Abstract
Monoamine oxidase inhibition is an important therapeutic approach for various neurodegenerative disorders. Reversible MAO inhibitors selectively targeting only one isoform possess substantial merit in terms of safety, efficacy, and side effect profile. This study aimed to isolate the secondary metabolites of Zanthoxylum flavum stems and evaluate their recombinant human MAO inhibition, antimicrobial, and antiprotozoal activities. As a result, fourteen compounds were isolated and identified (nine of them were reported from Z. flavum for the first time). Compound 3 (sesamin) exhibited potent selective MAO-B inhibition (IC50 value of 1.45 ± 0.05 µM) which reported herein for the first time. Compound 2 showed selective MAO-A inhibition activity, compound 5 exhibited good trypanocidal activity, and compound 7 displayed moderate antibacterial activity. The promising MAO-B inhibitory activity of sesamin provoked us to further explore the kinetic properties, the binding mode, and the underlying mechanism of MAO-B inhibition by this lignan. This detailed investigation substantiated a reversible binding and mixed MAO-B catalytic function inhibition via sesamin (Ki: 0.473 ± 0.076 μM). Selectivity and reversibility of sesamin on MAO-B provide exciting prerequisites for further in vivo investigation to confirm its therapeutic potentiality.
Keywords: Zanthoxylum flavum, Rutaceae, Sesamin, Monoamine oxidase inhibition, Antimicrobial, Antiprotozoal
1. Introduction
The interest of researchers in the discovery of selective MAO-A or MAO-B inhibitors is steadily increasing. This interest stems from the therapeutic potential of these inhibitors in neurodegenerative diseases management (Carradori et al., 2018). Selective MAO-A inhibitors have employed as effective antidepressants while several MAO-B inhibitors are clinically used in Alzheimer's and Parkinson's diseases therapy (Erdogan Orhan, 2016). The mitochondrial flavoprotein monoamine oxidases (MAOs), which are expressed widely in both neuronal and non-neuronal tissues, are responsible for the oxidative deamination of endogenous as well as exogenous amines. Their two isoforms have different tissue distributions, substrate preference, and inhibitor specificity (Yildiz et al., 2014). Non-selective and irreversible MAO inhibition is accompanied by a hypertensive crisis risk after ingestion of dietary tyramine, while selective MAO-B or MAO-A inhibitors are free from this potential threat (Di Stefano et al., 2013). Therefore, much effort has been devoted to finding new natural or synthetic selective MAO inhibitors. Prolonged therapy with the clinically used irreversible MAO-B inhibitors has demonstrated undesirable effects that evoking the urgent need for the discovery of reversible alternatives (Erdogan Orhan, 2016, Park et al., 2019). Park et al., 2019 developed a new reversible MAO-B inhibitor that efficiently lowering increased GABA levels in Alzheimer’s patients and consequently avoiding shortcomings of long-term treatment with current irreversible ones.
Various medicinal properties have been reported from several Zanthoxylum species (Rutaceae), including cytotoxic, hepatoprotective, antipyretic, diuretic, antioxidant, and tooth care (Nooreen et al., 2019, Ross et al., 2008, Ross et al., 2006). Z. flavum Vahl, a rich source of coumarins, alkaloids, and lignans, is an evergreen tree found in Cuba, Jamaica, Bahamas, Hispaniola, Bermuda, Lower Florida Keys, and Puerto Rico. This plant has shown to possess antioxidant, cytotoxic, and mild antimalarial activities (Ross et al., 2008, Ross et al., 2006).
Sesamin, a major lignan in Z. flavum, and its structurally-related derivatives have been reported to possess multifarious pharmacological activities encompassing anti-inflammatory, anti-carcinogenic, hepatic fatty acid oxidation enhancing, immune-modulatory, antihypertensive, and neuroprotective activities (Pathak et al., 2019).
The present study demonstrates, for the first time, selective reversible inhibition of MAO-B enzymatic activity by sesamin. MAO-B activity and oxidative stress are major factors for the occurrence of many neurodegenerative conditions (Niedzielska et al., 2016). Therefore, antioxidant and neuroprotective properties of sesamin along with its MAO-B inhibitory activity will enhance its potential efficacy. This conclusion should be confirmed through further in vivo and clinical studies.
2. Materials and methods
2.1. General experimental procedures
The 1H, 13C, and 2D NMR spectra were recorded on a Varian Mercury 400 MHz spectrometer, Bruker Avance DRX spectrometer at 600 MHz (1H) and 150 MHz (13C) using TMS as an internal standard. The HR-ESI-MS were done using a Bruker Bioapex-FTMS with electrospray ionization. Adsorbents for column Chromatography including Diaion HP-20, Silica gel 60 F254 (0.2 mm, Merck), MN-polyamide-SC-6, and Sephadex™ LH-20. Human recombinant MAO-A and -B were obtained from BD Biosciences. Clorgyline, R-(-)-deprenyl hydrochloride, Kynuramine dihydrobromide, phenelzine sulfate, 4-hydroxyquinoline, K2HPO4 buffer, and DMSO were purchased from Sigma.
2.2. Plant material
Stems of Z. flavum were collected and identified in August 2003 from Montgomery Botanical Centre, Old culture Road region in Florida by Dr. Charles L. Burnadt and a voucher specimen (518 Audubon, Oxford, MS-38655) has been deposited at his herbarium.
2.3. Extraction and isolation
Air-dried powdered stems (1.3 kg) were macerated at room temperature with 70% MeOH repeatedly till exhaustion. A dry residue (121.6 g) was obtained after the concentration of the combined extracts under reduced pressure. A water suspension of the crude extract was prepared and partitioning was performed using hexane, chloroform, and ethyl acetate to give three main fractions (F1-F3), respectively. Column chromatography CC (silica gel, 800 g, 3.5 × 150 cm) was used for the fractionation of F2 (22.98 g) eluted with a gradient of hexane-EtOAc of increasing polarities (95:5 to 90:10 to 85:15 to 80:20) to afford 8 sub-fractions (Fr. 1 to Fr. 8). Fr. 1 (316 mg) was fractionated by CC (silica gel, 12 g, 1 × 20 cm) using a gradient of hexane-EtOAc (95:5 to 80:20) to furnish compound 1 (10 mg). Fr. 2 (1 g) was subjected to CC (silica gel, 40 g, 2 × 60 cm) using hexane-EtOAc (85:15) then (70:30) to provide compound 2 (195 mg). Fr. 3 (2 g) was purified by CC (silica gel, 80 g, 1 × 100 cm) using hexane-EtOAc (7:3) to elute compound 3 (615 mg). Fr. 4 (750 mg) was separated using CC (silica gel, 30 g, 1 × 60 cm) eluted with hexane-EtOAc (7:3) affording compound 4 (86 mg). Fr. 6 (350 mg) was subjected to CC (silica gel, 12 g, 1 × 20 cm) eluted with hexane-EtOAc (4:6) to yield compound 5 (16.5 mg). Purification of Fr. 7 (460 mg) by CC (Sephadex™ LH-20, 50 g, 1 × 100 cm) using MeOH yielding compound 6 (7.9 mg). Diaion-HP20 was used for fractionation of the ethyl acetate fraction F3 (11.3 g) starting the elution with distilled H2O then MeOH. The methanol soluble fraction (10 g) was initially fractionated via CC (silica gel, 400 g, 5 × 100 cm) using a gradient of DCM-MeOH (98:2 to 7:3) to provide 236 fractions which grouped according to TLC profile into 8 subfractions (E1 to E8). E1 (268.4 mg) was loaded onto (silica gel, 15 g, 1 × 30 cm) using hexane-EtOAc (95:5) to give 10 subfractions from which subfraction 3 (37 mg) was further purified using CC (Sephadex™ LH-20, 30 g, 1 × 80 cm) to give compound 7 (11.8 mg). E2 (600 mg) was separated by CC (silica gel, 25 g, 1 × 30 cm) proceeding with DCM-MeOH (98:2) as an elution system to afford compound 8 (6 mg). Fractionation of E4 (420 mg) by CC (Sephadex™ LH-20, 50 g, 1 × 100 cm) using DCM-MeOH (1:1) affording compound 9 (92 mg) together with other promising subfractions which upon further purification over silica gel using EtOAc-DCM-MeOH (30:30:5.5) yield compound 10 (4.8 mg). Compound 11 (156.8 mg) was isolated via CC (silica gel, 50 g, 1.5 × 30 cm) from E5 (1.2 g) using DCM-MeOH system (80:20 to 75: 25). E6 (2.6 g) was fractionated by MN-polyamide SC-6 (250 g) starting the elution with 100% H2O then a gradient of H2O-MeOH till 100% MeOH to obtain 8 subfractions which upon further purification via Sephadex™ LH-20 afford compounds 12 (122.7 mg) and 13 (9.6 mg). Compound 14 (77.2 mg) was obtained from E7 (490 mg) by CC (Sephadex™ LH-20, 50 g, 1 × 100 cm) using MeOH.
2.4. Antiprotozoal assay
Compounds 1–5, 7–9, and 11–14 were evaluated for their antiprotozoal activities against Trypanosoma brucei brucei. The Alamar Blue assay was used on cell cultures of Trypanosoma brucei trypomastigotes (Manda et al., 2014). Three concentrations of the compounds ranging from 10 − 0.25 µg/mL were evaluated. The dose-response curves with the aid of XLfit software were used for computing IC50 and IC90 values which compared to DFMO as a reference.
2.5. Antimicrobial assay
Compounds 1–5, 7–9, and 11–14 were evaluated for antimicrobial activity against Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Mycobacterium intracellulare ATCC 23068, Staphylococcus aureus ATCC 29213, methicillin-resistant S. aureus ATCC 33591, Candida albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, Aspergillus fumigatus ATCC 204305, and Cryptococcus neoformans ATCC 90113. The compounds were tested at 20, 10, 5.0, … 0.02 µg/mL. Ciprofloxacin was a positive control for bacteria and amphotericin B was a positive control for fungi (Bharate et al., 2007).
2.6. Monoamine oxidases (MAO-A/-B) inhibition assay
The kynuramine oxidative deamination assay was utilized to evaluate the monoamine oxidases inhibitory effects of compounds 1–4, 7, 9 and 12–14 at concentrations of 0.001–100 μM for MAO-A and 0.01–100 μM for MAO-B (Chaurasiya et al., 2016). The assay is based on tracking and monitoring the MAO catalyzed conversion of kynuramine to 4-hydroxy quinolone fluorometrically. The conditions of the assay were optimized following the previously published procedure (Larit et al., 2018). The oxidized fluorescent product of kynuramine (4-hydroxyquinoline) was assessed at λem 380 nm and λex 320 nm following the published procedure using the SoftMax Pro program (Parikh et al., 2002). The standard inhibitors phenelzine, clorgyline, and deprenyl were used at a concentration range of 0.001 μM to 100 μM with the aid of XL-Fit© software.
2.7. MAO-B kinetics inhibition mechanism with sesamin
The determination of the MAO-B inhibition constant (Ki) by sesamin was carried out through enzymatic assays at different kynuramine concentrations ranging from 1.90 to 500 μM and confined sesamin concentrations. Two concentrations of the inhibitor, one lower and another one higher than its IC50 value along with a blank set (in absence of the inhibitor) were assayed to establish Km and Vmax values of sesamin. The findings were carefully examined for the inhibition category either competitive, uncompetitive, or mixed type (Chaurasiya et al., 2016, Pandey et al., 2018). The results were plotted as double reciprocal Lineweaver-Burk plots and the kinetic parameters Km, Vmax, and Ki (inhibition/binding affinity) were estimated via SigmaPlot 13.0 and the Enzyme-Kinetics module applying the Michaelis-Menten equation.
2.8. Investigation of sesamin's binding behavior with MAO-B
The sesamin's binding behavior with human MAO-B recombinant enzyme was studied through enzyme-inhibitor complex dissociation by equilibrium dialysis (Chaurasiya et al., 2017). Recombinant human MAO-B (50 μg/mL protein) incubated with sesamin (15.0 μM) in an enzyme incubation mixture of 1 mL buffered at pH 7.4 using 100 mM potassium phosphate. Following a 20 min incubation of the reaction mixture at 37 °C, the reaction was terminated by chilling on ice. Overnight dialysis of the formed complex mixture was carried out against potassium phosphate buffer at 4 °C for approximately 14–16 h (replacing the buffer three times). The difference between the catalytic activities of the human MAO-B prior to and following the dialysis process was analyzed. A parallel identical procedure was run for the enzyme control (in absence of the inhibitor) along with the standard inhibitors deprenyl (a selective irreversible MAO-B inhibitor) and phenelzine (a non-selective irreversible inhibitor).
3. Results and discussion
3.1. Identification of phytochemicals
The identification of the isolates was based on their spectroscopic analyses interpretation (MS, 1D, and 2D NMR), compared with the corresponding published data in the literature. The isolated compounds were identified as lupeol (1) (Jamal et al., 2008), isoimperatorin (2) (Ross et al., 2008), sesamin (3) (Ross et al., 2008), bergapten (4) (Ross et al., 2008), oxypeceudanin hydrate (5) (Shalaby et al., 2014), cubebin (6) (Matsuda et al., 2004), imperatorin (7) (Shalaby et al., 2014), pabulenol (8) (Shalaby et al., 2014), 4-hydroxy-2-methoxyphenyl-6-O-syringyl-β-ᴅ-glucopyranoside (9) (Bai et al., 2012), 5, 6, 3ʹ-trihydroxy-3, 7, 4′-trimethoxyflavone-6-O-β-ᴅ-glucopyranoside (10) (Bohm and Collins, 1979), marmesinin (11) (Kitajima et al., 1998), isorutarin (12) (Sharma et al., 1979), oxypeucedanin hydrate 3″-O-β-ᴅ-glucopyranoside (13) (Razavi et al., 2008), and 5, 4′-dihydroxy-3′-methoxyflavanone-7- (6″-O-α-ʟ-rhamnopyranosyl)-β-ᴅ-glucopyranoside (14) (Gohari et al., 2012). This study reveals the presence of compounds 5, 6, 8–14 in Z. flavum for the first time. Coumarins, lignans, and flavonoids are of common occurrence in the genus Zanthoxylum and the chemotaxonomic significance of their accumulation within the genus is undeniable. Therefore, the isolated compounds from the plant under study greatly support its chemotaxonomy.
3.2. Determination of MAO-A and -B inhibitory effects
This study investigated the in vitro inhibitory effects of sesamin and other isolated compounds on monoamine oxidases (MAOs) catalytic activities (Table 1). Sesamin exhibited maximal selective potency of MAO-B inhibition. Also, compound 2 showed selective MAO-A inhibition activity. Considering the relevant neuroprotective and antioxidant effects of sesamin through attenuation of oxidative stress, boosting the potential therapeutic value of sesamin in oxidative stress associated conditions is expected (Ahmad et al., 2016).
Table 1.
Inhibition of recombinant human MAO-A and -B by some isolated compounds.
| Compounds | MAO-A IC50 (µM)* |
MAO-B IC50 (µM)* |
|---|---|---|
| 1 (lupeol, C30H50O) | na | na |
| 2 (isoimperatorin, C16H14O4) | 6.98 ± 0.29 | na |
| 3 (sesamin, C20H18O6) | na | 1.45 ± 0.05 |
| 4 (bergapten, C12H8O4) | 4.43 ± 0.15 | 8.85 ± 1.17 |
| 7 (imperatorin, C16H14O4) | 30.0 ± 1.18 | 24.84 ± 2.41 |
| 9 (4-hydroxy-2-methoxyphenyl-6-O-syringyl-β-ᴅ-glucopyranoside, C22H26O12) | na | 85.69 ± 7.77 |
| 11 (marmesinin, C20H24O9) | na | na |
| 12 (isorutarin, C20H24O10) | 33.20 ± 1.23 | 75.52 ± 4.29 |
| 13 (oxypeucedanin hydrate 3″-O-β-ᴅ-glucopyranoside, C22H26O11) | na | na |
| 14 (5, 4′-dihydroxy-3′-methoxyflavanone-7- (6″-O-α-ʟ-rhamnopyranosyl)-β-ᴅ-glucopyranoside, C28H34O15) | na | na |
| Phenelzine | 0.221 ± 0.009 | 0.131 ± 0.023 |
| Clorgyline | 0.0075 ± 0.0002 | – |
| Deprenyl | – | 0.049 ± 0.003 |
The IC50 values computed from the dose response inhibition curves are mean ± SD of triplicate observations.na, not active at 100μM.
3.3. Evaluation of MAO-B inhibition kinetic mechanism by sesamin
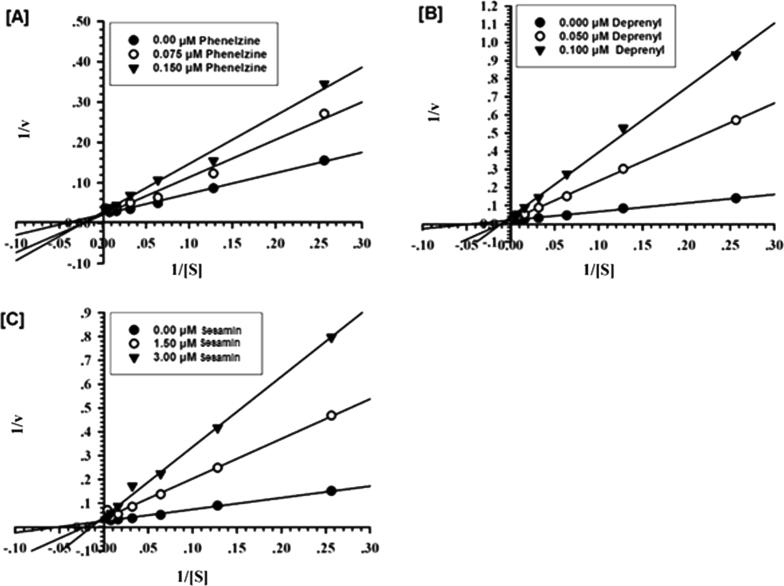
Analysis of the obtained data involving Ki, Km, and Vmax values of sesamin represented in Table 2 and Fig. 1 indicated that sesamin inhibited the catalytic function of MAO-B through a mixed manner which allowed binding of sesamin in the active catalytic site or an allosteric site on the enzyme, whether or not kynuramine has already been bound, with different affinities for these two binding sites.
Table 2.
Inhibition/binding affinity constants (Ki) values for inhibition of recombinant human MAO-B by sesamin, phenelzine, and deprenyl.
| Compounds | Monoamine oxidase-B |
|
|---|---|---|
| Ki (µM)* | Type of Inhibition | |
| Sesamin | 0.4730 ± 0.0760 | Mixed/Reversible |
| Phenelzine (ref.) | 0.0616 ± 0.0062 | Mixed/Irreversible |
| Deprenyl (ref.) | 0.0128 ± 0.0020 | Mixed/Irreversible |
Values are mean ± SD of triplicate experiments.
Fig. 1.
Kinetic characteristics of recombinant human MAO-B inhibition with [A] phenelzine, [B] deprenyl and [C] sesamin. [V = nmoles/min/mg protein and S = substrate kynuramine concentration (μM)].
3.4. Study of sesamin's binding behavior with MAO-B
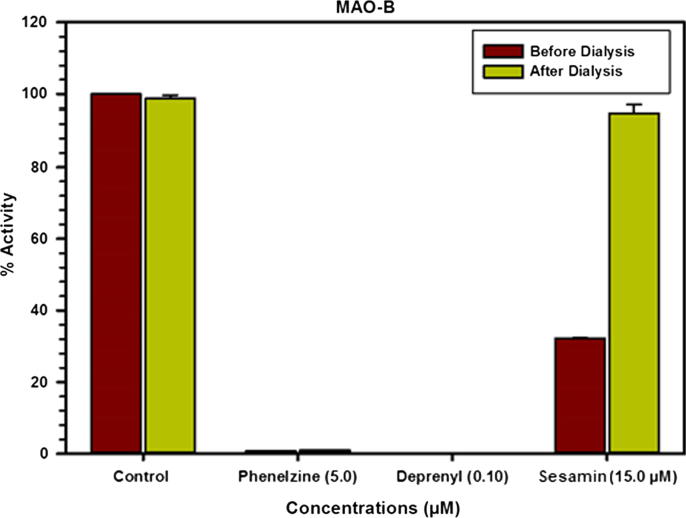
The equilibrium dialysis assay is based on incubating the recombinant enzyme protein with high inhibitor concentrations followed by comprehensive dialysis of the produced complex to provide important information concerning the nature of the sesamin binding with the enzyme based on the reconstituted proportion of the protein catalytic activity. Almost complete inactivation of the enzyme was achieved upon incubation however over 90% of functional recovery of the enzyme was recorded from the enzyme-sesamin incubation mixture next to the overnight dialysis. The blank reaction which was done on the recombinant human MAO-B protein incubated without the inhibitor lost about >5% of the enzymatic activity during overnight dialysis. Analogous treatment of the enzyme with deprenyl and phenelzine also showed irreversible inhibition/binding with the human MAO-B protein (Chaurasiya et al., 2017, Pandey et al., 2018). These observations clearly indicated the dialyzable nature of the enzyme-sesamin complexes. Thus, MAO-B enzyme is reversibly inactivated via this new interesting inhibitor (Fig. 2). Considering the previously reported pharmacokinetic properties of sesamin, which reveal its efficient absorption and wide distribution mainly in liver and kidney as a metabolic form, sesamin may serve as a safer alternative to the classical irreversible inhibitors (Tomimori et al., 2017).
Fig. 2.
Analysis of the nature of binding of sesamin with recombinant human MAO-B by recovery of the catalytic activity of the enzyme after dialysis dissociation. [Each bar shows mean ± SD of triplicate values].
3.5. Antimicrobial and antiprotozoal activities
Compound 5 showed good trypanocidal activity against Trypanosoma brucei with IC50 and IC90 values of 5.75 and 6.78 µg/mL, respectively compared to the positive control, DFMO with IC50 and IC90 values of 3.59 and 8.09 μg/mL, respectively. Compound 7 showed good antibacterial activity against M. intercellare with an IC50 of 10.96 µg/mL compared to the positive control ciprofloxacin (IC50 value of 0.399 µg/mL).
4. Concluding remarks and future perspective
Fourteen compounds were isolated from Z. flavum stems. Notably, compounds 5, 6, 8–14 were obtained from Z. flavum for the first time. Compound 3 (sesamin) was the most active as MAO-B selective inhibitor with an IC50 value of 1.45 ± 0.05 µM (Ki value of 0.473 ± 0.076 μM) through a mixed enzyme blockade mechanism. The reversible inactivation of MAO-B via sesamin was experimentally confirmed. A properly designed in vivo studies and clinical application of sesamin should be conducted. Additionally, structure-activity relationship studies that comparing sesamin with its analogues and derivatives worth careful examination to determine the essential functional groups conferring the desired activity of sesamin, unlock the therapeutic potentiality of these structurally-related candidates and may illuminate more active drugs that possess improved pharmaceutical characteristics.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the Egyptian Government, The University of Mississippi, the National Center for Natural Products Research, the United States and NIH COBRE grant P20GM104932 for financial support. We are appreciative to Dr. Charles L. Burnadt for collecting and identifying the plant. We are grateful to Dr. Baharthi Avula for doing HR-ESI-MS and Dr. M. Jacob for antibacterial screening.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2020.02.001.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ahmad S., ElSherbiny N.M., Jamal M.S., Alzahrani F.A., Haque R., Khan R., Zaidi S.K., AlQahtani M.H., Liou G.I., Bhatia K. Anti-inflammatory role of sesamin in STZ induced mice model of diabetic retinopathy. J. Neuroimmunol. 2016;295–296:47–53. doi: 10.1016/j.jneuroim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Bai J., Fang Z.F., Chen H., Yu S.S., Zhang D., Wei H.L., Ma S.G., Li Y., Qu J., Xu S., Ren J.H., Zhao F., Zhao N., Liu J.H. Antioxidant phenolic glycosides from the roots of Illicium dunnianum. Carbohydr. Res. 2012;361:206–211. doi: 10.1016/j.carres.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Bharate S.B., Khan S.I., Yunus N.A.M., Chauthe S.K., Jacob M.R., Tekwani B.L., Khan I.A., Singh I.P. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorganic Med. Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Bohm B.A., Collins F.W. Flavonoids of Some Species of Chrysosplenium*, Biochemical Systematics and Ecology. Pergamon Press Ltd; 1979. [Google Scholar]
- Carradori, S., Secci, D., Petzer, J.P., 2018. MAO inhibitors and their wider applications: a patent review. Expert Opin. Ther. Pat. [DOI] [PubMed]
- Chaurasiya N.D., Gogineni V., Elokely K.M., León F., Núñez M.J., Klein M.L., Walker L.A., Cutler S.J., Tekwani B.L. Isolation of acacetin from Calea urticifolia with inhibitory properties against human monoamine oxidase-A and -B. J. Nat. Prod. 2016;79:2538–2544. doi: 10.1021/acs.jnatprod.6b00440. [DOI] [PubMed] [Google Scholar]
- Chaurasiya N.D., León F., Ding Y., Gómez-Betancur I., Benjumea D., Walker L.A., Cutler S.J., Tekwani B.L. Interactions of Desmethoxyyangonin, a Secondary Metabolite from Renealmia alpinia, with Human Monoamine Oxidase-A and Oxidase-B. Evidence-based Complement. Altern. Med. 2017 doi: 10.1155/2017/4018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano A.F.D., Radicioni M.M., Rusca A. Pressor response to oral tyramine and monoamine oxidase inhibition during treatment with ralfinamide (NW-1029) Neurotox. Res. 2013;23:315–326. doi: 10.1007/s12640-012-9344-5. [DOI] [PubMed] [Google Scholar]
- Erdogan Orhan I. Potential of natural products of herbal origin as monoamine oxidase inhibitors. Curr. Pharm. Des. 2016;22:268–276. doi: 10.2174/1381612822666151112150612. [DOI] [PubMed] [Google Scholar]
- Gohari A.R., Ostad S.N., Moradi-Afrapoli F., Malmir M., Tavajohi S., Akbari H., Saeidnia S. Evaluation of the cytotoxicity of Satureja spicigera and its main compounds. Sci. World J. 2012 doi: 10.1100/2012/203861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A.K., Yaacob W.A., Din L.B. A chemical study on Phyllanthus reticulatus. J. Phys. Sci. 2008 [Google Scholar]
- Kitajima J., Okamura C., Ishikawa T., Tanaka Y. Coumarin glycosides of Glehnia littoralis root and rhizoma. Chem. Pharm. Bull. 1998;46:1404–1407. [Google Scholar]
- Larit F., Elokely K.M., Chaurasiya N.D., Benyahia S., Nael M.A., León F., Abu-Darwish M.S., Efferth T., Wang Y.H., Belouahem-Abed D., Benayache S., Tekwani B.L., Cutler S.J. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine. 2018;40:27–36. doi: 10.1016/j.phymed.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda S., Khan S.I., Jain S.K., Mohammed S., Tekwani B.L., Khan I.A., Vishwakarma R.A., Bharate S.B. Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-β-carbolines. Bioorganic Med. Chem. Lett. 2014;24:3247–3250. doi: 10.1016/j.bmcl.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Kawaguchi Y., Yamazaki M., Hirata N., Naruto S., Asanuma Y., Kaihatsu T., Kubo M. Melanogenesis stimulation in murine B16 melanoma cells by Piper nigrum leaf extract and its lignan constituents. Biol. Pharm. Bull. 2004;27:1611–1616. doi: 10.1248/bpb.27.1611. [DOI] [PubMed] [Google Scholar]
- Niedzielska E., Smaga I., Gawlik M., Moniczewski A., Stankowicz P., Pera J., Filip M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016 doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooreen Z., Tandon S., Yadav N.P., Kumar P., Xuan T.D., Ahmad A. Zanthoxylum: A review of its traditional uses, naturally occurring constituents and pharmacological properties. Curr. Org. Chem. 2019;23:1307–1341. [Google Scholar]
- Pandey P., Chaurasiya N.D., Tekwani B.L., Doerksen R.J. Interactions of endocannabinoid virodhamine and related analogs with human monoamine oxidase-A and -B. Biochem. Pharmacol. 2018;155:82–91. doi: 10.1016/j.bcp.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S., Hanscom S., Gagne P. A fluorescent-based, high-throughput assay for detecting inhibitors of human Monoamine Oxidase A and B. BD Biosci. Discov. Labware. 2002;5229 [Google Scholar]
- Park J.H., Ju Y.H., Choi J.W., Song H.J., Jang B.K., Woo J., Chun H., Kim H.J., Shin S.J., Yarishkin O., Jo S., Park M., Yeon S.K., Kim S., Kim Jeongyeon, Nam M.H., Londhe A.M., Kim Jina, Cho S.J., Cho S., Lee C., Hwang S.Y., Kim S.W., Oh S.J., Cho J., Pae A.N., Justin Lee C., Park K.D. Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer’s disease. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aav0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak, N., Bhaduri, A., Rai, A.K., 2019. Sesame: Bioactive Compounds and Health Benefits, pp. 181–200.
- Razavi S.M., Nazemiyeh H., Delazar A., Hajiboland R., Rahman M.M., Gibbons S., Nahar L., Sarker S.D. Coumarins from the roots of Prangos uloptera. Phytochem. Lett. 2008;1:159–162. [Google Scholar]
- Ross S.A., Krishnaven K., Radwan M.M., Takamatsu S., Burandt C.L. Constituents of Zanthoxylum flavum and their antioxidant and antimalarial activities. Nat. Prod. Commun. 2008;3:791–794. [Google Scholar]
- Ross S.A., Krishnaveni K.S., Burandt C.L. Two new benzofuran derivatives from the roots of Zanthoxylum flavum. J. Chem. Res. 2006:406–407. [Google Scholar]
- Shalaby N.M.M., Abd-Alla H.I., Aly H.F., Albalawy M.A., Shaker K.H., Bouajila J. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia boiss and its linear furanocoumarins. Biomed Res. Int. 2014 doi: 10.1155/2014/480545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B.R., Sharma P. Structure of leptophyllidin and identity of leptophyllin and leptophylloside with rutaretin and isorutarin. Counc. Sci. Ind. 1979 [Google Scholar]
- Tomimori N., Rogi T., Shibata H. Absorption, distribution, metabolism, and excretion of [14C] sesamin in rats. Mol. Nutr. Food Res. 2017:61. doi: 10.1002/mnfr.201600844. [DOI] [PubMed] [Google Scholar]
- Yildiz O., Karahalil F., Can Z., Sahin H., Kolayli S. Total monoamine oxidase (MAO) inhibition by chestnut honey, pollen and propolis. J. Enzyme Inhib. Med. Chem. 2014;29:690–694. doi: 10.3109/14756366.2013.843171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.