Abstract
Patients classified as idiopathic pulmonary arterial hypertension (defined as Group 1 on European Respiratory Society (ERS)/European Cardiac Society (ESC) criteria) may have evidence of minor co-existing lung disease on thoracic computed tomography. We hypothesised that these idiopathic pulmonary arterial hypertension patients (IPAH lung disease) are a separate subgroup of idiopathic pulmonary arterial hypertension with different phenotype and outcome compared with idiopathic pulmonary arterial hypertension patients without co-existing lung disease (IPAH no lung disease). Patients with ‘IPAH lung disease’ have been eligible for all clinical trials of Group 1 patients because they have normal clinical examination and normal spirometry but we wondered whether they responded to treatment and had similar survival to patients with ‘IPAH no lung disease’. We described the outcome of the cohort of patients with ‘IPAH no lung disease’ in a previous paper. Here, we have compared incident ‘IPAH lung disease’ patients with ‘IPAH no lung disease’ patients diagnosed concurrently in all eight Pulmonary Hypertension centres in the UK and Ireland between 2001–2009. Compared with ‘IPAH no lung disease’ (n = 355), ‘IPAH lung disease’ patients (n = 137) were older, less obese, predominantly male, more likely to be current/ex-smokers and had lower six-minute walk distance, lower % predicted diffusion capacity for carbon monoxide, lower mean pulmonary arterial pressure and lower pulmonary vascular resistance index. After three months of pulmonary hypertension-targeted treatment, six-minute walk distance improved equally in ‘IPAH lung disease’ and ‘IPAH no lung disease’. However, survival of ‘IPAH lung disease’ was lower than ‘IPAH no lung disease’ (one year survival: 72% compared with 93%). This survival was significantly worse in ‘IPAH lung disease’ even after adjusting for age, gender, smoking history, comorbidities and haemodynamics. ‘IPAH lung disease’ patients had similar short-term improvement in six-minute walk distance with anti-pulmonary arterial hypertension therapy but worse survival compared with ‘IPAH no lung disease’ patients. This suggests that ‘IPAH lung disease’ are a separate phenotype and should not be lumped with ‘IPAH no lung disease’ in clinical trials of Group 1 pulmonary arterial hypertension.
Keywords: idiopathic pulmonary arterial hypertension (IPAH), lung disease, treatment response, survival
Introduction
The demographics of idiopathic pulmonary arterial hypertension (IPAH) has changed over the past decade compared with IPAH patients from the era of the NIH registry.1,2 We have previously shown that younger subgroup of IPAH have different phenotype and survival compared with the older subgroup of IPAH.1 Patients with evidence of lung disease on thoracic computed tomography (CT) were excluded from that study. Recently, Trip et al. identified a subgroup of IPAH characterised by severely reduced diffusion capacity and associated the poor survival of those patients with old age, male gender and co-existing coronary disease.3 We hypothesised that IPAH patients with co-existing lung disease on thoracic CT (referred to as ‘IPAH lung disease’ in this study) are a separate subgroup of IPAH. The objective of this study was to describe the phenotype of ‘IPAH lung disease’ and compare them with IPAH patients without co-existing lung disease (referred to as ‘IPAH no lung disease’ in this study) diagnosed and treated concurrently over the study period in all eight pulmonary hypertension (PH) centre in the UK and Ireland. We also aimed to determine whether the presence of co-existing lung disease on thoracic CT without clinical signs of lung disease or spirometric abnormalities has any impact on the response to PH-targeted treatment and survival of ‘IPAH lung disease’.
Methods
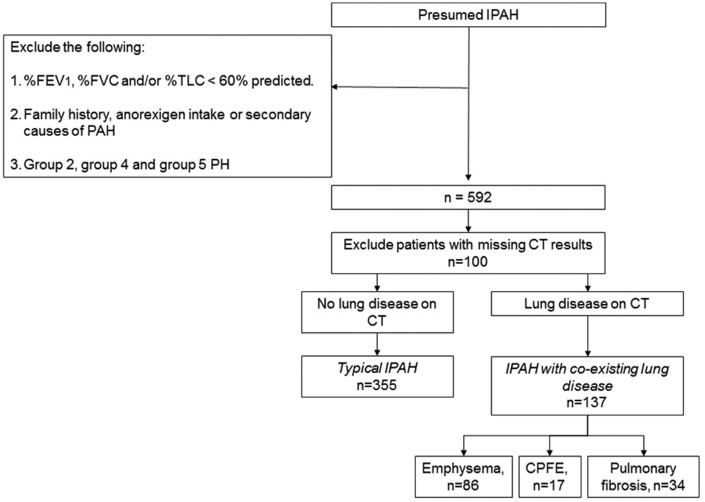
We identified all consecutive, treatment naïve, incident cases of IPAH diagnosed in all eight PH centres in the UK and Ireland between the 1 January 2001 and 31 December 2009. The diagnosis of IPAH was based on standard criteria used by all UK expert PH centres. All patients underwent multimodality assessment for their PH. This includes six-minute walk test, pulmonary function test, thoracic CT and right heart catheterisation (RHC). Patients were excluded if they had a family history or associated causes for their pulmonary arterial hypertension (PAH). We applied similar pulmonary function test exclusion criteria (defined in this study as forced expiratory volume in one second (FEV1), forced vital capacity (FVC) or total lung capacity <60% predicted) employed in randomised controlled trials of PAH to exclude Group 3 PH patients. Any patients who may also have had Group 2, Group 4 or Group 5 PH were also excluded. Patients were then divided into ‘IPAH no lung disease’ or ‘IPAH lung disease’ based on the presence or absence of minor co-existing lung disease on thoracic CT. Only emphysema and/or pulmonary fibrosis were included as co-existing lung disease in this study. These patients had no symptoms or signs of lung disease with the exception of breathlessness and had normal spirometry. Fig. 1 shows the flow chart of patients’ selection as ‘IPAH no lung disease’ or ‘IPAH lung disease’.
Fig. 1.
Flow diagram showing patient selection for ‘IPAH lung disease’ and ‘IPAH no lung disease’.
CPFE: combined pulmonary fibrosis and emphysema; CT: computed tomography; %FEV1: % predicted forced expiratory volume in second; % FVC: % predicted forced vital capacity; IPAH: idiopathic pulmonary arterial hypertension; % TLC: % predicted total lung capacity; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension.
The date of diagnostic right heart catheter (RHC) was taken as the date of diagnosis. Four patients were too unwell to have RHC at the time of diagnosis and were started on empirical PH treatment. All four patients had follow-up RHC confirming pre-capillary PH.
We compared the baseline characteristics, treatments and outcomes of ‘IPAH lung disease’ against ‘IPAH no lung disease’ diagnosed and treated concurrently over the study period. Due to the nature of the study, ethical approval was deemed unnecessary by the West of Scotland Research Ethics Committee.
Statistical analysis
Analyses were performed using SPSS version 22 (SPSS, Chicago, IL). For quantitative variables, mean ± standard deviation was used to describe parametric data, whereas median and interquartile range were used for non-parametric data. Comparisons between two independent groups were performed using Student’s t test or Mann–Whitney U test. Categorical variables were described by frequencies and percentages and comparisons between groups performed using χ2 or Fisher’s exact tests. Survival endpoint was taken as either date of death/transplant or censoring. Patients were censored if they were lost to follow up (date of last visit to PH centre was used as censor date) or if they were alive on the last day of the study (31 December 2009). Mortality was confirmed using PH centre records, general practitioner and NHS strategic tracing services. All-cause mortality was used in survival analyses. Survival from time of diagnosis was estimated using the Kaplan–Meier method. Multivariate Cox regression was used to compare the survival of ‘IPAH lung disease’ against ‘IPAH no lung disease’ after adjusting for potential confounders identified from univariate analyses. p-Values < 0.05 were considered to be significant.
Results
A total of 137 ‘IPAH lung disease’ and 355 ‘IPAH no lung disease’ patients were included in this study. Five patients were lost to follow up. The types of lung disease in patients with ‘IPAH lung disease’ were as follows: emphysema only (n = 86), pulmonary fibrosis only (n = 34) and emphysema and pulmonary fibrosis (n = 17). All ‘IPAH lung disease’ patients had FEV1 and FVC > 60% predicted.
Demographics, pulmonary function and haemodynamics
Baseline characteristics of ‘IPAH lung disease’ patients were compared with ‘IPAH no lung disease’ patients diagnosed over the same period in the UK and Ireland (Table 1). Compared with ‘IPAH no lung disease’, ‘IPAH lung disease’ patients were older, had lower body mass index, were predominantly male and current or ex-smokers; 21% (n = 28) of ‘IPAH lung disease’ patients had diabetes and 31% (n = 41) had ischaemic heart disease, compared with 13% (n = 44, p < 0.001) with diabetes and 12% (n = 42, p < 0.001) with ischaemic heart disease in ‘IPAH no lung disease’. However, left ventricular function was normal in all patients at Echo and RHC. Despite similar functional class distribution, ‘IPAH lung disease’ patients had lower mean six-minute walk distances (6MWDs) at the time of diagnosis compared with ‘IPAH no lung disease’. Mean values of spirometry and lung volumes were within normal limits in both groups of patients, whereas % predicted diffusion capacity for carbon monoxide (%DLCO) was impaired in ‘IPAH lung disease’ patients. Mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance index were significantly lower in ‘IPAH lung disease’ patients compared with ‘IPAH no lung disease’ patients; 96% (n = 129) of patients with ‘IPAH lung disease’ had severe PH (mPAP ≥ 35 mmHg or mPAP ≥ 25 mmHg with cardiac index < 2.0 L.min–1.m–2) as defined by Nice 2018 criteria.4
Table 1.
Comparison of baseline demographics, pulmonary function tests and haemodynamics parameters between patients with ‘IPAH lung disease’ and ‘IPAH no lung disease’.
| Baseline characteristics | ‘IPAH lung disease’ (n = 137) | ‘IPAH no lung disease’ (n = 355) | p-Values |
|---|---|---|---|
| Age, years (n = 492) | 68 (11.2) | 50 (17.1) | <0.001 |
| Gender, % female (n = 492) | 41% (56) | 71% (251) | <0.001 |
| Ethnicity, % non-white (n = 436) | 0% (0) | 11% (35) | <0.001 |
| Baseline functional class (n = 482) | |||
| I and II | 10% (14) | 15% (51) | 0.469 |
| III | 70% (93) | 67% (233) | |
| IV | 20% (27) | 18% (64) | |
| Smoking history (n = 404) | |||
| Current smoker | 14% (17) | 14% (39) | <0.001 |
| Ex-smoker | 75% (91) | 40% (114) | |
| Never smoke | 11% (13) | 46% (130) | |
| Baseline 6MWD, metres (n = 262) | 210 (117.1) | 292 (119.5) | <0.001 |
| Duration of symptoms, months, median (IQR) (n = 439) | 18 (10.8–36.0) | 18 (10.0–36.0) | 0.880 |
| Body mass index (n = 428) | 26 (6.0) | 29 (6.6) | <0.001 |
| % obesity (n = 428) | 17% (21) | 34% (105) | <0.001 |
| Haemoglobin, g/dL (n = 438) | 15.3 (1.8) | 15.1 (2.1) | 0.347 |
| % FEV1 (n = 399) | 88 (18.1) | 85 (14.3) | 0.070 |
| % FVC (n = 396) | 103 (20.0) | 94 (16.3) | <0.001 |
| FEV1/FVC ratio (n = 412) | 68 (9.6) | 75 (8.7) | <0.001 |
| % TLC (n = 232) | 107 (99.5) | 100 (64.1) | 0.517 |
| % DLCO (n = 374) | 38 (20.1) | 61 (21.0) | <0.001 |
| mRAP, mmHg (n = 467) | 9.5 (5.2) | 10 (5.8) | 0.468 |
| mPAP, mmHg (n = 479) | 48 (9.0) | 54 (14.1) | <0.001 |
| PCWP, mmHg (n = 445) | 9.4 (3.3) | 9.0 (3.7) | 0.386 |
| CI, L.min–1.m–2 (n = 404) | 2.0 (0.6) | 2.1 (0.7) | 0.258 |
| PVRI, WU.m–2 (n = 403) | 21 (8.3) | 23 (10.0) | 0.019 |
6MWD: six-minute walk distance; CI: cardiac index; % DLCO: % predicted diffusion capacity for carbon monoxide; % FEV1: % predicted forced expiratory volume in 1 second; % FVC: % predicted forced vital capacity; IPAH; idiopathic pulmonary arterial hypertension; IQR: interquartile range; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; PCWP: pulmonary capillary wedge pressure; PVRI: pulmonary vascular resistance index; % TLC: % predicted total lung capacity; WU: Woods unit.
Note: Results presented as mean ± SD or % (n) unless otherwise specified.
Treatment for ‘IPAH lung disease’ and ‘IPAH no lung disease’
All patients were treatment-naive at the time of diagnosis of PH. First-line treatment data for ‘IPAH lung disease’ and ‘IPAH no lung disease’ patients are shown in Table 2; 27% (n = 37) of ‘IPAH lung disease’ patients received combination therapy over the study period compared with 47% (n = 167) of ‘IPAH no lung disease’ patients (p < 0.001); 21% (n = 29) of ‘IPAH lung disease’ patients received prostanoids over the study period compared with 39% (n = 139) of ‘IPAH no lung disease’ patients (p < 0.001).
Table 2.
Comparisons of treatment between ‘IPAH lung disease’ and ‘IPAH no lung disease’.
| ‘IPAH lung disease’ (n = 137) | ‘IPAH no lung disease’ (n = 355) | p-Values | |
|---|---|---|---|
| Lung transplantation (n = 492) | 0% (0) | 2.5% (9) | 0.068 |
| Atrial septostomy (n = 492) | 0% (0) | 1.1% (4) | 0.580 |
| First-line treatment (n = 491) | |||
| No treatment | 0.7% (1) | 0.8% (3) | <0.001 |
| PD5i | 53% (73) | 30% (106) | |
| ERA | 32% (44) | 44% (155) | |
| Prostanoids | 7.4% (10) | 19% (66) | |
| Calcium channel blockers | 2.9% (4) | 5.1% (18) | |
| Combination therapy | 2.9% (4) | 2.0% (7) |
ERA: endothelin receptor antagonist; IPAH: idiopathic pulmonary arterial hypertension; PD5i: phosphodiesterase type V inhibitor.
Paired 6MWD at baseline and three months were available for 176 patients. 6MWD improved by 51 (95% CI: 22.6–78.7) metres after three months of disease-targeted therapies in ‘IPAH lung disease’ patients compared with 46 (95% CI: 32.4–59.9) metres in ‘IPAH no lung disease’ patients (p = 0.753). Absolute 6MWD at three months was 258 ( ± 144.4) metres in ‘IPAH lung disease’ patients compared with 347 ( ± 108.7) metres in ‘IPAH no lung disease’ patients (p < 0.001).
Functional class at three months was available for 299 patients. After three months of PH-targeted treatment, 17% (n = 14), 74% (n = 61) and 8.5% (n = 7) of ‘IPAH lung disease’ patients remained in functional class I/II, III and IV, respectively. In ‘IPAH no lung disease’ patients, after three months of PAH treatment, 32% (n = 69), 59.0% (n = 128) and 9.2% (n = 20) remained in functional class I/II, III and IV, respectively.
Survival
Eighty-nine ‘IPAH no lung disease’ and 61 ‘IPAH lung disease’ patients died over the study period. ‘IPAH lung disease’ patients had overall worse survival compared with ‘IPAH no lung disease’ patients. One-, two- and three-year survival of ‘IPAH lung disease’ patients were 72%, 52% and 42%, respectively. This is less compared with one-, two- and three-year survival of 93%, 82% and 71%, respectively, in ‘IPAH no lung disease’ patients (p < 0.001). Even after adjusting for age, gender, smoking history, co-morbidities and haemodynamics parameters, ‘IPAH lung disease’ patients were more likely to have a shorter time to death or transplantation compared with ‘IPAH no lung disease’ patients (Table 3).
Table 3.
Multivariate Cox regression models to determine whether ‘IPAH lung disease’ and ‘IPAH no lung disease’ patients differ after adjusting for confounders.
| p-Values | Hazard ratio | 95% confidence interval | |
|---|---|---|---|
| Model 1 | |||
| Diagnosis | |||
| ‘IPAH no lung disease’ | 1 | ||
| ‘IPAH lung disease’ | 0.01 | 1.71 | 1.108–2.631 |
| Age, years | <0.001 | 1.035 | 1.018–1.051 |
| Male gender | 0.94 | 1.02 | 0.688–1.498 |
| Smoking history | |||
| Never smoker | 1 | ||
| Current/ex-smoker | 0.04 | 1.56 | 1.023–2.445 |
| Ischaemic heart disease | 0.94 | 0.98 | 0.626–1.546 |
| Hypertension | 0.63 | 1.11 | 0.731–1.674 |
| Diabetes | 0.008 | 1.83 | 1.171–2.854 |
| Model 2 | |||
| Diagnosis | |||
| ‘IPAH no lung disease’ | 1 | ||
| ‘IPAH lung disease’ | <0.001 | 2.72 | 1.863–3.980 |
| mRAP, mmHg | 0.60 | 1.01 | 0.973–1.049 |
| SvO2, % | 0.1 | 0.98 | 0.955–1.004 |
| CI, L.min–1.m–2 | 0.02 | 0.64 | 0.440–0.938 |
CI: cardiac index; IPAH: idiopathic pulmonary arterial hypertension; mRAP: mean right atrial pressure; SvO2: mixed venous oxygen saturations.
Discussion
We report on the baseline characteristics and outcomes of a subgroup of IPAH patients characterised by severe pre-capillary PH, presence of minor co-existing lung disease on thoracic CT (IPAH lung disease), absence of ventilatory impairment on pulmonary function testing and normal respiratory examination who were treated with PH-targeted therapies. These patients were managed as IPAH no lung disease patients in real life. They would not be excluded by pulmonary function criteria in PAH clinical trials. Hence, many of these patients were probably recruited as IPAH in pivotal PAH clinical trials that form the evidence base for our current practice. However, our results show that the phenotype and response to treatment of these ‘IPAH lung disease’ patients are different compared with ‘IPAH no lung disease’ patients. The short-term improvement in 6MWD in response to PH disease-targeted therapies in ‘IPAH lung disease’ patients may have contributed to the positive results of PAH trials, many of which used change in 6MWD as the primary endpoint. However, functional class after three months of treatment and survival of ‘IPAH lung disease’ patients appeared to be significantly worse than ‘IPAH no lung disease’ patients and long-term survival for ‘IPAH lung disease’ was worse than ‘IPAH no lung disease’ despite similar haemodynamics at baseline.
‘IPAH lung disease’ patients described in our study had normal clinical features, spirometry and lung volumes. The most common parenchymal abnormality in our study cohort was emphysema. Emphysema may be noted on CT in otherwise asymptomatic healthy smokers and may not be associated with airflow obstruction on spirometry.5 Interstitial changes are also frequently seen on the CT of asymptomatic elderly patients and would not normally be of any clinical relevance.5 It is possible that this selective group of ‘IPAH lung disease’ patients described in our study simply represents IPAH patients where the predominant disease is PAH and the parenchymal abnormality is an incidental insignificant finding noted on thoracic CT done as part of the standard assessment of PH. However, the differences in phenotypes and the worse survival of this group of patients compared with ‘IPAH no lung disease’ suggest that ‘IPAH lung disease’ patients are indeed a separate subgroup different to ‘IPAH no lung disease’ patients.
Alternatively, some may argue that ‘IPAH lung disease’ patients in our study are Group 3 PH patients with severe PH. However, most COPD patients with severe PH also have mild- to moderate airflow obstruction,6,7 whereas spirometry was within normal limits in our ‘IPAH lung disease’ patients and fulfilled the inclusion criteria of PAH clinical trials as Group 1 PH patients. Indeed, spirometry was better in ‘IPAH lung disease’ than in ‘IPAH no lung disease’. A recent case report of three patients also described a similar phenotype to our study cohort: elderly male smokers with normal spirometry and lung volumes, emphysema on CT, severely reduced DLCO and severe precapillary PH.8 With our larger patient number, our study not only confirmed the presence of this separate PH phenotype but also allowed comparison of this selective group of patients with ‘IPAH no lung disease’ patients diagnosed and managed concurrently at the same PH centres.
Our study is multicentre and confirmed recent results from the Scottish Pulmonary Vascular Unit that showed different phenotype and poorer response to treatment of lung disease patients with severe PH compared with IPAH patients.9 In that study, 88 patients had severe PH, of which 32 patients had mild lung disease and the rest had severe lung disease.8 The ASPIRE registry also reported on the characteristics and outcomes of COPD patients with severe PH.11 In that study, mean % FEV1 was 65% in COPD patients with severe PH, whereas spirometry was normal in our ‘IPAHlung disease’ cohort.
The higher prevalence of smokers amongst the ‘IPAH lung disease’ patients may explain the lower DLCO observed in ‘IPAH lung disease’ patients. Smoking is associated with subclinical interstitial lung abnormalities,11,12 which in turn is associated with lower DLCO and 6MWD.13,14 Furthermore, tobacco smoke may have a direct effect on the pulmonary vasculature. Cigarette smoking has been associated with smooth muscle cell proliferation and extracellular matrix-protein deposition in pulmonary artery wall of smokers who have not yet developed COPD.15 Cigarette smoking was also associated with reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers with airway disease.16
The short-term improvement in 6MWD observed in ‘IPAH lung disease’ patients in our study cohort did not appear to translate into similar improvement in functional class or survival benefit to that observed in ‘IPAH no lung disease’ patients. This is important because short-term improvement in 6MWD has been used as primary end point in many clinical trials of PH-targeted therapies. The poor long-term prognosis of ‘IPAH lung disease’ patients may be explained by the lower absolute 6MWD achieved by ‘IPAH lung disease’ patients at three months. Absolute 6MWD after three months of PAH treatment predicts survival in IPAH, whereas change in 6MWD does not.17 PH-targeted therapies have vasodilatory and anti-proliferative properties and may help to unload the right ventricle to account for the short-term improvement in exercise capacity. However, other factors such as older age with associated co-morbidities and the effect of smoking may contribute to the worse survival of ‘IPAH lung disease’ patients compared with ‘IPAH no lung disease’ patients in our study. The male sex predominance amongst ‘IPAH lung disease’ patients may also account for the survival difference. Male sex is associated with worse pulmonary haemodynamics, reduced right ventricle response to treatment and worse overall survival in PAH.18–20 In addition, a higher proportion of ‘IPAH no lung disease’ patients received combination therapy and prostanoids compared with ‘IPAH lung disease’ patients in our study.
With the exception of the presence of incidental parenchymal lung abnormalities on thoracic CT, ‘IPAH lung disease’ patients described in this study otherwise satisfied the haemodynamic and pulmonary function criteria of IPAH and would have been – and, in many cases were – classified as IPAH in real life or in clinical trials. Disproportionately reduced DLCO as noted in our ‘IPAH lung disease’ cohort should raise the suspicion of co-existing parenchymal lung disease even in the absence of ventilatory impairment on pulmonary function testing. Trip et al. similarly noted higher proportion of co-existing lung disease on thoracic CT in IPAH patients with severely reduced DLCO.3 ‘IPAH lung disease’ patients need to be monitored closely because, despite similar short-term improvement in 6MWD compared with ‘IPAH no lung disease’ patients, the survival of ‘IPAH lung disease’ patients was relatively poor, and worse than ‘IPAH no lung disease’ despite treatment with PH-targeted therapies. On the face of it, our patients fit with the classification of Pulmonary Vascular Phenotype in COPD.21 They have severe precapillary PH and reduced DLCO but crucially they do not have airflow limitation and would be (were) recruited into clinical trials of patients with Group 1 PAH. More randomised controlled studies are needed to inform whether the use of PH-targeted therapies is beneficial in ‘IPAH lung disease’ patients. Better understanding of the phenotype of this subgroup of IPAH patients, as described in our study, may help to inform the selection of patients for future PAH trials. IPAH lung disease patients should be recognised and recruited as a separate subgroup to ‘IPAH no lung disease’ patients in future PAH clinical trials.
Limitations of our study
Our study has several limitations. This was a retrospective observational study and we have not controlled for treatment. It is possible that the presence of co-existing subclinical radiographic lung disease may have biased the choice of PH treatment and contributed to the worse outcome of ‘IPAH lung disease’ patients. Missing 6MWD data was unavoidable as not all centres used six-minute walk test to assess exercise capacity. Some patients were too unwell from their PH for their exercise capacity to be assessed. The small number of non-white subjects in our study may limit its generalisability. Our study population was comprised of a heterogeneous group of patients with different types and severity of parenchymal lung disease, although in all cases, the abnormalities were minor and associated with normal spirometry allowing classification by PH experts as Group 1 PAH. As CT images were not reviewed by an independent reviewer, potential misclassification bias cannot be excluded nor were the CT scans quantified. Chronic alveolar hypoxia may trigger pulmonary vascular remodelling. Although data were not collected for hypoxia or use of long-term oxygen therapy, there was no difference in haemoglobin level between ‘IPAH no lung disease’ and ‘IPAH lung disease’. We do not have information on the cause of death. That will be useful to confirm that the increased risk of death of ‘IPAH lung disease’ patients is not due to death unrelated to PH. Nevertheless, our results showed increased risk of death of ‘IPAH lung disease’ patients after adjusting for age, gender, smoking status and co-morbidities.
Conclusion
‘IPAH lung disease’ patients described in our study fulfilled current inclusion criteria for clinical trials of Group 1 PH patients and were treated as Group 1 PH patients in real life. However, they had different phenotype and outcomes compared with ‘IPAH no lung disease’ patients. Thoracic CT and disproportionately reduced DLCO help to differentiate ‘IPAH lung disease’ from ‘IPAH no lung disease’ patients. Despite similar baseline haemodynamic impairment and similar short-term response to PH-targeted therapies, survival of ‘IPAH lung disease’ was worse than ‘IPAH no lung disease’. Age, the effect of smoking and the presence of co-morbidities may account for the difference in long-term outcome between these two subgroups of patients. More emphasis should be put on differentiating between subgroups of IPAH patients with or without co-existing lung disease when designing future PH clinical trials. Scoring the extent of parenchymal changes on thoracic CT should be employed in addition to standard pulmonary function criteria when deciding whether a PH patient should be Group 1, a variant of Group 1 or Group 3 PH patient in future clinical trials and PH registries.
Author contributions
Andrew J Peacock and Yi Ling contributed equally. All other authors were involved in collecting data and read and approved the manuscript.
Conflict of interest
Andrew J. Peacock has received modest fees for research grant, modest support to travel to meetings and modest honoraria from Actelion, Bayer, GlaxoSmithKline, Merck Sharp & Dohme, Pfizer and United Therapeutics. Yi Ling has no disclosure to declare. Martin K. Johnson has received significant research grant and support for attending education meetings from Actelion and Merck Sharp & Dohme and significant fees for advisory board from Actelion. David G. Kiely has received significant fees from GlaxoSmithKline, Actelion and modest fees from Bayer Merck Sharp & Dohme for advisory boards and honoraria and his department has received significant research/educational grants from Bayer, GlaxoSmithKline and Actelion. Robin Condliffe has received modest fees for advisory boards and honoraria from Actelion, Bayer and GlaxoSmithKline. Charlie A. Elliot has received modest fees for advisory boards and honoraria from Actelion, GlaxoSmithKline and Bayer, and modest support for travel and attending conferences from Bayer and Actelion. J. Simon R. Gibbs has received significant fees for advisory boards from Actelion, Bayer, Merck Sharp & Dohme, Bellophoron, GlaxoSmithKline and significant educational grants from Amco, Merck Sharp & Dohme, GlaxoSmithKline and United Therapeutics. Luke S. Howard has received modest fees for advisory boards and steering committee work for clinical trials from Actelion, Bayer, GlaxoSmithKline, Bristol Myers Squibb and BTG PLC, significant honoraria from Actelion, Bayer and Merck Sharp & Dohme, and modest support to attend meetings and conference from Actelion, Merck Sharp & Dohme, Bayer and Endotronix and is a significant shareholder in The Physicians Clinic. Joanna Pepke-Zaba has received modest fees for research grant, support to travel to meetings and honoraria from Actelion, Bayer, GlaxoSmithKline and Merck Sharp & Dohme. Karen K.K. Sheares has received modest support to attend conference and scientific meetings from Actelion, Bayer and GlaxoSmithKline. Paul A. Corris has received significant funding from Actelion for advisory boards and clinical trials committee and significant funding from Bayer for advisory boards, lectures and clinical trials committee, and his department has received significant research funding from Bayer and Actelion. Andrew J.Fisher has no relevant disclosure to declare. James L. Lordan has received modest honoraria from Actelion and modest support to attend conference from Merck Sharp & Dohme. Sean Gaine has received modest fees for advisory board and honoraria from Actelion, GlaxoSmithKline, Merck Sharp & Dohme and Bayer, and modest fees for steering committee work for clinical trials from Actelion, GlaxoSmithKline, Novartis and United Therapeutics. J. Gerry Coghlan has received significant fees and/or research grant from Actelion, and moderate fees and/or research grant from GlaxoSmithKline, Bayer, Endotronix, United Therapeutics and Pfizer. S. John Wort has received modest honoraria from GlaxoSmithKline, Actelion and Bayer and has received significant research grants from Actelion and Bayer. Michael A. Gatzoulis has received modest research grants from Bayer, Pfizer and Actelion and has received modest funding from the Pulmonary Hypertension Association (UK) and the British Heart Foundation.
Funding
This study was funded by unrestricted educational grants from Pfizer, GSK, Actelion and Bayer.
References
- 1.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension. Results from the Pulmonary Hypertension Registry of the United Kingdom and Ireland. Am J Resp Crit Care Med 2012; 186: 790–796. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013; 168: 871–880. [DOI] [PubMed] [Google Scholar]
- 3.Trip P, Nossent EJ, de Man FS, et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J 2013; 42: 1575–1585. [DOI] [PubMed] [Google Scholar]
- 4.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark KD, Wardrobe-Wong N, Elliott JJ, et al. Are emphysema and airflow obstruction found together? Chest 2001; 120: 743–747. [DOI] [PubMed] [Google Scholar]
- 6.Chaouat A, Bugnet A, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. [DOI] [PubMed] [Google Scholar]
- 7.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127: 1531–1536. [DOI] [PubMed] [Google Scholar]
- 8.Adir Y, Shachner R, Amir O, et al. Severe pulmonary hypertension associated with emphysema. A new phenotype? Chest 2012; 142: 1654–1658. [DOI] [PubMed] [Google Scholar]
- 9.Brewis MJ, Church AC, Johnson MK, et al. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J 2015; 46: 1378–1389. [DOI] [PubMed] [Google Scholar]
- 10.Hurdman J, Condliffe R, Elliott CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013; 41: 1292–1301. [DOI] [PubMed] [Google Scholar]
- 11.Washko GR, Hunninghake G, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. New Eng J Med 2011; 364: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lederer DJ, Enright P, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease. The multi-ethnic study of atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med 2009; 180: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas IO, Ren P, Avila NA, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med 2007; 176: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle TJ, Washko G, Fernandez IE, et al. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med 2012; 185: 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos S, Peinado VI, Ramirez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J 2002; 19: 632–638. [DOI] [PubMed] [Google Scholar]
- 16.Barbera JA, Peinado VI, Santos S, et al. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med 2001; 164: 709–713. [DOI] [PubMed] [Google Scholar]
- 17.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension – prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780–788. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs W, van de Veerdonk MC, Trip P, et al. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 2014; 145: 1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ventetuolo CE, Praestgaard A, Palevsky HI, et al. Sex and haemodynamics in pulmonary arterial hypertension. Eur Respir J 2014; 43: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro S, Traiger GL, Turner M, et al. Sex differences in the diagnosis, treatment and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 2012; 141: 363–373. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs G, Agusti A, Barbera J, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Is there a pulmonary vascular phenotype? Am J Resp Crit Care Med 2018; 198: 1000–1011. [DOI] [PubMed] [Google Scholar]