Abstract
Tumor cells overexpress low-density lipoprotein (LDL) receptors (LDL-r). Hence, LDL is proposed as a targeting shuttle of anticancer drugs. Therefore, the objective of this study was to synthesize a dual inhibitor of heat shock protein 27 (HSP27) and human epidermal growth factor receptor 2 (HER2) conjugated with cholesterol and encapsulated into LDL for selective targeting of ovarian cancer cells. In the present study, the anticancer agent and its cholesterol conjugate were successfully prepared and characterized physically for color, shape, and melting point. Moreover, the compounds were chemically characterized for 1H NMR and 13C NMR spectra using FTIR and LCMS/MS. Our results revealed that the prepared anticancer agent and its cholesterol conjugate elicited dual HSP27 and HER2 inhibition, as confirmed using western blotting. The anticancer agent (compound D) entered cells and targeted the HSP27 function, thereby reducing HER2 expression. However, a cholesterol-conjugated anticancer agent (compound F) had high cellular uptake and inhibited the growth of SKOV3 cells after encapsulation into LDL. The obtained results concluded that the design of an LDL-encapsulated cholesterol-conjugated HSP27-HER2 dual inhibitor may be a promising approach to realize specific targeted achieve killing of ovarian cancer.
Keywords: Drug delivery, LDL, Cholesterol, Heat shock protein 27, Ovarian cancer
1. Introduction
Heat shock protein 27 (HSP27) is a chaperon protein involved in various cellular processes such as thermotolerance, signaling, proliferation, development, differentiation, and apoptosis (Jaragh-Alhadad, 2018, Garrido et al., 2006). Human epidermal growth factor receptor 2 (HER2) is another regulator of cell cycle. Both proteins are functionally regulated by phosphorylation/dephosphorylation mechanisms, and are overexpressed in different types of cancer, such as ovarian cancer (Grummer and Carroll, 1988, Haley et al., 2000, Garrido et al., 2006, Jaragh-Alhadad, 2018). Both HSP27 and HER2 proteins play an important role in cancer cell survival and resistance to antitumor agents (Jaragh-Alhadad, 2018, Haley et al., 2000, Garrido et al., 2006). In this context, using such proteins as anticancer targets represents a smart approach in the development of novel anticancer therapies (Jaragh-Alhadad, 2018, Haley et al., 2000, Garrido et al., 2006).
Tumor cells are fast growing cells with high potential of biomembrane formation; consequently, they require high level of cholesterol as an essential component in biomembrane assembly (Alanazi et al., 2003, Radwan and Alanazi, 2014a, Radwan and Alanazi, 2014b). Cholesterol is used not only in cell membrane assembly but also in steroid hormone biosynthesis. Ovarian cells import cholesterol from the blood by using low-density lipoproteins (LDL) as bionanoparticle delivery shuttles (Oktay and Oktem, 2010; Colombo et al., 2010, Ayako et al., 2012). It has been shown that the cholesterol demand of ovarian cells increases in the case of malignancies, including ovarian tumor (Oktay and Oktem, 2010; Colombo et al., 2010, Ayako et al., 2012). In biological systems, the trafficking of cholesterol and hydrophobic drugs between internal body compartments is mediated by LDL as delivery nanoshuttles (Harisa and Alanazi, 2014). By this manner, LDL can be utilized as a targeted shuttle that can deliver chemotherapeutics with cholesterol conjugate to tumor cells overexpressing LDL receptors (LDL-r) (Harisa and Alanazi, 2014). LDL traffics through the cell membrane by receptor-mediated endocytosis machinery. In the intracellular milieu, LDL cargoes are broken down by lysosomal enzymes, thus releasing chemotherapeutic agents from the cholesterol conjugates (Harisa and Alanazi, 2014). Therefore, LDL may serve as selective delivery shuttles for anticancer medicines (Ian and Gang, 2007). Although several studies have reported LDL as delivery shuttles of anticancer agents into tumor tissues (Martin and Theo, 1990, Yunfei and Millie, 2001, Harisa and Alanazi, 2014), further studies are required to realize their application in clinical setting.
Chaperone inhibitors are substantial cytotoxic agents; however, their clinical application is limited by their nonselectivity for tumor cells. Many researches are focused on the development of substances that reduce the chaperone activity in cancer cells in a selective manner (Lazarev et al., 2018). Hsp27 expression increases in response to various stress stimuli, including anticancer chemotherapy, and causes tumor drug resistance (Zhong et al., 2013). Conjugation of chemotherapeutics with cholesterol to improve the effectiveness of their cytotoxic activities is still a matter of research.
Therefore, the main goal of this study was to design and characterize an HSP27-HER2 dual inhibitor cholesterol conjugate. The inhibitory effect of this anticancer cholesterol conjugate was studied using western blotting, and the prepared inhibitor was subsequently encapsulated into LDL as delivery shuttle. Finally, the cytotoxic effect of the LDL-encapsulated cholesterol conjugate was investigated in SKOV3 ovarian cancer cells.
2. Materials and methods
2.1. Chemicals
All chemical spectral analyses were conducted at Kuwait University Research Center. Thin layer chromatography (TLC) was performed using Polygram SIL G/UV 254 TLC plates, and the results were visualized under ultraviolet light at 254 and 350 nm, with hexane/ethyl acetate as a solvent. Column chromatography was performed using silica gel 60A with a mesh size of 40–60 µm. 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained using a Bruker DPX 600 NMR spectrophotometer at 600 MHz and 150 MHz in DMSO and CDCL3, respectively. Mass spectra were detected on a GC-MS DFS–Thermo spectrometer. An IR spectrum was obtained with a Jasco 6300 FTIR spectrometer. Melting point was determined using a Netzsch DSC 204 F1 Phoenix differential scanning calorimeter.
SKOV3 human ovarian cancer cells were obtained from American Type Culture Collection. A cholesterol-conjugated HSP27 inhibitor was synthesized in our laboratory. Cell culture media and other supplements, such as 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), were obtained from Sigma-Aldrich (Milwaukee, WI). Chemicals and reagents are commercially available and ready for direct use, requiring no preparation. Biological parameters were examined at the Biology Department, Kuwait University.
2.2. Synthesis and characterization of anticancer cholesterol conjugate
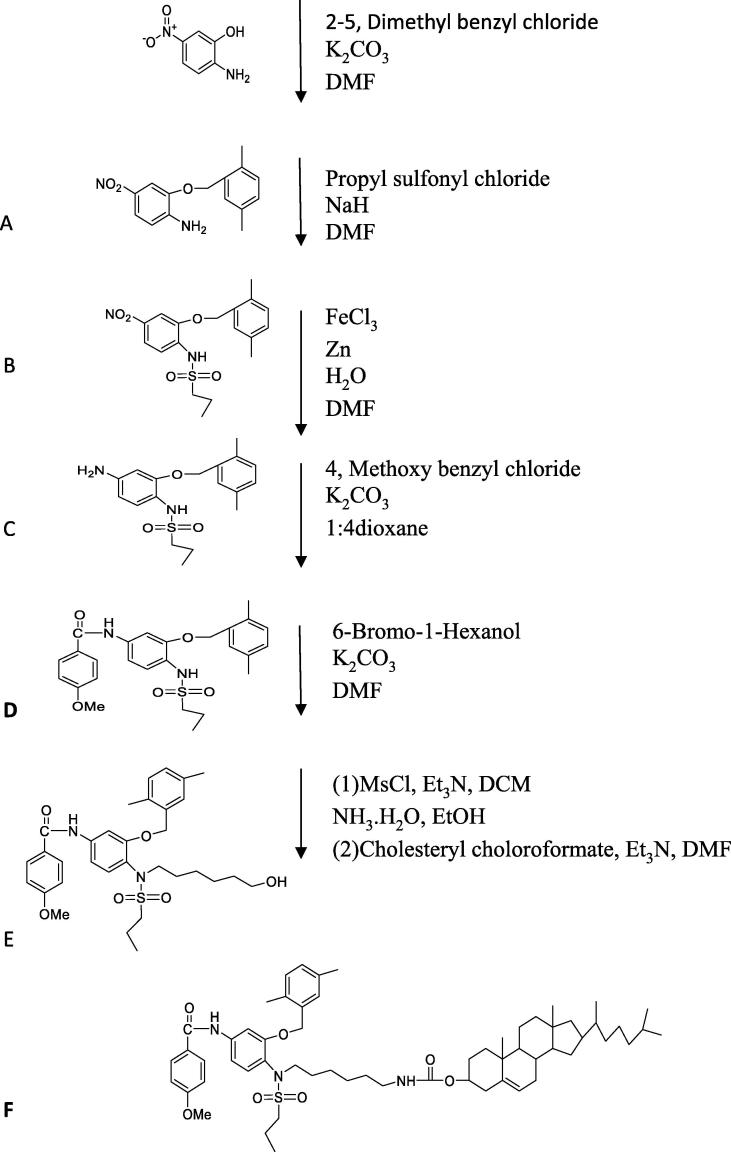
In the present study, multi-step synthesis was described in Scheme 1. 2-amino-5- nitrophenol used as a starting material (0.77 g, 5 mmol), which was mixed with K2CO3 (0.69 g, 5 mmol) and 2, 5-dimethyl benzyl chloride (0.77 g, 5 mmol) in presence of 5 ml DMF to obtain compound A. The next step we added on compound A, both sodium hydride (0.6 g, 25 mmol), and propane sulfonyl chloride (0.71 g, 5 mmol), in 5 ml dry dimethylformamide (DMF) at room temperature and the reaction mixture was left to stir overnight to obtain compound B. This followed by reduction step for the nitro group in compound B to an amine group to obtain compound C (in the presence of Zn (3.25 g, 50 mmol), FeCl3 (3.25 g, 20 mmol), 5 ml DMF and water solution. Then, 4 methoxy benzyl chloride (0.78 g, 5 mmol), K2CO3 (0.69 g, 5 mmol) and 5 ml 1:4 dioxane were added on compound C (1.74 g, 5 mmol) to obtain compound D, which is HSP27 inhibitor. Then, 6-Bromo-1-Hexanol (1.086 g, 6 mmol) was added to the mixture of compound D (2.41 g, 5 mmol) in presence of K2CO3 (0.69 g, 5 mmol) and 5 ml DMF to 5 obtain Compound E. The mixture left overnight to stir at room temperature. In the last step on mixture E (2.91 g, 5 mmol) Et3N (2.525 g, 25 mmol) and DCM (5 ml) were added at 0 °C and then added MsCl (1.71 g, 15 mmol). This mixture was left to stir until the reaction completed. Then, ice cold water added to wash the solution and evaporate it and get the organic layer. To this mixture add 20 ml from both ethanol and ammonium hydroxide and left the reaction to stir for two days at room temperature. The solution was concentrated to give the intermediate amine. On this intermediate cholesteryl chloroformate added (2.69 g 6 mmol), Et3N (1.01 g, 10 mmol) and DMF and left to stir for two hours at room temperature to obtain the desired compound F (Cholesterol conjugated to HSP27 inhibitor). Water was used to quench all the reactions steps except the reduction step to obtain compound C we used acetone. The compounds were first purified by column chromatography. The chemical characterization of the designed dual HSP27 and HER2 inhibitor is the term of 1H NMR and 13C NMR, melting point, FTIR and LCMS/MS. This reaction was repeated two times.
Scheme 1.
Synthesis steps for cholesterol conjugated HSP27 inhibitor
2.3. Encapsulation of cholesterol-conjugated anticancer agents into LDL
Commercial LDL 50 µl was obtained for Sigma Louis and used to encapsulate cholesterol conjugate 5 µl at ratio (5:1 ratio). The particle size was measured using dynamic light scattering (DLS) technique. Plain LDL 30 µl was mixed with 1.5 ml phosphate buffer saline (PBS) and the particles size was measured which had a diameter about 78.1 nm. Compound F (cholesterol conjugate) was encapsulated into LDL as following, LDL and Compound F were mixed into 1.5 ml PBS and then sample was sonicated and left for 24 h to rest and reform. Then, the sample particle size diameter was measured which was 249.6 nm. This is proof that the cholesterol conjugates encapsulated into LDL. Reaction steps were repeated three times. LDL encapsulated cholesterol conjugate was precipitated when treated with SKOV3 cells in the aqueous solution which is a direct evidence the cholesterol conjugate loaded into the LDL particles (Idippily et al, 2017).
2.4. Cytotoxicity and western blotting assays
Cancer cells were maintained in RPMI 1640 medium containing 10% FBS, 10 ml penicillin–streptomycin, and 100 µl ciprofloxacin. FBS was deactivated for 30 min in a 37 °C water bath before use. Cell cultures were grown at 37 °C in an incubator with humidified atmosphere of 5% CO2. MTT assay was performed by monitoring the reduction of yellow MTT into purple products. After culturing the cells in a monolayer culture, the cells were harvested, seeded on RPMI1640 medium in a 96-well plate, and incubated overnight. The cells were treated with different concentrations of anticancer agents for 72 h. The control wells received DMSO at equal concentrations to those in the drug-treated cell wells. Next, the cell medium was replaced with fresh medium, and cell viability was determined by addition of 100 µl of 0.5 mg/ml MTT reagent, a popular metabolic dye, for 1 h. The supernatants were removed from the wells, and 100 µl DMSO was then added on the reduced MTT dye. The final absorbency was measured using a plate reader at 570 nm, and the value was normalized to that of control. Western blotting analysis was performed on the SKOV3 cell lysates treated with the anticancer agent (compound D) or the cholesterol-conjugated anticancer agent (compound F).
2.5. Statistical analysis
The GraphPad Prism software (Graph Pad Software, Inc.) and Microsoft Excel (Microsoft Corporation) were used to analyze statistical and graphical data. IC50 values were normalized using nonlinear regression analysis. The experiments were conducted in quadruplicate and repeated at least three times.
3. Results
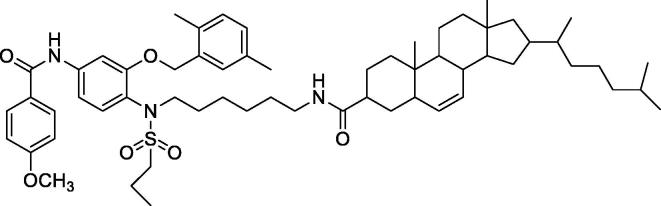
In the present study, an anticancer agent (compound D) and its cholesterol conjugate (compound F) were successfully prepared. The chemical structures of the synthesized anticancer agent D and its cholesterol conjugate (Compound F) were shown in Fig. 1A, Fig. 1B, respectively.
Fig. 1A.
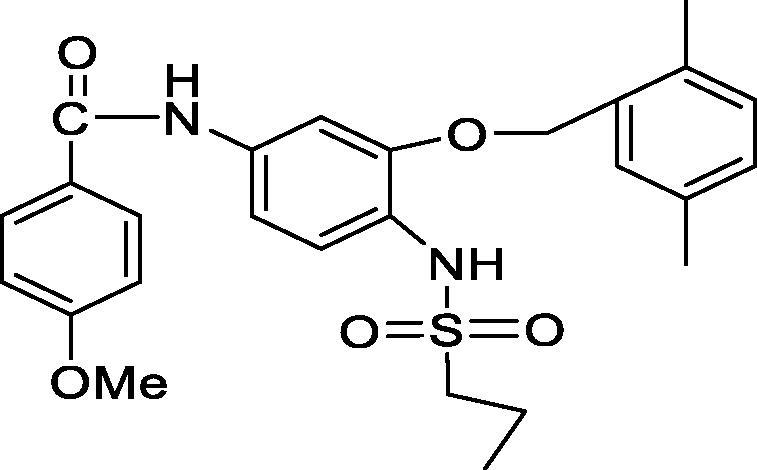
Chemical structure of Anticancer agent D.
Fig. 1B.
Chemical structure of Anticancer agent D conjugated to cholesterol.
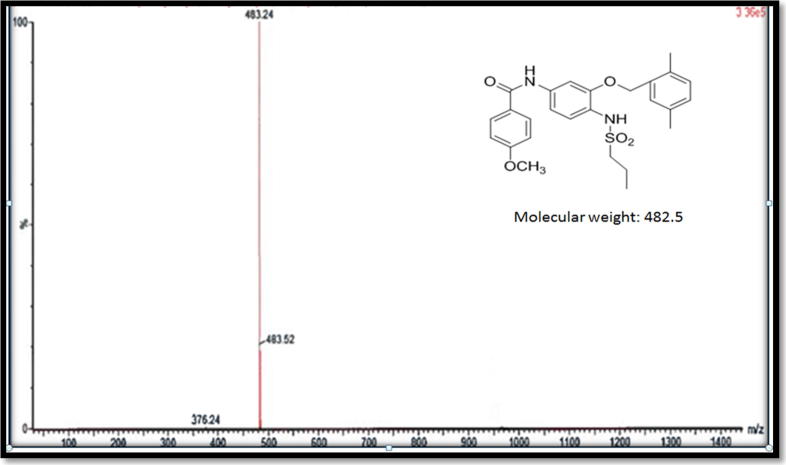
Compound D white solid, yield 2.12 g, 88%, DSC mp 183 °C; IR spectra (KBr, cm−1): 1141, 1225 (C—O), 1693 (C—O amide), 2938 (C—H aliphatic), 3180 (N—H amide). 1H NMR-600 MHz (DMSO‑d6, δ ppm): 0.8 (t, 3H, CH3), 1.6 (m, 2H, CH2), 2.3 (s, 3H, phenyl-CH3), 2.4 (s, 3H, phenyl-CH3) 3.0 (t, 2H, SO2—CH2—), 3.8 (s, 3H, CH3O—), 5.0 (s, 3H, ph-CH2—O—), 7.0–8.0 (m, 10H, aromatic Hs), 8.9 (s, 1H, NH). LCMS m/z (483, M+1).
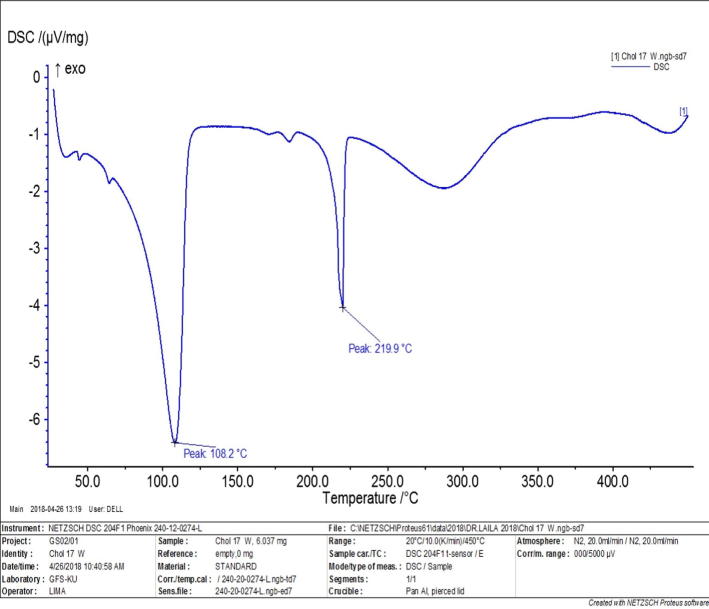
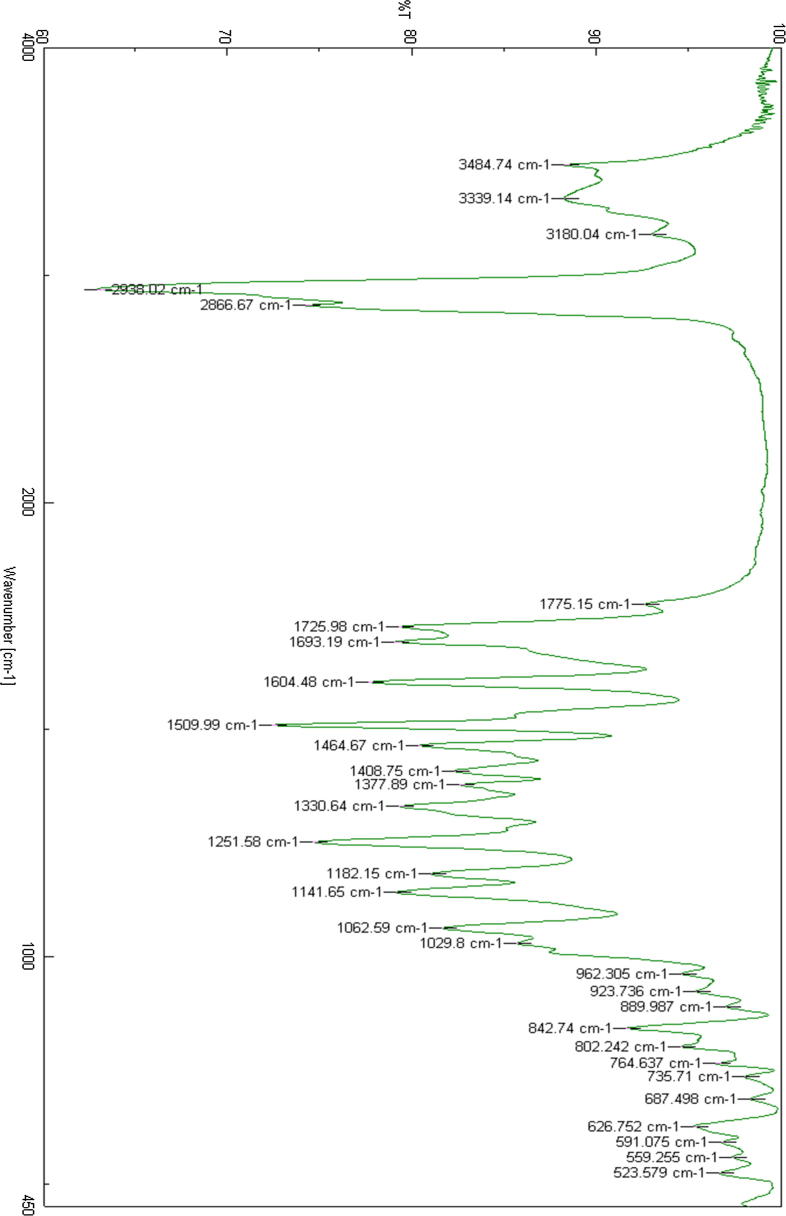
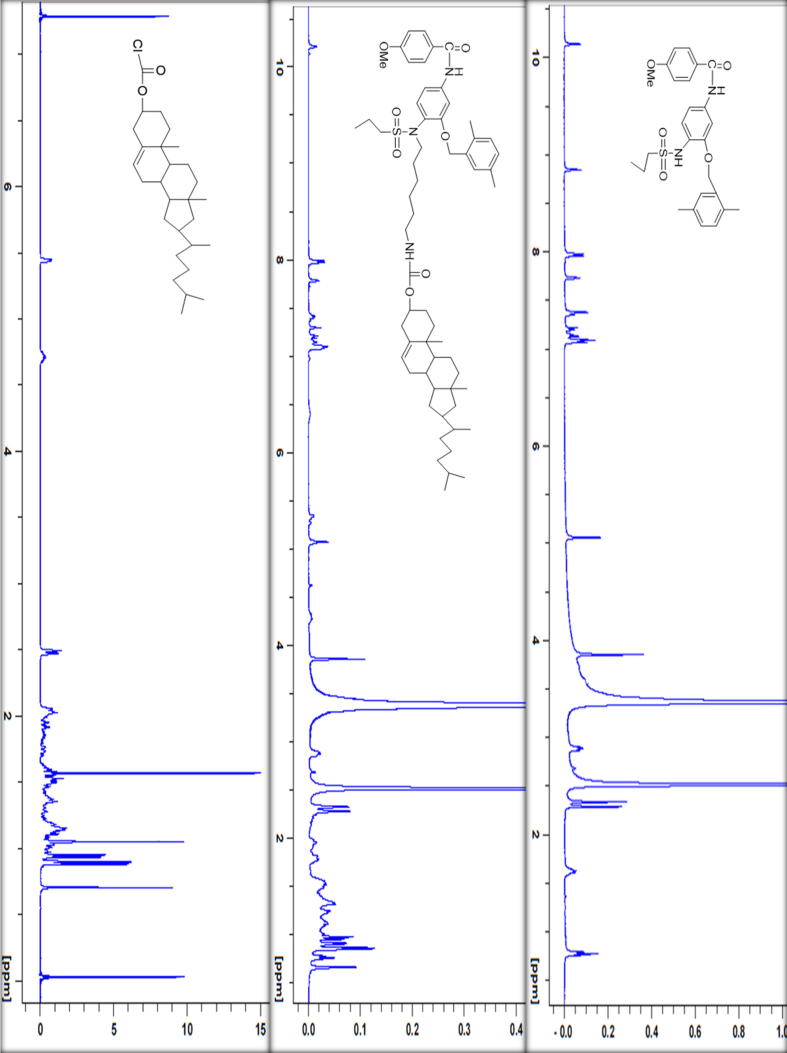
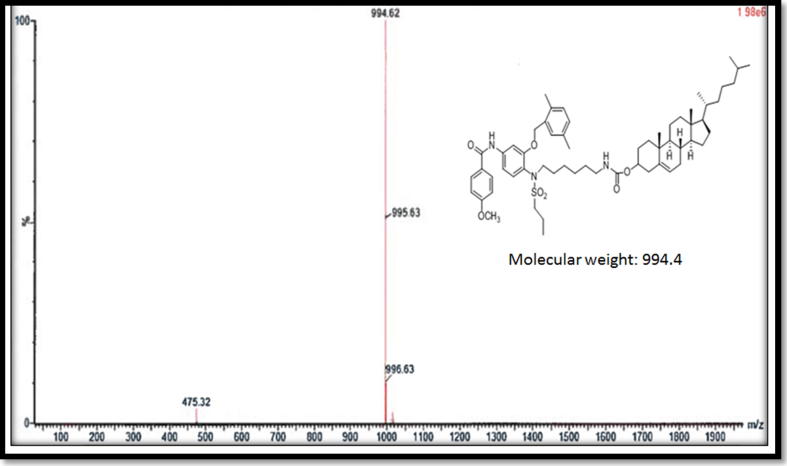
Compound F (cholesterol-conjugated) dual protein inhibitor was obtained as pale-yellow solid with 80% yield. Its melting point was 108.2 °C and 219 °C (Fig. 2). FTIR, vmax/cm−1 3484 (NH), 3339 (NH), 1725 (CO), 1693 (CO) (Fig. 3). This compound is pale yellow solid yield 3.975 g, 80%; DSC mp 108.2oC and 219.9 °C). 1H NMR-600 MHz (DMSO‑d6, δ ppm): 0.65–1.9 (m, 54H, cholesteryl-Hs, CH3CH2CH2SO2-, four CH2 hexyl), 2.3 (s, 3H, phenyl-CH3), 2.4 (s, 3H, phenyl-CH3) 2.9 (m, 6H, SO2—CH2—, two CH2-N), 3.8 (m, 4H, CH3O—, OCH cholesteryl), 5.0–5.5 (m, 4H, ph-CH2—O—, CCH cholesteryl), 7.0–8.0 (m, 10H, aromatic Hs). LCMS m/z (995.6, M+1). The 13C NMR spectra were as follows (150 MHz, CDCL3, 25 °C): δ165.6, 162.9, 156.6, 156.0, 149.9, 140.9, 139.8, 138.6, 135.8, 134.4, 133.8, 130.6, 130.4, 129.6, 129.2, 126.9, 124.0, 122.8, 122.4, 121.9, 114.2, 112.1, 105.0, 83.2, 74.9, 72.0, 69.1, 56.8, 56.3, 55.7, 54.2, 50.2, 42.5, 39.9, 39.7, 38.7, 38.5, 37.7, 37.4, 37.2, 36.9, 36.7, 36.3, 0.35.9, 32.0, 29.1, 28.4, 28.2, 27.5, 24.4, 24.0, 23.0, 22.7, 21.1, 19.5, 19.4, 18.9, 18.6, 17.1, 13.1, 12.0. HRMS [M]+ calcd for C60H87N3O7S: 994.41, found: 994.6. Fig. 4 shows the 1H NMR spectra for anticancer agent D, cholesterol-conjugated anticancer agent D, and pure cholesterol. As shown in Fig. 5A, Fig. 5B, respectively, LCMS/MS confirmed the molecular weight of compound D to be 483 m/z and that of cholesterol-conjugated anticancer compound F to be 995.6 m/z.
Fig. 2.
Melting point for cholesterol conjugated anticancer agent targeting dual proteins.
Fig. 3.
FTIR spectra for cholesterol conjugated anticancer agent D.
Fig. 4.
1H NMR spectra for anticancer agent D, cholesterol conjugated anticancer agent F, and pure cholesterol.
Fig. 5A.
LCMS/MS confirmed the molecular weight of the compound D at 483 m/z
Fig. 5B.
LCMS/MS confirmed the molecular weight of the compound conjugated to cholesterol at 995.6 m/z
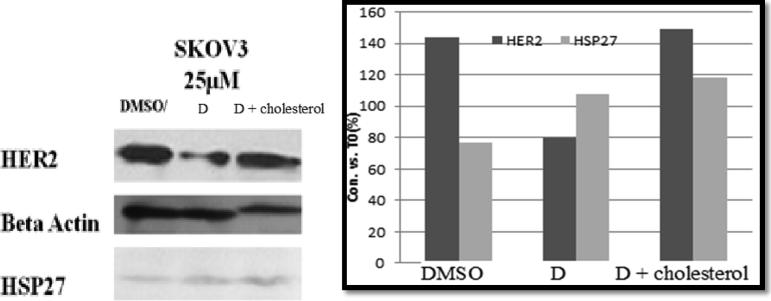
Western blotting analysis was performed on the lysates of SKOV3 cells treated with the anticancer agent (compound D) or cholesterol-conjugated anticancer agent (compound F). Western blotting results revealed that compound D had the ability to enter the cells and target the HSP27 function, thus reducing HER2 expression. However, compound F did not have the ability to target HSP27 and reduce HER2 expression. This result was expected and might have been attributed to the inability of compound F to pass through the cell membrane of SKOV3 cells (Fig. 6).
Fig. 6.
Westerm blot analysis of compound D and its cholesterol conjugated compound on HER2 and HSP27 expression The experiments were done quadruplicated and repeated at least three times; data were represented as Mean ± SD.
MTT assay was performed to evaluate the toxicity of the synthesized compounds to SKOV3 ovarian cancer cells. The current data showed that the free anticancer agent (compound D) at 25 µM inhibited cell growth, whereas cholesterol-conjugated compound D did not inhibit cancer cell growth (Fig. 7). These results were also expected because previous studies reported that cholesterol is always transported by LDL into cells, and that it cannot enter cells by itself. Table 1 shows the IC50 values of free compound D and cholesterol-conjugated compound D. Moreover, cholesterol-conjugated anticancer agent compound F showed no inhibitory activity on SKOV3 cell growth.
Fig. 7.
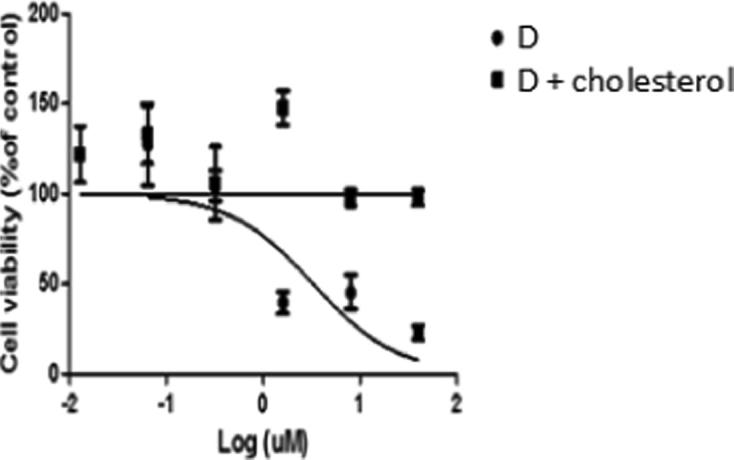
Anticancer agent D has the ability to reduce SKOV3 cell growth The experiments were done quadruplicated and repeated at least three times; data were represented as Mean ± SD.
Table 1.
The IC50 values for both anticancer agent D and its cholesterol conjugate. Anticancer agent D was able to inhibit SKOV3 cell growth while cholesterol conjugated anticancer agent D has no activity.
| Compounds | IC50 values to inhibit SKOV3 cell growth µM |
|---|---|
| Anticancer agent D | 0.07 ± 0.001 |
| Anticancer agent cholesterol conjugate | >100 |
The experiments were done quadruplicated and repeated at least three times; data were represented as Mean ± SD.
The particle size of plain LDL reached up to 78 nm, whereas that of LDL incorporating the cholesterol conjugate was 249 nm (Table 2). SKOV3 cells were treated with free cholesterol conjugate and LDL-encapsulated cholesterol conjugate, and the results revealed that the free agent did not inhibit cancer cell growth. Surprisingly, the cells treated with the LDL-encapsulated cholesterol-conjugated inhibitor showed inhibited growth. This finding indicated that LDL were endocytosed into the cells through LDL-r, which was overexpressed by the ovarian cancer cells (Table 3).
Table 2.
LDL particle size was measured using DLS technique in PBS solution.
| Particles | Diameter of sample |
|---|---|
| LDL | 78.10 ± 0.65 |
| LDL with the drug | 249.6 ± 12.5 |
The experiments were done quadruplicated and repeated at least three times, data were represented as Mean ± SD
Table 3.
SKOV3 cell growth inhibition data tested with LDL encapsulated cholesterol conjugated anticancer agent D.
| Compound | IC50 values to inhibit SKOV3 cell growth at 25 µM |
|---|---|
| Anticancer agent D + cholesterol | >100 |
| LDL + anticancer D + cholesterol | 22.5 ± 6.6 |
The experiments were done quadruplicated and repeated at least three times, data were represented as Mean ± SD
4. Discussion
Previous studies reported that cholesterol conjugates of chemotherapeutic agents show enhanced drug uptake by tumor cells and pronounced cytotoxic activity, compared to free anticancer agents (Harisa and Alanazi, 2014). Such conjugates can be transferred into LDL by mimicking of the native components of LDL. The LDL-encapsulated chemotherapeutic conjugates are taken up by LDL-r, which is upregulated on tumor cells (Yamamoto et al., 2017, Shi et al., 2017). The nanostructure feature, biodegradability, and biocompatibility of LDL inhibit their removal by the reticulo-endothelial system, compared to synthetic nanoparticles (Yamamoto et al., 2017, Shi et al., 2017).
Therefore, we aimed to design, LDL-encapsulated cholesterol-conjugated anticancer agents with dual protein-inhibitory capability against HSP27 and HER2. First, the anticancer agent was synthesized and the conjugated with cholesterol. Second, the cholesterol-conjugated anticancer agent was encapsulated by LDL as delivery vehicles targeting ovarian cancer cells to achieve improved cellular uptake.
In the present study, an anticancer agent and its cholesterol conjugate were successfully prepared, and their physical characteristics (color, shape, and melting point) were determined. Furthermore, their chemical characteristics, including 1H NMR and 13C NMR spectra, were determined by FTIR and LCMS/MS. Compound D was white solid with 88% yield while, compound F was pale yellow solid with 80% yield. 1H NMR and 13C NMR peaks of both compounds were confirmed from the spectrum. Compound F showed two melting points peaks at 108.2 °C and 219.9 °C. On the other hand, compound D showed one peak at 183°CAlso, LCMS data confirmed the molecular weight for compounds D was expected to see a peak at 482 m/z and we got a peak in the positive mode and plus proton at 483 m/z (Fig. 5A). Also, the molecular weight of cholesterol conjugated (compound F) was confirmed, we expected to see a peak at 994 m/z and plus proton in the positive mode we got that peak 995.6 m/z (Fig. 5B). FTIR spectra showed the NH and CO peaks (Fig. 3). The present data are concurrent with those of several published studies on cholesterol-conjugated anticancer agents as selective targeted anticancer agents (Grummer and Carroll, 1988, Alanazi et al., 2003, Radwan and Alanazi, 2014a, Radwan and Alanazi, 2014b). Cholesterol-conjugated anticancer agents represent prodrugs that can enhance the delivery of anticancer drugs to cancer cells; for example, cholesterol-conjugated 5-fluorouracil effectively targets and exerts its cytotoxic effect to cancer cells (Radwan and Alanazi, 2014a). Similarly, cholesterol-conjugated platinum compounds showed enhanced antitumor efficacy with selective targetability.
The dual protein-inhibitory effect of the prepared anticancer agents was examined using western blotting analysis. The results indicated that anticancer agent D entered cancer cells and targeted the HSP27 function, thus reducing HER2 expression. Likewise, many studies have shown that reduction of the HSP27 activity enhances selective killing of cancer cells (Lazarev et al., 2018). Moreover, dual inhibition on Hsp27 and tubulin increases the anticancer effect of chemotherapy and decreases tumor drug resistance (Zhong et al., 2013, Jaragh-Alhadad, 2018). In contrast, cholesterol-conjugated compound F could not target HSP27 and HER2 expression, which might be attributed to its inability to pass the cell membrane. Conversely, it has been reported that cholesterol-conjugated chemotherapy may increase drug cellular import and ovarian cancer targetability while improving drug safety (Stanislav et al., 2005, Huaimin et al., 2016).
Our results also confirmed that anticancer agent D exerted cytotoxic effect, inhibiting cancer cell growth, whereas cholesterol-conjugated anticancer compound F did not inhibit cancer cell growth. These results were expected because according to previous studies, encapsulation of anticancer agent-cholesterol conjugates into LDL is favorable to improve their passage through the plasma membrane via the LDL-r pathway (Gong et al., 2017, Idippily et al., 2017, Harisa and Alanazi, 2014). On the contrary, the cells treated with LDL-encapsulated cholesterol-conjugated compound showed inhibited growth, indicating that LDL passed the cell through the LDL-r, which is overexpressed on ovarian cancer cell membrane (Grummer and Carroll, 1988). These results are consistent with those of several studies. Gong et al., showed that drug encapsulation into LDL can be exploited as a novel approach for cancer chemotherapy (Gong et al., 2017, Harisa and Alanazi, 2014). Similarly, drug delivery shuttles that can imitate LDL are proposed as delivery systems for anticancer drugs with selective tumor cell targetability through an LDL-mediated pathway (Emami et al., 2012, Andalib et al., 2012, Alanazi et al., 2015). Taken together, cholesterol-conjugated anticancer agents were considered a potential strategy to realize selective tumor targeting (Huntosova et al., 2012, Radwan and Alanazi, 2014a, Radwan and Alanazi, 2014b).
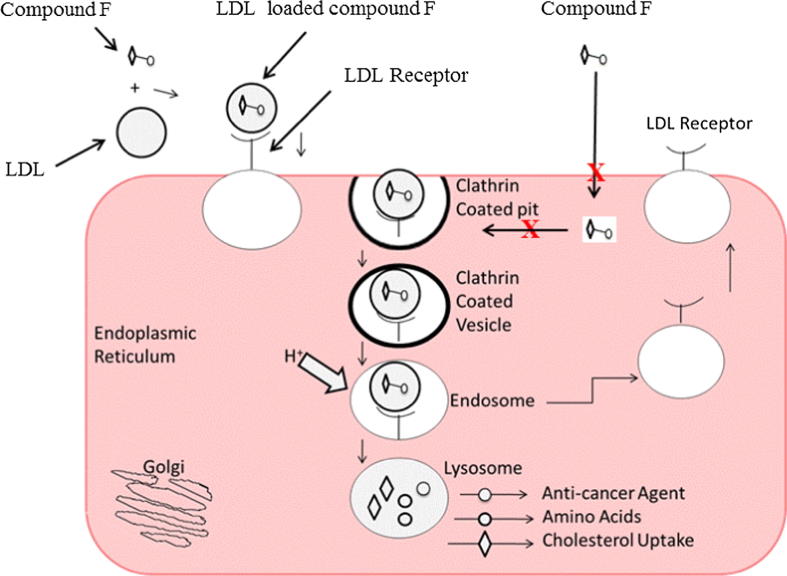
Such cholesterol-conjugated drugs may be effectively transported into LDL in vivo, and then taken up by the LDL-r overexpressed on tumor cells. In the intracellular environment, cholesterol will be removed, allowing the anticancer drug to be freed by lysosomal enzymes and then exert their therapeutic effect (Harisa and Alanazi, 2014, Idippily et al., 2017) (Fig. 8). This strategy may increase the selectively of chemotherapeutical agents for cancer tissues over normal tissues, which express LDL-r at a relatively lower level than that by tumor cells (Idippily et al., 2017). Thus, tumor targeting effect of cholesterol-conjugated anticancer drugs can be enhanced by using LDL as delivery shuttles with potential cytotoxic effect.
Fig. 8.
Binding hypothesis of LDL-r overexpressed on ovarian cancer cell with cholesterol conjugate loaded LDL binding. Up on entry into the cell then the lysosomes degrade the components of LDL and liberate the drug from the cholesterol into the intracellular milieu, however unloaded compound F not degraded by the lysosome.
5. Conclusion
In conclusion, in the present study, an anticancer agent and its cholesterol conjugate were successfully prepared and characterized for physical and chemical properties. Anticancer agent D showed the ability to enter cancer cells and target the HSP27 function, thereby reducing HER2 expression. Cholesterol-conjugated anticancer agent F had the potency to inhibit the growth of SKOV cells after encapsulation into LDL. Herein, the encapsulation of cholesterol-conjugated anticancer agent into LDL may be a promising strategy to target ovarian cancer cells. Quantitative evaluation of the encapsulation efficacy of the conjugate into LDL is still ongoing.
Acknowledgments
Acknowledgements
This work was supported by the grant (ZS06/17). Research facilities are provided by the Chemistry and Biology Departments at Kuwait University, Kuwait (instrument project No: GS01/03, GS01/05, and GS02/01). The authors also extend their appreciation to the Vice Research Chairs at King Saud University, Saudi Arabia for funding this work through the Kayyali Chair for Pharmaceutical Industry, Department of Pharmaceutics, College of Pharmacy, King Saud University.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alanazi F., Halpern D.S., Lu D.R. Development of cholesterol-based conjugates for targeted drug delivery. STP Pharma. Sci. 2003;13:27–35. [Google Scholar]
- Alanazi F., Haq N., Radwan A. Formulation and evaluation of cholesterol-rich nanoemulsion (LDE) for drug delivery potential of cholesteryl-maleoyl-5-fluorouracil. Pharm. Dev. Technol. 2015;20:266–270. doi: 10.3109/10837450.2013.860551. [DOI] [PubMed] [Google Scholar]
- Andalib S., Varshosaz J., Hassanzadeh F. Optimization of LDL targeted nanostructured lipid carriers of 5-FU by a full factorial design. Adv. Biomed. Res. 2012;1:45. doi: 10.4103/2277-9175.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayako K., Yutaka U., Tetsuji N. Therapeutic strategies in epithelial ovarian cancer. J. Exp. Clin. Cancer Res. 2012;31:14. doi: 10.1186/1756-9966-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo N., Peiretti M., Parma G. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;(Supplement 5):v23–v30. doi: 10.1093/annonc/mdq244. [DOI] [PubMed] [Google Scholar]
- Emami J., Rezazadeh M., Varshosaz J. Formulation of LDL targeted nanostructured lipid carriers loaded with paclitaxel: a Detailed study of preparation, freeze drying condition, and in vitro cytotoxicity. J. Nanomater. 2012:1–10. [Google Scholar]
- Garrido C., Brunet M., Didelot C. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Gong Y., Yin J.Y., Tong B.D. Low density lipoprotein – rosiglitazone – chitosancalcium alginate/nanoparticles inhibition of human tenon's fibroblasts activation and proliferation. Oncotarget. 2017;8:105126–105136. doi: 10.18632/oncotarget.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummer R.R., Carroll D.J. A review of lipoprotein cholesterol metabolism: importance to ovarian function. J. Animal Sci. 1988;66:3160–3173. doi: 10.2527/jas1988.66123160x. [DOI] [PubMed] [Google Scholar]
- Haley D.A., Bova M.P., Huang Q.L. Small heat-shock protein structures reveal a continuum from symmetric to variable assemblies. J. Mol. Biol. 2000;298:261–272. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- Harisa G., Alanazi F. Low density lipoprotein bionanoparticles: From cholesterol transport to delivery of anti-cancer drugs. Saudi Pharm. J. 2014;22:504–515. doi: 10.1016/j.jsps.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaimin W., Zhaoqianqi F., Dongdong W. Enzyme-regulated supramolecular assemblies of cholesterol conjugates against drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2016;138:10758–10761. doi: 10.1021/jacs.6b06075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntosova V., Buzova D., Petrovajova D. Development of a new LDL-based transport system for hydrophobic/amphiphilic drug delivery to cancer cells. Int. J. Pharm. 2012;436:463–467. doi: 10.1016/j.ijpharm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Ian R., Gang Z. Mimicking nature's nanocarrier: Synthetic low-density lipoprotein-like nanoparticles for cancer-drug delivery. Nanomedicine. 2007;2:375–380. doi: 10.2217/17435889.2.3.375. [DOI] [PubMed] [Google Scholar]
- Idippily N.D., Gan C., Orefice P. Synthesis of vorinostat and cholesterol conjugate to enhance the cancer cell uptake selectivity. Bioorg. Med. Chem. Lett. 2017;27:816–820. doi: 10.1016/j.bmcl.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Jaragh-Alhadad L. In-vitro evaluation of HSP27 inhibitors function through HER2 pathway for ovarian cancer therapy. Transl. Cancer Res. 2018;7:1510–1517. [Google Scholar]
- Martin K., Theo J.C. Native and modified lipoproteins as drug delivery systems. Adv. Drug Deliv. Rev. 1990;5:231–251. [Google Scholar]
- Lazarev F., Sverchinsky D., Mikhaylova E. Sensitizing tumor cells to conventional drugs: HSP70 chaperone inhibitors, their selection and application in cancer models. Cell Death Dis. 2018;18:41. doi: 10.1038/s41419-017-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K., Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil. Steril. 2010;93:762–768. doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Radwan A., Alanazi F. Design and synthesis of new cholesterol-conjugated 5-fluorouracil: a novel potential delivery system for cancer treatment. Molecules. 2014;19:13177–13187. doi: 10.3390/molecules190913177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan A., Alanazi F. Targeting cancer using cholesterol conjugates. Saudi Pharm. J. 2014;22:3–16. doi: 10.1016/j.jsps.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K., Xue J., Fang Y. Inorganic kernel-reconstituted lipoprotein biomimetic nanovehicles enable efficient targeting “Trojan Horse” delivery of STAT3-Decoy oligonucleotide for overcoming TRAIL resistance. Theranostics. 2017;7:4480–4497. doi: 10.7150/thno.21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislav J., Jin C., Larisa V. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg. Med. Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Takada T., Yamanashi Y. VLDL/LDL acts as a drug carrier and regulates the transport and metabolism of drugs in the body. Sci. Rep. 2017;7:633–639. doi: 10.1038/s41598-017-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunfei C., Hughes-F Millie. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int. J. Cancer. 2001;91:41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Zhong B., Chennamaneni S., Lama R. Synthesis and anticancer mechanism investigation of dual Hsp27 and tubulin inhibitors. J. Med. Chem. 2013;11:5306–5320. doi: 10.1021/jm4004736. [DOI] [PMC free article] [PubMed] [Google Scholar]