Abstract
Aim:
We aim to demonstrate that a local nanoparticle-mediated hyperthermia can effectively eliminate tumor-associated Tregs and thereby boost checkpoint blockade-based immunotherapy.
Materials & methods:
Photothermal therapy (PTT), mediated with systemically administered stealthy iron-oxide nanoparticles, was applied to treat BALB/c mice bearing 4T1 murine breast tumors. Flow cytometry was applied to evaluate both Treg and CD8+ T-cell population. Tumor growth following combination therapy of both PTT and anti-CTLA-4 was further evaluated.
Results:
Our data reveal that tumor-associated Tregs can be preferentially depleted via iron-oxide nanoparticles-mediated PTT. When combining PTT with anti-CTLA-4 immunotherapy, we demonstrate a significant inhibition of syngeneic 4T1 tumor growth.
Conclusion:
This study offers a novel strategy to overcome Treg-mediated immunosuppression and thereby to boost cancer immunotherapy.
Keywords: : anti-CTLA-4 immunotherapy, immunosuppression, photothermal therapy, Tregs, tumor microenvironment
Graphical abstract

One of the critical breakthrough findings in cancer research in the past decades is the identification of negative immune regulation in cancer patients, revealing that the patients’ own antitumor immunity is primarily inhibited by tumor-recruited immunosuppressive cells in the tumor microenvironment (TME) [1]. Using immune checkpoint inhibitors, such as anticytotoxic anti-CTLA-4, antiprogram death 1 and antiprogram death ligand 1 monoclonal antibodies (mAbs), recent clinical trials have witnessed unprecedented durable responses in some metastatic patients with a variety of cancers, indicating that cancer patients’ antitumor immunity can be restored by blocking immunosuppressive pathways [2]. However, only a small subset of patients benefit from these promising immunotherapeutic strategies [3], the majority of cancer patients do not respond or do develop acquired resistance after immunotherapeutic treatments [4]. Although a combination of different checkpoint inhibitors has been proposed to improve their blockade efficacy [5], a growing body of evidence suggests that the key therapeutic hurdle still lies in the insufficient addressing of the TME-mediated immunosuppression by these inhibitors alone [3].
CD4+FoxP3+ Tregs are key immunosuppressive cells that regulate antitumor immunity and have been found at high frequencies in tumor tissues of various types of cancers [6,7]. The expansion of Tregs in the TME can occur through migration from peripheral circulation followed by further polarization into a protumor phenotype through either tumor-derived cytokines or tumor-derived exosomes [8,9]. Those tumor-associated Tregs not only highly express suppressive proteins (e.g., CTLA-4) on their cellular surfaces [10], but also produce immunosuppressive cytokines and metabolites, such as TNF-α, TGF-β, IL-10 and adenosine in the TME, to effectively shut down cytotoxic effector T cells’ antitumor efficacy [11,12]. It is recorded that the presence of large proportions of Tregs among tumor-infiltrating lymphocytes is correlated with poor prognosis in various cancer patients [13]. However, although it is long-time believed that Tregs contribute a major hurdle facing current cancer immunotherapy for a widespread efficacy, addressing tumor-associated Tregs and the Treg-mediated immunosuppression still remains a great challenge.
Various strategies were proposed with the aim to target tumor-associated Tregs. For example, systemic administration of mAb (i.e., anti-CD25) was first proposed to target Tregs [14]. The circulating Treg population was significantly decreased following the treatment; however, such systemic Treg elimination strategies have proven to be less efficient at boosting immunotherapy, likely due to the concurrent elimination of activated effector lymphocytes [15]. In addition, two recent studies also revealed that tumor-resident Tregs show marked transcriptomic differences from Tregs in peripheral blood [16,17], suggesting the need to target the Tregs accumulated in the tumor tissues. Recent data from preclinical murine studies showed that anti-CTLA-4 mAbs, as a key negative immunity inhibitor, may preferentially deplete intratumoral Tregs via an Fc-dependent mechanism [18–20]. However, more data from clinical studies revealed that treatment with anti-CTLA-4 mAbs does not change Treg density within the human tumor microenvironment [10,21]. Furthermore, scientists found that tumor-associated Tregs are resistant to traditional antitumor strategies such as chemotherapy and radiotherapy [22]. For instance, one recent clinical study surprisingly found that chemotherapy, which was widely thought to enhance cancer immunotherapy, unfavorably enhanced Treg-mediated suppressive activity on effector T cells [23]. Also, radiotherapy, another widely used combination strategy to enhance checkpoint blockade therapy, results in a preferential increase of Tregs in tumors [24]; this increase is very likely attributable to Treg responses toward tumor-derived antigens released from treatment-induced dying tumor cells [25]. Therefore, it is essential to develop novel strategies that can effectively disrupt Tregs in the TME to overcome TME-mediated immunosuppression.
Using nanomedicine to address tumor-associated Tregs has also been explored. For instance, a recent study developed a nanoparticle carrier that can systemically deliver siRNA into Tregs to block tumor-induced immune inhibitory pathways [26]. Since Tregs in tumor tissues are more relevant for the immunosuppression, a local strategy to eliminate tumor-associated Tregs may work more effectively than systemic strategies as this strategy leaves conventional Tregs elsewhere in the body unaffected. Nanoparticle-mediated photothermal therapy (PTT) as a noninvasive local anticancer treatment has been widely explored [27]. However, traditional nanoparticle-mediated PTT applications have focused on photothermal ablation of bulk tumors with intratumoral injection of nanomediators [28–30], and PTT’s capability to disrupt Tregs has been largely neglected. Here, we hypothesize that nanoparticle-mediated PTT with systemically delivered ‘stealthy’ and small-sized nanomediators can effectively deplete tumor-associated Tregs, to thereby enhance checkpoint blockade-based cancer immunotherapy. This hypothesis is based on the following two rationales. First, tumor-recruited immune cells are present in high numbers at the margins of mammary tumors, with decreasing frequency throughout the tumor stromal layer moving deeper within the tumor [31]. Second, research in nanomedicine suggests that systemically administered nanoparticles stay close to the tumor-infiltrated suppressive immune cells after accumulation at the tumor stromal layer by taking advantage of the enhanced permeability and retention effect [32,33]. This hypothesis was further supported by a few recent reports revealing that nanoparticle-mediated hyperthermia boosts T-cell-based immunotherapy [34–37]. Other groups and we have recently reported that systemically administered iron-oxide nanoparticles (IONPs) can be used as biodegradable nanomediators for photothermal cancer therapy [38,39]. In this report, we further demonstrate that using IONP-mediated PTT in a sequential way helps in preferentially depleting tumor-recruited Tregs in 4T1 murine breast tumor models; thereby anti-CTLA-4-based cancer immunotherapy against nonresponding tumors, such as breast tumors, is boosted.
Materials & methods
Synthesis of IONPs coated with polysiloxane-containing diblock copolymer
Polymer-coated IONPs were prepared as previously described by our lab [38]. Briefly, IONPs (15 nm in diameter) were synthesized by thermal decomposition of iron oxide (III) (FeO[OH], hydrated, catalyst grade, 30−50 mesh) in the presence of oleic acid (technical grade, 90%) and 1-octadecene (technical grade, 90%) as reported previously [38]. Single-core nanoparticles were rendered soluble in aqueous solution after coating with diblock copolymer poly(ethylene oxide)-b-poly(γ-methacryloxypropyl trimethoxysilane) (PEO-b-PγMPS), which was synthesized by the reversible addition of fragmentation chain transfer polymerization, and purified by a magnetic separator (Frantz Isodynamic Separator-Model L-1, PA, USA) [40]. The average hydrodynamic diameter of resultant polymer-coated IONPs was measured using a dynamic light scattering instrument (Malvern Zeta Sizer Nano S-90, Malvern Panalytical Ltd, Malvern, UK). The magnetic nanocrystals and core–shell structure were viewed by transmission electron microscopy (TEM; JEOLJEM-1400Plus,120 kV, JEOL USA, MA, USA). The IONP concentration was determined using o-phenanthroline (American Chemical Society [ACS] reagent, 99%) after digestion with hydrochloric acid (ACS reagent, 37%) as previously described [40]. The polymer-coated IONPs were stored in refrigerator for in vitro and in vivo studies.
Cell culture
The 4T1 murine breast cancer cells were cultured under a 5% CO2 environment in RPMI-1640 media (Invitrogen, CA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, PA, USA) and 1% antibiotic–antimycotic (Invitrogen).
Photothermal effect of IONPs in vitro
For all in vitro studies, IONPs were suspended in phosphate-buffered saline (PBS; 0.1 mg Fe/ml, 200 μl/well) on a 96-well plate resting on a hot plate set to 37°C. Solutions were heated by an near-infrared (NIR) laser (885 nm, spot size, 5 × 8 mm2, MDL-III-885, OPTO Engine LLC, UT, USA) for 10 min with a laser fluence rate of 1.25 W cm-2 (laser power of 0.5 W or as specified). The temperatures of the solutions were measured by an infrared camera (FLIR Systems, i7, MA, USA). For 4T1 cell-viability studies using alamarBlue (AbD Serotec, NC, USA), the reduced laser powers were used as specified. The cancer cells were seeded at 1000 cells/well in 200 μl on a 96-well plate and were treated the following day. After returning to the incubator for 72 h, 20 μl of alamarBlue reagent was added to each well, and the fluorescence was measured 4 h later (540/25 nm excitation, 590/20 nm emission, 570 nm mirror) using a BioTek micro-plate reader (Synergy 2; BioTek, VT, USA).
Mouse tumor models
All studies involving mice were conducted in accordance with a standard animal protocol approved by the University Committee on the Use and Care of Animals at the University of Michigan. 6–8 week-old BALB/c mice were obtained from Envigo (MI, USA). Xenograft formation was generated by direct injection of 1 × 106 4T1 cells suspended in 100 μl of PBS, into the flanks of mice. Tumor detection was assessed by palpation; once identified, measurements of tumor volume were carried out using digital calipers and calculated by volume = (width)2 × length/2. Mice were randomly divided into different treatment groups once primary tumor volumes reached 50−90 mm3, using a free online service with six mice in each group.
MRI of tumor-bearing mice
An MRI scanner at 7.4-T field strength was used for MRI studies. A multiecho fast spin–echo sequence was used to simultaneously collect a series of datapoints at different echo times (TE = 15−90 ms with an increment of 15 ms). T2 relaxation time was then calculated by fitting the decay curve on a pixel-by-pixel basis by using a nonlinear monoexponential algorithm M(TE) = M0 exp(-TEi/T2), where TE is the echo time, and M(TE) is the MRI signal intensity at which TE is used. Mice were placed in a volume coil and anesthetized using 2% isoflurane throughout the MRI experiments. Tumor-bearing BALB/c mice were scanned to collect contrast-enhanced MRI data 24 h after systemic administration of IONPs at a dose of 20 mg Fe/Kg mouse bodyweight or PBS as a control. A T2 weighted fast spin echo sequence was used to obtain T2 relaxometry of the tumor tissues.
Flow cytometry
PTT for all in vivo studies was conducted as previously described [38,41]. Briefly, tumor-bearing mice (8 days after tumor cell challenge) were intravenously injected with IONPs by tail vein (20 mg Fe/kg mouse bodyweight). Sequential PTT (0.5 W laser power, 10 min each) was conducted 24 and 48 h, or specified time points after IONP injection under anesthesia by inhalation of isoflurane gas (n = 6). Tumor surface temperatures were monitored using an infrared camera. For analysis of various T-cell population changes following PTT in 4T1 tumors, mice were humanely euthanized at the designed timepoints after PTT (one dose of anti-CTLA-4 antibody was given) (n = 4). For the treatment of PTT-1X, mice were sacrificed after 24 and 48 h after PTT, respectively. For the treatment of PTT-2X, mice were sacrificed 48 h after PTT. Mice in the control group (no PTT) received the same dose of anti-CTLA-4 antibody and IONPs (n = 4) as in PTT group. Tumor tissues were harvested and digested into single cell suspension using a gentleMACS Octo Dissociator (MACS Miltenyi Biotec, CA USA) according to the manufacturer’s protocol before antibody staining. Cells were labeled for flow cytometry by incubation with the following fluorophore-conjugated antimouse antibodies: CD3-PE, CD4-APC, CD8-Brilliant Violet 421, FoxP3-Alexafluor 488 (BioLegend, CA, USA). LIVE/DEAD™ Fixable Far-Red Dead Cell Stain kit (Thermo Fisher Scientific, MA, USA) was used for cell viability staining. FoxP3 staining was performed using the FoxP3 staining buffer set (Catalog# 00–5523, eBioscience, CA, USA) [42]. In brief, after surface staining for 20 min at 4°C with the antibody (Ab) cocktails in Hank's balanced salt solution (HBSS) plus 2% fetal bovine serum (FBS), cells were washed and incubated with freshly prepared fixation/permeabilization solution for 40 min at 4°C, washed twice with permeabilization buffer, and then stained with Ab to FoxP3 for 30 min at 4°C. Flow cytometry data were acquired on a FACSCanto II (BD Biosciences, CA, USA) and analyzed with flow software Summit.
In vivo IONP-mediated PTT in combination with checkpoint blockade therapy
Three doses of anti-CTLA-4 antibody (100 μg/mouse; Clone 9D9; InVivoPlus from BioXCell, NH, USA) were intravenously administered every 3 days starting right after first PTT. PTT once was conducted 24 h after IONP injection with the same laser power but with irradiation for 20 min followed by the same dose of anti-CTLA-4 antibody. To demonstrate the immune responses against distal cancer cells, 1 × 105 4T1 cells in 50 μl of PBS were inoculated on the other side (contralateral side) 1 day before PTT-based treatment on the established primary tumors (n = 6). To demonstrate CD8+ T-cell-mediated immune responses, mice who received sequential PTT were given one dose of anti-CD8 antibody (100 μg/mouse; Clone 2.43; InVivoPure from BioXCell; n = 6). Tumor growth at distal sites was monitored every other day for 16 days. To demonstrate the generation of tumor antigen-specific memory T cells, tumor-cured mice from sequential PTT-based combination therapy (33 days after initial PTT treatment; n = 12) or naive mice (n = 6) were re-challenged with 1 × 105 4T1 cells in 50 μl of PBS and the tumor growth was monitored every other day for 16 days without giving any further anticancer treatment. In another study, the same amount of 4T1 cells in 100 μl of PBS was injected through tail vein into both tumor-cured (n = 9) and naive mice (n = 9). Mice were monitored every day to record mouse survival rate.
Histology
For analysis of 4T1 lung metastasis after various treatments, mice were euthanized 24 days after cancer cell challenge. The harvested lung tissues were formalin-fixed, embedded in paraffin and sectioned. Unstained slides were dewaxed using xylene and rehydrated using graded alcohol. Rehydrated slides were stained with hematoxylin and eosin for visualization of nucleic acids and cytoplasm. Pulmonary metastases were also enumerated by intratracheal injection of India ink (15%). India ink-injected lungs were excised, washed once in water and fixed in Fekete’s solution (100 ml 70% EtOH, 10 ml 37% formaldehyde and 5 ml glacial acetic acid) at room temperature. Tumor metastasis sites subsequently appeared as white nodules on the surface of black lungs. Prussian blue staining was used to determine the presence of IONPs in 4T1 tumor tissues. Tumor-bearing BALB/c mice were sacrificed 24 h after intravenous injection of IONPs (20 mg Fe/kg mouse bodyweight). Tumors and major organs were collected. The selected tissue blocks were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, CA, USA) and frozen using liquid nitrogen immediately. The frozen tissues were sectioned into 5-μm thick slices and examined by Prussian blue staining to confirm the presence of IONPs in the tissue sections. After counterstaining with nuclear fast red, the slides were examined under bright field microscopy.
Statistical analysis
All results were expressed as mean ± standard deviation. Biological replicates were used in all experiments unless stated otherwise. Unpaired two-tailed Student’s t-test was used for comparison of two groups. One-way analysis of variance was performed when more than two groups were compared, when determined significant (p < 0.05), multiple comparisons were performed using Tukey’s post-hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Results
Systemically delivered IONPs accumulate at tumor tissues for NIR light-triggered hyperthermia
Although PTT as a noninvasive anticancer treatment has gained considerable attention in recent years [27], most PTT-related efforts have focused on tumor photothermal ablation with intratumoral injection of nanomediators due to the challenge of effective tumor accumulation following systemic administration [29]. Here in this report, the nanomediators are composed of highly crystallized IONPs with a stealthy polymer coating [40]; consequently, they can effectively accumulate at the tumor stromal layer by enhanced permeability and retention effect, and can stay near tumor-associated Tregs in the TME [32]. NIR light-triggerable nanomediators were characterized by TEM and dynamic light scattering (DLS; Figure 1 & Supplementary Figure 1). As shown via negatively stained TEM (Figure 1A), all the IONPs are individually dispersed with a core–shell structure with an IONP core size around 18 nm and a polymer layer around 3−5 nm thick. Measuring their hydrodynamic size using DLS confirmed an average size of 30.7 ± 1.8 nm (Figure 1B), which is much smaller than traditional nanomediators [43]. The as-prepared polymer-coated IONPs have a slightly negatively charged surface (ζ-potential: -14.5 ± 0.6 mV) and are stable under various conditions, including 1 M NaCl and cell culture media [40]. These highly stable nanoparticles with small overall size are favorable for effective tumor accumulation following systemic administration [44].
Figure 1. . Systemically delivered iron-oxide nanoparticles accumulate at tumor tissues for NIR light-triggered hyperthermia.
(A) Transmission electron microscopy images of polymer-coated IONPs with negative staining. (B) Hydrodynamic size distribution of the resultant IONPs in aqueous solution. (C) Heating curves for photothermal therapy (IONPs + laser), compared with laser only. Laser power tested was 0.5 W. All the temperatures were measured during 10 min of illumination with a diode laser (λ = 885 nm) at a fluence rate of 1.25 W cm-2. (D) T2-weighted magnetic resonance images of mice bearing 4T1 tumors with (20 mg Fe/kg bodyweight) and without administration of IONPs (E) (two mice in each group). Circled areas mark the tumor sites. (F) Normalized T2-weighted intensities in tumor tissues with pre-scan and 24 h after tail-vein injection of IONPs (average of two mice). (G) Pictures of Prussian blue-stained frozen tumor tissues following systemic administration of the same dose of IONPs, and (H) the control tissue without IONP administration. Scale bar: 20 μm. (I) Two representative FLIR photos of a mouse before and after NIR light irradiation for 10 min. (J) Surface temperature of tumors in mice as monitored by infrared camera (FLIR® i7, MA, USA) after 10 min laser irradiation (laser fluence rate: 1.25 W cm-2) 24 h after intravenous injection of IONPs (circle, n = 5) or no injection as a control (square, n = 5). Data are presented as the mean ± standard deviation.
IONP: Iron-oxide nanoparticle; PBS: Phosphate-buffered saline.
Hyperthermia using these IONP-based nanomediators triggered by NIR light (885 nm) was first evaluated in vitro (Figure 1C). Heating curves on 96-well plates containing PBS spiked with IONPs (0.1 mg Fe/ml, 0.2 ml) or PBS (control) clearly revealed the enhanced photothermal effect under the irradiation of NIR light. Analysis of the in vitro cell viability after IONP-mediated hyperthermia revealed a significant cell death (p < 0.01; Supplementary Figure 2) in comparison to nontreated cells, IONP-only treated cells, or laser-only treated cells (no IONPs). NIR light-triggered IONP-mediated hyperthermia was further evaluated in 4T1 tumor-bearing BALB/c mice. IONP tumor accumulation following systemic administration was first confirmed using MRI and Prussian blue staining. Our MRI images from before and 24 h after IONP tail-vein injection revealed a great darkening effect at the tumor sites (Figure 1D) compared with the controls (Figure 1E), indicating the effective accumulation of IONPs in the tumor tissues. ImageJ software was used to compare the signal change in tumor tissues quantitatively. The data showed that the normalized intensity in tumors 24 h after IONP administration (average of two mice) dropped by 63% when compared with pre-scan, in clear contrast to the slight signal change in the control mice (Figure 1F). The accumulation of IONPs in tumor tissues was also confirmed by Prussian blue staining of the frozen tumor tissues (Figure 1G & H). Then, in vivo photothermal effects in 4T1 tumor-bearing mice under the irradiation of an 885 nm laser light were demonstrated. An infrared camera (FLIR i7, MA, USA) was used to monitor the surface temperature of mice and tumors (Figure 1I). The enhanced response of the photothermal effect from IONPs is shown in Figure 1J. The surface temperature of the tumor site in mice administered with IONPs increased from approximately 35–53°C after laser irradiation for 10 min. In contrast, the surface temperatures of tumors on control mice (no nanomediator administration) only show a slight increase after laser irradiation with the same laser power and duration of exposure. It is worth noting that the laser power in the current in vivo work was halved compared with our previously reported studies to better reveal the immunological responses triggered by IONP-mediated PTT [38,41].
IONP-mediated sequential PTT depletes Tregs
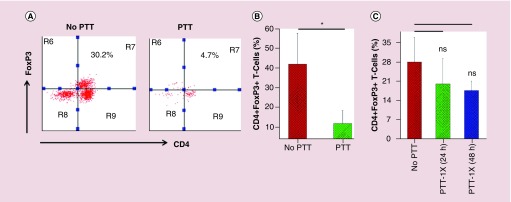
We next evaluated how IONP-mediated PTT affect tumor-associated Tregs in 4T1 murine breast tumor tissues in BALB/c mouse models, which were widely used to mimic metastatic human breast cancer [45]. Since Tregs play an important immunosuppressive role in human breast cancer [46], Tregs in mouse tumor tissues identified as CD3+CD4+FoxP3+ T cells, were analyzed by flow cytometry (Figure 2A & Supplementary Figure 3). Our data show that there is a significant presence of Tregs in 4T1 tumor tissues before PTT treatment (12 days after tumor inoculation), which is in line with previous findings [47,48]. Our flow data demonstrate that tumor-associated Tregs can be effectively depleted via IONP-mediated sequential PTT, with a significant reduction of the Treg percentage over all infiltrated T cells from 42.2 ± 15.8% (control) to 11.9 ± 6.5% (48 h after second PTT; p < 0.05; Figure 2B). Interestingly, we found that the effective tumor-associated Treg disruption only takes place with sequential PTT, not one administration of PTT. Our data revealed that after one administration of PTT, IONP-mediated hyperthermia is effective enough to cause tumor cell and resident tumor-associated immune cell death (Supplementary Figure 4). However, our data show that the Treg percentage at 24 h after PTT has no significant reduction, compared with the level in the control mice, and sustains a similar density at 48 h after treatment if there is no subsequent PTT applied (Figure 2C).
Figure 2. . Iron-oxide nanoparticle-mediated sequential photothermal therapy depletes Tregs.
(A) Representative FACS plots of CD4+FoxP3+ T cells from 4T1 tumor tissues after either no PTT or sequential PTT. (B) Quantification of Tregs proportion in tumor tissues (n = 4). (C) Percentage of Tregs over all infiltrated T cells in tumor tissues following one administration of PTT (n = 4). Cells were sequentially gated on singlets, live cells and CD3+ T cells. Error bars show standard deviation.
*p < 0.05.
ns: No significant difference; PTT: Photothermal therapy.
Since IONP-mediated PTT can effectively deplete tumor-associated Tregs, we wanted to know if that CD8+ T-cell activation can be significantly enhanced. It is worth noting that although CD8+ T cells are present in 4T1 tumor tissues before PTT treatment, these tumor-infiltrating CD8+ T cells are usually ‘exhausted’ due to the inhibitory microenvironment [49]. We hypothesize that newly recruited CD8+ T cells after IONP-mediated PTT might be easier to activate [50]. Our flow data showed that following IONP-mediated sequential PTT, the percentage of CD8+ T cells over all infiltrated T cells is indeed significantly increased from 5.6 ± 2.1% (control) to 37.5 ± 6.9% (PTT; p < 0.001; Figure 3A & B). Interestingly, our data further suggest that CD8+ T-cell activation can only be triggered through two doses of PTT, as single PTT revealed no significant CD8+ T-cell percentage change following treatment (Figure 3C). Our flow analysis of T cells in splenocytes revealed that the CD8+ T-cell percentage is also significantly increased from 24.7 ± 0.4% (control) to 27.4 ± 2.1% (PTT; p < 0.05) after sequential PTT (Figure 3D & E), but not from one dose of PTT (Figure 3F).
Figure 3. . Iron-oxide nanoparticle-mediated sequential photothermal therapy enhances CD8+ T-cell activation.
(A) Representative FACS plots of CD8+ T cells from tumor tissues after either no PTT or sequential PTT. (B) Quantification of CD8+ T-cell percentage over all infiltrated T cells at tumor tissues (n = 4). (C) Quantification of CD8+ T-cell percentage in tumor tissues following one administration of PTT (n = 4). (D) Representative FACS plots of CD8+ T cells in spleen 48 h after either no treatment or sequential PTT on tumors. (E) Quantification of CD8+ T-cell percentage in spleen (n = 4). (F) Quantification of CD8+ T-cell percentage in spleen following one administration of PTT (n = 4). Cells were sequentially gated on singlets, live cells and CD3+ T cells. Error bars show standard deviation.
*p < 0.05; ***p < 0.001.
ns: No significant difference; PTT: Photothermal therapy.
IONP-mediated sequential PTT enhances immune checkpoint therapy
We then wanted to know whether the effective depletion of Tregs and the subsequent enhanced CD8+ T-cell activation through IONP-mediated sequential PTT can enhance checkpoint inhibitor’s anticancer efficacy. The in vivo antitumor efficacy of combining IONP-mediated PTT with anti-CTLA-4 therapy was investigated (Figure 4A). Our data show that the combination therapy can effectively inhibit aggressive 4T1 tumor growth, while the immune checkpoint inhibitors alone or PTT alone fail to do so, compared with the tumor growth in nontreated mice (Figure 4B). Consistently, when only one administration of PTT is applied, the combination therapy shows no significant 4T1 tumor growth inhibition (Figure 4C), suggesting the treatment resistance very likely due to the remained Tregs. It is well documented that the 4T1 cell line can spontaneously metastasize to the lungs and other organs [51]. Our data show that sequential PTT, in combination with anti-CTLA-4 therapy, can dramatically reduce lung metastasis as revealed by hematoxylin and eosin staining of lung tissues (Figure 4D). Metastatic colonies were found in the lungs in mice that received either nontreatment, immune checkpoint Ab alone, or PTT-2X alone. However, these colonies were not found in the mice that received PTT-2X/Ab combination therapy. Quantitative analysis of metastatic nodules in lungs further shows that both PTT and PTT-based combination therapy significantly reduced 4T1 lung metastasis, but, no significant effect was observed in anti-CTLA-4 treated mice or nontreated mice (Figure 4E).
Figure 4. . Iron-oxide nanoparticle-mediated sequential photothermal therapy enhances checkpoint blockade immunotherapy against established 4T1 murine breast tumors.
(A) Schematic illustration for the combination therapy against established 4T1 tumors. (B) Tumor growth curves from mice that received either sequential PTT (PTT-2x with a 24-h interval) or PTT-1x (C) in combination with anti-CTLA-4 therapy (n = 5). (D) Representative hematoxylin and eosin staining of lung slices (two panels with a magnification of 10× and 40× , respectively) from mice with treatments indicated. Mice were sacrificed 24 days after cancer-cell challenge. Arrows point to metastatic nodules. Scale bar: 100 μm in top panel and 20 μm in bottom panel. (E) Number of lung metastasis nodules for mice that received different treatments 24 days after cancer-cell challenge (n = 5). Error bars show standard deviation.
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Ab: Antibody; IONP: Iron-oxide nanoparticle; iv.: Intravenous injection; ns: No significant difference; PTT: Photothermal therapy.
In addition to inhibiting primary tumor growth, IONP-mediated sequential PTT can generate an abscopal effect on inhibiting distal tumor growth (Figure 5). As schematically outlined in Figure 5A, a secondary tumor inoculation was performed at a distal site 1 day ahead of primary tumor treatment. Our data suggest that sequential PTT in combination with anti-CTLA-4 therapy against established primary tumors results in a complete rejection of 4T1 cancer cells at distal sites (Figure 5B). In clear contrast, all distal tumors developed following combination therapy but with single administration of PTT (Figure 5C). Our data further suggest that treating mice with sequential PTT alone can cause immune rejection of 4T1 cancer cells in 50% of BALB/c mice on days 8 and 12 after cancer inoculation, in comparison to the expected nonimmune rejection in the nontreatment group or anti-CTLA-4-treated mice throughout our study (Figure 5D). This dramatic immune rejection is lost when CD8+ T cells are depleted using anti-CD8 antibody, suggesting the cancer-cell rejection is likely due to CD8+ T-cell-mediated immune responses (Figure 5E). This enhanced anticancer effect from the combination therapy was also revealed by distal tumor growth curves, showing that sequential PTT-based combination therapy significantly inhibits tumor growth (p < 0.01) while anti-CTLA-4 therapy alone shows no differences when compared with the nontreatment group (Figure 5E).
Figure 5. . Photothermal therapy-based combination therapy triggers antigen-specific immune responses against distal cancer cells.
(A) Schematic illustration of the experimental design to demonstrate triggering of systemic tumor-specific T-cell response against distal cancer cells by combining iron-oxide nanoparticles-mediated sequential PTT with immune checkpoint inhibitors. (B & C) Individual mouse distal tumor growth curves following combination therapy with two doses of PTT (on days 1 and 2) (B) and one dose of PTT (on day 1) (C). (D) Table of the number of the mice that are distal tumor-free over the total number of mice under different treatment conditions at three representative days after distal tumor challenge. (E) Averaged distal tumor growth curves of different groups of mice after various treatments (n = 6). Data are presented as mean ± standard deviation.
**p < 0.01.
Ab: Antibody; ns: No significant difference; PTT: Photothermal therapy.
To further test if sequential PTT-based combination therapy can generate memory immune responses to prevent cancer recurrence, we re-challenged tumor cells to tumor-cured mice following PTT-based combination treatment (schematically shown in Figure 6A). Our data showed that the majority of the tumor-cured mice reject re-challenged 4T1 cancer cells without any further treatment (8/12; Figure 6B), indicating functional memory T-cell immune surveillance in these mice. In contrast, all naive mice experienced growth of the challenged 4T1 cancer cells, as expected (Figure 6C). Interestingly, we noticed that cancer cells grow visible tumors in some tumor-cured mice during the early days after rechallenge but were rejected a few days later, as summarized in Figure 6D, indicating the invisible ‘war’ between tumor-specific T cells and tumor cells. To demonstrate if the trained effector T cells following PTT-based combination therapy can reject the metastatic tumor cells, we investigated the survival rate of mice that received 4T1 cells through tail-vein injection to mimic metastasis. Our results revealed that 100% of naive mice died due to lung metastasis within 24 days after injection, while all the tumor-cured mice that received sequential PTT-based combination therapy were still alive with healthy conditions (Figure 6E). Our data clearly suggest that the as-developed novel combination therapy generates tumor-antigen-specific memory immune responses after primary tumor elimination.
Figure 6. . Photothermal therapy-based combination therapy generates antigen-specific memory immune responses.
(A) Schematic illustration of the experimental design for re-challenging cancer cells to tumor-cured mice following photothermal therapy and antibody combination treatment. (B & C) Individual tumor growth curve of all cured mice after re-challenge (n = 12) (B) and naive mice (control; n = 6) (C). (D) Table of the number of tumor-free mice over the total number of mice at three representative days after re-challenge. (E) Survival curves of mice over 30 days following tail-vein injection of 4T1 cells (1 × 105/mouse) to tumor-cured mice (n = 9) and control naive mice (n = 9).
Discussion
Although nanoparticle-mediated hyperthermia has long been explored as a noninvasive anticancer treatment [27,52–57], its impact on affecting immunosuppressive tumor immunity has rarely been evaluated. Furthermore, previously reported hyperthermia treatments generally used nanomediators that were administered through intratumoral injection, which is less effective at distributing nanomediators within the stromal layer [28–30,58], thereby less effective at disrupting tumor-associated Tregs.
Immunosuppressive Tregs in established tumors become dominant in response to tumor-derived cytokines [59]. Therefore, it is reasonable to assume that when dying tumor cells quickly release cytokines due to the initial antitumor treatment (i.e., PTT), Tregs would respond more efficiently than cytotoxic CD8+ T cells to these steep increases of antigens. This potential difference in response dynamics to tumor cytokines may provide a therapeutic ‘window’ in which the second dose of PTT selectively eliminates tumor-homing replenished Tregs compared with newly activated cytotoxic CD8+ T cells that have not yet reached the tumor tissue. This therapeutic window was further supported by our in vivo antitumor efficacy study by testing the impact of the timing of the second dose of PTT on distal tumor growth (Supplementary Figure 5). Our data suggest that a second dose of PTT at 24 h after the first one has the best antitumor efficacy (complete rejection of distal tumor growth) than either at 6 h (too early to fully eliminate Tregs) or 48 h (too late, damaging CD8+ T cells as well). This proposed mechanism is also supported by the previous observations indicating that the frequency of inhibitory Tregs was increased following a single dose of radiation [60] but decreased after fractionated dose delivery [61], and that a synergistic antitumor effect arises from fractioned but not single-dose radiotherapy [61–63].
Previous studies also suggest that Tregs could dynamically respond toward tumor tissues when tumor cells re-release tumor-derived cytokines, especially due to initial antitumor treatment [6,23,24,64–66]. For instance, it was found that immune suppression could be quickly resumed between days 2 and 5 due to the rebound Tregs from peripheral circulation following IL-12-mediated antitumor treatment; this immunosuppression resulted in the short-lived adjuvant activity of IL-12 [65]. These findings clearly suggest that although immunogenic cell death to promote cytotoxic T-cell activation could happen under certain circumstances [67], immunosuppressive cell death may also occur; this immunosuppressive cell death could thwart the immune stimulatory effect [25] and eventually lead to therapeutic resistance [68,69]. Given the fact that cancer patients often develop treatment resistance following regular front-line therapies – including chemotherapy, radiotherapy and immunotherapy [70] – the dynamic responses of these immunosuppressive cells may account for a key mechanism for developing resistance following various antitumor treatments [14,15,23,71].
Our data suggest that IONP-mediated PTT can preferentially deplete tumor-associated Tregs but not tumor-associated myeloid cells (Supplementary Figure 6). Given that the administered nanomediators (IONPs) are not specifically targeting Tregs, we would rather believe that Tregs are more sensitive to PTT-induced hyperthermia than myeloid cells [12]. Further research aiming to fully understand the factors that control the dynamics of different host immune cells responding to tumor antigen is needed to better understand the selective elimination of tumor-associated Tregs that we found in this work. However, it is worth noting that this novel strategy using IONP-mediated PTT to deplete tumor-associated Tregs preferentially is in clear contrast to other antitumor strategies (e.g., chemotherapy, radiotherapy, immune checkpoint blockade-based immunotherapy), which have been found to unfavorably enhance Treg-mediated suppressive activity on effector T cells [23] or induce a preferential increase of Tregs in tumors [60,72]. Taken together, our substantial in vivo data suggest that IONP-mediated sequential PTT provides an innovative strategy to effectively regulate suppressive tumor immunity and enhance antigen-specific CD8+ T-cell activation, thereby synergistically enhancing immune checkpoint blockade-based cancer immunotherapy.
Conclusion
In conclusion, we demonstrate that PTT mediated with systemically administered IONPs can effectively deplete immunosuppressive regulatory T cells in 4T1 murine breast tumor tissues to overcome TME-mediated immunosuppression, and can significantly boost anticancer efficacy of checkpoint blockade-based cancer immunotherapy. Our study further suggests that IONP-mediated PTT in a sequential way can eliminate tumor-associated Tregs. We demonstrate that IONP-mediated sequential PTT when combined with immune checkpoint blockade therapy can significantly inhibit established 4T1 tumor growth and generate abscopal effect on inhibiting distal tumor growth. Furthermore, we demonstrate that combination therapy based on IONP-mediated sequential PTT can generate memory tumor antigen-specific CD8+ T cells to prevent tumor cells from recurring. This study provides new insight into the mechanisms by which tumor-associated Tregs, especially the newly responding ones following initial antitumor treatments, contribute to TME-mediated immunosuppression and immune resistance. This study will facilitate the development of novel anticancer strategies for patients with either poor immunotherapeutic responses or developed immune resistances.
Future perspective
Future research should focus on further confirming this selective elimination of tumor-associated Tregs in other tumor models, such as B16 melanoma. Additional research in understanding how Tregs respond to tumor antigen burst release should be further explored. Future research can also be performed using targeted nanomediators to specifically deplete tumor-associated immunosuppressive cells.
Summary points.
Photothermal therapy (PTT) mediated with systemically administered iron-oxide nanoparticles (IONPs) can effectively deplete immunosuppressive regulatory T cells in 4T1 murine breast tumor tissues.
IONP-mediated sequential PTT when combined with immune checkpoint blockade therapy can significantly inhibit established 4T1 tumor growth and generate abscopal effect on inhibiting distal tumor growth.
Combination therapy based on IONP-mediated sequential PTT can generate memory tumor antigen-specific CD8+ T cells to prevent tumor cells from recurring.
Supplementary Material
Acknowledgments
The authors thank the technical support from the Center for Molecular Imaging and Biomedical Research Core Facilities at the University of Michigan.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/nnm-2019-0190
Financial & competing interests disclosure
This work in part was supported by the University of Michigan Comprehensive Cancer Center grant (NIH 2P30CA046592-24 to D Sun), Upjohn Research Award (G021782 to H Chen), UMOR Award (29208 to H Chen), and MCubed Award (U064006 to H Chen). This work was also partially supported by the Gillson Longenbaugh Foundation (to Q Li and A Chang). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Couzin-Frankel J. Cancer immunotherapy. Science 342(6165), 1432–1433 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Emens LA, Ascierto PA, Darcy PK. et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 81, 116–129 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Zou WP, Wolchok JD, Chen LP. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8(328), 328rv4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168(4), 707–723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J. Immunother. Cancer 5, 16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Workman CJ, Vignali DA. Targeting regulatory T cells in tumors. Febs J. 283(14), 2731–2748 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 27(1), 109–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A comprehensive review of Treg functions in tumor tissues and stragegies to target Tregs.
- 8.Piao YJ, Kim HS, Hwang EH, Woo J, Zhang M, Moon WK. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget 9(7), 7398–7410 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood 108(3), 804–811 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh B, O'Brien S, Lee D. et al. CTLA4 blockade expands FoxP3(+) regulatory and activated effector CD4(+) T cells in a dose-dependent fashion. Blood 112(4), 1175–1183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 4(3), E28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maj T, Wang W, Crespo J. et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 18(12), 1332–1341 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao JL, Savage PA. Unlocking the complexities of tumor-associated regulatory T cells. J. Immunol. 200(2), 415–421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An interesting work of reviewing the advances in the understanding of tumor-associated Treg diversity.
- 14.Rech AJ, Mick R, Martin S. et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci. Transl. Med. 4(134), 134ra62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs JFM, Punt CJA, Lesterhuis WJ. et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a Phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 16(20), 5067–5078 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Plitas G, Konopacki C, Wu K. et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45(5), 1122–1134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important work demonstrating the potent immunosuppressive features of the tumor-resident Tregs in human breast carcinomas.
- 17.De Simone M, Arrigoni A, Rossetti G. et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity 45(5), 1135–1147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargas FA, Furness AJS, Litchfield K. et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 33(4), 649–663 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An interesting work demonstrating the Fc-gamma receptor-mediated intratumoral Treg depletion by anti-CTLA-4 antibodies.
- 19.Selby MJ, Engelhardt JJ, Quigley M. et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 1(1), 32–42 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Romano E, Kusio-Kobialka M, Foukas PG. et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl Acad. Sci. USA 112(19), 6140–6145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A, Subudhi SK, Blando J. et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3(+) regulatory T cells (Tregs) in human cancers. Clin. Cancer Res. 25(4), 1233–1238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An interesting work of reporting that the anti-CTLA-4 anbibodies do not deplete Tregs in human tumors.
- 22.Postow MA, Chesney J, Pavlick AC. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372(21), 2006–2017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koumarianou A, Christodoulou MI, Patapis P. et al. The effect of metronomic versus standard chemotherapy on the regulatory to effector T-cell equilibrium in cancer patients. Exp. Hematol. Oncol. 3(1), 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SS, Dong H, Liu X. et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol. Res. 3(6), 610–619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou JJ, Greten TF, Xia Q. Immunosuppressive cell death in cancer. Nat. Rev. Immunol. 17(6), 401–401 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li SY, Liu Y, Xu CF. et al. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Rel. 231, 17–28 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Hussein EA, Zagho MM, Nasrallah GK, Elzatahry AA. Recent advances in functional nanostructures as cancer photothermal therapy. Int. J. Nanomed. 13, 2897–2906 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An important review of the progresses in nanoparticle-mediated photothermal cancer therapy.
- 28.Yang K, Hu LL, Ma XX. et al. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 24(14), 1868–1872 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Guo LR, Yan DD, Yang DF. et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 8(6), 5670–5681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang C, Diao S, Wang C. et al. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 26(32), 5646–5652 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Wyckoff JB, Wang Y, Lin EY. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67(6), 2649–2656 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat. Mater. 12(11), 958–962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen AE, Petersen AL, Henriksen JR. et al. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano 9(7), 6985–6995 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Toraya-Brown S, Sheen MR, Zhang P. et al. Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine 10(6), 1273–1285 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 26(48), 8154–8162 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cano-Mejia J, Burga RA, Sweeney EE. et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomed. Nanotechnol. 13(2), 771–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen HW, Burnett J, Zhang FX, Zhang JM, Paholak H, Sun DX. Highly crystallized iron oxide nanoparticles as effective and biodegradable mediators for photothermal cancer therapy. J. Mater. Chem. B 2(7), 757–765 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Zhou ZG, Sun YA, Shen JC. et al. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 35(26), 7470–7478 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Chen HW, Wang LY, Yeh J. et al. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-P γ MPS copolymer coating. Biomaterials 31(20), 5397–5407 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paholak HJ, Stevers NO, Chen HW. et al. Elimination of epithelial-like and mesenchymal-like breast cancer stem cells to inhibit metastasis following nanoparticle-mediated photothermal therapy. Biomaterials 104, 145–157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao X, Ahmadzadeh M, Lu YC. et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 119(24), 5688–5696 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports the clinical correlation between the density of circulating Tregs and the therapeutic outcomes following adoptive immunotherapy.
- 43.Hirsch LR, Stafford RJ, Bankson JA. et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl Acad. Sci. USA 100(23), 13549–13554 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabral H, Matsumoto Y, Mizuno K. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6(12), 815–823 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Gu Y, Liu YF, Fu L. et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat. Med. 25(2), 312–322 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Plitas G, Konopacki C, Wu KM. et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45(5), 1122–1134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu ZQ, Kim JH, Falo LD, You ZY. Tumor regulatory T cells potently abrogate antitumor immunity. J. Immunol. 182(10), 6160–6167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goudin N, Chappert P, Megret J, Gross DA, Rocha B, Azogui O. Depletion of regulatory T cells induces high numbers of dendritic cells and unmasks a subset of anti-tumour CD8(+)CD11c(+)PD-1(lo) effector T cells. PLoS ONE 11(6), e0157822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology 129(4), 474–481 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crespo J, Sun HY, Welling TH, Tian ZG, Zou WP. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25(2), 214–221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 8, 228 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melancon MP, Zhou M, Li C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc. Chem. Res. 44(10), 947–956 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 114(21), 10869–10939 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C. Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano 10(2), 2436–2446 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Sheng ZH, Zheng MB, Cai LT. Translational nanomedicine for imaging-guided photothermal therapy. Nanomed. Nanotechnol. 12(2), 568–568 (2016). [Google Scholar]
- 56.Zou LL, Wang H, He B. et al. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 6(6), 762–772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 9(1), 1074 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong WJ, Li YS, Niu DC. et al. Facile synthesis of monodisperse superparamagnetic Fe3O4 Core@hybrid@Au shell nanocomposite for bimodal imaging and photothermal therapy. Adv. Mater. 23(45), 5392–5397 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS ONE 6(11), e27690 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kachikwu EL, Iwamoto KS, Liao YP. et al. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 81(4), 1128–1135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang T, Yu HF, Ni C. et al. Hypofractionated stereotactic radiation therapy activates the peripheral immune response in operable stage I non-small-cell lung cancer. Sci. Rep. 7, 4866 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An interesting work revealing the effect of radiation frequency on antitumor efficacy.
- 62.Dewan MZ, Galloway AE, Kawashima N. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15(17), 5379–5388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaue D, Ratikan JA, Iwamoto KS, Mcbride WH. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. 83(4), 1306–1310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lecciso M, Ocadlikova D, Sangaletti S. et al. ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front. Immunol. 8, 1918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li QS, Virtuoso LP, Anderson CD, Egilmez NK. Regulatory rebound in IL-12-treated tumors is driven by uncommitted peripheral regulatory T cells. J. Immunol. 195(3), 1293–1300 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J. Exp. Med. 210(11), 2435–2446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17(2), 97–111 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Medler TR, Cotechini T, Coussens LM. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends Cancer 1(1), 66–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 25(4), 198–213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15(7), 409–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2, 15025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martens A, Wistuba-Hamprecht K, Foppen MG. et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin. Cancer Res. 22(12), 2908–2918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.