Short abstract
Objective
This study assessed the efficacy of conbercept for patients with non-proliferative diabetic retinopathy (NPDR).
Methods
In this retrospective clinical study, 54 patients with NPDR (54 eyes) were treated with intravitreal injection of conbercept using a 3+ pro re nata regimen and followed up for 12 months. Best corrected visual acuity (BCVA), central foveal thickness (CFT), area of hard exudate (HE), and number of microaneurysms (MAs) were used as indicators of therapeutic effects. Systemic adverse reactions were recorded to assess safety.
Results
During the 12-month follow-up period, the mean number of injections was 6.12 ± 1.89 on demand. From baseline to the 12-month follow-up, the BCVA of patients with NPDR increased from 0.71 ± 0.20 logMAR to 0.43 ± 0.16 logMAR, CFT decreased from 424.26 ± 64.89 μm to 269.27 ± 44.79 μm, and the number of MAs declined from 79.53 ± 27.18 to 33.34 ± 16.53. Moreover, the area of HE was significantly reduced after 9 months of treatment. There were no serious systemic adverse events during the follow-up.
Conclusions
Intravitreal injection of conbercept has a stable and robust effect on patients with NPDR over a 12-month follow-up period. Thus, conbercept is an effective and feasible treatment for NPDR.
Keywords: Non-proliferative diabetic retinopathy, conbercept, intravitreal injection, efficacy, microaneurysm, visual acuity, central foveal thickness, hard exudate
Introduction
The worldwide incidence of diabetes mellitus is high1 and its complications are complex and diverse, including diabetic retinopathy,2 diabetic cardiovascular disease,3 and diabetic nephropathy.4 These complications are the main causes of disability and death in patients with diabetes.5 Diabetic retinopathy is a common microvascular complication of diabetes mellitus,6 which is induced by high glucose metabolic stress.7 Because diabetic retinopathy can cause a degree of blindness, it is regarded as a serious danger to human health.8
Diabetic retinopathy can be divided into two clinical stages: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy.9 NPDR is considered the early stage of diabetic retinopathy; the incidence of NPDR development into proliferative diabetic retinopathy is approximately 14%10 and is increasing each year. Notably, NPDR is characterized by complex pathogenesis and a lack of obvious symptoms, as well as difficult clinical treatment.11 Thus far, there have been few effective treatments. Panretinal photocoagulation is mainly used for treatment of severe late-stage NPDR, but this treatment can only maintain visual acuity and may damage other visual functions (e.g., driving); moreover, the recurrence rate after treatment remains high.12,13 Thus, there is an urgent need to develop a local drug for the treatment of NPDR to prevent its progression to a stage that may impact visual acuity.
Some studies have shown that vascular endothelial growth factor (VEGF) participates in retinal neovascularization.14 Intravitreal injection of anti-VEGF drugs has become an adjuvant therapy before vitrectomy.15,16 Conbercept (KH902; Chengdu Kanghong Biotechnology Co., Ltd., Sichuan, China), a new anti-VEGF reagent, has been developed and used in clinical treatment.17 Previous studies have shown that conbercept can improve a variety of clinical symptoms, such as best corrected visual acuity (BCVA), central foveal thickness (CFT), and choroidal neovascularization area.18 Conbercept has been used in the treatment of diabetic macular edema,17 wet age-related macular degeneration,19 chronic central serous chorioretinopathy,20 and proliferative diabetic retinopathy.14 However, conbercept has not been reported as a common treatment for NPDR. In this retrospective clinical study, we evaluated the efficacy of intravitreal injection of conbercept in patients with severe NPDR, with the aim of expanding clinical treatment options for these patients.
Patients and methods
Patients
This study included patients with NPDR who were admitted to the Department of Ophthalmology, Qilu Hospital of Shandong University from January 2016 to January 2018. This retrospective study protocol was approved by the Ethics Review Committee of Qilu Hospital, Shandong University. Because of the retrospective nature of the study, the requirement for informed consent was waived by the Ethics Review Committee. Patients with type 2 diabetes mellitus were included; in these patients, the glucose level was controlled by medication, the glycosylated hemoglobin level was <10%, and blood pressure was <160/90 mmHg. Severe NPDR was confirmed by ocular funduscopy, fundus fluorescein angiography, and optical coherence tomography (OCT); for included patients, at least 20 bleeding points were observed in a single retinal quadrant, while at least two quadrants exhibited beads of veins or more than five optic disc areas in retinal capillaries without perfusion. Finally, included patients had undergone no fundus treatment (e.g., retinal photocoagulation or intravitreal injection of anti-VEGF drugs or hormones) before this study. Exclusion criteria were poor imaging quality due to media turbidity and/or complications of non-diabetic retinal vascular diseases.
Methods
All patients were treated with a 3+ pro re nata regimen under aseptic conditions with conbercept: they received one intravitreal injection per month for 3 months, and then continued or stopped treatment based on their clinical progression.20 Retreatment was performed if either of the following criteria were met: BCVA loss was ≤0.2 logarithm of minimum resolution angle (logMAR) or OCT showed that the CFT value exceeded 280 μm. Standard ophthalmic examinations were performed before treatment and at 1, 3, 6, 9, and 12 months after initiation of treatment; these examinations included visual acuity measurement, OCT, fundus color photography, and fundus fluorescein angiography.
The efficacy of therapy was evaluated using four indicators: BCVA, CFT, hard exudate (HE), and microaneurysm (MA). A standard logarithmic visual acuity chart was used to assess BCVA and the results were converted to logMAR units for statistical analysis. Spectral-domain OCT (Heidelberg Engineering, Heidelberg, Germany) was used to measure the CFT. Simultaneously, OCT B-scans were used to quantify the area of HE in the eyes of patients with severe NPDR. The number of MAs was automatically measured on OCT images by RetmarkerDR (Retmarker SA, Coimbra, Portugal). Safety was evaluated during the study by recording the occurrence of severe diseases (e.g., choroidal neovascularization, endophthalmitis, vitreous hemorrhage, and cerebrovascular disease).
Statistical Analysis
IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA) was used for data analyses. Mean ± standard deviation values were used to indicate the distributions of measurement data. The χ2 test was performed to compare qualitative variables and paired-sample t-tests were performed to compare changes in BCVA, CFT, HE, and MAs during the follow-up period. Differences were considered to be statistically significant when P < 0.05.
Results
Patient clinical characteristics and baseline statistics
Fifty-four patients with NPDR were included (26 men and 28 women; mean age, 60.1 ± 8.3 years). Fifty-four eyes were selected from these 54 patients, including 30 right eyes and 24 left eyes. The baseline patient characteristics are described in Table 1. The mean baseline BCVA was 0.71 ± 0.20 logMAR and mean baseline CFT was 424.26 ± 64.89 μm. Treatment was performed with a 3+ pro re nata regimen; the mean number of injections during the follow-up period was 6.12 ± 1.89. No adverse reactions occurred during treatment or follow-up.
Table 1.
Baseline demographic and clinical characteristics of patients with non-proliferative diabetic retinopathy.
| Parameters | |
| Patients/eyes (n) | 54/54 |
| Sex (n, male/female) | 26/28 |
| Eyes (n, left/right) | 24/30 |
| Mean age (years) | 60.1 ± 8.3 |
| Baseline BCVA (logMAR, mean ± standard deviation) | 0.71 ± 0.20 |
| Baseline CFT (µm, mean ± standard deviation) | 424.26 ± 64.89 |
| Number of injections (mean ± standard deviation) | 6.12 ± 1.89 |
Abbreviations: BCVA, best-corrected visual acuity; CFT, central foveal thickness; logMAR, logarithm of the minimum angle of resolution.
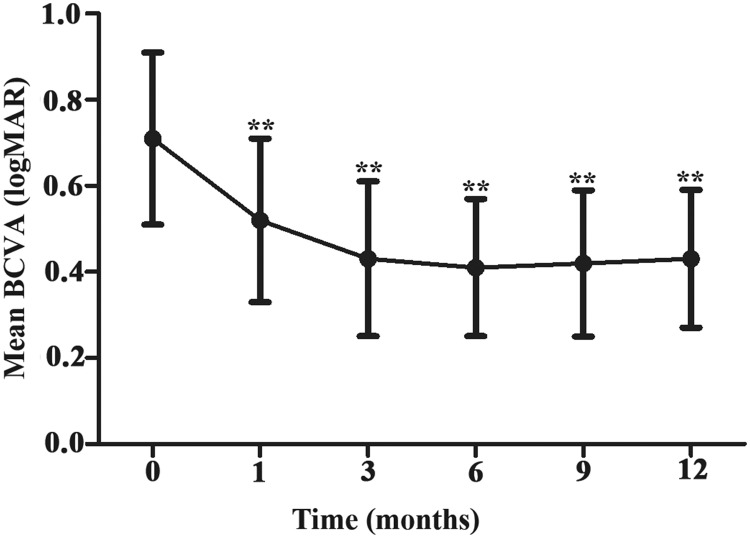
Alteration of BCVA
The mean BCVA at each follow-up time point significantly differed from the baseline value (Figure 1 and Table 2, P < 0.001). These findings suggested that BCVA significantly improved at the 1-, 3-, and 6-month follow-up time points, compared with the baseline value; it then remained stable through the 12-month follow-up time point.
Figure 1.
Mean logMAR BCVA ( ± standard deviation) from baseline to the 12-month follow-up time point. **P < 0.001 vs. baseline.
Abbreviations: BCVA, best-corrected visual acuity; logMAR, logarithm of the minimum angle of resolution.
Table 2.
BCVA (logMAR) values over time in patients with non-proliferative diabetic retinopathy who underwent intravitreal injection of conbercept
| Before treatment | 1 month | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|---|
| n = 35 | 0.71 ± 0.20 | 0.52 ± 0.19 | 0.43 ± 0.18 | 0.41 ± 0.16 | 0.42 ± 0.17 | 0.43 ± 0.16 |
| t | – | 5.06 | 7.65 | 8.61 | 8.12 | 8.03 |
| P | – | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Values are shown as mean ± standard deviation.
P value vs. before treatment.
Abbreviations: BCVA, best-corrected visual acuity; logMAR, logarithm of the minimum angle of resolution.
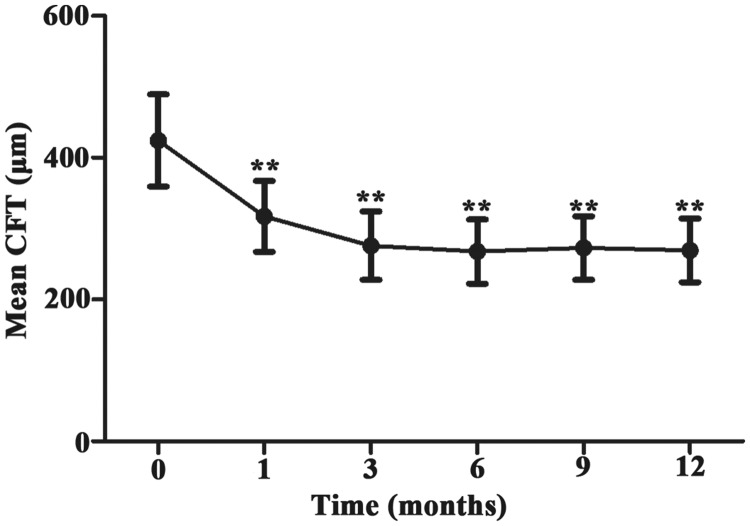
Alteration of CFT
The mean CFT markedly decreased at each follow-up time point, compared with the baseline value (Figure 2 and Table 3, P < 0.001). These results indicated that intravitreal injection of conbercept led to significant reduction of CFT; moreover, this effect was stable.
Figure 2.
Mean CFT ( ± standard deviation) from baseline to the 12-month follow-up time point. **P<0.001 vs. baseline.
Abbreviation: CFT, central foveal thickness.
Table 3.
CFT (µm) values over time in patients with non-proliferative diabetic retinopathy who underwent intravitreal injection of conbercept.
| Before treatment | 1 month | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|---|
| n = 54 | 424.26 ± 64.89 | 317.33 ± 50.06 | 275.91 ± 48.29 | 267.56 ± 45.15 | 272.63 ± 44.77 | 269.27 ± 44.79 |
| t | 9.59 | 13.48 | 14.57 | 14.13 | 14.44 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Values are shown as mean ± standard deviation.
P value vs. before treatment.
Abbreviation: CFT, central foveal thickness.
Alteration of HE
To evaluate the efficacy of conbercept treatment on HE, patients were divided into four groups according to the area of HE at baseline (first group, patients with no HE; second group, patients with <0.5 mm2 area of HE; third group, patients with 0.5 to 2.5 mm2 area of HE; fourth group, patients with >2.5 mm2 area of HE). As shown in Table 4, the numbers of patients in the second, third, and fourth groups significantly decreased at the 9-month follow-up time point, compared with the numbers at baseline; conversely, the number of patients in the first group increased significantly (P < 0.05). The findings at the 12-month follow-up time point were similar (P < 0.05). These results indicated that intravitreal injection of conbercept significantly reduced the area of HE and that this effect was stable.
Table 4.
Numbers of patients with different areas of HE among patients with non-proliferative diabetic retinopathy who underwent intravitreal injection of conbercept.
| Area of HE | Before treatment | 1 month | 3 months | 6 months | 9 months | 12 months |
|---|---|---|---|---|---|---|
| 0 | 10 | 11 | 13 | 18 | 20 | 21 |
| <0.5 mm2 | 31 | 35 | 34 | 31 | 30 | 29 |
| 0.5–2.5 mm2 | 11 | 6 | 5 | 4 | 3 | 3 |
| >2.5 mm2 | 2 | 2 | 2 | 1 | 1 | 1 |
| χ2 | – | 1.76 | 2.78 | 5.89 | 8.25 | 8.87 |
| P | – | 0.62 | 0.43 | 0.12 | 0.04* | 0.03* |
Values are shown as numbers of patients.
P value vs. before treatment.
Abbreviation: HE, hard exudate.
Alteration of MA
The number of MAs markedly declined at each follow-up time point, compared with the number at baseline (Figure 3 and Table 5, P < 0.001). These results indicated that the number of MAs was significantly reduced at the 1-month follow-up time point, compared with the baseline; during the follow-up period, this reduction persisted.
Figure 3.
Mean number of MAs ( ± standard deviation) from baseline to the 12-month follow-up time point. **P < 0.001 vs. baseline.
Abbreviation: MA, microaneurysm.
Table 5.
Number of MAs in patients with non-proliferative diabetic retinopathy who underwent intravitreal injection of conbercept.
| Before treatment | 1 month | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|---|
| n = 54 | 79.53 ± 27.18 | 44.37 ± 19.88 | 38.60 ± 18.24 | 35.11 ± 17.02 | 33.63 ± 16.36 | 33.34 ± 16.53 |
| t | – | 7.67 | 9.19 | 10.18 | 10.63 | 10.67 |
| P | – | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Values are shown as mean ± standard deviation.
P value vs. before treatment.
Abbreviation: MA, microaneurysm.
Discussion
NPDR is more common than proliferative diabetic retinopathy in patients with type 2 diabetes mellitus;2 therefore, it is particularly important to diagnose and treat NPDR as early as possible to prevent progression to vision-threatening proliferative diabetic retinopathy. In this study, a 3+ pro re nata regimen was used for intravitreal injection of conbercept in patients with NPDR. During the 12-month follow-up period, BCVA significantly increased, CFT significantly decreased, and the number of MAs significantly declined; moreover, the area of HE was significantly reduced after 9 months of follow-up.
VEGF can accelerate retinal neovascularization and is presumed to play an important role in the pathogenesis of diabetic retinopathy.21 Recently, anti-VEGF therapy has become an important aspect of diabetic retinopathy treatment.22 Intravitreal injection of anti-VEGF has been used for clinical treatment of many vision-threatening diseases.23 Notably, patients with diabetic retinopathy greatly benefit from this vision-saving therapy.23,24 Thus far, many studies have shown that anti-VEGF drugs can effectively block the development of retinopathy and significantly improve BCVA in affected patients. Intravitreal injection of methotrexate improved persistent diabetic macular edema and 16.6% of patients showed significant improvement in BCVA.25 Intravitreal injection of ranibizumab is also regarded as an effective treatment for diabetic retinopathy because it can significantly improve BCVA in affected patients.26 In addition, Gross et al.27 showed that anti-VEGF therapy could reduce the severity of diabetic retinopathy; moreover, the therapeutic effect was superior to that of panretinal photocoagulation for at least 2 years.
Conbercept is a new type of anti-VEGF drug that can bind to all subtypes of VEGF;28 it also exhibits high affinity and strong durability.29 Intravitreal injection of conbercept has been successful in treatment of many vision-related diseases, such as diabetic macular edema,30 neovascular age-related macular degeneration,31 chronic central serous chorioretinopathy,20 central retinal vein occlusion,32 and proliferative diabetic retinopathy.33 The findings in the present study further support the results of the prior analyses. Specifically, the BCVA of patients with NPDR was significantly improved after 1 month of follow-up; BCVA decreased from 0.71 ± 0.20 logMAR at baseline to 0.43 ± 0.16 logMAR at the 12-month follow-up time point. CFT also showed a marked reduction at the 12-month follow-up time point, relative to baseline. These results indicated that injection of conbercept has a robust deterrent effect on visual deterioration in patients with NPDR, suggesting that conbercept can slow the progression of NPDR to proliferative diabetic retinopathy.
HEs occur in the early stage of diabetic retinopathy (i.e. NPDR), and an increased area of HE has been associated with blindness due to diabetic retinopathy.34,35 Moon et al.36 proposed the use of HE severity as an index for evaluation of the pharmacological effects of diabetic retinopathy. In our study, patients with NPDR exhibited significant improvement in the area of HE at the 9-month follow-up time point after intravitreal injection of conbercept. In addition, prior reports have shown that the number of MAs is closely related to the progression of diabetic retinopathy and may be useful for monitoring therapeutic effects in affected patients.37 Our findings indicated that the number of MAs in patients with NPDR was markedly reduced at the 1-, 3-, and 6-month follow-up time points; this parameter remained stable through the 12-month follow-up time point. Taken together, these results suggested that intravitreal injection of conbercept has a considerable effect on NPDR and exhibits stable efficacy in affected patients.
In conclusion, our findings demonstrated that intravitreal injection of conbercept exhibits an obvious and stable therapeutic effect on NPDR in affected patients. However, the therapeutic mechanism requires further in-depth analysis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Hong Wang https://orcid.org/0000-0002-8276-683X
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018; 14: 88–98. [DOI] [PubMed] [Google Scholar]
- 2.Yang QH, Zhang Y, Zhang XM, et al. Prevalence of diabetic retinopathy, proliferative diabetic retinopathy and non-proliferative diabetic retinopathy in Asian T2DM patients: a systematic review and meta-analysis. Int J Ophthalmol 2019; 12: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun D, Man W, Zhang L. Roles of insulin resistance, endothelial dysfunction and lifestyle changes in the development of cardiovascular disease in diabetic patients. Curr Drug Targets 2017; 18: 1792–1799. [DOI] [PubMed] [Google Scholar]
- 4.Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol 2017; 13: 311–318. [DOI] [PubMed] [Google Scholar]
- 5.Sen S, Chakraborty R. Treatment and diagnosis of diabetes mellitus and its complication: advanced approaches. Mini Rev Med Chem 2015; 15: 1132–1133. [DOI] [PubMed] [Google Scholar]
- 6.Sivaprasad S, Pearce E. The unmet need for better risk stratification of non-proliferative diabetic retinopathy. Diabet Med 2019; 36: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahsan H. Diabetic retinopathy–biomolecules and multiple pathophysiology. Diabetes Metab Syndr 2015; 9: 51–54. [DOI] [PubMed] [Google Scholar]
- 8.Sumarriva K, Uppal K, Ma C, et al. Arginine and carnitine metabolites are altered in diabetic retinopathy. Invest Ophthalmol Vis Sci 2019; 60: 3119–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park YG, Roh Y. New diagnostic and therapeutic approaches for preventing the progression of diabetic retinopathy. J Diabetes Res 2016; 2016: 1753584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato Y, Lee Z, Hayashi Y. . Subclassification of preproliferative diabetic retinopathy and glycemic control: relationship between mean hemoglobin A1C value and development of proliferative diabetic retinopathy. Jpn J Ophthalmol 2001; 45: 523–527. [DOI] [PubMed] [Google Scholar]
- 11.Gong H, Song Q, Wang L. . Manifestations of central retinal artery occlusion revealed by fundus fluorescein angiography are associated with the degree of visual loss. Exp Ther Med 2016; 11: 2420–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royle P, Mistry H, Auguste P, et al. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: systematic review and economic evaluation. Health Technol Assess 2015; 19: v–xxviii, 1–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deschler EK, Sun JK, Silva PS. Side-effects and complications of laser treatment in diabetic retinal disease. Semin Ophthalmol 2014; 29: 290–300. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Lu L, Chen B, et al. Efficacy comparison of intravitreal injections of conbercept and ranibizumab for severe proliferative diabetic retinopathy. Can J Ophthalmol 2019; 54: 291–296. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118: 615–625. [DOI] [PubMed] [Google Scholar]
- 16.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology 2010; 117: 1078–1086.e2. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Qu Y, Suo Y, et al. Correlation of retinal layer changes with vision gain in diabetic macular edema during conbercept treatment. BMC Ophthalmol 2019; 19: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su L, Ren X, Wei H, et al. Intravitreal conbercept (kh902) for surgical treatment of severe proliferative diabetic retinopathy. Retina 2016; 36: 938–943. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Liang Y, Xie J, et al. Conbercept for patients with age-related macular degeneration: a systematic review. BMC Ophthalmol 2018; 18: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao J, Zhang C, Liu C, et al. The efficacy of intravitreal conbercept for chronic central serous chorioretinopathy. J Ophthalmol 2019; 2019: 7409426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant MB, Afzal A, Spoerri P, et al. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs 2004; 13: 1275–1293. [DOI] [PubMed] [Google Scholar]
- 22.Heng LZ, Comyn O, Peto T, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med 2013; 30: 640–650. [DOI] [PubMed] [Google Scholar]
- 23.Hurley B. Therapeutic revolution in the management of diabetic retinopathy. Can J Ophthalmol 2017; 52: S1–S2. [DOI] [PubMed] [Google Scholar]
- 24.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006; 26: 352–354. [DOI] [PubMed] [Google Scholar]
- 25.Falavarjani KG, Golabi S, Modarres M. Intravitreal injection of methotrexate in persistent diabetic macular edema: a 6-month follow-up study. Graefes Arch Clin Exp Ophthalmol 2016; 254: 2159–2164. [DOI] [PubMed] [Google Scholar]
- 26.Zucchiatti I, Bandello F. Intravitreal ranibizumab in diabetic macular edema: long-term outcomes. Dev Ophthalmol 2017; 60: 63–70. [DOI] [PubMed] [Google Scholar]
- 27.Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015; 314: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Zhang J, Yan M, et al. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology 2011; 118: 672–678. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Sun X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther 2015; 9: 2311–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Wang D, Chen F, et al . Hyperreflective foci in OCT image as a biomarker of poor prognosis in diabetic macular edema patients treating with conbercept in China. BMC Ophthalmol 2019; 19: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Khersan H, Hussain RM, Ciulla TA, et al . Innovative therapies for neovascular age-related macular degeneration. Expert Opin Pharmacother 2019; 20: 1879–1891. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y, Zhong QW, Zhang AQ, et al. Microvascular changes after conbercept therapy in central retinal vein occlusion analyzed by optical coherence tomography angiography. Int J Ophthalmol 2019; 12: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren X, Bu S, Zhang X, et al. Safety and efficacy of intravitreal conbercept injection after vitrectomy for the treatment of proliferative diabetic retinopathy. Eye (Lond) 2019; 33: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 1996; 114: 1079–1084. [DOI] [PubMed] [Google Scholar]
- 35.Lammer J, Bolz M, Baumann B, et al. Detection and analysis of hard exudates by polarization-sensitive optical coherence tomography in patients with diabetic maculopathy. Invest Ophthalmol Vis Sci 2014; 55: 1564–1571. [DOI] [PubMed] [Google Scholar]
- 36.Moon SW, Shin YU, Cho H, et al . Effect of grape seed proanthocyanidin extract on hard exudates in patients with non-proliferative diabetic retinopathy. Medicine (Baltimore) 2019; 98: e15515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son G, Kim YJ, Sung YS, et al. Analysis of quantitative correlations between microaneurysm, ischaemic index and new vessels in ultrawide-field fluorescein angiography images using automated software. Br J Ophthalmol 2019; 103: 1759–1764. [DOI] [PubMed] [Google Scholar]