Abstract
Background
Different therapies have been suggested for polycystic ovary syndrome (PCOS), but changes in lifestyle and diet have been considered. Diet and dietary factors can be very effective in modifying the disease. The positive effects of probiotic and synbiotics supplementation on improving lipid profiles and anthropometric indices have been examined in various diseases. This study was conducted to evaluate the effects of synbiotics supplementation on lipid and anthropometric profiles in infertile women with PCOS.
Methods
PCOS patients aged 19–37 years old were randomized to receive either synbiotics supplement (n = 50) or placebo (n = 49) for 12 weeks.
Results
Consumption of synbiotics compared to the placebo, resulted in a significant decrease in Low-density lipoprotein cholesterol (LDL) value (Change Mean Difference (CMD): 4.66, 95%CI: 0.20, 9.13) and a significant increase in high-density lipoprotein cholesterol (HDL) (CMD: 1.80, 95%CI: 0.34, 3.26). Although we failed to find a significant effect of synbiotics consumption on total cholesterol (TC) and triglyceride (TG) levels. We did not find differences in anthropometric indices between groups.
Conclusions
Overall, 12 weeks of synbiotics supplementation among PCOS women resulted in beneficial effects on LDL and HDL, although it is not yet clear how much our findings are clinically significant and more clinical studies with larger sample sizes are still needed.
Trial registration
Iranian Registry of clinical Trial, IRCT.ir, ID: IRCT2014110515536N2. Registered on 19 December 2015.
Graphical abstract
Keywords: Polycystic ovary syndrome; Synbiotics; Cholesterol, LDL; Cholesterol, HDL; Triglycerides
Background
PCOS is one of the most common polygenic endocrine disorders in women of reproductive age [1, 2]. The prevalence of this syndrome varies in different countries [3]. It may be influenced by race and body composition [4]. Based on the Rotterdam consensus, the prevalence of women with PCOS is more than 15% [5–7]. It is over 30% in women with overweight and obesity [4, 6]. There is a great deal of cost on health systems resulting from complications associated with PCOS [8]. More than half of the patients with PCOS are obese. Obesity increases the risk of diabetes mellitus and cardiovascular diseases [9]. Insulin resistance and increased serum insulin are commonly found in PCOS. One third of women with PCOS have impaired glucose tolerance and 10–70% of them are with type 2 diabetes [10]. One of other complications in PCOS is abnormalities in the metabolism of lipoproteins, which includes increased cholesterol, TG, and LDL and decreased HDL [11]. Various studies have shown that correction of the lipid pattern in patients with PCOS can be very helpful [12]. Different therapies have been suggested for PCOS, but changes in lifestyle and diet have been considered [13]. Diet and dietary factors can be very effective in modifying the disease. Probiotics and prebiotics dietary are considerations [14, 15].
Probiotics are living microorganisms originate from the gastrointestinal tract. They affect the human’s metabolic and inflammatory indices. Prebiotics are indigestible food compounds that selectively stimulate the growth and activity of some bacteria in intestinal flora. Synbiotics is a blend of prebiotic food and probiotic bacteria [16, 17]. Animal studies have shown that intestinal bacterial flora changes in PCOS and goes to pathogenic bacteria [18]. The positive effects of probiotic and synbiotics supplementation on improving lipid profiles and anthropometric indices have been examined in various diseases [19, 20]. This study was conducted to evaluate the effects of synbiotics supplementation on lipid and anthropometric profiles in infertile women with PCOS.
Material and methods
Design of the trial
This was a double blind placebo controlled trial conducted at the Arash hospital, Tehran, Iran. Women with PCOS were allocated to receive synbiotics supplement (n = 44) or placebo (n = 44) in a 1:1 ratio for a period of 12 weeks. The study protocol was approved by the Tehran University of Medical Sciences Ethics Committee, which granted full ethical approval for the trial in September 2015. This trial was registered at Iranian Registry of clinical Trial (www.IRCT.ir) by the number of IRCT2014110515536N2. Written informed consent was obtained from all study participants.
Participants
Women diagnosed with PCOS aged 19–37 years old were identified at their first visit to Arash hospital, Tehran, Iran. Inclusion criteria were as follows: confirmed PCOS based on the 2003 Rotterdam criteria (two of the following features: Oligo-ovulation and/or anovulation, clinical and biochemical hyperandrogenism, and polycystic ovaries in ultrasonography). Women were unsuitable for inclusion if their body mass index (BMI) was below the specified range (BMI < 25), they had a history of thyroid disorders, hyperprolactinemia, Cushing’s syndrome, liver and kidney disease, cardiovascular disease, digestive diseases (food allergies, celiac disease, irritable bowel disease), high blood pressure and diabetes, autoimmune disease, allergy to probiotic capsules or placebo, current or previous consumption of antibiotic, multivitamin mineral supplements, probiotics, perebiotic, synbiotics and specific diet or physical activity programs (within the last 3 months), or they were pregnant or breast-feeding.
Intervention
Synbiotics and placebo capsules were produced and supplied by Zist Takhmir Company. Each active synbiotics capsule contained 500 mg of seven strains beneficial bacteria (Lactobacillus acidophilus 3× 1010 CFU/g, Lactobacillus casei 3× 109 CFU/g, Lactobacillus bulgaricus 5× 108 CFU/g, Lactobacillus rhamnosus 7× 109 CFU/g, Bifidobacterium longum 1× 109 CFU/g, Bifidobacterium breve 2× 1010 CFU/g, Streptococcus thermophilus) 3× 108 CFU/g) and prebiotic Inulin (fructooligosaccharide). Placebo capsules contained 500 mg starch and maltodextrins without bacteria. Both capsules were identical in appearance, smell and taste, and packaged in compartment labeled as either drug A or drug B, and these capsules were stored at − 5 °C until dispensed to participants. Capsule identification for patients and research staff was not possible. The viability of the synbiotics was confirmed by regular analysis of capsules in the hospital laboratory.
Randomization and concealment
Allocation to treatment groups was conducted by an independent researcher using a random number sequence, generated with a computer-generated randomization scheme, according to a randomised block design. The block size was six. The randomization list was concealed from the primary researcher who enrolled and assessed participants in sequentially numbered, sealed, opaque envelopes. The study period consisted of a 12-week consumption period, in which initiation of the test and consumption of either the synbiotics or placebo was designated as week 0 (W0). Compliance with the drug consumption guidelines was monitored via phone call once per week. Patients were requested not to alter their diets or normal physical activity and avoid consuming other synbiotics and fermented products during the study. The compliance was also guaranteed by the use of three-day dietary records completed during the study and Nutritionist IV program was used to estimate dietary intake of patients.
Study outcomes and clinical investigations
Anthropometric measurements, dietary intakes and biochemical indices were evaluated in all participants at baseline and after 12 weeks of intervention in each separate arm. Body weight was measured with a digital scale (model 220; Seca, Hamburg Germany; weighing accuracy of 0.1 kg) with subjects in underwear, in an overnight fasting status, without shoes and in a minimal clothing and after voiding their bladder. Body height was measured in centimeters, standing upright, using a wall mounted stadiometer (model 220; Seca) and the reading was taken to the last completed 1 mm (0.1 cm). BMI was calculated as weight (kilograms) divided by height (meters) squared. Waist circumference (WC) was measured with a flexible tape measure, at the uppermost lateral border of the hip crest (ilium), and was recorded to the nearest millimeter. Hip circumference (HC) measured at the largest posterior extension of the buttocks. Waist hip ratio (WHR) was calculated as waist/height. Trained research staff took all measurements.
A volume of 15 CC blood sample were obtained in Vacutainers after 12–14 h of fasting and sera were separated, processed and stored at − 80 °C for the determination of serum TC, TG and HDL. Fasting plasma TC, TG and HDL were measured by standard techniques. TC was determined by an enzymatic colorimetric method using cholesterol esterase, cholesterol oxidase, peroxidase and the chromagen 4-aminophenazone/phenol. TG levels were determined by an enzymatic colorimetric method using lipoprotein lipase glycerokinase, glycerophosphate oxidase and the chromagen 4-aminophenazone/N-ethyl-N-(3-sulphopropyl)-m-amisidine. HDL was determined by immunoinhibition assay. LDL was calculated according to the Friedewald formula as follow: LDL = TC – HDL − (TG/5). For FSH, LH, E2, Progesterone and Testosterone measurements, immunometric assays based on enhanced luminescence were used (electrochemical luminescence analyzer, E411; Roche Diagnostics, Mannheim, Germany). The results are expressed as IU/L. All samples were analyzed at the Clinical Chemistry Laboratory of the Arash Hospital, Tehran, Iran.
Sample size and statistical analysis
It was assumed that a total sample of 100 subjects (50) women per group, (which included a 15% dropout factor) would provide 80% power to detect a difference in mean of TG between the synbiotics and placebo groups. Assuming means of 250(mg/dl) in synbiotics group and 190(mg/dl) in the placebo group; a standard deviation (SD) of 100; and a two-sided test having a type I error of 0.05.
All statistical analyses were performed with IBM SPSS software for Windows (version 20.0; IBM). Baseline characteristics were compared among the two intervention groups by using the independent sample t– test for continuous data and a chi-square test for categorical data. The changes in anthropometric measurements, nutrient intakes, and blood lipid parameters of the patients between the beginning and end of the intervention were compared by paired sample t-tests. The magnitude of the effect is presented as mean difference and its 95% confidence interval. Linear mixed effects model were fit to assess changes from baseline within the treatment groups and differences of those changes between treatment groups with respect to continuous outcomes over time. Data were analysed according to the intention-to-treat principle.
Results
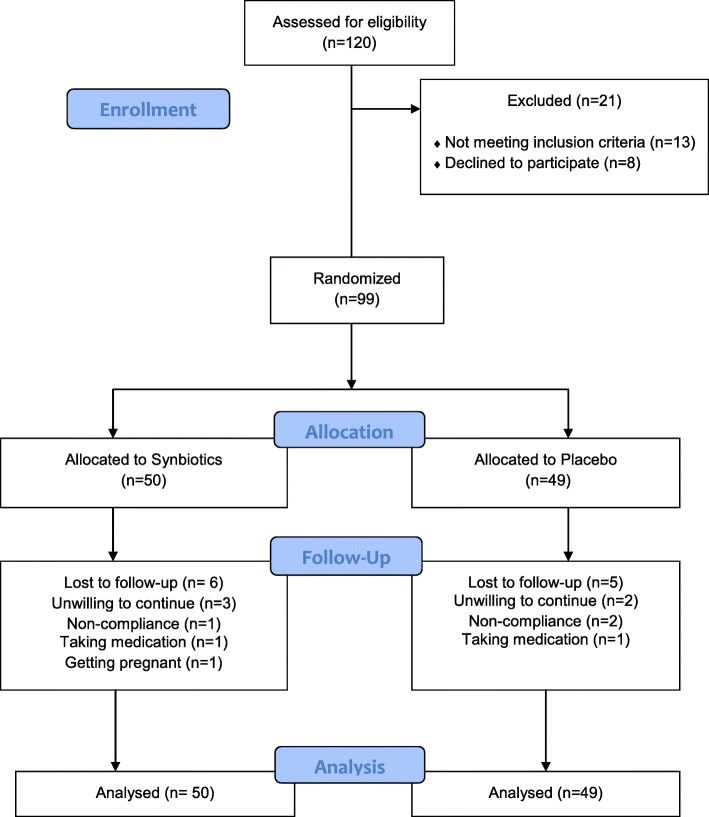
Recruitment of study participants commenced in September 2015 and ended in July 2016. During two months, 120 women were screened for eligibility; 99 women had inclusion criteria and were randomly assigned to either the placebo or the intervention groups. Eleven patients dropped out during the study period because of non-compliance to the allocated intervention (n = 3), no drug intake (n = 2), unwillingness to continue (n = 5) and pregnancy (n = 1). Finally, 88 subjects (synbiotics [n = 44], placebo [n = 44]) completed the study (Fig. 1). There were no significant differences between the patients of the two groups in maternal age, BMI, marital status, history of previous pregnancy, infertility, history of type2 diabetes, or menstruation (Table 1).
Fig. 1.
Flowchart showing participants’ recruitment. Non-compliance of the allocated intervention (n = 3), taking medication (n = 2), unwilling to continue (n = 5) and getting pregnant (n = 1).The analysis was intention-to-treat approach (ITT)
Table 1.
Patient’s characteristic after random assignment
| Total | Groups | P | ||
|---|---|---|---|---|
| Intervention | Control | |||
| Maternal age (years) | ||||
| Mean ± SD | 28.5 ± 5.3 | 28.1 ± 5.5 | 29 ± 5.1 | 0.471† |
| Median (range) | 28 (19 to 37) | 27 (19 to 37) | 28 (19 to 37) | |
| BMI(Kg/m2) | ||||
| Mean ± SD | 32.46 ± 5.27 | 32.89 ± 6.11 | 32 ± 4.23 | 0.435† |
| Median (range) | 31.54 (28.01 to 35.59) | 30.6 (28.01 to 36.16) | 31.76 (28.1 to 35.15) | |
| Marriage | ||||
| Single | 18 (20.5%) | 10 (22.2%) | 8 (18.6%) | 0.674* |
| Married | 70 (79.5%) | 35 (77.8%) | 35 (81.4%) | |
| Widow | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Divorced | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| History of previous pregnancy | ||||
| Yes | 41 (46.6%) | 21 (46.7%) | 20 (46.5%) | 0.988* |
| No | 47 (53.4%) | 24 (53.3%) | 23 (53.5%) | |
| Infertility | ||||
| Yes | 35 (39.8%) | 17 (37.8%) | 18 (41.9%) | 0.696* |
| No | 53 (60.2%) | 28 (62.2%) | 25 (58.1%) | |
| History of type2 diabetes | ||||
| Yes | 17 (19.3%) | 9 (20.0%) | 8 (18.6%) | 0.868* |
| No | 71 (80.7%) | 36 (80.0%) | 35 (81.4%) | |
| Menstruation | ||||
| Normal | 30 (34.1%) | 14 (31.1%) | 16 (37.2%) | 0.834* |
| Oligoamenorhea | 43 (48.9%) | 23 (51.1%) | 20 (46.5%) | |
| Amenorrhea | 15 (17.0%) | 8 (17.8%) | 7 (16.3%) | |
† Based on t-test. * Based on Chi-Square test
Tables 2 and 3 present baseline, post intervention and values of changes for anthropometric measurements, nutrient intakes, and blood lipid parameters in synbiotics and placebo group, respectively. No significant difference was detected in nutritional intake (average calorie, macronutrients, and fiber intake) from pre intervention to post intervention between synbiotics and placebo groups. No statistically significant differences existed in anthropometric measurements and blood lipid parameters between the synbiotics and placebo groups at baseline.
Table 2.
Dietary intakes of study participants throughout the study
| Intervention | Control | Diff a | 95% CI | P | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Lower | Upper | |||
| Energy intake (kcal) | ||||||
| Pre | 2317.27 ± 542.81 | 2210.05 ± 360.47 | 107.22 | −88.99 | 303.43 | 0.28 |
| Post | 2322.6 ± 550.2 | 2204.8 ± 336.4 | 117.72 | −76.61 | 312.04 | 0.344 |
| Change | 5.33 ± 66.89 | −5.25 ± 54.59 | −10.50 | − 36.44 | 15.44 | 0.423 |
| Carbohydrate intake (% of TE) b | ||||||
| Pre | 52.18 ± 5.09 | 52.95 ± 4.61 | − 0.78 | −2.84 | 1.29 | 0.457 |
| Post | 52.22 ± 4.17 | 53.28 ± 4.42 | −1.06 | −2.88 | 0.76 | 0.276 |
| Change | −0.04 ± 2.14 | − 0.33 ± 2.09 | 0.28 | − 0.62 | 1.18 | 0.535 |
| Protein intake (% of TE) | ||||||
| Pre | 11.62 ± 2.38 | 12.28 ± 2.21 | − 0.66 | −1.63 | 0.32 | 0.183 |
| Post | 11.67 ± 2.36 | 12.3 ± 2.41 | − 0.64 | −1.65 | 0.38 | 0.859 |
| Change | −0.04 ± 1.19 | − 0.02 ± 1.26 | −0.02 | − 0.54 | 0.50 | 0.936 |
| Fat intake (% of TE) | ||||||
| Pre | 36.2 ± 4.47 | 34.81 ± 4.18 | 1.39 | − 0.45 | 3.22 | 0.137 |
| Post | 36 ± 4 | 34 ± 4 | 1.74 | 0.18 | 3.30 | 0.067 |
| Change | 0.04 ± 2.22 | 0.4 ± 1.85 | −0.35 | −1.22 | 0.52 | 0.424 |
| Fiber Intake(g) | ||||||
| Pre | 11.86 ± 1.55 | 12.19 ± 1.7 | −0.33 | −1.02 | 0.36 | 0.343 |
| Post | 12.02 ± 1.52 | 12.35 ± 1.32 | −0.33 | −1.02 | 0.36 | 0.622 |
| Change | −0.17 ± 0.87 | −0.16 ± 0.9 | −0.33 | −1.02 | 0.36 | 0.984 |
a Intervention minus control group
bTE total energy
Table 3.
Comparison of anthropometric measurements and blood lipid parameters between two Groups
| Intervention | Control | Diff a | 95% CI | P | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Lower | Upper | |||
| Weight1 | ||||||
| Pre | 84 ± 18 | 80.3 ± 12.1 | 3.67 | −2.87 | 10.21 | 0.267† |
| Post | 83.81 ± 18.66 | 80.23 ± 11.39 | 3.58 | −3.01 | 10.17 | 0.891§ |
| Change | 0.18 ± 2.34 | 0.08 ± 8.7 | 0.09 | −2.58 | 2.77 | 0.944 |
| BMI1 | ||||||
| Pre | 32.89 ± 6.11 | 32 ± 4.23 | 0.89 | −1.37 | 3.14 | 0.435† |
| Post | 32.81 ± 6.33 | 32.04 ± 4.58 | 1.19 | − 1.60 | 3.13 | 0.524§ |
| Change | −0.07 ± 0.91 | 0.5 ± 3.77 | − 0.12 | − 1.27 | 1.02 | 0.829 |
| Waist circumference | ||||||
| Pre | 90.5 ± 12.3 | 90 ± 9 | 0.55 | −4.04 | 5.14 | 0.814† |
| Post | 89.87 ± 13.03 | 89.56 ± 8.87 | 0.32 | −4.43 | 5.06 | 0.832§ |
| Change | 0.65 ± 3.21 | 0.42 ± 5.43 | 0.23 | −1.65 | 2.11 | 0.810 |
| Hip circumference | ||||||
| Pre | 112 ± 10 | 110.55 ± 8.76 | 1.72 | −2.34 | 5.79 | 0.400† |
| Post | 111.59 ± 10.34 | 110.47 ± 7.48 | 1.12 | − 2.72 | 4.96 | 0.66§ |
| Change | 0.69 ± 2.67 | 0.09 ± 5.06 | 0.60 | −1.10 | 2.31 | 0.482 |
| WHR1 | ||||||
| Pre | 0.81 ± 0.06 | 0.82 ± 0.07 | −0.01 | −0.04 | 0.02 | 0.478† |
| Post | 0.8 ± 0.07 | 0.81 ± 0.06 | −0.01 | − 0.03 | 0.02 | 0.705§ |
| Change | 0.001 ± 0.03 | 0.004 ± 0.03 | −0.003 | −0.01 | 0.01 | 0.599 |
| Blood pressure systolic | ||||||
| Pre | 113 ± 25 | 114 ± 10 | −0.88 | −9.17 | 7.41 | 0.833† |
| Post | 117 ± 12 | 115 ± 10 | 1.98 | −2.79 | 6.74 | 0.28§ |
| Change | −3.46 ± 22.49 | −0.6 ± 5.55 | −2.86 | −9.96 | 4.24 | 0.425 |
| Blood pressure diastolic | ||||||
| Pre | 75.67 ± 9.81 | 73.36 ± 7.99 | 2.31 | −1.52 | 6.14 | 0.234† |
| Post | 73 ± 10 | 74 ± 8 | − 1.43 | −5.29 | 2.42 | 0.025§ |
| Change | 2.91 ± 8.14 | −0.83 ± 4.79 | 3.74 | 0.87 | 6.62 | 0.011 |
| Total cholesterol (mg/dl) | ||||||
| Pre | 175.2 ± 27.5 | 175.1 ± 28.7 | 0.08 | −11.82 | 11.99 | 0.989† |
| Post | 170 ± 24 | 173 ± 32 | −3.03 | −14.85 | 8.78 | 0.433§ |
| Change | 5 ± 18.53 | 1.88 ± 20.76 | 3.12 | −5.22 | 11.45 | 0.459 |
| High density lipoprotein cholesterol(mg/dl) | ||||||
| Pre | 46.44 ± 7.69 | 45.19 ± 8.14 | 1.26 | −2.10 | 4.61 | 0.458† |
| Post | 45 ± 8 | 45 ± 8 | −0.55 | −3.79 | 2.70 | 0.023§ |
| Change | 1.71 ± 3.75 | −0.09 ± 3.09 | 1.80 | 0.34 | 3.26 | 0.016 |
| Low density lipoprotein cholesterol(mg/dl) | ||||||
| Pre | 97 ± 19 | 96 ± 20 | 0.80 | −7.52 | 9.12 | 0.849† |
| Post | 92 ± 19 | 95 ± 20 | −3.87 | −11.99 | 4.26 | 0.038§ |
| Change | −5.27 ± 11.32 | 0.6 ± 9.63 | 4.66 | 0.20 | 9.13 | 0.041 |
| Triglyceride(mg/dl) | ||||||
| Pre | 139 ± 78 | 134 ± 87 | 4.92 | −29.97 | 39.81 | 0.78† |
| Post | 141 ± 78 | 130 ± 83 | 10.53 | −23.56 | 44.63 | 0.469§ |
| Change | −2.24 ± 44.59 | 3.37 ± 41.48 | −5.62 | −23.89 | 12.66 | 0.543 |
a Intervention minus control group
† Based on t-test
§ Based on Linear mixed effects model (the included variables were: basic value of dependent variable, treatment type, BMI and Maternal age)
Results of linear mixed effects models showed statistically significant differences between the two groups in LDL (P = 0.041) and HDL (P = 0.016) at the end of study, adjusted for BMI, maternal age, and baseline values. Consumption of a synbiotics, compared to the placebo, resulted in a significant decrease in LDL value (Change Mean Difference: 4.66, 95%CI: 0.20, 9.13) and a significant increase in HDL (Change Mean Difference: 1.80, 95%CI: 0.34, 3.26). Although we failed to find a significant effect of synbiotics consumption on TC (Change Mean Difference: 3.12, 95% CI: − 5.22, 11.45, P = 0.459) and TG (Change Mean Difference: -5.62, 95% CI: − 23.89, 12.66, P = 0.543) levels. We did not find differences in weight (Change Mean Difference: 0.09, 95% CI: − 2.58, 2.77, P = 0.944), BMI (Change Mean Difference: -0.12, 95% CI: − 1.27, 1.02, P = 0.829), WC(Change Mean Difference: 0.23, 95% CI: − 1.65, 2.11, P = 0.810), HC (Change Mean Difference: 0.60, 95% CI: − 1.10, 2.31, P = 0.482) or WHR (Change Mean Difference: -0.003, 95%CI: − 0.01, 0.01, P = 0.599) between groups.
Discussion
The results of current study showed that synbiotics supplementation for 12 weeks can increase HDL and decrease LDL in patients with PCOS. This effect was also significant after adjusting for confounding variables, including gestational age, BMI and baseline indices. However, no significant between-group difference was found for anthropometric indices such as BMI, WC, HC and WHR in women with PCOS.
In line with the results of this trial, a recent 12-week clinical trial among 60 women aged 18–40 years old diagnosed with PCOS demonstrated that the administration of one serving/day of synbiotics capsule (500 mg), resulted in significant decreases in TG, VLDL, while no alterations in anthropometric indices were reported. In the mentioned study, three probiotic strain was similar to the present study (Lactobacillus acidophilus strain T16 (IBRC-M10785), Lactobacillus casei strain T2 (IBRC-M10783), and Bifidobacterium bifidum strain T1 (IBRC-M10771) (2 × 109 CFU/g each)) [21]. On the contrary, Ahmadi et al. showed reduction effects of probiotic on weight and BMI in patients with PCOS. In this randomized, double-blind, placebo-controlled trial, 60 women with PCOS were randomized to receive probiotic capsule (n = 30) or placebo (n = 30) for 12 weeks. In their study, dry and freeze probiotics of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum were used. Compared to the present study, a more limited range of probiotics was used and prebiotic was not used [14]. Furthermore, a 2019 meta-analysis (N = 8 trials, nine treatment arms), reported beneficial effects of pro−/synbiotics supplementation on insulin resistance indices without any significant effects on anthropometric measurements [22]. In a systematic review and meta-analysis, Hadi et al. evaluated the effects of synbiotics supplementation on anthropometric indices among participants with overweight or obesity. This meta-analysis of 23 randomized trials indicated that supplementation with synbiotics can decrease body weight and WC. In contrast, synbiotics did not have favorite effects on BMI and body fat compared with the placebo group [23]. A study by Gomes et al. in 2017 showed that probiotic supplement reduces abdominal fat in women with obesity and overweight. In this study, a probiotic mixture, without prebiotics, was used. The probiotic composition of the Gomez’s study included Lactobacillus acidophilus, Casei Lactobacillus, Lactococcus lactis, Bifidum bifidobacterium, Bifidobacterium lactis, which is different from the probiotic composition of the present study. Also, the study was conducted on women with obesity who received diet alongside probiotics [24]. Another study revealed that the synbiotics supplementation reduces weight and improves the BMI in people with diabetes. In this study, the probiotic mixture of the Lactobacillus family, the Bifidobacterium family and Streptococcus thermophiles was used. Prebiotics consisted of freto-oligosaccharides and lactose, as well as vitamin group B supplement, maltodextrin and magnesium. Different compound of probiotic and prebiotic supplements could lead to the difference between findings of this study and the present study [25]. In a systematic review study of clinical trials, it was shown that probiotic supplement could cause weight loss and improve BMI when a variety of strains was used. The supplement also more affected people with overweight. The duration of supplement should be more than 8 weeks [26]. Ferolla et al. in 2016 showed that probiotic supplement could lead to weight loss in patients with nonalcoholic steatohepatitis (NASH). They also used Lactobacillus reuteri strain as probiotic supplement, and guar gum and inulin as prebiotic supplement. Diet was also prescribed for all participants [27]. In a systematic review study, Maria Jose Sáez-Lara et al. evaluated the effects of synbiotics supplementation on the prevention and treatment of obesity, IRS, DM2, and non-alcoholic fatty liver disease (NAFLD). Supplementations of synbiotics and probiotic were associated with weight loss and BMI in people with obesity. This effect has been observed in different strains and in different prebiotic combinations [28]. Bernini, L.J.a et al. in 2016 showed that supplement of Lactobacillus lactis strain decreases BMI in patients with metabolic syndrome. In their study, the probiotic strain as fermented milk was consumed by participants. The length of the study was 45 days [29]. It seems that some divergence between these trials described in these two reviews is potentially due to differences in the probiotic strain, study duration and severity of disease.
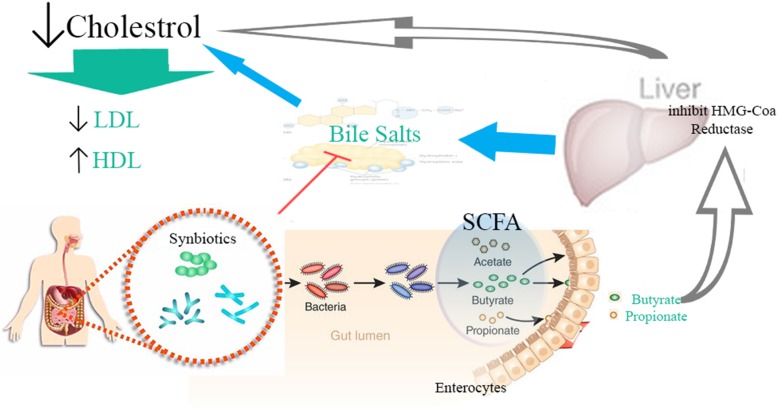
The present study showed that synbiotics supplement could reduce LDL and increase HDL in patients with PCOS. Although these changes were statistically significant, but their values are not clinically significant, and it seems that synbiotics may have complementary therapies role in LDL and HDL managements. Previous studies have confirmed a decrease in LDL and increased HDL through probiotic supplement [30]. Decreased LDL and TC and TG have been observed in previous studies [31]. In a clinical trial on men and women with hypercholesterolemia, a significant decrease was observed in LDL and TC after a 12-week probiotic and prebiotic supplement [32]. A systematic review investigated the use of probiotics and prebiotics simultaneously and their effect on lipid profile. Synbiotics supplement effectiveness is better than probiotic effectiveness only in improving lipid profiles [33, 34]. In addition to the combination of probiotics and prebiotics, the probiotic strain also may be effective in reducing serum cholesterol. Another systematic review and meta-analysis conducted in 2015 showed that the strain of Lactobacillus Acidophilus had the highest effect on lowering LDL compared to other probiotic strains [35]. Bernini et al. found that probiotic supplement reduced LDL and TC [29]. In a systematic review study in 2011, it was concluded that probiotic supplement decreased TC and LDL. The suggested mechanism in this study was to integrate cholesterol into the cell wall of probiotic bacteria in the intestinal tissue and to prevent the absorption of cholesterol [36, 37]. Another suggested mechanism of probiotic supplement for the reduction of cholesterol was production of hydrolytic enzymes by these microorganisms. It causes deconjugation and reduction in absorption of bile acids in the intestine; thereby eliminating the acids through feces and consequently, reducing cholesterol levels [38]. On the other hand, probiotics in the intestine increase the Short-chain fatty acids (SCFA), such as acetate and propionate. Propionate has a variety of metabolic effects and in the liver, inhibits the enzyme HMG-COA reductase, a restriction enzyme in the production of cholesterol in the body. In this way, probiotics supplement may also reduce total cholesterol and LDL levels [39].
The strengths of the study are the inclusion of all PCO phenotypes, which allowed a much greater possibility of drawing generalizable conclusions and small number of participants’ drop out. However, this trial has several limitations that need to be addressed. First, lack of an objective method for measuring patient compliance like fecal bacterial profiles. Second, probability of recall bias due to dietary intake was assessed by using 3-day food records. Third, it would be better to compare four groups: placebo, synbiotic alone, probiotic alone, and the combination of probiotic and synbiotic, but we have resource limitation to conduct this design. And finally maybe longer duration could make us able to find more reliable and clinical worthwhile outcomes.
Conclusions
The present study showed that the use of synbiotics in reducing LDL and also increasing HDL in women with PCOS could be effective. Considering that lipid profile disorders are one of the common problems in PCOS patients, it seems that synbiotics supplement in these patients could reduce the symptoms of the disease. Based on this initial study, 12 weeks of synbiotics supplementation among PCOS women resulted in beneficial effects on LDL and HDL. The clinical impact - both short term and long term - is unknown. It is recommended that more clinical studies with a larger sample size be conducted in the future.
Acknowledgements
The authors thank the participants, staff of Arash women’s hospital and Arash research development center for their assistance during data collection and data cleaning.
Abbreviations
- PCOS
Polycystic ovary syndrome
- LDL
Low-density lipoprotein cholesterol
- CMD
Change Mean Difference
- HDL
High-density lipoprotein cholesterol
- TC
Total cholesterol
- TG
Triglyceride
- WC
Waist circumference
- HC
Hip circumference
- WHR
Waist hip ratio
- SD
Standard deviation
- BMI
Body mass index
- NASH
Nonalcoholic steatohepatitis
- NAFLD
Non-alcoholic fatty liver disease
Authors’ contributions
MS and EK: designed the experiments, performed the experiments, analysed, interpreted data and prepared the manuscript; MA: assisted with study design, performed the study protocol and revised the manuscript; NS and SV: assisted with study design, performed the study protocol and revised the manuscript; MH and AM: verified all obtained results and revised the manuscript; JH: designed the experiments, provided the samples, analysed the data and revised the manuscript; and all authors took part in preparation and modification of figures and the manuscript. The author(s) read and approved the final manuscript.
Funding
The authors have no support of funding to report.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The study protocol was approved by the institutional review board of Tehran University of Medical Sciences. All participants completed written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pasquali R, Gambineri A. Polycystic ovary syndrome: a multifaceted disease from adolescence to adult age. Ann N Y Acad Sci. 2006;1092:158–174. doi: 10.1196/annals.1365.014. [DOI] [PubMed] [Google Scholar]
- 2.ESHRE TT, Group A-SPCW Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89:505–522. doi: 10.1016/j.fertnstert.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Boyle JA, Cunningham J, O’Dea K, Dunbar T, Norman RJ. Prevalence of polycystic ovary syndrome in a sample of indigenous women in Darwin, Australia. Med J Aust. 2012;196:62–66. doi: 10.5694/mja11.10553. [DOI] [PubMed] [Google Scholar]
- 4.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2009;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 5.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome-part 1. Endocr Pract. 2015;21:1291–1300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 6.Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected caucasian women from Spain 1. J Clin Endocrinol Metabol. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 7.ESHRE TR, Group A-SPCW Revised 2003 Consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–4658. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 9.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elnashar A. An evidence based approach for diagnosis of adolescent polycystic ovarian syndrome. Middle East Fertil Soc J. 2016;21:194–195. doi: 10.1016/j.mefs.2016.05.003. [DOI] [Google Scholar]
- 11.Palomba S, Santagni S, Falbo A, La Sala GB. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Women's Health. 2015;7:745. doi: 10.2147/IJWH.S70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol. 1994;41:463–471. doi: 10.1111/j.1365-2265.1994.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 13.Costello MF, Misso ML, Wong J, Hart R, Rombauts L, Melder A, Norman RJ, Teede HJ. The treatment of infertility in polycystic ovary syndrome: a brief update. Aust N Z J Obstet Gynaecol. 2012;52:400–403. doi: 10.1111/j.1479-828X.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadi S, Jamilian M, Karamali M, Tajabadi-Ebrahimi M, Jafari P, Taghizadeh M, Memarzadeh MR, Asemi Z. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Hum Fertil. 2017;20:1–8. [DOI] [PubMed]
- 15.Rashad NM, Amal S, Amin AI, Soliman MH. Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J Funct Foods. 2017;36:317–324. doi: 10.1016/j.jff.2017.06.029. [DOI] [Google Scholar]
- 16.Gatlin III D, Peredo AM: Prebiotics and probiotics: definitions and applications Prebiotics and probiotics: definitions and applications 2012.
- 17.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am J Clin Nutr. 2001;73:361s–364s. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, Tang L. Association between polycystic ovary syndrome and gut microbiota. PLoS One. 2016;11:e0153196. doi: 10.1371/journal.pone.0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38:38. [PMC free article] [PubMed] [Google Scholar]
- 20.Hütt P, Songisepp E, Rätsep M, Mahlapuu R, Kilk K, Mikelsaar M. Impact of probiotic Lactobacillus plantarum TENSIA in different dairy products on anthropometric and blood biochemical indices of healthy adults. Benefic Microbes. 2014;6:233–243. doi: 10.3920/BM2014.0035. [DOI] [PubMed] [Google Scholar]
- 21.Shoaei T, Heidari-Beni M, Tehrani HG. Effects of probiotic supplementation on pancreatic β-cell function and c-reactive protein in women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Int J Prev Med. 2015;6:27-33. [DOI] [PMC free article] [PubMed]
- 22.Hadi A, Moradi S, Ghavami A, Khalesi S, Kafeshani M. Effect of probiotics and synbiotics on selected anthropometric and biochemical measures in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Clin Nutr. 2019;3:1-5. [DOI] [PubMed]
- 23.Hadi A, Alizadeh K, Hajianfar H, Mohammadi H, Miraghajani M. Efficacy of synbiotic supplementation in obesity treatment: a systematic review and meta-analysis of clinical trials. Crit Rev Food Sci Nutr. 2018;60:1–13. [DOI] [PubMed]
- 24.Gomes AC, de Sousa RGM, Botelho PB, Gomes TLN, Prada PO, Mota JF. The additional effects of a probiotic mix on abdominal adiposity and antioxidant status: a double-blind, randomized trial. Obesity. 2017;25:30–38. doi: 10.1002/oby.21671. [DOI] [PubMed] [Google Scholar]
- 25.sadat Ebrahimi Z, Nasli-Esfahani E, Nadjarzade A, Mozaffari-khosravi H. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: a randomized, double-blind, clinical trial. J Diabetes Metab Disord. 2017;16:23. doi: 10.1186/s40200-017-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Wu Y, Fei X. Effect of probiotics on body weight and body-mass index: a systematic review and meta-analysis of randomized, controlled trials. Int J Food Sci Nutr. 2016;67:571–580. doi: 10.1080/09637486.2016.1181156. [DOI] [PubMed] [Google Scholar]
- 27.Ferolla SM, Couto CA, Costa-Silva L, Armiliato GN, Pereira CA, Martins FS, MdLA F, Vilela EG, Torres HO, Cunha AS. Beneficial effect of Synbiotic supplementation on hepatic Steatosis and anthropometric parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients. 2016;8:397. doi: 10.3390/nu8070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci. 2016;17:928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernini LJ, Simão ANC, Alfieri DF, Lozovoy MAB, Mari NL, de Souza CHB, Dichi I, Costa GN. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Effects of probiotics on metabolic syndrome. Nutrition. 2016;32:716–719. doi: 10.1016/j.nut.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Fabian E, Elmadfa I. Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Ann Nutr Metab. 2006;50:387–393. doi: 10.1159/000094304. [DOI] [PubMed] [Google Scholar]
- 31.Klein A, Friedrich U, Vogelsang H, Jahreis G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur J Clin Nutr. 2008;62:584. doi: 10.1038/sj.ejcn.1602761. [DOI] [PubMed] [Google Scholar]
- 32.Ooi L-G, Ahmad R, Yuen K-H, Liong M-T. Lactobacillus acidophilus CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J Dairy Sci. 2010;93:5048–5058. doi: 10.3168/jds.2010-3311. [DOI] [PubMed] [Google Scholar]
- 33.DiRienzo DB. Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutr Rev. 2014;72:18–29. doi: 10.1111/nure.12084. [DOI] [PubMed] [Google Scholar]
- 34.Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. Effect of probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: a randomized controlled single-blind pilot study. J Cardiovasc Pharmacol Ther. 2015;20:289–298. doi: 10.1177/1074248414555004. [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2015;47:430–440. doi: 10.3109/07853890.2015.1071872. [DOI] [PubMed] [Google Scholar]
- 36.Brashears M, Gilliland S, Buck L. Bile salt deconjugation and cholesterol removal from media by Lactobacillus casei1. J Dairy Sci. 1998;81:2103–2110. doi: 10.3168/jds.S0022-0302(98)75785-6. [DOI] [PubMed] [Google Scholar]
- 37.Greany K, Bonorden M, Hamilton-Reeves J, McMullen M, Wangen K, Phipps W, Feirtag J, Thomas W, Kurzer M. Probiotic capsules do not lower plasma lipids in young women and men. Eur J Clin Nutr. 2008;62:232. doi: 10.1038/sj.ejcn.1602719. [DOI] [PubMed] [Google Scholar]
- 38.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang G, Liu X-M, Zhang Q-X, Tian F-W, Zhang H, Zhang H-P, Chen W. Research advances with regards to clinical outcome and potential mechanisms of the cholesterol-lowering effects of probiotics. Clin Lipidol. 2012;7:501–507. doi: 10.2217/clp.12.40. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.