–30 years old.25 Therapy did not show dose-limiting toxicities; patients seroconverted while viral replication was detected in peripheral blood. Two patients showed disease stabilization after therapy. Icovir-5, the oncolytic adenovirus used in our trial, was tested in a first-in-human, dose-escalation phase I clinical trial (CT) as a single intravenous (i.v.) infusion in patients with advanced malignant melanoma (ClinicalTrials.gov: NCT01864759).26 The results in 12 patients treated with a dose up to 1013 vp showed a dose-limiting hepatic toxicity (transaminitis), establishing a recommended phase II dose of 3.3 × 1012 vp. Detection of Icovir-5 in biopsies indicated that the i.v. route allows minimal systemic tumor targeting, but clinical benefits were not observed in any patient. The main conclusions of these trials were that oncolytic virotherapies are feasible and well tolerated and that the antiviral immune response may limit the effects of the therapy, more importantly, in a multidose schedule.
In our trial, repeated infusions of significant numbers of cells and viral particles in each patient were followed by autolimited mild or minor viral-related toxicities (fever). In searching for optimum dosage or schedule of oncolytic viruses in the clinic, our experience indicates that the use of MSC may allow an increasing amount of oncolytic virus by repeated administration, avoiding or minimizing emergent toxicities. We did not pursue a dose-escalation approach in this trial but chose a fixed one, based on our previous clinical experience.9,19 We had previously infused doses as high as 5 million cells/,g, 2 × 104 vp per cell,19 with no adverse effects or evidence of a better outcome with doses higher than that used in this trial.
All patients recruited in the trial had a recurrent/refractory metastatic solid tumor to ≥2 lines of conventional treatment at the time of inclusion. Two pediatric patients with neuroblastoma showed disease stabilization by International Neuroblastoma Risk Group (INRG) criteria, including metaiodobenzylguanidine (MIBG) scintigraphy, magnetic resonance imaging (MRI) imaging, and bone marrow analysis. We had previously seen responses among patients with neuroblastoma treated with Celyvir.9,19
Adenoviral detection in peripheral blood was done by PCR. Samples were obtained immediately before the administration of the medicinal product, this meaning 7 days after a previous Celyvir infusion. We have previously studied the replicative cycle of Icovir-5 in human MSCs and found that it takes 72 h to complete the total lyse of in vitro-infected MSCs,9 a time in which MSCs home into metastases in vivo. It is highly unlikely that the limited amount of adenovirus released from the infused MSCs can be circulating in peripheral blood samples collected 7 days after the infusion. We rather think that a positive PCR at that time point represents a second wave of adenovirus replication that took place in the patients. Nevertheless, since PCR can amplify minute amounts of DNA, the possibility that the positive PCR was only detecting genomic fragments that may have represented leftover or a degrading input virus cannot be completely dismissed. A positive PCR was found in most pediatric patients (78%) at a median of 2 infusions (range: 1–4) after initiating Celyvir, and most of them maintained adenoviral replication once detected for the first time (71%). These results strongly suggest that the carrier cells maintained the capacity for targeted delivery of the oncolytic adenoviruses upon repeated infusions. We have previously seen that Celyvir is clinically effective, and the virus can be detected in the tumor masses, even in the presence of pre-existing antiadenoviral immunity, particularly in dogs vaccinated against canine adenovirus that participated in a veterinary trial with the canine version of Celyvir.18 These results support that MSCs may provide shield to the virus from the systemic immune attack, permitting its homing into the tumor lesions. We never found detectable adenoviral replication in one pediatric patient nor in any of the adult ones that were studied at all time points. These differences between pediatric and adult patients may be related to the fact that adults received lower total amount of viruses compared to pediatric patients. The differences in weight between kids and adults and the limitations of expanding MSCs from the marrow of heavily treated patients made it difficult to obtain the target cell dose for all adult patients. It is also possible that some important aspects of the Celyvir mechanism of action were impaired among this cohort of adult patients: increased immune destruction of the medicinal product before it could be effective, insufficient tumor-homing capacity, resistance to oncolysis. We do not have data on the serological status of the patients, information that might have helped in deciding whether an enhanced antiadenoviral response could affect mainly adult patients. Confirming these differences and understanding their causes will be particularly important in future studies.
We also monitored possible changes in circulating immune cells during therapy. There were variations in the kinetics of major immune cell subpopulations considering individual patients, but no major changes were seen when considering both cohorts globally. Even though no conclusion can be drawn from such a small sample, it appeared that pediatric patients showed increased numbers of circulating leukocyte subpopulations of the adaptive immune response, B and T lymphocytes. In addition, the 2 pediatric patients that had disease stabilization also showed B and T lymphocyte subsets above those of patients who progressed. This might suggest that an active immune system is needed in order to obtain clinical benefits from Celyvir.
The use of autologous MSCs imposes a 6-weeks delay in manufacturing Celyvir, a condition that may be unacceptable for patients with aggressive disease. The screening failure rate was high (>50%), mainly because of disease progression before the medicinal product could be released from manufacturing (82%). Given that allogeneic MSCs have shown the same safety profile and efficacy results as autologous cells in animal models, we plan to use off-the-shelf MSCs from healthy donors in the next trial. We have already identified intrinsic characteristics in MSCs from healthy donors in animal models (unpublished) that associate with better clinical outcomes. In patients treated with Celyvir, we have found differences in the expression of homing- and immune-related genes by MSCs of responder versus nonresponder patients.19 Further research will allow us to prepare an optimized version of Celyvir to be used in the patients as soon as the therapy is indicated.
The results from this trial confirm that Celyvir is a safe strategy for administering repeated doses of oncolytic virotherapy systemically and warrant further evaluation in the phase 2 setting. We plan to combine Celyvir with other antitumoral agents that can act synergistically: radiotherapy increases homing of MSCs into irradiated areas,27 nonlymphodepleting chemotherapeutics destroy cancer cells by mechanisms of action different to those of oncolytic viruses28 without hampering the contributing role of the adaptive immune system, and oncolytic virotherapy enhances tumor infiltration by immune cells, which optimizes the subsequent effects of checkpoint inhibitors.29 We are currently focused on understanding the effect of the type of carrier cells in the immune response and optimizing the cellular vehicle to maximize the oncolytic potential of the medicinal product. The safety of Celyvir will allow us to maximize antitumor effects without adding unacceptable toxicities to any of these combinations. Other groups have recently started to explore the possibilities of combining MSCs with oncolytic viruses (the Celyvir strategy) for glioblastoma (ClinicalTrials.gov: NCT03896568; M.D. Anderson Cancer Center) and for ovarian carcinomas (ClinicalTrials.gov: NCT02068794; Mayo Clinic), revealing a growing interest in the field. All of these works will expand a new area of advanced therapy medicinal products of manipulated cells that may open new strategies against relapsed/refractory cancers.
Materials and Methods
Patient Eligibility
Patients were included in three cohorts (cohort A pediatric, cohort B adult, and cohort C pediatric CNS tumors). For this report, pediatric patients from cohorts A and C will be presented together. Children in these two cohorts were recruited if the following criteria were met: age between 6 months and 18 years, performance status ≥60%, life expectancy of at least 6 months, recurrent/refractory metastatic solid tumor to ≥2 lines of conventional treatment, measurable disease, and signature of appropriate informed consent by the parents or legal representatives and informed assent for children aged 12 to 18 years. The same criteria applied for patients in cohort B with the exception of age, which was 18 to 75 years, and Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2.
Exclusion criteria were the following: pregnant or lactating women; symptomatic, uncontrolled CNS metastases; any limitation to the parents’/guardians’ ability to provide informed consent; adequate washout from prior therapies (30 days for any investigational medicinal product, 3 weeks from prior chemotherapy, 2 weeks from biological agents); any unresolved prior treatment-related toxicity except alopecia or gamma-glutamyltransferase (GGT) elevation; inadequate bone marrow function (hemoglobin < 9 g/dL, transfusions allowed, absolute neutrophil count < 1,500/μL, platelet count < 100,000/μL, and lymphocyte count < 500/μL) and biochemistry (alanine transaminase/aspartate transaminase [ALT/AST] > 2.5 upper limit of normal [ULN], total serum bilirubin > 1.5 × ULN, albumin < 2.5 g/dL, creatinine > 1.5 × ULN, or calculated glomerular filtration rate < 60 mL/min); grade ≥ 2 peripheral neuropathy per Common Terminology Criteria for Adverse Events (CTCAE) version 4.0; and severe, uncontrolled conditions, including infections, HIV seropositivity, documented liver cirrhosis, significant cardiac conditions (New York Heart Association [NYHA] grade ≥ I, congestive heart failure, unstable angina, severe arrhythmia, myocardial infarction or clinically relevant valvular disorder in the previous 6 months, prolonged corrected QT [QTc] interval > 480 ms confirmed in two determinations separated by 24 h), dyspnea at rest, or requirement for chronic use of oxygen, and any other condition that could place the patients at additional, unbearable risks or affect the patient’s compliance to the study schedule. Fertile women of child-bearing potential had to agree to use a medically acceptable form of birth control, including abstinence, while on this study and after 1 month.
The institutional review board of Hospital Universitario Nino Jesus approved this study, and continued approval was maintained throughout the study. This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Study Design
This was a phase I/Ib single-center, open-label, nonrandomized feasibility study. The primary objective was to evaluate the safety and tolerability of repeated weekly infusions of Celyvir. Secondary objectives were to evaluate the antitumor activity, to evaluate the immune response to treatment, and to identify biomarkers associated with clinical response. Both adult and pediatric cohorts recruited concurrently.
Icovir-5
Icovir-5 is a conditionally replicative, oncolytic adenovirus in which viral replication is controlled by an E2F-responsive promoter. The Arg-Gly-Asp (RGD) motif has been incorporated to enhance infectivity. Deletion of the retinoblastoma protein (pRB)-binding site of E1a restricts adenoviral replication to cells with an activated RB pathway, a distinction between cancer cells and healthy quiescent ones. This oncolytic adenovirus has been previously described.7 We showed replication of Icovir-5 in ex vivo-proliferating MSCs containing free E2F. Viral production by MSCs was 3-log below that from cancer cells.9 Icovir-5 was manufactured under GMP (good manufacturing practice) conditions at the Baylor College of Medicine facilities.
Manufacturing and Administration of Celyvir
Autologous MSCs were collected from a bone marrow aspirate (BMA), and Celyvir was manufactured within 6 weeks from BMA at our GMP facility, accredited by the national competent authority AEMPS (Agencia Española de Medicamentos y Productos Sanitarios), as previously reported.14 We show the general workflow for autologous Celyvir manufacture (Figure S1) and release criteria (Table S2) as Supplemental Information. A stability study was conducted on the final product (i.e., Icovir-5-infected MSCs, conditioned and packed in its primary container) to establish its expiry toward its use in patients. On the day of Celyvir infusion, MSCs were irradiated with 30 Gy and infected during 2 h with Icovir-5 at 200 MOI (multiplicity of infection) before infusion. Patients received weekly infusions for 6 weeks at a target dose of 2 × 106 cells/kg (pediatric cohort) or 0.5–1 × 106 cells/kg (adults), containing 2 × 104 vp per cell. Infusions were given over 10–20 min in the outpatient facilities. Patients were premedicated with i.v. hydrocortisone, diphenhydramine, and metamizole prior to each infusion.
Study Assessments
During screening, patients underwent standard clinical workup to assess their eligibility for the study and baseline tumor evaluation. With each weekly Celyvir infusion, full blood count, biochemistry, tumor markers (when applicable), peripheral blood for flow cytometry, and adenoviral PCR were collected. After six doses, the initial workup was repeated, along with tumor evaluation. A bone marrow biopsy or tumor biopsy at the end of treatment was optional.
Safety assessments were performed throughout the study. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events. Response assessment for solid tumors was performed, according to RECIST (version 1.1), for glioma and neuroblastoma, according to neuro-oncology criteria (Response Assessment in Neuro-Oncology [RANO]) and INRG, respectively.
After the six planned doses, patients with stable disease or better experiencing clinical benefit were offered to receive treatment on the compassionate-use program. Patients were followed up clinically after the end of the 6 cycles or until progression.
Biomarkers
Biomarker studies in peripheral blood samples included PCR detection of adenoviral DNA and flow cytometry for analysis of lymphocyte subpopulations.
Adenoviral Replication
The presence of circulating adenoviral particles was assessed with the RealCycler ADNV diagnostic kit for clinical samples (Progenie Molecular, Valencia, Spain; https://www.progenie-molecular.com). The target gene is the adenovirus hexon gene, and the specificity corresponds with the 57 known serotypes of adenoviruses. The sensitivity of this semiquantitative PCR-based method is 10 copies/μL. PCRs were performed 7 days after Celyvir infusions.
Leukocyte Subpopulations by Flow Cytometry
Peripheral blood leukocyte subtypes were studied as previously described by our group.15 Flow cytometry analysis was performed with FACSCanto II and FACSDiva software, version 6.1.2 (BD Biosciences, Franklin Lakes, NJ). Fluorochrome-conjugated monoclonal antibodies were from BD Biosciences (CD3-Pacific Blue, CD45-fluorescein isothiocyanate [FITC], lineage-FITC, HLADR-allophycocyanin [APC]-Cy7, CCR7-phycoerythrin [PE], CD4-PerCp.Cy5, CD8-APC-Cy7) and from Beckman Coulter (Fullerton, CA, USA) (CD19-PE, CD56-APC). The following leukocyte populations were identified by flow cytometry: T lymphocytes (CD45+CD3+CD56−), B lymphocytes (CD45+CD19+), NK cells (CD45+CD3−CD56+), NKT cells (CD45+CD3+CD56+), T4 lymphocytes (CD3+CD4+CD8−), T4 regulators (CD3+CD4+CD25+CD127low/negative), T4 effectors (CD3+CD4+CCR7−), T8 lymphocytes (CD3+CD4−CD8+), T8 effectors (CD3+CD8+CCR7−), and dendritic cells (lineagenegativeHLADRhigh).
Statistics
All statistical analyses were performed using Stata/IC 11.0 (StataCorp LP, College Station, TX; http://www.stata.com/). The nonparametric Wilcoxon rank-sum test was used to compare quantitative variables.
Author Contributions
Conceptualization, R.A., J.G.-C., and M.R.; Methodology, D.R., A.L., M.A., C.H., J.A.L.-M., G.M., and A.G.-M.; Validation, L. Moreno and F.B.; Formal Analysis, D.R., F.B., L. Moreno, and M.R.; Investigation, D.R., A.L., M.A., C.H., J.A.L.-M., G.M., and A.G.-M.; Resources, L. Madero, R.A., J.G.-C., and M.R.; Data Curation, D.R., L.M., and F.B.; Writing – Original Draft, M.R., D.R., L. Moreno, and F.B.; Writing – Review & Editing, D.R., J.A.L.-M., L. Moreno, A.L., F.B., M.A., C.H., A.G.-M., G.M., R.A., L. Madero, J.G.-C., and M.R.; Supervision, M.R.; Project Administration, M.R.; Funding Acquisition, M.R. and L. Madero.
Conflicts of Interest
R.A. is founder and holds equity in VCN Biosciences. All other authors declare no competing interests.
Acknowledgments
The trial was sponsored by Fundación de Investigacion Biomedica del Hospital Nino Jesus (EudraCT 2008-000364-16; NCT01844661). This work was funded by grants EC11/061, EC08/00094, and EC07/90591 from Instituto de Salud Carlos III and Fondos FEDER. M.R. is supported by Asociación Pablo Ugarte, Asociación NEN, and Fundación Neuroblastoma.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.01.019.
Supplemental Information
References
- 1.Packiam V.T., Lamm D.L., Barocas D.A., Trainer A., Fand B., Davis R.L., 3rd, Clark W., Kroeger M., Dumbadze I., Chamie K. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018;36:440–447. doi: 10.1016/j.urolonc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Poh A. First oncolytic viral therapy for melanoma. Cancer Discov. 2016;6:6. doi: 10.1158/2159-8290.CD-NB2015-158. [DOI] [PubMed] [Google Scholar]
- 3.Rothermel L.D., Zager J.S. Engineered oncolytic viruses to treat melanoma: where are we now and what comes next? Expert Opin. Biol. Ther. 2018;18:1199–1207. doi: 10.1080/14712598.2018.1544614. [DOI] [PubMed] [Google Scholar]
- 4.Cripe T.P., Ngo M.C., Geller J.I., Louis C.U., Currier M.A., Racadio J.M., Towbin A.J., Rooney C.M., Pelusio A., Moon A. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015;23:602–608. doi: 10.1038/mt.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington K.J., Hingorani M., Tanay M.A., Hickey J., Bhide S.A., Clarke P.M., Renouf L.C., Thway K., Sibtain A., McNeish I.A. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 2010;16:4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 6.Lang F.F., Conrad C., Gomez-Manzano C., Yung W.K.A., Sawaya R., Weinberg J.S., Prabhu S.S., Rao G., Fuller G.N., Aldape K.D. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018;36:1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascallo M., Alonso M.M., Rojas J.J., Perez-Gimenez A., Fueyo J., Alemany R. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol. Ther. 2007;15:1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- 8.Alonso M.M., Cascallo M., Gomez-Manzano C., Jiang H., Bekele B.N., Perez-Gimenez A., Lang F.F., Piao Y., Alemany R., Fueyo J. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007;67:8255–8263. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 9.García-Castro J., Alemany R., Cascalló M., Martínez-Quintanilla J., Arriero Mdel.M., Lassaletta A., Madero L., Ramírez M. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther. 2010;17:476–483. doi: 10.1038/cgt.2010.4. [DOI] [PubMed] [Google Scholar]
- 10.de Witte S.F.H., Luk F., Sierra Parraga J.M., Gargesha M., Merino A., Korevaar S.S., Shankar A.S., O’Flynn L., Elliman S.J., Roy D. Immunomodulation by therapeutic Mesenchymal Stromal Cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36:602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 11.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., von Bonin M., Barbieri L., Halai K., Ward S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;9:eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 12.Schweizer M.T., Wang H., Bivalacqua T.J., Partin A.W., Lim S.J., Chapman C., Abdallah R., Levy O., Bhowmick N.A., Karp J.M. A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived Mesenchymal Stem Cells in men with localized prostate cancer. Stem Cells Transl. Med. 2019;8:441–449. doi: 10.1002/sctm.18-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crenshaw B.J., Jones L.B., Bell C.R., Kumar S., Matthews Q.L. Perspective on adenoviruses: epidemiology, pathogenicity, and gene therapy. Biomedicines. 2019;7:61. doi: 10.3390/biomedicines7030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garofalo M., Villa A., Rizzi N., Kuryk L., Rinner B., Cerullo V., Yliperttula M., Mazzaferro V., Ciana P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control. Release. 2019;294:165–175. doi: 10.1016/j.jconrel.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Rincón E., Cejalvo T., Kanojia D., Alfranca A., Rodríguez-Milla M.Á., Gil Hoyos R.A., Han Y., Zhang L., Alemany R., Lesniak M.S., García-Castro J. Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget. 2017;8:45415–45431. doi: 10.18632/oncotarget.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales-Molina Á., Gambera S., Cejalvo T., Moreno R., Rodríguez-Milla M.Á., Perisé-Barrios A.J., García-Castro J. Antitumor virotherapy using syngeneic or allogeneic mesenchymal stem cell carriers induces systemic immune response and intratumoral leukocyte infiltration in mice. Cancer Immunol. Immunother. 2018;67:1589–1602. doi: 10.1007/s00262-018-2220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco-Luzón L., González-Murillo A., Alcántara-Sánchez C., García-García L., Tabasi M., Luis Huertas A., Chesler L., Ramírez M. Systemic oncolytic adenovirus delivered in mesenchymal carrier cells modulate tumor infiltrating immune cells and tumor microenvironment in mice with neuroblastoma. Oncotarget. 2020;11:347–361. doi: 10.18632/oncotarget.27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cejalvo T., Perisé-Barrios A.J., Del Portillo I., Laborda E., Rodriguez-Milla M.A., Cubillo I., Vázquez F., Sardón D., Ramirez M., Alemany R. Remission of spontaneous canine tumors after systemic cellular viroimmunotherapy. Cancer Res. 2018;78:4891–4901. doi: 10.1158/0008-5472.CAN-17-3754. [DOI] [PubMed] [Google Scholar]
- 19.Melen G.J., Franco-Luzón L., Ruano D., González-Murillo Á., Alfranca A., Casco F., Lassaletta Á., Alonso M., Madero L., Alemany R. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 2016;371:161–170. doi: 10.1016/j.canlet.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Martínez A., González-Vicent M., Valentín J., Aleo E., Lassaletta A., Sevilla J., Vicario J.L., Ramírez M., Díaz M.A. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplant. 2012;47:1419–1427. doi: 10.1038/bmt.2012.43. [DOI] [PubMed] [Google Scholar]
- 21.Kolb E.A., Sampson V., Stabley D., Walter A., Sol-Church K., Cripe T., Hingorani P., Ahern C.H., Weigel B.J., Zwiebel J., Blaney S.M. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group Phase I Consortium report. Pediatr. Blood Cancer. 2015;62:751–758. doi: 10.1002/pbc.25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke M.J., Ahern C., Weigel B.J., Poirier J.T., Rudin C.M., Chen Y., Cripe T.P., Bernhardt M.B., Blaney S.M. Phase I trial of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors: a report of the Children’s Oncology Group. Pediatr. Blood Cancer. 2015;62:743–750. doi: 10.1002/pbc.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstock J.D., Wright Z., Bag A.K., Gessler F., Gillespie G.Y., Markert J.M., Friedman G.K., Johnston J.M. Stereotactic placement of intratumoral catheters for continuous infusion delivery of herpes simplex virus −1 G207 in pediatric malignant supratentorial brain tumors. World Neurosurg. 2019;122:e1592–e1598. doi: 10.1016/j.wneu.2018.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streby K.A., Geller J.I., Currier M.A., Warren P.S., Racadio J.M., Towbin A.J., Vaughan M.R., Triplet M., Ott-Napier K., Dishman D.J. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin. Cancer Res. 2017;23:3566–3574. doi: 10.1158/1078-0432.CCR-16-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streby K.A., Currier M.A., Triplet M., Ott K., Dishman D.J., Vaughan M.R., Ranalli M.A., Setty B., Skeens M.A., Whiteside S. First-in-Human Intravenous Seprehvir in Young Cancer Patients: A Phase 1 Clinical Trial. Mol. Ther. 2019;27:1930–1938. doi: 10.1016/j.ymthe.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García M., Moreno R., Gil-Martin M., Cascallò M., de Olza M.O., Cuadra C., Piulats J.M., Navarro V., Domenech M., Alemany R., Salazar R. A phase 1 trial of oncolytic adenovirus ICOVIR-5 administered intravenously to cutaneous and uveal melanoma patients. Hum. Gene Ther. 2019;30:352–364. doi: 10.1089/hum.2018.107. [DOI] [PubMed] [Google Scholar]
- 27.Thomas J.G., Parker Kerrigan B.C., Hossain A., Gumin J., Shinojima N., Nwajei F., Ezhilarasan R., Love P., Sulman E.P., Lang F.F. Ionizing radiation augments glioma tropism of mesenchymal stem cells. J. Neurosurg. 2018;128:287–295. doi: 10.3171/2016.9.JNS16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson G.R., Relph K., Harrington K., Melcher A., Pandha H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances. Oncolytic Virother. 2016;5:1–13. doi: 10.2147/OV.S66083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.