Short abstract
Objectives
Oxidative stress within the idiopathic pulmonary fibrosis microenvironment decreases the survival of lung mesenchymal stem cells (LMSCs), resulting in disease progression. Herein, the effects of curcumin (CUR) against hydrogen peroxide (H2O2)-mediated damage to murine LMSCs were examined.
Methods
Apoptosis, reactive oxygen species, and mitochondrial membrane potential were detected by flow cytometry. Protein levels of B-cell lymphoma-2 (Bcl-2), Bcl-2 associated x (Bax), cleaved caspase-3, protein kinase B (PKB/Akt), phosphorylated-Akt, nuclear factor erythroid-2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) were evaluated by western blot analysis.
Results
Apoptosis rates in the 2.5, 5, and 10 µM CUR groups were 23.27% ± 0.31%, 14.87% ±0.41%, and 6.47% ± 0.50%, respectively, all of which were lower than in the H2O2 group (24.46% ± 1.35%). Reactive oxygen species levels were decreased, while mitochondrial membrane potential levels were increased in concentration-dependent manners in the CUR groups compared with the H2O2 group. Compared with the H2O2 group, all CUR groups showed reduced cleaved caspase-3 expression, increased Nrf2 and HO-1 expression, and increased Bcl-2/Bax and p-Akt/Akt ratios.
Conclusions
The protective effects of CUR against H2O2-mediated damage in murine LMSCs may be mediated through the Akt/Nrf2/HO-1 signaling pathway.
Keywords: Curcumin, lung mesenchymal stem cells, Akt/Nrf2/HO-1 signaling pathway, oxidative stress, apoptosis, idiopathic pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease with a mean survival time of 2–5 years.1 Although two agents, pirfenidone and nintedanib, have been shown to decrease the physiological progression of IPF and are likely to improve progression-free survival, patient outcomes remain unsatisfactory.2 Recently, cell replacement therapies have attracted significant attention as a better alternative method of treating IPF.3–5 Mesenchymal stem cells (MSCs) are a type of adult stem cell than can repair tissues, regulate immunity, and inhibit fibrosis.6 As such, lung MSCs (LMSCs) are considered the most effective treatment for IPF; however, they show limited survival after transplantation, which may be related to the internal environment.7 Additionally, the harsh pulmonary microenvironment inhibits the repair of damaged tissue by autologous LMSCs. Oxidative stress is one of the key underlying factors of IPF pathogenesis and is also a critical factor that leads to apoptosis.8,9 Thus, agents that enhance the antioxidant capacity of LMSCs are needed.
Curcuma longa L. (curcuma aromatica) is a yellow substance that is commonly used for food coloring, an ingredient in curry, and a Chinese medicinal herb. Curcumin (CUR) is one of the main chemical constituents of curcuma aromatica. A multi-effective monomer, CUR has been shown to have antioxidant, antithrombotic, and anti-inflammatory activites.10 Furthermore, CUR has been used in many preclinical studies, for which both internal sickness and external wounds have been reported.11–13 Several investigations have indicated that CUR decreased the release of superoxide and nitric oxide (NO), contributing to its protective effect against oxidative stress.14–16 However, there is some evidence that CUR acts as a growth suppressor of partially malignant cells by inducing apoptosis via inactivating protein kinase B (PKB/Akt), downregulating inhibitor of apoptosis proteins (IAPs), and activating the intrinsic apoptotic pathway via generating reactive oxygen species (ROS).17 Moreover, recent studies of IPF have demonstrated that CUR has potential antifibrotic activity, which was accompanied by increased apoptosis in primary epithelial cells and fibroblasts.18 Thus, CUR may play a dual role in oxidative stress. This study investigated the effects and possible mechanisms of action of CUR in LMSCs. Additionally, the efficacy of CUR was compared with that of the classical antioxidant N-Acetyl-L-cysteine (NAC).
Materials and methods
Animals and reagents
Healthy male C57BL/6 mice (n = 3, weight: 18 ± 2 g, age: 5-week-old) were provided by Guangdong Provincial Hospital of Traditional Chinese Medicine (Guangdong, China). CUR, NAC, 3% hydrogen peroxide (H2O2), dimethyl sulfoxide (DMSO), and methylthiazolyldiphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Alpha-Minimum Eagle’s Medium (α-MEM), fetal bovine serum (FBS), penicillin-streptomycin, and 0.25% trypsin-EDTA were obtained from Gibco (Grand Island, NY, USA). Primary antibodies against B-cell lymphoma-2 (Bcl-2), Bcl-2 associated x (Bax), cleaved caspase-3, Akt, phosphorylated-Akt (p-Akt), heme oxygenase-1 (HO-1), and nuclear factor erythroid-2-related factor 2 (Nrf2) were obtained from Cell Signaling Technology (Danvers, MA, USA). The Annexin V-FITC Apoptosis Detection Kit and Mitochondrial Membrane Potential Detection Kit (5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine, JC-1) were purchased from Beyotime Biotechnology Research Institute (Shanghai, China). This study was approved by Animal Ethics Committee of Guangdong Provincial Hospital of Traditional Chinese Medicine.
Cell extraction, culture, and identification
The mice were sacrificed by cervical dislocation and immersed in 75% ethanol for 10 minutes. Then the chest cavity was opened using sterile scissors to obtain the lungs. The trachea and bronchi were removed, and lung tissue was cut into small pieces. The chopped lung tissue was then placed in α-MEM containing 0.5 mg/mL of type II collagenase (Sigma-Aldrich) and incubated at 37°C with 5% CO2 for 90 minutes. The incubated tissue was then gently ground and filtered through a 70-µm cell strainer, and the filtrate was centrifuged at 377 ×g for 15 minutes. Subsequently, the supernatant was removed, and the cell pellet was cultured in α-MEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2. The culture medium was changed every 3 days. When adherent cells reached 70%–80% confluency, they were passaged following digestion with 0.25% trypsin-EDTA. Phenotypic evaluations were performed at passage 10 by flow cytometry, and tenth- through fifteenth-generation cells were used for subsequent studies.
Cell viability assay
Murine LMSCs were plated in 96-well plates at a seeding density of 8 × 105 cells/mL and incubated for 48 hours. The cells were then exposed to 200, 400, 600, 800, and 1000 µM H2O2 for 6 hours. Subsequently, 10 µL of MTT was added to each well and incubated at 37°C for 4 hours. After replacing the liquid in the wells with 150 µL of DMSO, the plates underwent gentle shaking for 10 minutes in the dark, after which the absorbance was measured at 490 nm. Additionally, cells were plated for 24 hours and treated with media supplemented with 2.5, 5, 10, and 20 µM CUR or 5 mM NAC for another 24 hours. Subsequently, these cells were exposed to an appropriate dose of H2O2 for 6 hours, and then cell viability was measured as above.
Apoptosis assay
Cells were plated in 6-well plates at a seeding density of 8 × 105 cell/mL, incubated for 24 hours, and then divided into groups. After the corresponding treatments, the cells were collected and centrifuged at 168 × g for 5 minutes. Next, the supernatant was discarded, and the cells were washed three times with phosphate-buffered saline (PBS). Subsequently, the cells were resuspended in 195 µL of Annexin V-FITC binding solution, followed by adding 5 µL of Annexin V-FITC and 10 µL of propidium iodide. The cell suspensions were gently mixed and incubated at room temperature for 20 minutes in the dark. Finally, apoptotic cells were detected by FC500 flow cytometry (Beckman Coulter, Brea, CA, USA).
Intracellular ROS assay
Cells in each group were inoculated into 6-well plates and cultured for 24 hours. For the assay, 1 mL of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; diluted 1000-fold in α-MEM) was added to each well and incubated with the cells a 37°C with 5% CO2 for 20 minutes. Extracellular DCFH-DA was removed by washing with PBS, and then the cells were collected and centrifuged at 168 ×g 5 minutes. Finally, the cells were resuspended in 200 µL of PBS and detected by FC500 flow cytometry.
Mitochondrial membrane potential (MMP)
After the cells from each group had adhered to 6-well plates, 1 mL of JC-1 staining solution and 1 mL of basic medium were added to the plates and incubated at 37°C with 5% CO2 for 20 minutes. Next, the plates were centrifuged to separate the upper clear liquid, and the cells were resuspended in JC-1 staining buffer. JC-1 is an ideal fluorescent probe that is widely used to detect MMP and in this study, was measured by FC500 flow cytometry.
Western blotting
Cells were incubated in radio-immunoprecipitation assay (RIPA) buffer overnight with rocking at 4°C and were then centrifuged at 21913 ×g for 30 minutes at 4°C. The protein concentration of lysates was measured using the Protein Concentration Assay kit (LIANKE Biotech, Co. Ltd, Hangzhou, China). The lysates were then separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with western blocking buffer (Beyotime Biotechnology, Shanghai, China) for 90 minutes at room temperature. After incubation with primary antibodies overnight at 4°C, and then secondary antibodies for 1 hour at room temperature, immunoreactive bands were detected by chemiluminescence, and the grey values were analyzed with IMAGE J software.
Statistical analysis
All acquired data are expressed as mean ±standard deviation, and data between groups were evaluated by the one-way ANOVA test using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
Molecular characteristics of CUR and the identification of MLMSCs
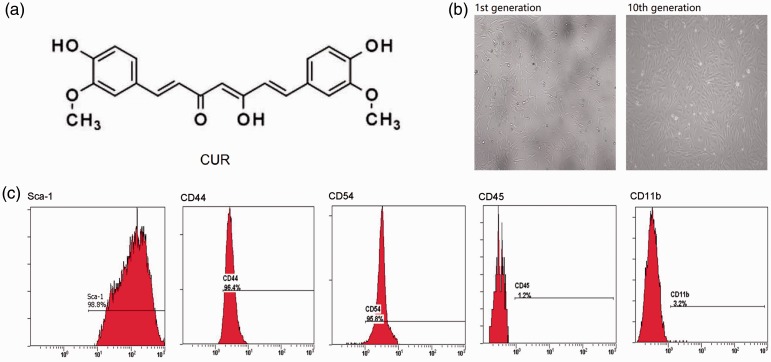
The molecular weight of CUR is 368.39, and its structure is shown in Figure 1a. Additionally, first generation murine LMSCs were indistinct and mixed with a few epithelioid cells (Figure 1b). However, when these cells were passaged to the tenth generation, they presented as typical fusiform-type. Furthermore, as shown by flow cytometry analysis, Sca-1, CD54, and CD44 were expressed in the tenth generation murine LMSCs, while CD45 and CD11b were absent (Figure 1c).
Figure 1.
Characteristics of curcumin (CUR) and murine LMSCs. (a) Chemical structure of CUR. (b) Morphological characteristics of murine LMSCs (magnification: 10×). (c) Identification of murine LMSCs by flow cytometry.
CUR restored the viability of H2O2-treated LMSCs
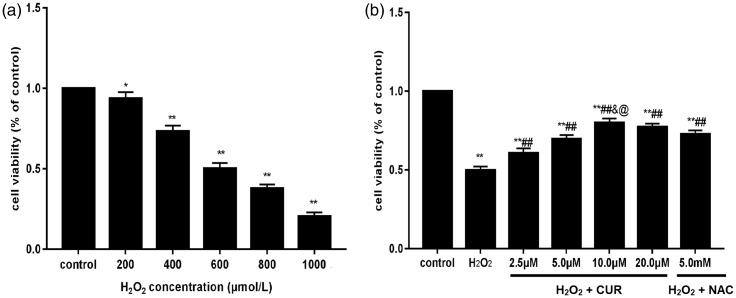
Compared with the control group, the survival of murine LMSCs was gradually reduced as the H2O2 dose increased, becoming 51% at 600 µM H2O2, which was selected as the appropriate dose for modeling (Figure 2a; *P < 0.05, **P < 0.01). In contrast, CUR and NAC treatment significantly increased the cell viability compared with H2O2-treated cells (##P < 0.01); however, all treatment groups showed decreased viability compared with control cells (**P < 0.01) (Figure 2b). There was no significant difference between the 10 and 20 µM CUR groups, and the NAC group showed lower viability than the 10 µM CUR group (@P < 0.05).
Figure 2.
Effects of H2O2, CUR, and NAC on the viability of murine LMSCs. (a) The cytotoxicity of various concentrations of H2O2 on murine LMSCs was determined by the MTT assay. (b) The protective effects of CUR and NAC on the viability of murine LMSCs. Data were obtained from three independent experiments and are expressed as mean ± SD; *P<0.05, **P<0.01 vs. control; ##P < 0.01 vs. H2O2 groups; &P > 0.05 vs. 20 µM CUR; @P < 0.05 vs. NAC.
CUR mitigated H2O2-induced apoptosis
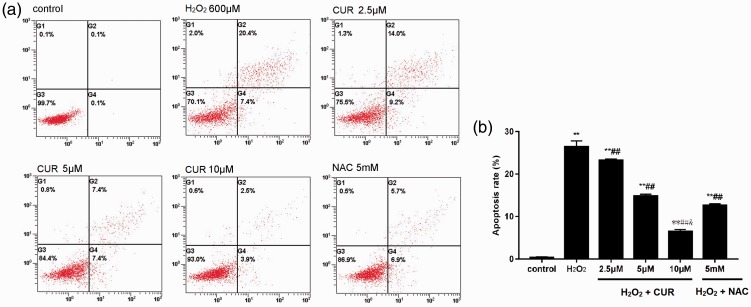
As shown in Figure 3, the apoptosis rate of the H2O2 group was 24.46% ± 1.35%, the NAC group was 12.63% ± 0.35%, and the 2.5, 5, and 10 µM CUR groups were 23.27% ± 0.31%, 14.87% ± 0.41%, and 6.47% ± 0.50%, which were all higher than the control group (0.33% ± 0.15%; **P < 0.01). Furthermore, the CUR and NAC groups were significantly different than the H2O2 group (##P < 0.01). CUR inhibited apoptosis in a dose-dependent manner, and 10 µM CUR was lower than NAC (&P < 0.01).
Figure 3.
Effects of CUR and NAC on H2O2-induced apoptosis. (a) The apoptosis rates of murine LMSCs were detected by flow cytometry. (b) Quantitative analysis of the percentage of apoptotic cells. Percentage of apoptotic cells was calculated as the sum of the percentages of early- and late-stage apoptotic cells. Data were obtained from three independent experiments and are expressed as mean ± SD; **P < 0.01 vs. control; ##P < 0.01 vs. H2O2; &P < 0.01 vs. NAC.
CUR reduced intracellular ROS levels
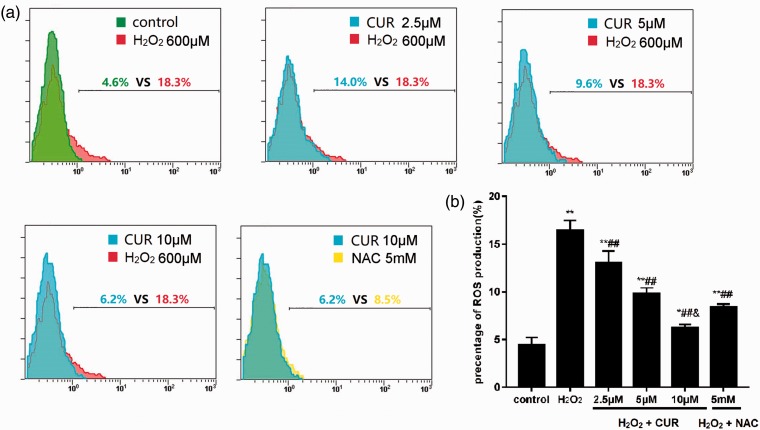
Intracellular ROS levels were 16.37% ±1.11% in the H2O2 group, 8.43% ± 0.29% in the NAC group, and 13.00% ± 1.28%, 9.83% ± 0.59%, and 6.23% ± 0.35% in the 2.5, 5, and 10 µM CUR groups, respectively, which were all significantly increased compared with the intracellular ROS levels of the control group (4.40% ± 0.82%; **P < 0.01, *P < 0.05). The CUR and NAC groups were significantly different compared with the H2O2 group (##P < 0.01), and the ROS level in the 10 µM CUR group was significantly lower than in the NAC group (&P < 0.01) (Figure 4).
Figure 4.
Effects of CUR and NAC on intracellular ROS production. (a) ROS levels in murine LMSCs were evaluated by flow cytometry. (b) Quantitative analysis of the percentage of intracellular ROS production. Percentages of ROS were calculated as intracellular oxidized matter. Data were obtained from three independent experiments and are expressed as mean ± SD; **P < 0.01, *P < 0.05 vs. control; ##P < 0.01 vs. H2O2; &P < 0.01 vs. NAC.
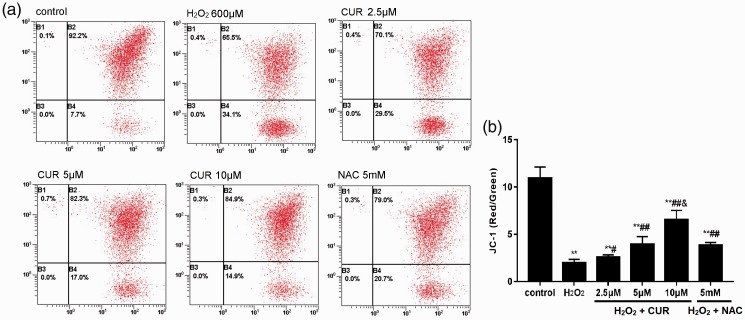
CUR improved mitochondrial function
As shown in Figure 5, the MMP level of the control group was 19.92% ± 1.20%, which rapidly decreased to 1.97% ± 0.37% in the H2O2 group. MMP levels were 2.57% ± 0.25%, 3.95% ± 0.81%, and 6.55% ± 0.97% in the 2.5, 5, and 10 µM CUR groups, respectively, and 3.84% ±0.28% in the NAC group. Compared with the control group, all treatment groups were significantly decreased (**P < 0.01), and these results were more pronounced in the CUR and NAC groups (#P < 0.05, ##P < 0.01).
Figure 5.
Effects of CUR and NAC on MMP. (a) The MMP levels of murine LMSCs were measured by flow cytometry. (b) Quantitative analysis of the percentage of MMP levels. Percentages of MMP were calculated as the ratio of JC-1 aggregates to monomers. Data were obtained from three independent experiments and are expressed as mean ± SD; **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. H2O2; &P < 0.01 vs. NAC.
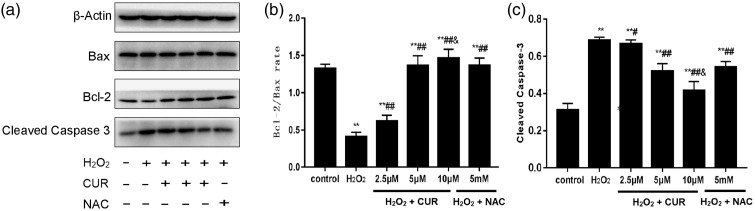
Effects of CUR on Bax, Bcl-2, and cleaved caspase-3 protein levels
As shown in Figure 6, compared with the control group, cleaved caspase-3 protein levels and the Bcl-2/Bax ratio were significantly increased in the H2O2, NAC, and CUR groups. The H2O2 and 2.5 µM CUR groups were lower than the control group, and the 5 and 10 µM CUR groups and the NAC group showed more pronounced differences. All CUR groups and the NAC group showed higher Bcl-2/Bax ratios and lower cleaved caspase-3 expression than the H2O2 group (#P < 0.05, ##P < 0.01). Compared with the NAC group, the Bcl-2/Bax ratio was significantly increased and cleaved caspase-3 expression was significantly decreased in the 10 µM CUR group (&P < 0.01).
Figure 6.
Effects of CUR and NAC on Bax, Bcl-2, and cleaved caspase-3 levels in murine LMSCs. (a) Protein levels of Bax, Bcl-2, and cleaved caspase-3 were assessed by western blotting. (b) Quantitative analysis of the Bax/Bcl-2 ratios. (c) Quantitative analysis of cleaved caspase-3 levels. Data were obtained from three independent experiments and are expressed as mean ± SD; **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. H2O2; &P < 0.01 vs. NAC.
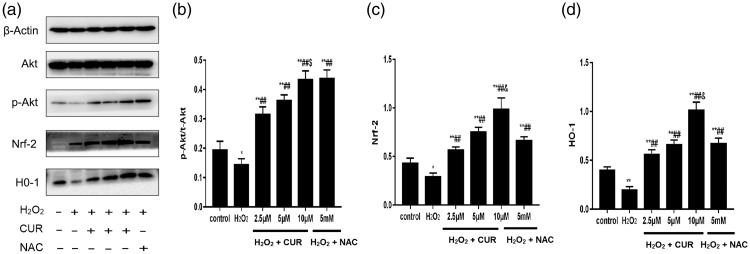
Effects of CUR on the Akt/Nrf2/HO-1 signaling pathway
The p-Akt/Akt ratio and Nrf2 and HO-1 levels were significantly increased in the CUR groups and the NAC group compared with the control group (*P < 0.05, **P < 0.01), but were significantly decreased in the H2O2 group (##P < 0.01). The p-Akt/Akt ratio and levels of Nrf2 and HO-1 were increased in a dose-dependent manner in all CUR groups. Compared with the NAC group, the 10 µM CUR group showed higher levels of Nrf2 and HO-1 (&P < 0.01), but the p-Akt/Akt ratio was not significantly different (Figure 7).
Figure 7.
Effects of CUR and NAC on the Akt/Nrf2/HO-1 pathway in murine LMSCs. (a) Protein levels of Akt, p-Akt, Nrf2, and HO-1 were assessed by western blotting. (b) Quantitative analysis of p-Akt/Akt ratios. (c) Quantitative analysis of Nrf2 levels. (d) Quantitative analysis of HO-1 levels. Data were obtained from three independent experiments and are expressed as mean ± SD; *P < 0.05, **P < 0.01 vs. control; ##P < 0.01 vs. H2O2; $P > 0.05, &P < 0.01 vs. NAC.
Discussion
IPF is a progressive fibrosing interstitial pneumonia, in which healthy lung tissue is gradually replaced by growing scars. Furthermore, the alveolar structure is destroyed, which disrupts gas exchange to a degree that ultimately becomes life-threatening.19 Currently, epithelial cell dysfunction and abnormal epithelial-mesenchymal signaling is thought to activate fibroblasts to deposit and remodel the extracellular matrix, eventually leading to fibrotic changes.20 This chronic activation is possibly related to oxidative stress, inflammatory reactions, aging, and immunity.21–24 Currently, there is no effective drug for IPF, and the optimal treatment is lung transplantation. MSCs are a type of mesodermal adult stem cell that are widely distributed in connective tissues and organ stroma. MSCs show self-replicating, multidirectional differentiation, and regenerative capacities in damaged organs and tissues.6 They can also migrate to injured site, repair defective tissue through proliferation and directional differentiation, and secrete factors that activate other repair mechanism.25 Thus, MSC therapy may replace lung transplantation as a better treatment for IPF. However, the pathological environment of IPF can affect the survival of MSCs, of which oxidative stress is a key factor.26
Oxidative stress refers to an imbalance between oxidation and anti-oxidation in vivo that causes oxidation and produces a large number of oxidative intermediates, including ROS, which can negatively impact signal transduction and the maintenance of physiological function. As H2O2 is a strong oxidant, we chose it in this study to simulate the oxidative stress of the IPF microenvironment. The viability of murine LMSCs was significantly reduced after H2O2 exposure, decreasing to 51% with 600 µM H2O2, which was selected as the modeling density. Additionally, excessive ROS resulted in apoptosis, which was closely related to mitochondrial dysfunction.27 This study found that CUR could restore the viability of murine LMSCs and reduce their level of oxidative stress-induced apoptosis to a better degree than NAC. Furthermore, CUR pretreatment reduced intracellular ROS production and improved the MMP of murine LMSCs. MMP is required for oxidative phosphorylation and ATP production; thus, MMP indirectly reflects mitochondrial status. Taken together, CUR was shown to enhance the antioxidant capacity of murine LMSCs, and was superior to the classical and powerful antioxidant NAC.
Bax and Bcl-2 belong to the Bcl-2 gene family, which plays key roles in regulating apoptosis. Bax and Bcl-2 regulate the stability of the mitochondrial structure, and function through synergistic action with other apoptotic proteins. The Bcl-2 family includes two types of proteins: anti-apoptotic proteins, such as Bcl-2, and pro-apoptotic proteins, such as Bax. In the early stage of apoptosis, the pro-apoptotic proteins form pores in the outer mitochondrial membrane by homologous oligomerization, resulting in cytochrome-c release and eventually caspase activation and apoptosis.28,29 This study found that levels of the anti-apoptotic protein Bcl-2 were decreased, while levels of the pro-apoptotic proteins Bax and cleaved caspase-3 were increased under oxidative stress. These results suggested that oxidative stress weakened the inhibition of apoptosis in LMSCs, while proteins that promoted apoptosis were enhanced. With CUR pretreatment, the Bcl-2/Bax ratio was increased, and caspase-3 expression was decreased, which further confirmed the protective effect of CUR on the viability of LMSCs.
Nrf2 belongs to the alkaline leucine zipper family and is a widely expressed transcription factor in vivo that has strong antioxidant capacity, primarily through HO-1 activation. Akt plays an important role in cell survival and apoptosis and participates in the activation and nuclear translocation of Nrf2 through phosphorylation.30 The Akt/Nrf2/HO-1 pathway is closely related to oxidative stress and apoptosis. Several studies have described agents that alleviate cellular oxidative damage through this pathway.31–33 In this study, CUR was found to promote Akt phosphorylation, increase the p-Akt/Akt ratio, and increase Nrf2 expression, leading to increased HO-1 release. Thus, this pathway may underlie the antioxidant effect of CUR.
Conclusion
In summary, CUR was demonstrated to have an antioxidant protective effect on murine LMSCs that may be related to the Akt/Nrf2/HO-1 signaling pathway. Additionally, H2O2-mediated mitochondrial damage was observed, which could potentially affect mitochondrial fusion, function, and/or autophagy; these potential effects will be the focus of future studies.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81560765, No. 81660760, and No. 81860826), the Technology Research and Development Program of the Education Department of Jiangxi Province (No. GJJ150851), the Technology Research and Development Program of the Science and Technology Department of Jiangxi Province (No. 20171BAB215056), and the Postgraduate Innovation Project of Jiangxi Province (YC2018-B075).
ORCID iD
Liangji Liu https://orcid.org/0000-0001-9567-0063
References
- 1.Xaubet A, Ancochea J, Molina-Molina M. Idiopathic pulmonary fibrosis. Med Clin (Barc) 2017; 148: 170–175. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 2017; 20: 17074. [DOI] [PubMed] [Google Scholar]
- 3.Akram KM, Samad S, Spiteri MA, et al. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir Res 2013; 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappetta D, De Angelis A, Spaziano G, et al. Lung mesenchymal stem cells ameliorate elastase-induced damage in an animal model of emphysema. Stem Cells Int 2018; 2018: 9492038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan YW, Choo KB, Chen CM, et al. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther 2015; 6: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harpole M, Davis J, Espina V. Current state of the art for enhancing urine biomarker discovery. Expert Rev Proteomics 2016; 13: 609–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 2015; 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandl A, Meyer M, Bechmann V, et al. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res 2011; 317: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 9.Cheresh P, Kim SJ, Tulasiram S, et al. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 2013; 1832: 1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med 2017; 22: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelli D, Sahebkar A, Johnston TP, et al. Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol Res 2017; 115: 133–148. [DOI] [PubMed] [Google Scholar]
- 12.Kunnumakkara AB, Bordoloi D, Harsha C, et al. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci (Lond) 2017; 131: 1781–1799. [DOI] [PubMed] [Google Scholar]
- 13.Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today 2017; 22: 1582–1592. [DOI] [PubMed] [Google Scholar]
- 14.Samarghandian S, Azimi-Nezhad M, Farkhondeh T, et al. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 2017; 87: 223–229. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Wang F, Gao Y, et al. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J Pharmacol Sci 2016; 132: 192–200. [DOI] [PubMed] [Google Scholar]
- 16.Rashid K, Chowdhury S, Ghosh S, et al. Curcumin attenuates oxidative stress induced NFκB mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochem Pharmacol 2017; 143: 140–155. [DOI] [PubMed] [Google Scholar]
- 17.Kuttikrishnan S, Siveen KS, Prabhu KS, et al. Curcumin induces apoptotic cell death via inhibition of PI3-Kinase/AKT pathway in B-Precursor acute lymphoblastic leukemia. Front Oncol 2019; 9: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez LR, Bui SN, Beuschel RT, et al. Curcumin induced oxidative stress attenuation by N-acetylcysteine co-treatment: a fibroblast and epithelial cell in-vitro study in idiopathic pulmonary fibrosis. Mol Med 2019; 25: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017; 389: 1941–1952. [DOI] [PubMed] [Google Scholar]
- 20.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014; 9: 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseinzadeh A, Javad-Moosavi SA, Reiter RJ, et al. Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis. Expert Opin Ther Targets 2018; 22: 1049–1061. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Cooley MA, Jarnicki AG, et al. Fibulin-1c regulates transforming growth factor-β activation in pulmonary tissue fibrosis. JCI Insight 2019; 5: 124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardo A, Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2019; 13: S417–S421. [DOI] [PubMed] [Google Scholar]
- 24.Chanda D, Otoupalova E, Smith SR, et al. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med 2019; 65: 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birbrair A. Stem cell microenvironments and beyond. Adv Exp Med Biol 2017; 1041: 1–3. [DOI] [PubMed] [Google Scholar]
- 26.Fois AG, Paliogiannis P, Sotgia S, et al. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir Res 2018; 19: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veith C, Boots AW, Idris M, et al. Redox imbalance in idiopathic pulmonary fibrosis: a role for oxidant cross-talk between NADPH oxidase enzymes and mitochondria. Antioxid Redox Signal 2019; 31: 1092–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nkpaa KW, Awogbindin IO, Amadi BA, et al. Ethanol exacerbates manganese-induced neurobehavioral deficits, striatal oxidative stress, and apoptosis via regulation of p53, caspase-3, and Bax/Bcl-2 ratio-dependent pathway. Biol Trace Elem Res 2019; 191: 135–148. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Yanagihara T, Yokoyama T, et al. Bax-inhibiting peptide attenuates bleomycin-induced lung injury in mice. Biol Open 2017; 6: 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai W, Deng S, Wu W, et al. Rapamycin attenuates the paraquat-induced pulmonary fibrosis through activating Nrf2 pathway. J Cell Physiol 2019; 235: 1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Cai ZN, Mehmood S, et al. Polysaccharide FMP-1 from Morchella esculenta attenuates cellular oxidative damage in human alveolar epithelial A549 cells through PI3K/AKT/Nrf2/HO-1 pathway. Int J Biol Macromol 2018; 120: 865–875. [DOI] [PubMed] [Google Scholar]
- 32.Cui W, Leng B, Wang G. Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharmacol 2019; 97: 370–376. [DOI] [PubMed] [Google Scholar]
- 33.Dai H, Wang P, Mao H, et al. Dynorphin activation of kappa opioid receptor protects against epilepsy and seizure-induced brain injury via PI3K/Akt/Nrf2/HO-1 pathway. Cell Cycle 2019; 18: 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]