Abstract
Aim:
Digital ELISA-based assays for blood biomarkers of neurological disease are on the verge of clinical use. Here, we aimed to determine whether different preanalytical blood processing techniques influence results.
Materials & methods:
Concentrations of neurofilament light chain (NfL), Tau and amyloid beta (Aβ) were measured in human plasma and serum specimens using digital ELISA and compared between blood products. Measured levels of NfL were highly equivalant between serum and plasma in all analyses, however, measured levels of Tau and Aβ were consistently lower in serum relative to plasma.
Conclusion:
Tau and Aβ are likely lost during clotting in serum preparations, and should be assayed in plasma to get an accurate measure of circulating levels.
Keywords: : brain injury, methods, neurodegeneration, NFL, SiMoA, single molecule array, techniques
Due to recent technological advances in proteomic detection, blood-based biomarker assays with utility for diagnosis and monitoring of neurological conditions are on the verge of clinical use. The proteomic composition of the brain is highly unique relative to other organs, and damage to neural tissue results in the release of brain-specific proteins into peripheral circulation. Thus, the detection of these proteins in the blood can serve as biomarkers of acute and chronic neural pathology. For example, circulating levels of brain-specific proteins have been shown to be elevated in a wide variety of acute and chronic neural conditions including traumatic brain injury [1], stroke [2], multiple sclerosis [3], HIV-related neurodegeneration [4], dementia [5] and Alzheimer's disease [6]. Unfortunately, these proteins are typically only present in circulation at low-picogram to subpicogram concentrations, and the lower limits of detection associated with conventional ELISA techniques traditionally used to assay blood are not robust enough to detect them at early enough time-points in most pathologies to make them clinically informative.
However, recent advances in proteomic techniques are allowing for detection of these markers at low enough concentrations to allow for the development of assays with true clinical utility. Digital ELISA is a recently developed immunoassay methodology which allows for femtogram-level detection of protein analytes in biofluids. First, antibody conjugated paramagnetic beads are used to capture single molecules of target protein, and protein–bead complexes are labeled with fluorophore-conjugated detection antibody. Beads are then assessed for the presence or absence of target protein using a precision-fabricated microwell array capable of capturing one bead per well. This technique has been shown to be as much as 1000-times more sensitive than traditional ELISA methods [7], and is now being rapidly adopted for measurement of neuro biomarkers blood. For example, digital ELISA has now been successfully implemented to detect blood biomarkers of neural damage in individuals with subclinical concussion [8], Alzheimer's disease in individuals prior to onset of symptoms [9], and in relapsing-remitting multiple sclerosis patients prior to development of new lesions [10]. As a result, there has been a rapid push for the development of digital ELISA-based clinical assays targeting brain biomarkers, and there now tests which are close to consideration by regulatory bodies for in vitro diagnostic use.
As these tests become closer to clinical use, it is important to understand the preanalytical factors which need to be considered for constant and reliable measurements. In particular, blood preparation methods can affect the results of a wide range of clinical tests; most notably, assayed levels of many biomarkers can differ between serum and plasma, simply as a result of true differences in analyte concentration between the two blood products, or due to interference from the anticoagulants used to prepare plasma [11]. It is currently unknown whether blood processing methods affect the levels of neuro biomarkers measured with digital ELISA. Here, we measured the levels of three commonly tested neuro biomarkers in both serum and plasma using digital ELISA to determine if different blood processing techniques influence results.
Materials & methods
Experimental design
Parallelly-drawn paired plasma and serum specimens were obtained from a small group of heathy human donors (n = 8), and the concentrations of neurofilament light chain (NfL), Tau and amyloid beta (Aβ) were measured using digital ELISA; measured concentrations were subsequently compared between the two different blood products. In order to confirm our results, digital ELISA was used to measure the levels of the same biomarkers in a larger but unpaired pool of serum (n = 85) and plasma (n = 47) samples collected from donors with a variety of health statuses.
Subjects
Blood donors of varying health statuses were recruited as part of various different clinical investigations at Case Western Reserve University (OH, USA), University Hospitals Cleveland Medical Center (OH, USA), and West Virginia University (WV, USA). Demographic information was collected from either the subject or significant other by a trained clinician. All procedures were approved by the institutional review boards of University Hospitals (IRB protocol #20181112) and West Virginia University (IRB protocol #1410450461R001). Written informed consent was obtained from all subjects or their authorized representatives prior to any study procedures.
Blood collection & processing
Blood for serum specimens was drawn via venipuncture and collected in clot-activating serum separator tubes (Becton Dickinson, NJ, USA). Blood was allowed to clot for 30 min at room temperature, and spun at 2500 × g for 10 min to separate serum. Resultant serum was aliquoted and stored at -80 °C until analysis. Blood for plasma specimens was drawn via venipuncture and collected in either spray coated K2ETDA or lithium heparin tubes (Becton Dickinson). Blood was spun at 2500 × g for 10 min to separate plasma. Resultant plasma was aliquoted and stored at -80 °C until analysis.
Digital ELISA
Levels of NfL, Tau and Aβ in serum and plasma specimens were measured via the Quanterix HD-1 Single Molecule Array (SiMoA) platform at the Quanterix Accelerator Laboratory (Quanterix Corporation, MA, USA) by a technician blinded to specimen information. NfL was measured via a single-plex assay kit targeting total NfL (Quanterix catalog# 103186), while Tau and Aβ were measured via a multiplex assay kit (Quanterix catalog #101995) targeting total Tau and the 40 amino acid isoform of Aβ specifically. All assays were performed using manufacture recommended protocols. Standard curves were generated via triplicate measurements of recombinant proteins (Quanterix catalog #102255, #103221) and all specimens were assayed in duplicate. Intra-assay coefficient of variation (CV) values were calculated from technical replicate measures of specimens assayed within a single run. Interassay CV values were calculated from technical replicate measures of high- and low-range control specimens (Quanterix catalog #102256, #102257, #103222, #103223) assayed across multiple runs.
Statistics
Statistics were performed via R version 2.14. T-test or analysis of covariance (ANCOVA) was used for comparison of continuous variables where appropriate. Strength of correlational relationships were assessed via Spearman's ρ. The null hypothesis was rejected when p < 0.05. The parameters of all statistical tests performed are outlined in detail within the figure legends.
Results
Levels of neuro biomarkers in paired serum & plasma specimens
Demographic characteristics of healthy donors are indicated in Table 1. Healthy donors included three males and five females, with an average age of 50.0 ± 4.0. All plasma specimens were anticoagulated with K2EDTA.
Table 1. . Demographic characteristics of paired serum and plasma specimens donors.
| Demographic | Healthy donors (n = 8) |
|---|---|
| Age; mean ± standard deviation | 50 ± 4.0 |
| Female; n (%) | 5 (62.5) |
| Male; n (%) | 3 (37.5) |
| African–American; n (%) | 8 (100) |
| Caucasian; n (%) | 0 (0.0) |
Measured biomarker levels for all technical replicates for all specimens used in the paired analysis fell within the quantitative ranges for each assay. Intra-assay CVs for NfL, Tau and Aβ measurements in plasma were 3.5, 9.3 and 2.6% respectively, while intra-assay CVs for NfL, Tau and Aβ measurements in serum were 4.3, 9.8 and 2.7%, respectively.
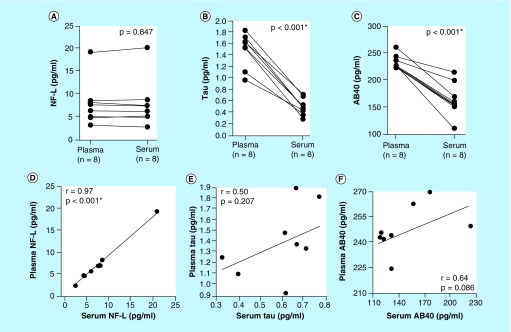
Measured levels of NfL were highly consistent between serum and plasma; we observed a negligible 5.4 ± 4.1% average percent difference in measured levels between paired specimens, which was not statistically significant (p = 0.847). Furthermore, measured NfL levels in serum and plasma exhibited a linear relationship and were highly correlated (r = 0.97; Figure 1A & E). Conversely, measured levels of Tau were on average 65.7 ± 8.7% lower in serum specimens compared with paired plasma specimens. This difference was highly statistically significant (p < 0.001), and levels were positively correlated (r = 0.50; Figure 1B & F). Similar to Tau, measured Aβ levels were on average 29.5 ± 12.1% lower in serum specimens versus paired plasma specimens. This difference was also highly statistically significant (p < 0.001), and levels were again positively correlated (r = 0.64; Figure 1C & G).
Figure 1. . Measured levels of neuro biomarkers in paired serum and plasma specimens.
(A–C) Measured levels of NfL, Tau and Aβ in parallelly-drawn paired serum and plasma specimens. Lines connect paired specimens. Means were compared via two-tailed paired t-test. (D–F) Correlation of measured levels of NfL, Tau and Aβ between serum and plasma. Strength of correlations were tested via Spearman's ρ.
NfL: Neurofilament light chain.
Levels of neuro biomarkers in unpaired serum & plasma specimens
Clinical and demographic characteristics of donors associated with serum and plasma specimens are indicated in Table 2. Donors associated with serum specimens were of similar age to those associated with plasma specimens, and exhibited similar gender and ethnic demographics. A total of 47% of plasma specimens were anticoagulated with K2EDTA, and the remaining 53% with lithium heparin.
Table 2. . Clinical and demographic characteristics of unpaired serum and plasma specimens donors.
| Characteristic | Plasma donors (n = 47) | Serum donors (n = 85) |
|---|---|---|
| Age; mean ± standard deviation | 54.9 ± 13.3 | 50.8 ± 15.3 |
| Female; n (%) | 23 (48.9) | 57 (67.1) |
| Male; n (%) | 24 (51.1) | 28 (32.9) |
| African–American; n (%) | 32 (68.1) | 52 (61.2) |
| Caucasian; n (%) | 15 (31.9) | 33 (38.8) |
| Healthy; n (%) | 0 (0.0) | 26 (30.6) |
| Cardiovascular disease; n (%) | 22 (46.8) | 49 (57.6) |
| HIV positive; n (%) | 25 (53.2) | 3 (3.5) |
| Acute brain injury; n (%) | 14 (29.8) | 13 (15.3) |
Measured neuro biomarker levels for all technical replicates for all specimens in the unpaired analysis fell within the quantitative ranges for each assay, and interassay CVs were all less than 15%. Intra-assay CVs for NfL, Tau and Aβ measurements in plasma were 4.6, 9.1 and 9.5%, respectively, while intra-assay CVs for NfL, Tau and Aβ measurements in serum were 4.7, 11.4 and 2.7%, respectively.
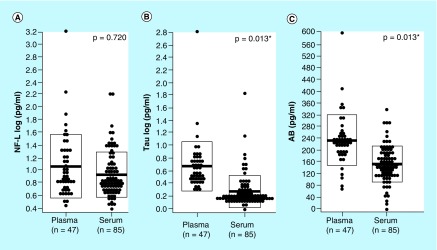
Mean levels of NfL were 19.1% lower in serum relative to plasma, however, constant with our analysis of paired specimens, this difference was not statistically significant after controlling for donor health status and the type of anticoagulant used in plasma preparation (p = 0.720; Figure 2A). Mean levels of Tau and Aβ were respectively 54.1 and 33.9% lower in serum specimens relative to plasma specimens. These differences were both statistically significant while controlling for donor health status and the type of anticoagulant used in plasma preparation (p = 0.013, p = 0.013; Figure 2B & C).
Figure 2. . Measured levels of neuro biomarkers in unpaired serum and plasma specimens.
(A–C) Measured levels of NfL, Tau and Aβ in unpaired serum and plasma specimens. Means were compared via one-way ANCOVA controlling for donor health status and type of anticoagulant used in plasma preparation. NfL and Tau values were log transformed prior to comparison.
ANCOVA: Analysis of covariance; NfL: Neurofilament light chain; Aβ: Amyloid beta.
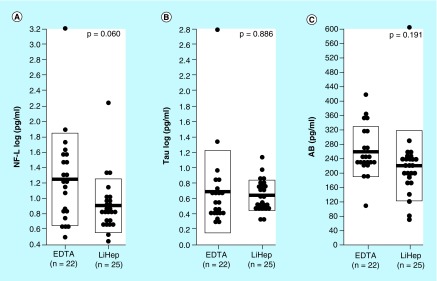
Mean levels of NfL, Tau and Aβ were respectively 32.9, 2.2 and 14.6% lower in plasma prepared using lithium heparin versus K2EDTA (Figure 3B). However, none these differences were statistically significant after controlling for donor health status (p = 0.060, p = 0.886, p = 0.191; Figure 3A–C).
Figure 3. . Measured levels of neuro biomarkers in plasma specimens anticoagulated with EDTA and lithium heparin.
(A–C) Measured levels of NfL, Tau and Aβ in plasma specimens anticoagulated with either EDTA or lithium heparin. Means were compared via one-way ANCOVA controlling for donor health status. NfL and Tau values were log transformed prior to comparison.
Aβ: Amyloid beta; ANCOVA: Analysis of covariance; LiHep: Lithium heparin; NfL: Neurofilament light chain.
Discussion
The aim of this work was to better understand what preanalytical factors may affect the levels of commonly-tested neuro biomarkers measured via digital ELISA; specifically, we wanted to determine whether the use of serum or plasma specimens influences results.
We observed negligible differences in measured NfL levels between serum and plasma in both our analyses of paired and unpaired specimens, suggesting that the use of serum or plasma has little effect on levels of NfL measured with digital ELISA. This is encouraging, as it suggests that different blood processing protocols can produce consistent results when measuring NfL in the clinical setting, and that retrospective research studies using archived specimens can likely do pooled analysis using a mix of different blood products without having to be concerned about confounds. Conversely, we observed up to a 65% difference in measured Tau and Aβ levels between blood products in some instances, suggesting that levels of these markers are not directly comparable between serum and plasma, and that consistent blood processing protocols need to be implemented to produce reliable results.
In terms of the mechanism which drove the observed differences in measured Tau and Aβ levels between serum and plasma, we believe it is a result of true differences in their concentrations in the blood products, and not due to a falsely-high signal caused by the presence of anticoagulants in plasma. It is unlikely that the difference was driven by an anticoagulant interfering with assay chemistry for two reasons, first, we did not observe a similar effect when measuring NfL using the same platform, and second, the effect was observed independently of the type of anticoagulant used in plasma preparation. Due to their propensity to aggregate, it is most probable that Tau and Aβ become trapped in the fibrin-platelet matrix during clotting, which would account for the lower levels which we observed in serum. Interestingly, the degree of correlation between serum and plasma levels with respect to both markers was relatively weak, suggesting that there is a high degree of interindividual variability regarding the degree to which they are lost during serum preparation. Such variability could be due differences in the levels of various clotting components and overall clotting kinetics between individuals. Thus, our collective results suggest that measurement of Tau and Aβ in plasma is likely to give a better indication of true circulating levels.
It is important to note that we only analyzed plasma prepared using K2EDTA and lithium heparin. While these are two of the most commonly used anticoagulants used to prepare plasma for immunoassays, there are other anticoagulants which are used clinically such as sodium citrate and potassium oxalate. It is well known that anticoagulants can cause interference in immunoassays, and that such interference is highly dependent on the type of anticoagulant, analyte and assay [12]. While we did not notice any dramatic or statistically significant differences in measured neuro biomarker levels between plasma prepared using K2EDTA and lithium heparin, future work should more thoroughly examine whether the use of different anticoagulants affects results. Such work will be essential in developing the standardized blood processing protocols which are so clearly needed for reliable measurement of these markers.
It is also important to note that we only reported on three commonly measured neuro biomarkers, and that our results do not extend to others not directly assessed here. For example, we specifically assayed the 40 amino acid isoform of Aβ, as is the most biologically prevalent in humans [13]. However, there are other isoforms of Aβ, such as 38 and 42 amino acid variants, that are produced in some neurodegenerative conditions [14]; these other isoforms may not exhibit similar differences in concentration between serum and plasma as the 40 amino acid isoform. However, our results clearly indicate that preliminary experiments assessing the possible effects of different blood processing methods on measured levels of these other isoforms would have be completed in order to justify any type of combined analysis using a mix of different blood products.
Collectively, our results clearly indicate that different blood preparation methods can affect the levels of neuro biomarkers assayed by digital ELISA, and highlight the importance of considering what preanalytical factors need to be standardized in order to produce reliable measures. With respect to the specific markers which we assessed here, our findings suggest that serum and plasma levels of NfL are likely directly comparable, however, measurements of Tau and Aβ are not. Tau and Aβ are likely lost during clotting, and should be assayed in plasma to get an accurate measure of circulating levels.
Future perspective
Within the next decade, we fully expect digital ELISA-based blood assays measuring neuro biomarkers to be in clinical use for the diagnosis and monitoring of neurological damage. The findings reported here clearly indicate that the use of serum versus plasma can produce dramatically different measures, and that standardized blood processing methodologies need to be established in order to produce accurate and consistent results. Future studies should more thoroughly examine whether the type of anticoagulant used in plasma preparation influences the levels of these biomarkers. This work is essential if these assays are going to make the jump from the research environment to the clinic.
Summary points.
Background
Digital ELISA-based assays for blood biomarkers with utility for diagnosis and monitoring of neurological conditions are on the verge of clinical use.
It is important to understand the preanalytical factors which need to be considered for reliable measurements.
Here, we measured the levels of three commonly tested neuro biomarkers in both serum and plasma using digital ELISA to determine if different blood processing techniques influence results.
Methods
Parallelly-drawn paired plasma and serum specimens were obtained from a small group of heathy human donors (= 8), and the concentrations of neurofilament light chain (NfL), Tau and amyloid beta (Aβ) were measured using digital ELISA and compared.
Levels of the same biomarkers were also measured in a larger but unpaired pool of serum (= 85) and plasma specimens (= 47) collected from donors with a variety of health statuses to confirm results.
Results
Measured levels of NfL were highly consistent between blood products in both analyses.
Measured levels of Tau and Aβ were up to 65% lower in serum specimens relative to plasma specimens.
Conclusion
Serum and plasma levels of NfL measured with digital ELISA are likely directly comparable, however, measurements of Tau and Aβ are not.
Tau and Aβ are likely lost during clotting, and should be assayed in plasma to get an accurate measure of circulating levels.
Acknowledgments
The authors would like to thank the Chantler lab at West Virginia University for providing access to acute brain injury and healthy donor blood samples, as well as the SMART Center in the FPB School of Nursing at Case Western Reserve University for critical review of the manuscript and general research support.
Footnotes
Financial & competing interests disclosure
Research reported in this publication was supported by the National Institute of Nursing Research and the National Institute on Aging of the National Institutes of Health under Award Number P30NR015326 issued to SM Moore. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Case Western Reserve University FPB School of Nursing start-up funds issued to GC O'Connell. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Shahim P, Gren M, Liman V. et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 6(1), 36791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiedt S, Duering M, Barro C. et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology 91(14), e1338–e1347 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Disanto G, Barro C, Benkert P. et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis: serum NfL as a biomarker in MS. Ann. Neurol. 81(6), 857–870 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gisslén M, Price RW, Andreasson U. et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 3, 135–140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Aβ1-40 and Aβ1-42 and the risk of dementia: a prospective case–cohort study. Lancet Neurol. 5(8), 655–660 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Nakamura A, Kaneko N, Villemagne VL. et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature 554(7691), 249–254 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Rissin DM, Kan CW, Campbell TG. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28(6), 595–599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the development and application of digital ELISA.
- 8.Joseph JR, Swallow JS, Willsey K. et al. Elevated markers of brain injury as a result of clinically asymptomatic high-acceleration head impacts in high-school football athletes. J. Neurosurg. 130(5), 1642–1648 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Dominantly Inherited Alzheimer Network; Preische O, Schultz SA. et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat. Med. 25(2), 277–283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports use of digital ELISA to detect early Alzheimer's disease pathology.
- 10.Kuhle J, Kropshofer H, Haering DA. et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 92(10), e1007–e1015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barelli S, Crettaz D, Thadikkaran L, Rubin O, Tissot J-D. Plasma/serum proteomics: pre-analytical issues. Expert Rev. Proteomics 4(3), 363–370 (2007). [DOI] [PubMed] [Google Scholar]; • Reviews preanalytical factors to be considered for protein measures in serum and plasma.
- 12.Bowen RAR, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem. Med. 24(1), 31–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardy ERLC, Catto AJ, Hooper NM. Proteolytic mechanisms in amyloid-β metabolism: therapeutic implications for Alzheimer's disease. Trends Mol. Med. 11(10), 464–472 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Kummer MP, Heneka MT. Truncated and modified amyloid-beta species. Alzheimers Res. Ther. 6(3), 28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]