Abstract
Maternal bonding early postpartum lays an important foundation for child development. Changing brain structure and function during pregnancy and postpartum may underscore maternal bonding. We employed connectome-based predictive modeling (CPM) to measure brain functional connectivity and predict self-reported maternal bonding in mothers at 2 and 8 months postpartum. At 2 months, CPM predicted maternal anxiety in the bonding relationship: Greater integration between cerebellar and motor–sensory–auditory networks and between frontoparietal and motor–sensory–auditory networks were associated with more maternal anxiety toward their infant. Furthermore, greater segregation between the cerebellar and frontoparietal, and within the motor-sensory-auditory networks, was associated with more maternal anxiety regarding their infant. We did not observe CPM prediction of maternal bonding impairments or rejection/anger toward the infant. Finally, considering 2 and 8 months of data, changes in network connectivity were associated with changes in maternal anxiety in the bonding relationship. Our results suggest that changing connectivity among maternal brain networks may provide insight into the mother–infant bond, specifically in the context of anxiety and the representation of the infant in the mother’s mind. These findings provide an opportunity to mechanistically investigate approaches to enhance the connectivity of these networks to optimize the representational and behavioral quality of the caregiving relationship.
Keywords: cerebellum, connectome, connectomics, parenting, postpartum bonding
Introduction
The quality of the mother–infant bond is critical for child development and well-being (Bowlby 1969). Structural and functional brain changes during the perinatal period putatively lay the foundation for optimal maternal bonding (Kim et al. 2010; Hoekzema et al. 2017). Therefore, studies of the maternal brain may provide new insight into potential neural pathways of optimal, and sub-optimal, caregiving to optimize outcomes for mothers and children (Squire and Stein 2003). Studies of human maternal neurocircuitry were motivated by rich preclinical literature evidencing neural reorganization during the postpartum period in cortical (e.g., prefrontal and orbitofrontal), limbic (e.g., amygdala), hypothalamic (including the medial preoptic area), and striatal (e.g., ventral tegmental area, nucleus accumbens) regions (e.g., Barrett and Fleming 2011; Pereira 2016). Critically, neural reorganization becomes more distributed across the postpartum period with increasing maternal experience (Pereira and Morrell 2011).
Accumulating research has employed functional magnetic resonance imaging (fMRI) to identify neural circuitry underscoring maternal caregiving (Rutherford and Mayes 2013). Most studies have examined regional activation in the maternal brain following the presentation of visual and/or auditory infant stimuli (e.g., Leibenluft et al. 2004; Strathearn et al. 2008; Kim et al. 2011). Convergent findings suggest key networks are activated in response to infant stimuli, and these networks include brain regions implicated in reward processing, saliency detection, emotion regulation, and social cognition (Swain et al. 2014; Feldman 2015; Barba-Müller et al. 2018). However, meta-analytic examination of a subset of these fMRI studies reveals a more limited subset of brain regions underlying maternal responsiveness, namely the uncus and amygdala, caudate, precentral gyrus, and thalamus (Paul et al. 2018).
Efforts to complement activation-based fMRI studies of maternal caregiving examine the task-based functional connectivity of distinct brain regions during maternal processing of infant affective cues (Moses-Kolko et al. 2010; Atzil et al. 2011; Ho and Swain 2017; Swain et al. 2017). Measuring the synchrony of activation across brain regions may provide greater insight into networks underscoring maternal caregiving, and postpartum bonding, than regional brain activation alone. Notably, these maternal studies employing task-based functional connectivity encapsulate brain activation during processing of infant affective cues in contrast to intrinsic functional connectivity approaches in studying the maternal brain, which instead examine synchronous brain activation at rest in the absence of external stimuli (e.g., Chase et al. 2013; Atzil et al. 2017).
Task-based functional connectivity studies have also been conducted in the context of maternal psychopathology. Two studies have examined functional connectivity in the context of maternal depression. While engaged in a negative emotional face-matching task, there was significant connectivity between the left amygdala and left dorsomedial prefrontal cortex (dmPFC) in mothers without depression, which was absent in mothers with depression (Moses-Kolko et al. 2010). Maternal depression was also associated with differences in connectivity between the left extended amygdala and nucleus accumbens, as well as dmPFC, during an infant cry perception task (Ho and Swain 2017). A third exploratory study suggested that psychotherapy may alter functional connectivity of the maternal brain during an infant cry perception task (Swain et al. 2017).
In summary, research has indicated the utility of task-based functional connectivity in tracking maternal attunement (Atzil et al. 2011, 2017) and the impact of maternal psychopathology to infant cue processing (Moses-Kolko et al. 2010; Ho and Swain 2017; Swain et al. 2017), motivating further study of the functional connectivity of the maternal brain. Here, we employ connectome-based predictive modeling (CPM) as a machine-learning approach to probe functional connectivity measured across the whole brain rather than being restricted to specific seed regions like previous functional connectivity studies of the maternal brain (Shen et al. 2017). In addition, unlike explanatory models based on correlation or regression, CPM with built-in cross-validation increases the likelihood of replication in future studies by limiting overfitting (Shmueli 2010; Whelan and Garavan 2014; Gabrieli et al. 2015). CPM is actualized through examination of the functional connection of predefined nodes throughout the brain and their associations with observed behaviors or cognitive measures and, then, by predicting the strength of these brain–behavior associations. For instance, CPM has been employed to predict attention (Rosenberg et al. 2016), personality traits (Hsu et al. 2018), and cocaine abstinence (Yip et al. 2019). Here, we examine whether CPM applied to the maternal brain can predict a critical element of caregiving, namely the mother–infant bond. Furthermore, we examine whether connectivity-bonding associations persist across the postpartum period. Given prior functional connectivity associations between maternal brain and behavior, we hypothesized that CPM would predict maternal bonding across the postpartum period. To our knowledge, this is the first application of CPM to identify maternal brain networks predicting maternal bonding.
Methods
Participants
The Yale Human Investigations Committee approved all procedures before recruitment commenced. Sixty-two mothers were recruited from the local community of New Haven, CT, as part of a larger study on parenting. Inclusion/exclusion criteria included recent delivery and no contraindications to MRI. Seven mothers were removed from further analyses because of excess motion leaving a sample of 55 mothers (mean age = 28 years; SD =5 years; 26 primiparous; 26 multiparous; 3 did not report parity) early postpartum (M = 2 months; SD = 1 month) who completed the Postpartum Bonding Questionnaire (PBQ; Brockington et al. 2001, 2006) and MRI scanning. Maternal ethnicity consisted of Caucasian (n = 43), African American (n = 8), American Indian/Alaskan (n = 2), and Other (n = 2). Maternal education was employed as a proxy for socioeconomic status (Mayes and Bornstein 1995; Landi et al. 2012), and mean education was 15 years (SD = 4 years). Marital status was single (n = 25) or married (n = 28; 2 did not report). Mothers also completed a trait anxiety measure (M = 48; SD = 3; Spielberger et al. 1970) and a depression measure (M = 12; SD = 8; Beck et al. 1996). Mothers were invited to participate in a second lab and MRI visit at 8 months postpartum. At the second postpartum time point (M = 8 months; SD = 2 months), 37 mothers completed the PBQ and 29 mothers completed the MRI scan. Of these mothers, seven did not complete either the PBQ or the MRI scan at the first time point. Overall, 55 mothers completed the first PBQ and first MRI scan, 32 mothers completed the second PBQ and first MRI scan, 30 mothers completed the second PBQ and second MRI scan, and 23 mothers completed all four. All mothers provided written informed consent and were compensated $80 for their participation in each of the scan visits. Their infants were also provided with a small gift.
The PBQ is the central dependent measure in the study (Brockington et al. 2001, 2006) is a 25-item questionnaire designed to measure a mother’s experience of bonding with her child and her representation of this bonding in mind. Each item is scored on a Likert scale where “0” refers to “always” and “4” refers to “never.” The PBQ consists of four subscales. The impaired bonding subscale reflects items that represent impairments in bonding (e.g., “I feel happy when my baby smiles or laughs”) and originally identified over 90% of mothers with bonding impairments compared to healthy mothers and depressed mothers with normal bonding (cut-off score ≥12). The rejection subscale reflects items that measure rejection and anger toward the infant (e.g., “I feel angry with my baby”) and is useful in identifying mild and severe bonding disorders (cut-off score ≥13). The anxiety subscale includes items related to anxiety in caring for the infant (e.g., “My baby makes me anxious”) and is useful in identifying anxious mothers (cut-off score ≥10). The final scale includes items related to risk of abuse (e.g., “I have done harmful things to my baby”; cut-off score ≥2). No mother endorsed any items on this latter scale, and it was not included further. Impaired bonding (M = 5; SD = 5), rejection (M = 3; SD = 2), and anxiety (M = 3; SD = 2) subscale scores were calculated separately for the analysis. The PBQ shows good internal reliability and satisfactory validity (Brockington et al. 2001; Brockington et al. 2006; Wittkowski et al. 2007).
Experimental Design: Infant Face and Cry Functional Task
Unfamiliar infant face and cry stimuli were presented randomly during experimental trials using E-Prime 1.2 software. Although a number of studies incorporate infant faces that are familiar and unfamiliar (e.g., Strathearn et al. 2009; Kim et al. 2011), our selection of unfamiliar infant faces reflected the difficulty in acquiring variation in infant emotional expressions very early postpartum (within 1–2 months) as well as providing continuity in the stimuli set across the postpartum period, without needing to adjust infant face stimuli to reflect their current age (which may introduce additional confounding factors to our findings). Twenty-one photographs of infant faces were taken from six infants, aged 5–10 months, with each expressing happy, neutral, and sad expressions (126 total unique stimuli). While all face stimuli were provided from a prior maternal brain study (Strathearn and McClure 2002), they were re-rated to validate the emotional expression (Landi et al. 2011). Infant faces were balanced for sex (male, female) and race (Caucasian and African American). The infant faces were presented on a gray background, and the size, luminance, and contrast were kept constant. Two-second cry samples (44 100 Hz sampling frequency) were recorded from healthy infants aged 27–32 days (Gustafson and Green 1989). From these samples, cries from two infants were selected: one cry was a high-distress cry and the other was a low-distress cry (four cry samples in total). Praat software (http://www.fon.hum.uva.nl/praat/) was employed to normalize the cries to the same relative peak intensity. All cry stimuli were also rated to confirm their distress intensity (Landi et al., 2011). As well as these cry stimuli, a “neutral” 220 Hz pure tone was also presented.
Infant faces were presented centrally for 1000 ms and auditory stimuli were presented through headphones with a blank visual display in a passive viewing paradigm. There were 42 trials for each functional run, consisting of six trials for each of the face and cry conditions, as well as a one-back memory trial, that was not included in any analyses. The intertrial interval was jittered between 4000 and 14 000 ms.
Data Acquisition
All data were acquired with a Siemens Trio 3T MRI system employing a standard 12-channel head coil. Functional data were collected with a gradient echo, echoplanar sequence: repetition time = 2000 ms, echotime = 30 ms, flip angle = 80°, field of view = 220 × 220 mm, matrix = 64 × 64, in-plane resolution = 3.4 mm × 3.4 mm, slice thickness = 4 mm, and 32 slices. Each block of trials consisted of 163 volumes, including an initial 12-s rest period to achieve signal stability, which was removed from analyses.
Common Space Registration
First, images were skull stripped using FSL (https://fsl.fmrib.ox.ac.uk/fsl/), and any remaining nonbrain tissue was manually removed. All further analyses were performed using BioImage Suite (Joshi et al. 2011). The Shen 268 functional atlas was warped from MNI space to single participant space through the concatenation of a series of linear and nonlinear registrations. The functional series were linearly registered to the T1 axial–oblique (2D anatomical) image. The 2D anatomical image was linearly registered to the MPRAGE (3D anatomical) image. The 3D anatomical image was nonlinearly registered to the template MNI brain using a previously validated algorithm (Scheinost et al. 2017). All transformation pairs were calculated independently and combined into a single transform warping the single participant results into common space. This single transformation allows the single participant images to be transformed to common space with only one transformation, reducing interpolation error.
Functional Connectivity Preprocessing
The first 10 volumes of each functional run were discarded to allow for the magnetization to reach a steady state. Slice-time and motion correction was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). All further analyses were performed using BioImage Suite (Joshi et al. 2011). Several covariates of no interest were regressed from the data including linear and quadratic drifts, mean cerebrospinal fluid signal, mean white matter signal, and mean global signal. For additional control of possible motion-related confounds, a 24-parameter motion model (including six rigid body motion parameters, six temporal derivatives, and these terms squared) was regressed from the data. The data were temporally smoothed with a Gaussian filter (approximate cutoff frequency = 0.12 Hz). A canonical gray matter mask defined in common space was applied to the data, so only voxels in the gray matter were used in further calculations. Finally, for each participant, all preprocessed runs were variance normalized and concatenated.
Connectivity Matrices
Whole-brain functional connectivity was assessed as described previously (Finn et al. 2017; Greene et al. 2018). Briefly, network nodes were defined using the Shen 268-node functional brain atlas that included the cortex, subcortex, and cerebellum. The atlas was warped from MNI space into single-subject space, as described above. Task connectivity was calculated on the basis of the “raw” task time courses, with no removal of task-evoked activity. We have previously shown that connectivity matrices calculated in this manner emphasize individual differences in connectivity (Finn et al. 2017) and increase CPM performance (Greene et al. 2018). For every node, a mean time course was calculated by averaging the time courses of all of its constituent voxels. Pairwise correlations were computed between all pairs of nodes, and Pearson correlation coefficients were Fisher z-transformed to yield symmetric 268 × 268 connectivity matrices.
Connectome-Based Predictive Modeling
CPM was conducted to predict each of the PBQ subscales using previously validated custom MATLAB scripts (Shen et al. 2017). We were not able to predict the impaired bonding or the rejection subscales of the PBQ and therefore these were not considered further and we focused on maternal anxiety toward her infant. CPM takes connectivity matrices and phenotypic data from individuals as input to generate a predictive model of the behavioral data from connectivity matrices. Edges and phenotypic data from the training data set are correlated using regression analyses using either Pearson’s correlation or partial correlation (when controlling for possible confounds) to identify positive and negative predictive networks. Positive networks are networks for which increased edge weights (increased connectivity) are associated with the variable of interest, and negative networks are those for which decreased edge weights (decreased connectivity) are associated with the variable of interest. Single-subject summary statistics are then created as the sum of the significant edge weights in each network and are entered into predictive models that assume linear relationships with behavioral data. The resultant linear equation is then applied to the test data set to predict the phenotypic data. In the case of leave-one-out cross-validation, a single participant’s predicted value (i.e., the “left-out” participant) is generated by taking the data from all other participants as the training data set in an iterative manner until all participants have a predicted value. Our final predictive model was only composed of edges that appeared in every round of cross-validation (c.f., Greene et al. 2018).
Localization of Predictive Networks
Predictive networks identified using CPM are complex and composed of multiple brain regions and networks. Similar to previous CPM studies, predictive networks were summarized using parcellation of nodes either by spatial overlap with 10 macroscale brain regions (e.g., prefrontal cortex, cerebellum) and/or by overlap with 10 canonical functional networks (e.g., frontoparietal, motor/sensory). Macroscale brain regions were based on anatomical labels presented in Finn et al. (2015). Canonical functional network localizations were based on the functional networks presented in Noble et al. (2017). Additionally, for each node, the network theory measure degree was calculated as the sum of the number of edges for each node that belonged to the predictive networks. Finally, networks were summarized based on length-of-connection (short- vs. long-range connectivity). Euclidean distance between the centroids of each brain region in the Shen atlas was used to classify short- and long-range edges. First, distance was calculated for each pair of regions as: 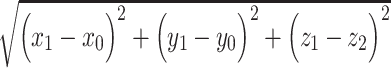 , where
, where 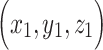 and
and 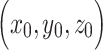 represent the centroid for any two regions. Pair-wise distances were median separated into short- and long-range connections. Visualizations of predictive edges were created using BioImage Suite Web (https://bioimagesuiteweb.github.io/webapp/connviewer.html).
represent the centroid for any two regions. Pair-wise distances were median separated into short- and long-range connections. Visualizations of predictive edges were created using BioImage Suite Web (https://bioimagesuiteweb.github.io/webapp/connviewer.html).
Motion Analysis
As head motion has been shown to confound connectivity studies, we calculated the average frame-to-frame displacement for each mother’s data. In line with current reports, for each mother, we selected the first two runs with an average frame-to-frame displacement less than 0.20. All other runs were removed from the analysis. In addition, we controlled for frame-to-frame displacement in our CPM analysis using partial correlation as described in Hsu et al. (2018)
Statistical Analysis
The correspondence between predicted and actual values, or model performance, was assessed using Spearman’s rank correlation (ρ), mean square error (defined as: 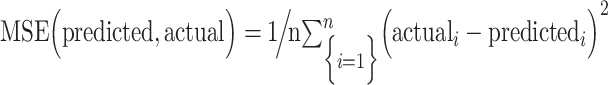 , cross-validation explained variance (defined as:
, cross-validation explained variance (defined as:  , where
, where 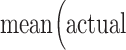 ) is the average maternal anxiety toward her infant from the PBQ across the sample). When using leave-one-out cross-validation, analyses in the leave-one-out folds are not independent, and the number of degrees of freedom is thus overestimated for parametric P values. Instead of parametric testing, we therefore performed permutation testing. To generate null distributions for significance testing, we randomly shuffled the correspondence between behavioral variables and connectivity matrices 5000 times and re-ran the CPM analysis with the shuffled data. Based on these null distributions, the P values for leave-one-out predictions were calculated as in previous work (Shen et al. 2017). As we expect a positive association between predicted and actual values, one-tailed P values are reported.
) is the average maternal anxiety toward her infant from the PBQ across the sample). When using leave-one-out cross-validation, analyses in the leave-one-out folds are not independent, and the number of degrees of freedom is thus overestimated for parametric P values. Instead of parametric testing, we therefore performed permutation testing. To generate null distributions for significance testing, we randomly shuffled the correspondence between behavioral variables and connectivity matrices 5000 times and re-ran the CPM analysis with the shuffled data. Based on these null distributions, the P values for leave-one-out predictions were calculated as in previous work (Shen et al. 2017). As we expect a positive association between predicted and actual values, one-tailed P values are reported.
Results
Prediction of Maternal Anxiety at 2 Months
The overall CPM model successfully predicted maternal anxiety toward bonding with their infant (ρ = 0.34, q2 = 0.11, MSE = 6.55, P = 0.018, permutation testing, 5000 iteration, one-tailed; Fig. 1A). Follow-up comparisons controlling for head motion (ρ = 0.38, q2 = 0.10, MSE = 6.61, P = 0.0082, permutation testing, 5000 iteration, one-tailed), maternal age (ρ = 0.3, q2 = 0.08, MSE = 6.78, P = 0.02, permutation testing, 5000 iteration, one-tailed), depression (ρ = 0.33, q2 = 0.11, MSE = 6.60, P = 0.011, permutation testing, 5000 iteration, one-tailed), trait anxiety (ρ = 0.28, q2 = 0.06, MSE = 6.92, P = 0.027, permutation testing, 5000 iteration, one-tailed), maternal education (ρ = 0.19, q2 = 0.03, MSE = 7.16, P = 0.09, permutation testing, 5000 iteration, one-tailed), and parity (ρ = 0.16, q2 = 0.00, MSE = 7.01, P = 0.15, permutation testing, 5000 iteration, one-tailed) demonstrated similar prediction performances. Similarly, to further investigate the specificity of the model to maternal, rather than trait, anxiety, the network strength of the CPM model did not correlate with trait anxiety and we were not able to predict trait anxiety with CPM.
Figure 1.
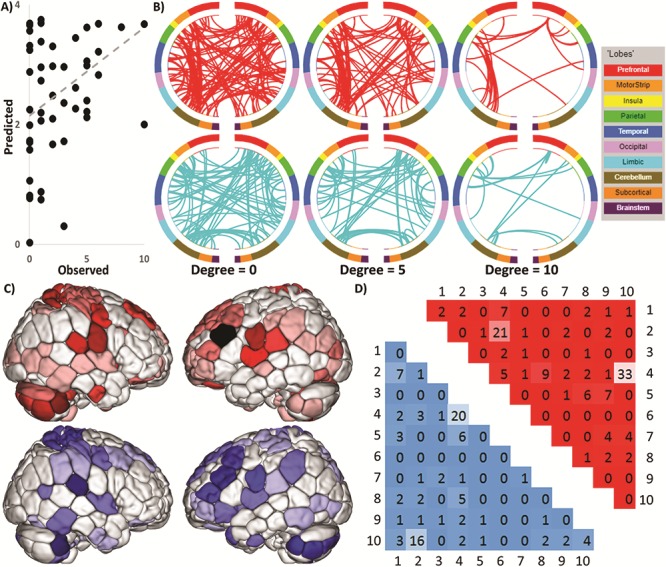
CPM predicts maternal anxiety toward her infant. (A) Scatter plot showing observed values plotted against actual values. (B) Edges that contributed to the CPM model organized by macroscopic brain regions. The positive network is in red (top) and negative network is in blue (bottom). To help in visualizing these complex networks, edges only belonging to nodes with five or more edges (degree ≥5; middle) and 10 or more edges (degree ≥10; right) are also shown. Visualization created using BioImage Suite Web, http://bisweb.yale.edu/. (C) Visualization of node degree (i.e., the sum of predictive edges for a node) for the positive (top; warm colors) and negative networks (bottom; cool colors). Darker color indicates higher degree. (D) The positive network is in red in the upper triangle and the negative network is in blue in the lower triangle. Each number corresponds to one canonical network (methods): 1 = medial frontal, 2 = frontoparietal, 3 = default mode, 4 = motor–sensory–auditory, 5 = visual A, 6 = visual B, 7 = visual association, 8 = salience, 9 = subcortical, and 10 = cerebellum.
Anatomical Localization
The predictive edges were widely distributed throughout the brain (Fig. 1B), yet only contain a small portion of the total edges in the connectome (220 edges total out of 35 778 or 0.6%). About 126 edges positively predicted maternal anxiety, consisting of 41 ipsilateral connections in the right hemisphere, 28 ipsilateral connections in left hemisphere, and 57 connections between the right and left hemispheres. A total of 94 edges negatively predicted maternal anxiety, consisting of 22 ipsilateral connections in the right hemisphere, 25 ipsilateral connections in left hemisphere, and 47 connections between the right and left hemispheres. For both positive and negative networks, nodes with the greatest number of edges were primarily located in the cerebellum, motor cortex, and left prefrontal cortex (Fig. 1C). Both networks included short- and long-range connections. However, the positive network was characterized by a greater number of long-range connections (67% long range, 33% short range, χ2 = 6.5, P = 0.01), whereas the negative network included an equal number of long- and short-range connections (58% long range, 42% short range, χ2 = 0.77, P = 0.38).
Network Localization
The positive and negative networks were further summarized by the number of edges within and between large-scale functional networks (Fig. 1D). By definition, edges within the positive and negative networks cannot be overlapping connections as a single edge cannot be both a positive and negative predictor. However, positive and negative networks included connections within and between similar large-scale functional networks. The positive network was characterized primarily by connections between the cerebellar and motor–sensory–auditory networks and between the frontoparietal and motor–sensory–auditory networks. The negative network was characterized primarily by connections between the frontoparietal and cerebellar networks and connections within the motor–sensory–auditory networks.
Virtual Lesion Analysis
Given that 80 out of the 220 total edges in the overall CPM model were within and between the frontoparietal, motor–sensory–auditory, and cerebellar networks, we used a virtual lesion analysis to evaluate the sensitivity of the edges within and between these networks versus edges from every other network. First, we retained edges only within and between the frontoparietal, motor/sensory/auditory, and cerebellar networks (i.e., removing all other edges) and performed CPM using only these edges. Using only these three networks, we achieved superior prediction performance than the CPM analysis using all 10 networks (ρ = 0.42, q2 = 0.15, MSE = 6.29). Second, we removed edges only within and between the frontoparietal, motor/sensory/auditory, and cerebellar networks (i.e., retaining all other edges) and performed CPM using only these edges. Using the remaining seven networks, we achieved worse prediction performance than the CPM analysis using all 10 networks (ρ = 0.31, q2 = 0.09, MSE = 6.74). Using Steiger’s test to compare dependent correlation coefficients using edges only within and between the frontoparietal, motor/sensory/auditory, and cerebellar networks for CPM resulted in significantly better prediction performance than using edges not in these networks for CPM (Z = 2.051, P = 0.04).
Prediction of Maternal Anxiety at 8 Months Postpartum
To test the generalization of our CPM model for future anxiety toward the infant, individual participant network summary scores were created as the difference of connectivity strengths within positive and negative networks for both the 2- and 8-month connectivity data. The CPM model, when applied to the 2 months of connectivity data, moderately predicted maternal anxiety toward the infant at 8 months (ρ = 0.3016, P = 0.047, df = 30, one-tailed). The CPM model, when applied to the 8 months of connectivity data, did not predict maternal anxiety toward the infant at 8 months (ρ = 0.13, P = 0.25, df = 28, one-tailed). Finally, we calculated the change in anxiety toward the infant between 2 and 8 months and the change in connectivity strength between 2 and 8 months to test whether a reduction in connectivity strength in the predictive networks correlated with a reduction in anxiety toward the infant. These changes were strongly correlated (ρ = 0.62, P < 0.01, df = 21, one-tailed, Fig. 2).
Figure 2.
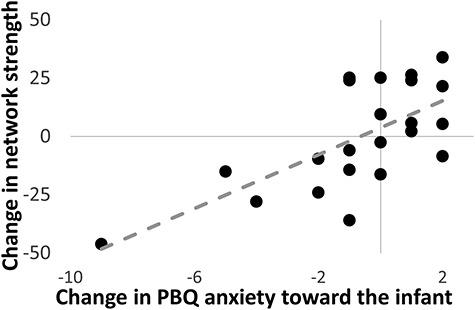
Scatter plot illustrating the association between the change in CPM network strength and the change in anxiety toward her infant from 2 to 8 months postpartum (ρ = 0.62, P < 0.01, df = 21, one-tailed). A greater reduction in network strength was positively correlated with a greater reduction of anxiety.
Discussion
In this study, we examined whether CPM would predict mother’s bonding with her child at 2 and 8 months postpartum. Our CPM approach predicted one specific facet of postpartum bonding, maternal anxiety toward the child, measured at 2 months postpartum. Greater “integration” between the cerebellar and motor–sensory–auditory networks and between the frontoparietal and motor–sensory–auditory networks was associated with more maternal anxiety regarding the infant. In contrast, greater “segregation” between the cerebellar and frontoparietal and within the motor-sensory-auditory networks was associated with more maternal anxiety regarding the infant. Furthermore, we observed that changes in network connectivity across the postpartum period were associated with changes in levels of postpartum bonding, specifically in the context of maternal anxiety toward her child. Finally, we were unable to model the other postpartum bonding subscales regarding impaired bonding and rejection. Taken together, these data indicate the value of CPM to studying the maternal brain with implications for bonding at 2 months postpartum, particularly in how mother’s internally represent the relationship with their infant in the context of maternal anxiety.
The specificity of the CPM to maternal anxiety toward the child may reflect the salience of this construct to maternal brain networks. Indeed, maternal anxiety symptoms are heightened during the perinatal period (Wenzel et al. 2003, 2005; Lee et al. 2007; Goodman et al. 2014). Importantly, we found that CPM predicted infant-directed maternal anxiety even when controlling for measures of anxiety (and depression). Our longitudinal findings reinforce the plasticity of the maternal brain and the value of repeated measures over time to understand functional and structural brain changes during pregnancy and the postpartum period and their implications for parenting (Kim et al. 2010; Hoekzema et al. 2017). Given that changes in network strength were associated with changes in maternal bonding, it is possible that perinatal interventions influencing connectivity within these networks (e.g., cognitive behavioral or pharmacological therapies, Rosenberg et al. 2016) may be helpful in increasing the representational quality of maternal bonding with the infant through decreasing anxiety in this context.
Like previous CPM studies (Shen et al. 2017; Greene et al. 2018), the generated predictive networks were complex, spanned the whole brain, and contained both short- and long-range connections. This complexity is consistent with the diverse brain regions and cognitive processes (e.g., reward processing, saliency detection, emotion regulation, and social cognition) associated with infant stimuli in the maternal brain (Swain et al. 2014; Feldman 2015; Barba-Müller et al. 2018). Nevertheless, a majority of the predictive edges were located within and between the frontoparietal, cerebellar, and motor–sensory–auditory networks, and only using these edges for CPM resulted in significantly better prediction than including edges spanning the whole brain. Figure 3 shows a model of the interaction between the frontoparietal, cerebellar, and motor–sensory–auditory networks in predicting a mother’s bonding with her child. Next, we offer potential interpretations of this data-driven model to provide a framework for future research on maternal bonding to mechanistically test.
Figure 3.
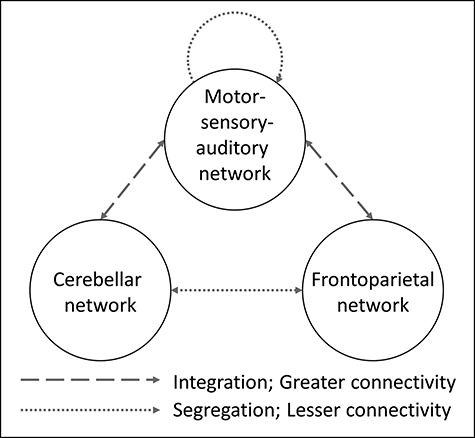
Schematic of simplified and best performing CPM model. When restricted to only edges within and between the frontoparietal, cerebellar, and motor–sensory–auditory networks, prediction results significantly improved. This model was characterized by greater integration between the cerebellar and motor–sensory–auditory networks and between the frontoparietal and motor–sensory–auditory networks and greater segregation between the cerebellar and frontoparietal and within the motor–sensory–auditory networks.
Although long implicated in motor control, increasing research suggests the cerebellum contributes to the perception, recognition, and processing of emotion, potentially translating emotional experiences into behavioral actions (Adamaszek et al. 2017). Notably, the cerebellum has also been linked to empathy (Singer et al. 2004), as well as emotion regulation (Turner et al. 2007; Adamaszek et al. 2017)—both capacities important for early caregiving (Swain et al. 2014; Rutherford et al. 2015). Similarly, fMRI studies of the maternal brain during the presentation of infant faces and cries implicate the cerebellum (Ranote et al. 2004; Strathearn et al. 2008; Swain et al. 2008; Laurent and Ablow 2012). Concurrently, most maternal fMRI studies report increased activation during processing of infant affective cues in motor–sensory–auditory regions (Swain 2008). Consequently, integration of these latter networks with cerebellar networks may increase prioritization of processing infant cues in the brain promoting caregiving behavior. Critically, given that increased activation of these networks was associated with more anxiety toward the child, perhaps this represents a hyperactivation of the network and an interaction between greater integration and heightened anxiety toward their infant in our maternal sample. Consistent with this notion, hypervigilance in parenting has been observed typically in the context of maternal anxiety (e.g., Ostlund et al. 2017); however, it has also been reported in other clinically vulnerable maternal populations (e.g., Schechter and Willheim 2009), perhaps speaking to a broader role for this network in motherhood. Nevertheless, mean maternal anxiety scores toward the infant were below the recommended clinical cut-off range (Brockington et al. 2001, 2006), suggesting further work is needed to more fully explore this network in mothers with more clinically significant anxiety toward bonding with their child.
Frontoparietal networks have been implicated in attentional control (Scolari et al. 2015) and may be modulated by emotional arousal (Moratti et al. 2004). Mothers with higher levels of anxiety toward bonding with their child may be more reactive to infant affective cues, including the infant cries and faces that were employed in this study, increasing attentional processing of these stimuli. Concurrently, increased attention toward salient infant cues may also drive increased connectivity between the frontoparietal and motor–sensory–auditory networks in the presence of anxiety. Given the frontoparietal network’s role in attention (Scolari et al. 2015), in conjunction with cerebellar engagement in emotion processing (Adamaszek et al. 2017), perhaps the observed decreased connectivity between the networks reflects more dysregulation between these two systems resulting in more maternal anxiety toward bonding with her child. Future research with repeated measurements of infant cue reactivity and bonding across the postpartum period will be important in the beginning to disentangle these findings. Finally, increased segregation within the motor–sensory–auditory networks suggests less integration of visual (infant facial expressions) and auditory (infant cries and tone) sensory information with increasing maternal anxiety toward the child, though this interpretation currently remains speculative.
Our study has several strengths. It acquired data prospectively during the early postnatal period, incorporating task-based fMRI in the context of salient infant cues (faces and cries) and an important caregiving measure of mothers’ perception of bonding with their child. Task-based connectivity analyses may be superior to resting-state connectivity in emphasizing individual differences (Finn et al. 2017) and increasing CPM performance (Greene et al. 2018). Understanding neural predictors of postpartum bonding in the context of infant-relevant stimuli may be optimal compared to comparable data collected in the absence of such caregiving cues. Indeed, finding that CPM predicted maternal anxiety in bonding—but not impaired bonding and rejection—may relate to the brain state elicited by the infant cries, as well as infant negative and neutral facial expressions, which may provoke anxiety in mothers. Finally, we used prediction, rather than explanation, to reduce overfitting and exaggerated effect sizes often reported in neuroimaging studies (Shmueli 2010; Whelan and Garavan 2014; Gabrieli et al. 2015).
Our goal was to investigate individual differences in the internal representation of maternal bonding during the postpartum period using predictive modeling. For this goal, we used task-based functional connectivity rather than traditional approaches based on general linear models (GLM). Previous studies have used GLM to delineate task-related activity from distinct and unique brain regions, but the magnitude of these signal changes are weak predictors of individual differences in behavior (Rosenberg et al. 2017; Gabrieli et al. 2015) as many processes or disorders cannot be localized to a single region. Meta-analyses suggest that many behaviors rely on the orchestrated activity of a distributed array of regions (Laird et al. 2005; Yarkoni et al. 2011). Thus, the best predictive models from task data may rely not on the magnitude of activation in a single area, but rather on the degree to which activity is coordinated across large-scale networks. Future analyses may examine the task-evoked responses to investigate the neural correlates of attending to positive versus negative infant faces or high versus low distress sounds.
Our study also has several limitations. The use of predictive models typically involves larger samples than this study. As a result, we used leave-one-out cross-validation rather than the emerging standard of 5- or 10-fold cross validation. The smaller sample size may also reduce the generalizability of our findings; therefore, replication studies involving larger, and more diverse, samples are needed. To aid these efforts, our CPM model is shared as open source from https://nitrc.org/projects/bioimagesuite/. While the CPM model was fairly robust when controlling for demographic and mood variables (including trait anxiety), the model became less sensitive when parity was included. Although less well studied, prior reproductive experience may impact caregiving at neural, hormonal, and behavioral levels (Bridges 2015; Maupin et al. 2016), warranting further investigation. While the CPM model applied to connectivity data at 2 months postpartum predicted maternal anxiety toward the child measured at 8 months, connectivity data at 8 months did not predict bonding at the same time point, indicating the importance of understanding the temporal dynamics of maternal brain and bonding associations. Overall, our results suggest that the CPM model for maternal anxiety does not relate to trait anxiety in our sample. However, a larger sample would likely be needed to fully characterize the association between the neural correlates of trait anxiety and maternal anxiety to provide more conclusive findings of the specificity of our model. Our CPM model was derived from unfamiliar infant face and cry stimuli; therefore, it will be important in advancing this work to examine whether maternal responses to their own infant’s affective cues also track future maternal bonding with her child (e.g., Strathearn et al. 2009; Kim et al. 2011) or whether these findings represent a more general response to infant cues in the prediction of postpartum bonding. Indeed, employing own infant cues may yield prediction of the other components of maternal bonding not found here. Relatedly, we also employed a self-reported measure of maternal bonding with her child. Although beneficial in providing insight into the mother’s internal representation of the infant at the level of anxiety, further work is needed to address whether maternal anxiety toward her child impacts the mother–infant relationship more generally. This may be achieved in part through employing objective measures of caregiving, including behaviorally coded maternal sensitivity, as well as assessments of children’s socio-emotional responding during interactions with their mother. Finally, we did not have access to imaging or assessments of the infants to ascertain the implications of these findings for child development. Future studies should investigate how maternal anxiety in the bonding relationship, the maternal brain, and the developing brain all interact.
In conclusion, we employed CPM to examine the maternal brain and associations with postpartum bonding. Our central findings suggest that cerebellum, frontoparietal, and motor–sensory–auditory networks may interact and contribute to increased maternal anxiety toward the child over the first 8 months postpartum.
Funding
National Institutes of Health (P01 DA022446, R01 DA026437, and R03 DA045289).
Notes
M.N.P. has received financial support or compensation for the following: M.N.P. has consulted for Shire, INSYS, RiverMend Health, Lakelight Therapeutics/Opiant, and Jazz Pharmaceuticals; has received research support to Yale from Mohegan Sun Casino, the National Center for Responsible Gaming, and Pfizer; and has consulted for legal entities on issues related to addictive disorders. Conflict of Interest: The authors report no conflicts of interest with respect to the content of this manuscript.
References
- Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Mariën P, Molinari M, Moulton E et al. . 2017. Consensus paper: cerebellum and emotion. Cerebellum. 16:552–576. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. 2011. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 36:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Touroutoglou A, Rudy T, Salcedo S, Feldman R, Hooker JM, Dickerson BC, Catana C, Barrett LF. 2017. Dopamine in the medial amygdala network mediates human bonding. Proc. Natl. Acad. Sci. 114:2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Müller E, Craddock S, Carmona S, Hoekzema E. 2018. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch. Womens Ment. Health. 22:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. 2011. Annual research review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J. Child Psychol. Psychiatry. 52:368–397. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck Depression Inventory-II. Vol 1 San Antonio, TX: Psychological Corporation, p. 82. [Google Scholar]
- Bowlby J. 1969. Attachment and Loss: Volume 1 Attachment. Sydney: Pimlico. [Google Scholar]
- Bridges RS. 2015. Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 36:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington IF, Fraser C, Wilson D. 2006. The postpartum bonding questionnaire: a validation. Arch. Womens Ment. Health. 9:233–242. [DOI] [PubMed] [Google Scholar]
- Brockington IF, Oates J, George S, Turner D, Vostanis P, Sullivan M, Loh C, Murdoch C. 2001. A screening questionnaire for mother-infant bonding disorders. Arch. Womens Ment. Health. 3:133–140. [Google Scholar]
- Chase HW, Moses-Kolko EL, Zevallos C, Wisner KL, Phillips ML. 2013. Disrupted posterior cingulate–amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc. Cogn. Affect. Neurosci. 9:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. 2015. The adaptive human parental brain: implications for children's social development. Trends Neurosci. 38:387–399. [DOI] [PubMed] [Google Scholar]
- Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, Constable RT. 2017. Can brain state be manipulated to emphasize individual differences in functional connectivity? NeuroImage. 160:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT. 2015. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Ghosh SS, Whitfield-Gabrieli S. 2015. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. 85:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JH, Chenausky KL, Freeman MP. 2014. Anxiety disorders during pregnancy: a systematic review. J. Clin. Psychiatry. 75:1153–1184. [DOI] [PubMed] [Google Scholar]
- Greene AS, Gao S, Scheinost D, Constable RT. 2018. Task-induced brain state manipulation improves prediction of individual traits. Nat. Commun. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson GE, Green JA. 1989. On the importance of fundamental frequency and other acoustic features in cry perception and infant development. Child Dev. 60:772–780. [PubMed] [Google Scholar]
- Ho SS, Swain JE. 2017. Depression alters maternal extended amygdala response and functional connectivity during distress signals in attachment relationship. Behav. Brain Res. 325:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Soliva JC, Tobeña A, Desco M, Crone EA. 2017. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20:287–296. [DOI] [PubMed] [Google Scholar]
- Hsu W-T, Rosenberg MD, Scheinost D, Constable RT, Chun MM. 2018. Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc. Cogn. Affect. Neurosci. 13:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, Papademetris X. 2011. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 9:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. 2011. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J. Child Psychol. Psychiatry. 52:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. 2010. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav. Neurosci. 124:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. 2005. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 3:65–78. [DOI] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJV, Mencl E, Worhunsky P, Potenza MZ and Mayes LC, 2011. Maternal neural responses to infant cries and faces: relationships with substance use. Frontiers in Psychiatry. 2, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Crowley MJ, Wu J, Bailey CA, Mayes LC. 2012. Deviant ERP response to spoken non-words among adolescents exposed to cocaine in utero. Brain Lang. 120:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. 2012. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc. Cogn. Affect. Neurosci. 7:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Lam SK, Lau SMSM, Chong CSY, Chui HW, Fong DYT. 2007. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet. Gynecol. 110:1102–1112. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. 2004. Mothers' neural activation in response to pictures of their children and other children. Biol. Psychiatry. 56:225–232. [DOI] [PubMed] [Google Scholar]
- Maupin AN, Roginiel A, Rutherford H, Mayes L. 2016. A preliminary review of whether prior reproductive experience influences caregiving. New Dir. Child Adolesc. Dev. 73–86. [DOI] [PubMed] [Google Scholar]
- Mayes L, Bornstein MH. 1995. Infant information-processing performance and maternal education. Early Dev Parenting. 4:91–96. [Google Scholar]
- Moratti S, Keil A, Stolarova M. 2004. Motivated attention in emotional picture processing is reflected by activity modulation in cortical attention networks. NeuroImage. 21:954–964. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. 2010. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am. J. Psychiatr. 167:1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D. 2017. Influences on the test-retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb. Cortex. 27:5415–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Measelle JR, Laurent HK, Conradt E, Ablow JC. 2017. Shaping emotion regulation: attunement, symptomatology, and stress recovery within mother–infant dyads. Dev. Psychobiol. 59:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Austin J, Elliott R, Ellison-Wright I, Wan MW, Drake R, Downey D, Elmadih A, Mukherjee I, Heaney L. 2018. Neural pathways of maternal responding: systematic review and meta-analysis. Arch. Womens Ment. Health. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. 2016. Structural and functional plasticity in the maternal brain circuitry. New Dir. Child Adolesc. Dev. 2016:23–46. [DOI] [PubMed] [Google Scholar]
- Pereira M, Morrell JI. 2011. Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J. Neuroendocrinol. 23:1020–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JFW, Appleby L. 2004. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 15:1825–1829. [DOI] [PubMed] [Google Scholar]
- Rosenberg MD, Finn ES, Scheinost D, Constable RT, Chun MM. 2017. Characterizing attention with predictive network models. Trends Cogn. Sci. 21(4):290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MD, Zhang S, Hsu W-T, Scheinost D, Finn ES, Shen X, Constable RT, Li C-SR, Chun MM. 2016. Methylphenidate modulates functional network connectivity to enhance attention. J. Neurosci. 36:9547–9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, Mayes LC. 2013. Minds shaped through relationships: the emerging neurobiology of parenting In: Emde R, Leuzinger-Bohleber M, editors. Early Parenting Research and the Prevention of Disorder: Interdisciplinary Challenges and Opportunities. London: Karnac, pp. 51–70. [Google Scholar]
- Rutherford HJV, Wallace NS, Laurent HK, Mayes LC. 2015. Emotion regulation in parenthood. Dev. Rev. 36:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter DS, Willheim E. 2009. When parenting becomes unthinkable: intervening with traumatized parents and their toddlers. J Am Acad Child Adolesc Psychiatry. 48:249–253. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, Ment LR. 2017. Alterations in anatomical covariance in the prematurely born. Cereb. Cortex. 27:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari M, Seidl-Rathkopf KN, Kastner S. 2015. Functions of the human frontoparietal attention network: evidence from neuroimaging. Curr. Opin. Behav. Sci. 1:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, Constable RT. 2017. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 12:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli G. 2010. To explain or to predict? Stat. Sci. 25:289–310. [Google Scholar]
- Singer T, Seymour B, O'doherty J, Kaube H, Dolan RJ, Frith CD. 2004. Empathy for pain involves the affective but not sensory components of pain. Science (New York, N.Y.). 303:1157–1162. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. 1970. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Squire S, Stein A. 2003. Functional MRI and parental responsiveness: a new avenue into parental psychopathology and early parent-child interactions? Br. J. Psychiatry. 183:481–483. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. 2009. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 34:2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. 2008. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 122:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, SM McClure. 2002. A Functional MRI Study of Maternal Responses of Infant Facial Cues . Washington DC: Annual ScientificMeeting of the Society for Neuroscience.
- Swain JE. 2008. Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry (Edgmont). 5:28. [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS, Rosenblum KL, Morelen D, Dayton CJ, Muzik M. 2017. Parent–child intervention decreases stress and increases maternal brain activity and connectivity during own baby-cry: an exploratory study. Dev. Psychopathol. 29:535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho S, Dayton CJ, Elmadih A, Abel K. 2014. Approaching the biology of human parental attachment: brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Res. 1580:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Todd Constable R, Leckman JF. 2008. Maternal brain response to own baby-cry is affected by cesarean section delivery. J. Child Psychol. Psychiatry. 49:1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM, Paradiso S, Marvel CL, Pierson R, Ponto LLB, Hichwa RD, Robinson RG. 2007. The cerebellum and emotional experience. Neuropsychologia. 45:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Haugen E, Jackson L, Robinson K. 2003. Prevalence of generalized anxiety at eight weeks postpartum. Arch. Womens Ment. Health. 6:43–49. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Haugen EN, Jackson LC, Brendle JR. 2005. Anxiety symptoms and disorders at eight weeks postpartum. J. Anxiety Disord. 19:295–311. [DOI] [PubMed] [Google Scholar]
- Whelan R, Garavan H. 2014. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol. Psychiatry. 75:746–748. [DOI] [PubMed] [Google Scholar]
- Wittkowski A, Wieck A, Mann S. 2007. An evaluation of two bonding questionnaires: a comparison of the mother-to-infant bonding scale with the postpartum bonding questionnaire in a sample of primiparous mothers. Arch. Womens Ment. Health. 10:171–175. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Scheinhost D, Nich C, Potenza M, Carroll K. 2019. Connectome-based prediction of future cocaine abstinence. Am. J. Psychiatry. 176(2):156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]


