Short abstract
Objective
This study was performed to analyze 22 cases of Mycoplasma pneumoniae pneumonia (MPP) associated with bronchial casts (BCs) in children.
Methods
We retrospectively reviewed all cases of MPP in children treated at our institution from November 2015 to December 2016. Demographic information, laboratory parameters, radiologic and fiberoptic bronchoscopy findings, treatment outcomes, and follow-up results were analyzed.
Results
Among 161 patients with MPP, 22 had BCs and 139 had no BCs. All BCs occurred in a segmental or subsegmental bronchus and were removed by fiberoptic bronchoscopy. Patients with BCs had a longer duration of fever after admission and higher incidence of refractory MPP. Substantially more children with than without BCs had a high M. pneumoniae load in the bronchoalveolar lavage fluid. All patients with BCs but only 55.4% without BCs were given methylprednisolone in addition to the standard antibiotic treatment. A significantly higher proportion of children with than without BCs received oxygen therapy. After discharge, complete radiological resolution took significantly longer in children with than without BCs.
Conclusions
In children with MPP, prompt removal of BCs may be necessary to prevent BC propagation. MPP with BCs is more severe than that without BCs, and treatment and recovery are more difficult.
Keywords: Mycoplasma pneumoniae pneumonia, bronchial cast, bronchoalveolar lavage fluid, fiberoptic bronchoscopy, methylprednisolone, children
Introduction
Mycoplasma pneumoniae (MP) is a significant pathogen of community-acquired pneumonia.1–3 During the past decade, there were several reports on MP epidemics that occurred in Europe and Asia.4–10 Increased incidence of MP infection in children was observed during these epidemics, indicating that MP frequently affects children.7–10 Clinical manifestations of MP infection in children range from mild tracheobronchitis to severe atypical MP pneumonia (MPP) that may be refractory to antibiotic treatment and require intensive care.11–13
The formation of bronchial casts (BCs) in large airways, a characteristic of plastic bronchitis (PB), has been described in both adults and children. BCs in children are often seen after surgical repair of congenital cardiac defects or in the presence of inflammatory or allergic diseases of the lung.14–17 Seear et al.18 classified BCs into two types: type 1 is caused by inflammatory diseases and consists of mainly fibrin and inflammatory cells, and type 2 (acellular) occurs only in children with congenital heart disease and consists of mainly mucin with little or no cellular infiltrate. Although some reports have described BCs in pediatric patients with PB, the occurrence of BC in children with MPP has not been documented. In this article, we present 22 pediatric patients with MPP associated with BCs.
Methods
This study was approved by the Medical Ethics Committee of Children’s Hospital of Hebei Province Affiliated with Hebei Medical University (reference number: 2019009). Consent to participate in the study was not required because this was a retrospective study.
We reviewed the medical records of all children with MPP admitted to our department from November 2015 to December 2016, when the incidence of MP infection peaked in our area. All cases of MPP were diagnosed based on clinical manifestations, laboratory testing, radiological examination, detection of MP DNA, and measurement of serum MP-specific antibodies. BCs were observed and removed by flexible fiberoptic bronchoscopy (FOB). Demographic information, blood test results, radiologic and FOB findings, and treatment and follow-up results were collected and analyzed. All patients included in this study underwent follow-up computed tomography (CT) examinations once every 4 weeks to determine whether complete radiological resolution had occurred.
Bronchoalveolar lavage fluid (BALF) was collected by FOB, and MP DNA in BALF was detected using a real-time polymerase chain reaction kit (DaAn Gene Co., Ltd., Guangzhou, China) that was approved by the State Food and Drug Administration of China for the detection of MP.19 Polymerase chain reaction was performed according to the manufacturer’s instructions. Using a standard control sample provided with the kit, the MP DNA level in each sample was determined as the DNA copy number/mL of BALF, and a titer of ≥107 copies/mL was defined as a high MP load. Serum MP antibodies were measured using the SERODIA-MYCO II Kit (Fujirebio Inc., Tokyo, Japan) following the protocol provided with the kit, and an antibody titer of ≥1:160 was considered positive.
Data were compared between patients with MPP who did and did not have BCs. All statistical analyses were performed using SPSS version 17.0 software (IBM Corporation, Armonk, NY, USA). Data normality was assessed by the Shapiro–Wilk test. Normally distributed data are expressed as mean ± standard deviation and were analyzed by Student’s t test, while non-parametric data are presented as median (1st quartile, 3rd quartile) and were analyzed by the Mann–Whitney test. Categorical variables are expressed as percentages and were analyzed with the chi-square test. A P value of <0.05 was considered statistically significant.
Results
In total, 184 children with MPP were hospitalized. Of these patients, 24 had BCs and 160 did not. A mixed viral or bacterial infection was found in 10 patients, including 2 with BCs and 8 without BCs. Ten patients without BCs were lost to follow-up, and three patients without BCs also had asthma. These 23 patients were excluded from the study, and comparisons were performed between the remaining 161 patients.
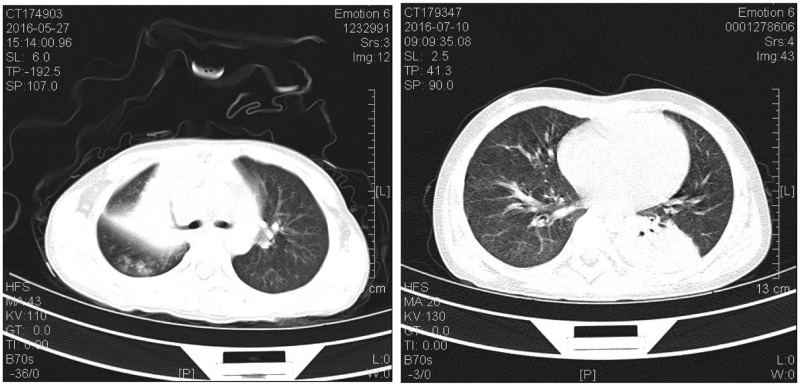
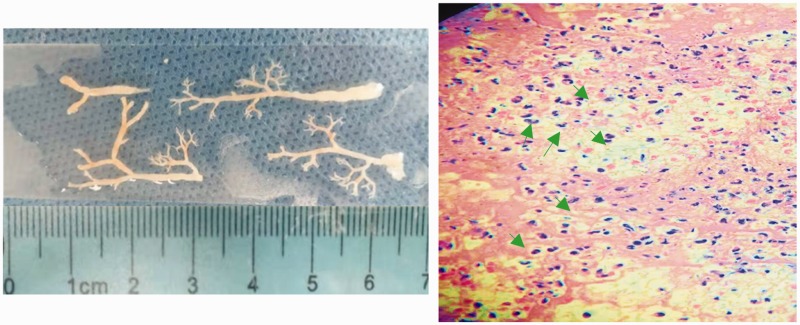
The mean age and the sex distribution, which showed no statistically significant differences between the two groups, are presented in Table 1. As shown by CT, all 22 children with BCs had lobar consolidation, which occurred in a single lobe in 17 patients and multiple lobes in 5. Representative CT images are presented in Figure 1. Six children also had atelectasis, and one developed sub-bronchial obliteration. Of the 22 patients with BCs, 8 had BCs in a segmental bronchus while 14 had BCs in a sub-segmental bronchus as observed by FOB. The BCs were removed by flexible FOB. Two children developed BC recurrence that required a second FOB procedure for treatment. BCs extracted from a patient are shown in Figure 2 (left panel). Hematoxylin and eosin (H&E) staining demonstrated that the BCs contained fibrin and numerous polymorphonuclear leukocytes (Figure 2, right panel).
Table 1.
Demographic information, key clinical characteristics, and laboratory test results of patients in the two study groups.
| BC group (n = 22) | Non-BC group (n = 139) | P value | |
|---|---|---|---|
| Age, years | 6.5 ± 2.5 | 6.0 ± 2.5 | 0.175 |
| Male/female | 9/13 | 79/60 | 0.168 |
| Length of fever, days | 4 (3, 6) | 3 (1, 4) | 0.009 |
| Length of hospital stay, days | 14 (12, 22) | 13 (11, 18) | 0.125 |
| Decreased breath sounds | 18 (81.8) | 70 (50.4) | 0.025 |
| Refractory MPP | 13 (59.1) | 29 (20.9) | 0.001 |
| WBC count, ×109/L | 8.7 (6.5, 12.3) | 10.4 (7.9, 13.0) | 0.176 |
| CRP, mg/L | 38.0 (16.3, 61.7) | 39.7 (13.0, 70.0) | 0.742 |
| LDH, U/L | 402.5 (312.0, 488.3) | 324.0 (246.0, 405.0) | 0.005 |
| High MP load in BALF | 15 (68.2) | 40 (28.8) | 0.001 |
| ALT level of >80 U/L | 5 (22.7) | 7 (5.0) | 0.006 |
Data are presented as mean ± standard deviation, n, n (%), or median (1st quartile, 3rd quartile).
BC: bronchial cast; MPP: Mycoplasma pneumoniae pneumonia; WBC: white blood cell; CRP: C-reactive protein; LDH: lactate dehydrogenase; MP: Mycoplasma pneumoniae; BALF: bronchoalveolar lavage fluid; ALT: alanine transaminase.
Figure 1.
Computed tomography images. The left panel shows right upper lobar consolidation and atelectasis in a patient, and the right panel shows left lower lobar consolidation in another child.
Figure 2.
Representative images of bronchial casts and hematoxylin and eosin (H&E) staining results. The left panel shows bronchial casts removed from a patient. H&E staining (right panel) shows that the major components of the bronchial casts were fibrin (pink) and infiltrations of inflammatory cells (blue). Arrows indicate polymorphonuclear leukocytes.
Compared with patients without BCs, those with BCs had a longer duration of fever after admission and a higher incidence rate of refractory MPP (P = 0.009 and 0.001, respectively) (Table 1). More children in the BC group had decreased breath sounds (P = 0.025) (Table 1). Laboratory testing performed within 24 hours of admission revealed that patients with BCs had a lactate dehydrogenase level of 402.5 (312.0, 488.3) U/L, which was remarkably higher than the level of 324.0 (246.0, 405.0) U/L in patients without BCs (P = 0.005). Additionally, substantially more children in the BC than non-BC group had a high MP load in BALF and an abnormally high alanine transaminase level (P = 0.001 and 0.006, respectively) (Table 1).
All patients with and without BCs were administered the macrolide antibiotic azithromycin. All children with BCs were also treated with methylprednisolone; however, only 55.4% (77 of 139) patients without BCs were treated with methylprednisolone (P < 0.0001). Oxygen therapy was administered to a significantly higher proportion of children with BCs (40.9%, 9 of 22) than without BCs (7.9%, 11 of 139) (P = 0.001). After discharge, children with BCs took 11.0 (7.5, 16.0) weeks to achieve complete radiological resolution, while children without BCs took 8.0 (6.0, 12.0) weeks (P = 0.023). No recurrence of BCs was observed after discharge until complete radiological resolution was achieved.
Discussion
Most reports of BCs have described them in association with PB. BCs in patients with PB form in large airways, which can have life-threatening consequences in children, leading to high mortality.16,20 Kunder et al.16 recently reported a 7% mortality rate in children with PB who developed BCs. Ding et al.20 studied nine cases of pediatric PB caused by influenza virus infection and reported that two children died of acute respiratory distress syndrome because of failed BC extraction. In contrast to the BCs that form in large airways in patients with PB, we observed BCs in smaller airways in the present study; specifically, we found BCs in a segmental or subsegmental bronchus causing lobar consolidation and/or atelectasis, which prompted us to perform FOB. Although all children with BCs in this study had a more severe clinical condition than those without BCs, none had respiratory failure, which we speculated might have been due to 1) BC formation in smaller airways and 2) the prompt removal of BCs, preventing propagation of the BCs to large airways. One patient developed sub-bronchial obliteration that was probably due to delayed FOB intervention.
Soyer et al.21 reported the treatment of BCs in five children with asthma. Of these patients, two (40.0%) required several bronchoscopy procedures to remove the BCs because of recurrence. In the present study, two children (9.1%) underwent a second FOB procedure for the extraction of recurrent BCs. We strongly believe that the low recurrence rate of BCs in the present study is attributable to anti-inflammatory and antibiotic treatments. Interestingly, Soyer et al.21 found Charcot–Leyden crystals (clustering of galectin-10 protein from eosinophils) in BCs from all five patients. We reviewed all H&E slides but found no Charcot–Leyden crystals in the present study. Notably, however, H&E staining has limited power for the identification of Charcot–Leyden crystals, especially with the presence of apoptotic and necrotic cells in the background.22
MPP is considered refractory if clinical and radiological deterioration occurs despite appropriate antibiotic therapy for ≥7 days.11 We found that significantly more patients in the BC than non-BC group had refractory MPP. Additionally, a higher number of children in the BC than non-BC group had a high MP load in BALF. Wang et al.23 measured the concentrations of MP DNA in BALF from children with refractory MPP and found a positive association between the MP load and inflammatory cell numbers in BALF. These authors also detected a markedly higher serum tumor necrosis factor-α level in patients with refractory MPP than non-refractory MPP.23 In view of these findings and previous reports showing that antibiotic treatment combined with corticosteroids constituted a better treatment option for children with refractory MPP than antibiotic treatment alone,24,25 Wang et al.23 suggested that MP induces excessive inflammation rather than antibiotic resistance contributing to refractory MPP. In agreement with this notion, all patients with BCs in the present study received methylprednisolone in addition to standard antibiotic treatment. Notably, we did not see a significant difference in the length of hospitalization between patients with and without BCs, which might be attributed to the facts that 1) FOB was promptly applied to remove BCs and 2) methylprednisolone was administered to all patients with BCs, which suppressed the immune response and decreased the inflammatory reaction.
To the best of our knowledge, this is the first description of BCs in children with MPP. Although BCs occur in a segmental or subsegmental bronchus in patients with MPP, we believe that prompt removal of BCs should be performed to prevent BC propagation. MPP with BCs is more severe than that without BCs, and treatment and recovery are more difficult.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Sukun Lu https://orcid.org/0000-0002-5970-7487
Jianhua Liu https://orcid.org/0000-0002-3951-3917
Zhigang Cai https://orcid.org/0000-0003-3671-1763
Jinfeng Shuai https://orcid.org/0000-0003-1487-0229
Kunling Huang https://orcid.org/0000-0001-9495-8751
References
- 1.Dorigo-Zetsma JW, Verkooyen RP, van Helden HP, et al. Molecular detection of Mycoplasma pneumoniae in adults with community-acquired pneumonia requiring hospitalization. J Clin Microbiol 2001; 39: 1184–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: a potentially severe infection. J Clin Med Res 2018; 10: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waites KB, Xiao L, Liu Y, et al. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev 2017; 30: 747–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uldum SA, Bangsborg JM, Gahrn-Hansen B, et al. Epidemic of Mycoplasma pneumoniae infection in Denmark, 2010 and 2011. Euro Surveill 2012; 17: 20073. [DOI] [PubMed] [Google Scholar]
- 5.Polkowska A, Harjunpää A, Toikkanen S, et al. Increased incidence of Mycoplasma pneumoniae infection in Finland, 2010-2011. Euro Surveill 2012; 17: 20072. [DOI] [PubMed] [Google Scholar]
- 6.Nir-Paz R, Abutbul A, Moses AE, et al. Ongoing epidemic of Mycoplasma pneumoniae infection in Jerusalem, Israel, 2010 to 2012. Euro Surveill 2012; 17: 20095. [PubMed] [Google Scholar]
- 7.Yan C, Sun H, Zhao H. Latest surveillance data on Mycoplasma pneumoniae infections in children, suggesting a new epidemic occurring in Beijing. J Clin Microbiol 2016; 54: 1400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalker VJ, Stocki T, Litt D, et al. Increased detection of Mycoplasma pneumoniae infection in children in England and Wales, October 2011 to January 2012. Euro Surveill 2012; 17: 20081. [PubMed] [Google Scholar]
- 9.Eibach D, Casalegno JS, Escuret V, et al. Increased detection of Mycoplasma pneumoniae infection in children, Lyon, France, 2010 to 2011. Euro Surveill 2012; 17: 20094. [PubMed] [Google Scholar]
- 10.Gadsby NJ, Reynolds AJ, McMenamin J, et al. Increased reports of Mycoplasma pneumoniae from laboratories in Scotland in 2010 and 2011-impact of the epidemic in infants. Euro Surveill 2012; 17: 20110. [PubMed] [Google Scholar]
- 11.Tamura A, Matsubara K, Tanaka T, et al. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect 2008; 57: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhou Y, Li S, et al. The clinical characteristics and predictors of refractory Mycoplasma pneumoniae pneumonia in children. PLoS One 2016; 11: e0156465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang RS, Wang SY, Hsieh KS, et al. Necrotizing pneumonitis caused by Mycoplasma pneumoniae in pediatric patients: report of five cases and review of literature. Pediatr Infect Dis J 2004; 23: 564–567. [DOI] [PubMed] [Google Scholar]
- 14.Singhi AK, Vinoth B, Kuruvilla S, et al. Plastic bronchitis. Ann Pediatr Cardiol 2015; 8: 246–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher KR, Singh TP, Kuebler J, et al. Risk factors and outcome of Fontan-associated plastic bronchitis: a case-control study. J Am Heart Assoc 2014; 3: e000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunder R, Kunder C, Sun HY, et al. Pediatric plastic bronchitis: case report and retrospective comparative analysis of epidemiology and pathology. Case Rep Pulmonol 2013; 2013: 649365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan RA, Black C, Reddy R. Plastic bronchitis in children. Fetal Pediatr Pathol 2012; 31: 87–93. [DOI] [PubMed] [Google Scholar]
- 18.Seear M, Hui H, Magee F, et al. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med 1997; 155: 364–370. [DOI] [PubMed] [Google Scholar]
- 19.Xu D, Li S, Chen Z, et al. Detection of Mycoplasma pneumoniae in different respiratory specimens. Eur J Pediatr 2011; 170: 851–858. [DOI] [PubMed] [Google Scholar]
- 20.Ding XF, Zhong LL, Zhang B, et al. Clinical features and pathogens of plastic bronchitis in children: an analysis of 9 cases. Chin J Contemp Pediatr 2014; 16: 729–733. [PubMed] [Google Scholar]
- 21.Soyer T, Yalcin Ş, Emiralioğlu N, et al. Use of serial rigid bronchoscopy in the treatment of plastic bronchitis in children. J Pediatr Surg 2016; 51: 1640–1643. [DOI] [PubMed] [Google Scholar]
- 22.Staribratova D, Belovejdov V, Staikov D, et al. Demonstration of Charcot-Leyden crystals in eosinophilic cystitis. Arch Pathol Lab Med 2010; 134: 1420. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Wang Y, Yan Y, et al. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. Int J Infect Dis 2014; 29: 18–23. [DOI] [PubMed] [Google Scholar]
- 24.Luo Z, Luo J, Liu E, et al. Effects of prednisolone on refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 2014; 49: 377–380. [DOI] [PubMed] [Google Scholar]
- 25.Lu A, Wang L, Zhang X, et al. Combined treatment for child refractory Mycoplasma pneumoniae pneumonia with ciprofloxacin and glucocorticoid. Pediatr Pulmonol 2011; 46: 1093–1097. [DOI] [PubMed] [Google Scholar]