Abstract
Aims
Ischaemic heart disease persists as the leading cause of death in both men and women in most countries and sex disparities, defined as differences in health outcomes and their determinants, may be relevant. We examined sex disparities in presenting characteristics, treatment and all-cause mortality in patients hospitalized with myocardial infarction (MI) or angina.
Methods and results
We conducted a cohort study of all patients admitted with MI or angina (01 October 2013 to 30 June 2016) from a secondary care acute coronary syndrome e-Registry in NHS Scotland linked with national registers of community drug dispensation and mortality data. A total of 7878 patients hospitalized for MI or angina were prospectively included; 3161 (40%) were women. Women were older, more deprived, had a greater burden of comorbidity, were more often treated with guideline-recommended therapy preadmission and less frequently received immediate invasive management. Men were more likely to receive coronary angiography [adjusted odds ratio (OR) 1.52, confidence interval (CI) 1.37–1.68] and percutaneous coronary intervention (adjusted OR 1.68, CI 1.52–1.86). Women were less comprehensively treated with evidence-based therapies post-MI. Women had worse crude survival, primarily those with ST-elevation myocardial infarction (14.3% vs. 8.0% at 1 year, P < 0.001), but this finding was explained by differences in baseline factors. Men with non-ST-elevation myocardial infarction had a higher risk of all-cause death at 30 days [adjusted hazard ratio (HR) 1.72, CI 1.16–2.56] and 1 year (adjusted HR 1.38, CI 1.12–1.69).
Conclusion
After taking account of baseline risk factors, sex differences in treatment pathway, use of invasive management, and secondary prevention therapies indicate disparities in guideline-directed management of women hospitalized with MI or angina.
Keywords: Sex disparities, Myocardial infarction, Coronary angiography, Percutaneous coronary intervention, Outcomes
Introduction
Ischaemic heart disease persists as the leading global cause of death.1 Myocardial infarction (MI) accounts for a large proportion of death due to cardiovascular disease. Between 2007 and 2016, age-sex standardized mortality for MI in Scotland fell by 42.5% from 129 to 74 per 100 000 population2—a trend also apparent in other countries.3,4 Despite improvements in survival, considerable disparities exist according to sex in terms of delivery of guideline-recommended treatments and outcomes following MI, suggesting women may be disadvantaged.5
Use of high-sensitivity troponin assays with sex-specific thresholds increases the detection of MI in women.6 However, women are less likely to undergo percutaneous coronary intervention (PCI) and are more often subject to underutilization of evidence-based secondary preventative pharmacotherapy.5,7,8 Differences in adoption of invasive management may, in part, be explained by a perception held by clinicians and patients that outcomes are worse for women receiving PCI, as well as differences in symptoms and baseline risk profile which may impact clinical decision-making.9 Adverse events post-MI, including cardiogenic shock, heart failure, and death, remain more common in women than in men, most notably in those with ST-elevation myocardial infarction (STEMI).10,11 Whether sex remains an independent predictor of adverse events despite adjustments for the higher risk-profile of women, notably age, is less clear.
We hypothesized that sex-related differences in demographics and comorbidity underpin disparities in the management and outcomes of women and men hospitalized with MI or angina. We investigated this hypothesis by analysis of a contemporary secondary care electronic registry (e-Registry) using electronic patient records (EPRs) for patients admitted to a complex regional healthcare network.12
Methods
Setting
Seven acute hospitals in the National Health Service (NHS) in Glasgow and the West of Scotland form a complex healthcare system serving a population of approximately 1.2 million. The Golden Jubilee National Hospital is a regional cardiothoracic centre that provides invasive cardiology services for this population. Electronic patient records were implemented across all secondary care clinical and administration systems in NHS Greater Glasgow and Clyde (GGC) and the Golden Jubilee National Hospital by June 2012 enabling capture of key components of hospital care. These EPRs have been combined into an e-Registry for quality improvement and research.12
The Information Services Division is part of NHS National Services Scotland and holds a range of health-related administrative data, including information relating to medicines dispensed in the community within its Prescribing Information System (PIS) database, morbidity collected from all hospital admissions in the Scottish Morbidity Record 01 (SMR01) database and all deaths registered by National Records of Scotland (NRS). Once data were extracted, identifiers were removed and replaced with a pseudonymous identifier. The research team accessed these pseudonymized datasets within a Safe Haven analytical platform.13
Ethics and governance
The project was supported by the National Advisory Committee for Coronary Heart Disease on behalf of the Scottish Government. The Joint Working Project received ethical approval from the NHS GGC Local Privacy Advisory Committee and was approved by hospital management and the Caldicott Guardian for clinical governance in each health board.
Design and methodology
Data were extracted from EPRs for all admissions (01 October 2013 to 30 June 2016) with an International Statistical Classification of Diseases (ICD-10) diagnosis of angina (I200-I209), MI (I210-I229), other ischaemic heart disease (I240-I249), or heart failure (I50) to ensure complete capture of events. Data were deposited within an existing repository for electronic health data and linked to electronic referrals for cardiovascular procedures performed in the invasive centre. An executable system was developed to identify, link and classify these records into episodes of care as detailed in a previous project.12 Patients with a final diagnosis of MI or angina were isolated and linked to PIS prescribing data, SMR01 data for comorbidities and mortality data from NRS. This linked dataset was analysed to look at patient characteristics, invasive cardiovascular procedures, service delivery metrics, drug treatment and mortality. The pre-specified primary outcomes were 30-day and 1-year all-cause mortality (from date of admission). The receipt of cardiac interventions and medical therapy at discharge, 6 months and 1 year post-discharge were the pre-specified secondary outcomes.
Statistical analysis
Baseline characteristics were described using means with standard deviations, total numbers with percentages, or medians with interquartile ranges. Where all patients were analysed, this included unspecified MI. Comparisons between men and women were made using appropriate statistical tests (t-test/Mann–Whitney/χ2/Fisher’s exact). Deprivation status was identified based on home postcode and measured using quintiles of the Scottish Index of Multiple Deprivation (SIMD) 2012 measure.14 Quintile 1 represents the highest level of deprivation with quintile 5 representing the least deprived. The top 20% most deprived data zones in Scotland are in quintile 1, and the distribution of Glasgow’s data zones is 49%, 19%, 13%, 10.5% and 8.5% (Q1–Q5).15 A Charlson comorbidity score was derived using standard procedures and ICD-10 codes included the hospital admission records (SMR01).16 Pre-admission medical therapy and medical therapy at discharge were defined as fulfilment of prescription within 90 days pre-admission and post-discharge, respectively. Medical therapy at 6 months and at 1 year were defined as fulfilment of prescription at 6 months or 1 year post-discharge ±45 days.
To analyse the relationship between sex and medical treatment, three analyses using mixed effects logistic models were performed for each drug and drug combination: (i) for patients alive at discharge, fulfilling a prescription claim within 90 days of discharge, (ii) for patients discharged with treatment and alive at 6 months post-discharge, fulfilling a prescription claim at 6 months post-discharge, and (iii) for patients discharged with treatment and alive at 1 year post-discharge, fulfilling a prescription claim at 1 year post-discharge. Analyses were adjusted for age, SIMD quintile, use of the respective drug within 90 days pre-admission, comorbidities, and PCI. Furthermore, we adjusted for clustering at the discharge hospital level. When analysing the association of sex with use of drug combinations, pre-admission drug use was not adjusted for. Multivariable logistic regression was used to evaluate the association of sex and baseline factors with invasive management. Cox proportional hazards regression was used to evaluate the association of sex with all-cause mortality. Kaplan–Meier survival curves were generated for all-cause death and sex differences were assessed using a log-rank test. Analyses were conducted using SAS Enterprise Guide (v5.1).
Results
Baseline characteristics
There were 7878 patients admitted with MI or angina between 1 October 2013 and 30 June 2016, including 3161 (40.1%) women (Table 1). Diagnosis of STEMI was made in 2042 (25.9%) patients, non-ST-elevation myocardial infarction (NSTEMI) in 3957 (50.2%) patients, hospitalized angina in 1425 (18.1%) patients, and in 454 (5.8%) patients the MI type was unspecified. Women were older than men (69.7 years vs. 64.0 years, P < 0.001) and were relatively more deprived (75.7% vs. 72.5% in SIMD Q1–3, P = 0.002). Diagnosis of STEMI was less common in women than men (20.3% vs. 29.7%, P < 0.001), but women had a higher proportion of NSTEMI and hospitalized angina. Comorbidity differed according to sex both in terms of higher Charlson scores and an increased proportion of individual comorbid diseases in women, who more frequently had hypertension, atrial fibrillation, renal failure, respiratory disease, cerebrovascular disease, stroke, heart failure, dementia, and depression. Compared to men, women were more often treated with statins (46.9% vs. 43.2%, P = 0.001), beta-blockers (34.9% vs. 30.5%, P < 0.001), and anticoagulants or antiplatelets (48.5% vs. 42.1%, P < 0.001) pre-admission.
Table 1.
Baseline demographics and management for all patients according to sex
| All (n = 7878) | Men (n = 4717) | Women (n = 3161) | P-value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 66.3 ± 13.7 | 64.0 ± 13.0 | 69.7 ± 13.9 | <0.001 |
| SIMD quintile, n (%) | <0.001 | |||
| 1 (most deprived) | 3265 (41.5) | 1865 (39.5) | 1400 (44.3) | 0.002a |
| 2 | 1418 (18.0) | 833 (17.7) | 585 (18.5) | |
| 3 | 1126 (14.3) | 720 (15.3) | 406 (12.8) | |
| 4 | 993 (12.6) | 623 (13.2) | 370 (11.7) | |
| 5 | 1074 (13.6) | 675 (14.3) | 399 (12.6) | |
| Diagnosis, n (%) | <0.001 | |||
| STEMI | 2042 (25.9) | 1399 (29.7) | 643 (20.3) | |
| NSTEMI | 3957 (50.2) | 2322 (49.2) | 1635 (51.7) | |
| HA | 1425 (18.1) | 749 (15.9) | 676 (21.4) | |
| Unspecified MI | 454 (5.8) | 247 (5.2) | 207 (6.5) | |
| Comorbidities, n (%) | ||||
| Hypertension | 1920 (24.4) | 986 (20.9) | 934 (29.5) | <0.001 |
| Diabetes | 1172 (14.9) | 672 (14.2) | 500 (15.8) | 0.055 |
| Atrial fibrillation | 822 (10.4) | 431 (9.1) | 391 (12.4) | <0.001 |
| Renal failure | 836 (10.6) | 416 (8.8) | 420 (13.3) | <0.001 |
| Respiratory disease | 1186 (15.1) | 579 (12.3) | 607 (19.2) | <0.001 |
| Cerebrovascular disease | 511 (6.5) | 253 (5.4) | 258 (8.2) | <0.001 |
| Peripheral vascular disease | 572 (7.3) | 340 (7.2) | 232 (7.3) | 0.826 |
| Heart failure | 794 (10.1) | 442 (9.4) | 352 (11.1) | 0.012 |
| Previous myocardial infarction | 1571 (19.9) | 967 (20.5) | 604 (19.1) | 0.130 |
| Dementia | 152 (1.9) | 63 (1.3) | 89 (2.8) | <0.001 |
| Depression | 165 (2.1) | 68 (1.4) | 97 (3.1) | <0.001 |
| Charlson score | <0.001 | |||
| 0 | 4322 (54.9) | 2701 (57.3) | 1621 (51.3) | |
| 1–3 | 2979 (37.8) | 1708 (36.2) | 1271 (40.2) | |
| ≥4 | 577 (7.3) | 308 (6.5) | 269 (8.5) | |
| Pre-admission medical therapy, n (%) | ||||
| Anticoagulant | ||||
| Warfarin | 340 (4.3) | 175 (3.7) | 165 (5.2) | 0.001 |
| Any anticoagulant | 463 (5.9) | 239 (4.9) | 224 (7.1) | <0.001 |
| Antiplatelet | ||||
| Aspirin | 2595 (32.9) | 1524 (32.3) | 1071 (33.9) | 0.145 |
| Clopidogrel | 743 (9.4) | 381 (8.1) | 362 (11.5) | <0.001 |
| Ticagrelor | 110 (1.5) | 66 (1.4) | 52 (1.6) | 0.379 |
| Any antiplatelet | 3134 (39.8) | 1792 (38.0) | 1342 (42.5) | <0.001 |
| Dual antiplatelet | 312 (4.0) | 173 (3.7) | 139 (4.4) | 0.104 |
| Statin | 3523 (44.7) | 2040 (43.2) | 1483 (46.9) | 0.001 |
| Beta-blocker | 2542 (32.3) | 1440 (30.5) | 1102 (34.9) | <0.001 |
| ACE inhibitor or ARB | 2739 (34.8) | 1612 (34.2) | 1127 (35.7) | 0.177 |
| Mineralocorticoid receptor antagonist | 145 (1.8) | 86 (1.8) | 59 (1.9) | 0.889 |
| Combined therapy | ||||
| Anticoagulant or antiplatelet | 3517 (44.6) | 1984 (42.1) | 1533 (48.5) | <0.001 |
| Anticoagulant or dual antiplatelet | 770 (9.8) | 407 (8.6) | 363 (11.5) | <0.001 |
| Anticoagulant or anticoagulant + antiplatelet | 463 (5.9) | 239 (5.1) | 224 (7.1) | <0.001 |
| Anticoagulant or anticoagulant + dual antiplatelet | 463 (5.9) | 239 (5.1) | 224 (7.1) | <0.001 |
| ≥3 medications | 2488 (31.6) | 1483 (31.4) | 1005 (31.8) | 0.740 |
| Type of admission, n (%) | ||||
| Emergency to invasive centre | 1473 (18.7) | 1035 (21.9) | 438 (13.9) | <0.001 |
| Non-emergency to invasive centre | 997 (12.7) | 636 (13.5) | 361 (11.4) | 0.007 |
| Emergency to local hospital | 4986 (63.3) | 2783 (59.0) | 2203 (69.7) | <0.001 |
| Non-emergency to local hospital | 422 (5.4) | 263 (5.6) | 159 (5.0) | 0.292 |
| Coronary angiography, n (%) | ||||
| All | 4866 (61.8) | 3219 (68.2) | 1647 (52.1) | <0.001 |
| PCI | 3149 (40.0) | 2192 (46.5) | 957 (30.3) | <0.001 |
| 64.7b | 68.1b | 58.1b | <0.001 | |
| Length of stay (days), median (IQR) | 4 (2–7) | 4 (2–7) | 5 (2–8) | <0.001 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; HA, hospitalized angina; IQR, interquartile range; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation; SIMD, Scottish Index of Multiple Deprivation; STEMI, ST-elevation myocardial infarction.
P-value for SIMD Q1–Q3 vs Q4–Q5.
Those who had PCI as a proportion of those who underwent coronary angiography.
Invasive management
Approximately 16% fewer women than men underwent coronary angiography (52.1% vs. 68.2%, P < 0.001) and PCI (30.3% vs. 46.5%, P < 0.001) (Table 1). Amongst those who had a coronary angiogram, women received PCI 10% less frequently than men (58.1% vs. 68.1%, P < 0.001). The difference in median duration of hospital stay was 1 day (5 days for women vs. 4 days for men, P < 0.001). In patients with STEMI, 6.2% fewer women than men were transferred for immediate invasive management (63.6% vs. 69.8%, P = 0.005) and the median door-to-balloon time was longer for women (23 min vs. 21 min, P < 0.001) (Supplementary material online, Table S1A). We also examined the effect of age on door-to-balloon time; in those 65 years and older, the median time was 3 min longer for women than for men (24 min vs. 21 min, P < 0.001), whereas no difference existed in those under 65 years (21 min vs. 21 min, P = 0.229) (data not shown).
The sex differences in demographic characteristics were similar for patients with STEMI and NSTEMI (Supplementary material online, Table S1A and B). In patients hospitalized with angina, there were fewer differences although women were older and less frequently received invasive management (Supplementary material online, Table S1C).
Predictors of coronary angiography and percutaneous coronary intervention
After adjusting for differences in age, deprivation and comorbidities, sex was an independent predictor of both coronary angiography and PCI in all patients (Table 2). For patients with STEMI, men were more likely to receive coronary angiography [adjusted odds ratio (OR) 1.44, confidence interval (CI) 1.05–1.97] and PCI (adjusted OR 1.62, CI 1.28–2.05). The same was true for patients with NSTEMI (coronary angiography adjusted OR 1.48, CI 1.26–1.75, PCI adjusted OR 1.52, CI 1.32–1.76).
Table 2.
Association of sex with coronary angiography and PCI according to diagnosis (odds ratio and 95% confidence interval shown for men vs. women)
| Coronary angiography |
PCI |
|||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | C-statistic | OR (95% CI) | P-value | C-statistic | |
| All | ||||||
| Age-adjusteda | 1.57 (1.42–1.73) | <0.001 | 0.699 | 1.70 (1.54–1.88) | <0.001 | 0.659 |
| Multivariable-adjustedb | 1.52 (1.37–1.68) | <0.001 | 0.733 | 1.68 (1.52–1.86) | <0.001 | 0.696 |
| STEMI | ||||||
| Age-adjusteda | 1.29 (0.96–1.73) | 0.086 | 0.690 | 1.51 (1.21–1.89) | <0.001 | 0.626 |
| Multivariable-adjustedb | 1.44 (1.05–1.97) | 0.023 | 0.775 | 1.62 (1.28–2.05) | <0.001 | 0.686 |
| NSTEMI | ||||||
| Age-adjusteda | 1.55 (1.33–1.81) | <0.001 | 0.761 | 1.57 (1.37–1.81) | <0.001 | 0.645 |
| Multivariable-adjustedb | 1.48 (1.26–1.75) | <0.001 | 0.790 | 1.52 (1.32–1.76) | <0.001 | 0.670 |
| HA | ||||||
| Age-adjusteda | 1.44 (1.05–1.99) | 0.026 | 0.610 | 2.18 (1.30–3.68) | 0.003 | 0.625 |
| Multivariable-adjustedb | 1.43 (1.02–1.99) | 0.037 | 0.675 | 2.25 (1.32–3.84) | 0.003 | 0.696 |
HA, hospitalized angina; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Adjusted for age only.
Adjusted for age, Scottish Index of Multiple Deprivation quintile, hypertension, diabetes, atrial fibrillation, renal failure, respiratory disease, cerebrovascular disease, peripheral vascular disease, heart failure, previous myocardial infarction, dementia, and depression.
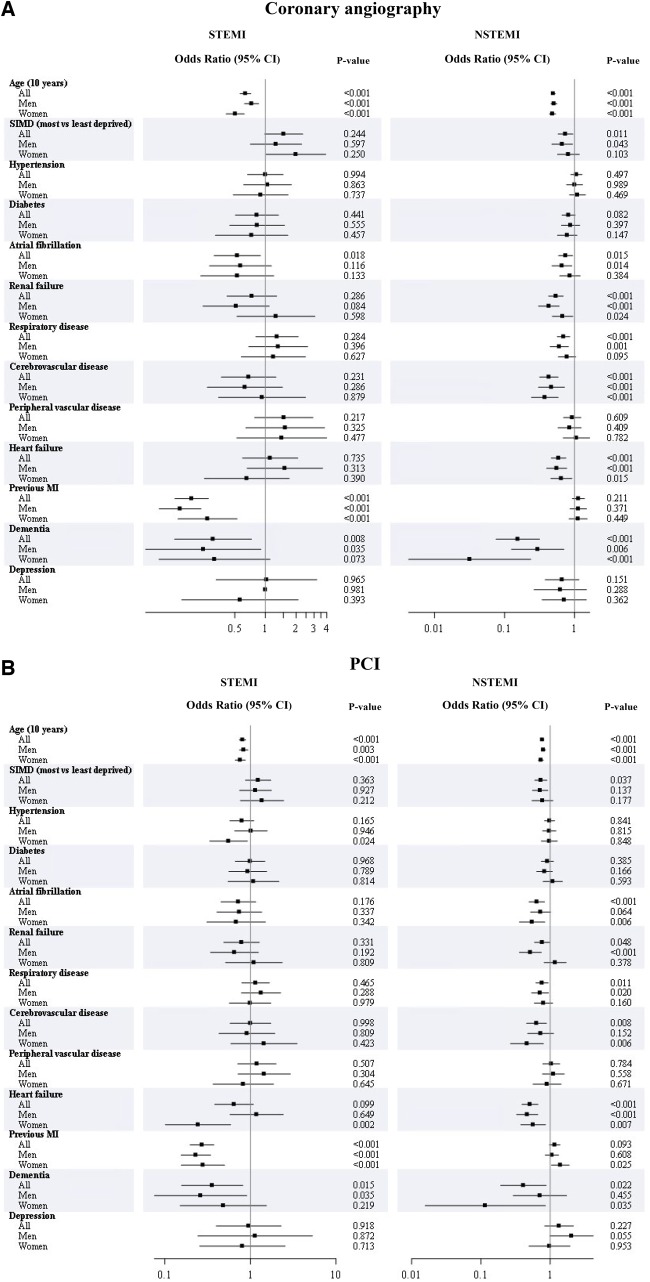
Several baseline characteristics were found to be independently associated with lower use of coronary angiography and PCI in patients with MI including older age, prior MI in STEMI, and heart failure in NSTEMI (Figure 1AandB). There were few major sex differences within subgroups; most notably, in those with NSTEMI and renal failure men were less likely than women to receive PCI, and in those with NSTEMI and dementia women were less likely than men to receive coronary angiography and PCI.
Figure 1.
(A) Association of baseline characteristics with coronary angiography according to sex for ST-elevation myocardial infarction and non-ST-elevation myocardial infarction (adjusted odds ratioa and 95% confidence interval shown for 10-year increase in age, most vs. least deprived, presence vs. absence of comorbidity). (B) Association of baseline characteristics with percutaneous coronary revascularization according to sex for ST-elevation myocardial infarction and non-ST-elevation myocardial infarction (adjusted odds ratioa and 95% confidence interval shown for 10-year increase in age, most vs. least deprived, presence vs. absence of comorbidity). aAdjusted for age, Scottish Index of Multiple Deprivation quintile, hypertension, diabetes, atrial fibrillation, renal failure, respiratory disease, cerebrovascular disease, peripheral vascular disease, heart failure, previous myocardial infarction, dementia, depression, plus sex in the ‘all’ group (excluding the variable being examined).
Medical therapy post-myocardial infarction
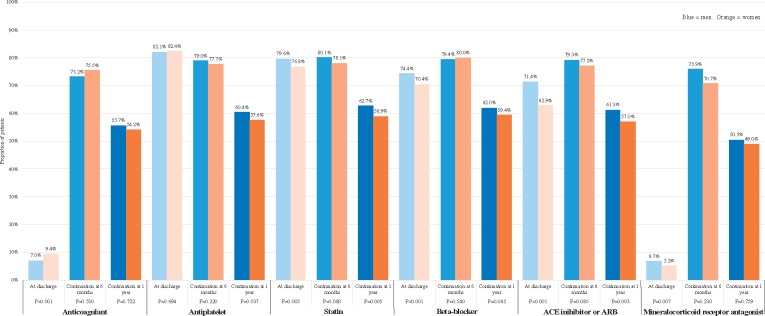
Women were less frequently treated with antiplatelets than men (with no greater treatment with anticoagulants), with a difference at 1 year of 2.8% (P = 0.037) (Figure 2). At 1 year, women were also less often prescribed statins (3.8% difference, P = 0.005) and angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) (4.3% difference, P = 0.003). A similar pattern was seen in the NSTEMI group (Supplementary material online, Figure S1B). In this group, women were also less frequently treated with beta-blockers at 1 year. Drug therapy was similar for men and women at 1 year in the STEMI and hospitalized angina groups, other than anticoagulants, with which fewer women than men hospitalized with angina were treated (Supplementary material online, Figure S1A and C). In patients with STEMI or hospitalized angina, sex was not an independent predictor of treatment with antiplatelets, statins, ACE inhibitors or ARBs, or beta-blockers at 1 year (Supplementary material online, Table S2). Conversely, in NSTEMI men were 20–32% more likely than women to be treated with statins, ACE inhibitors or ARBs, or beta-blockers at 1 year.
Figure 2.
Medical therapy at dischargea, at 6 monthsb, and at 1 yearb for all patients according to sex and medication. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker. aAt discharge is defined as within 90 days post-discharge. bProportions shown for 6 months and 1 year are of those on the drug(s) at discharge and still alive.
Death
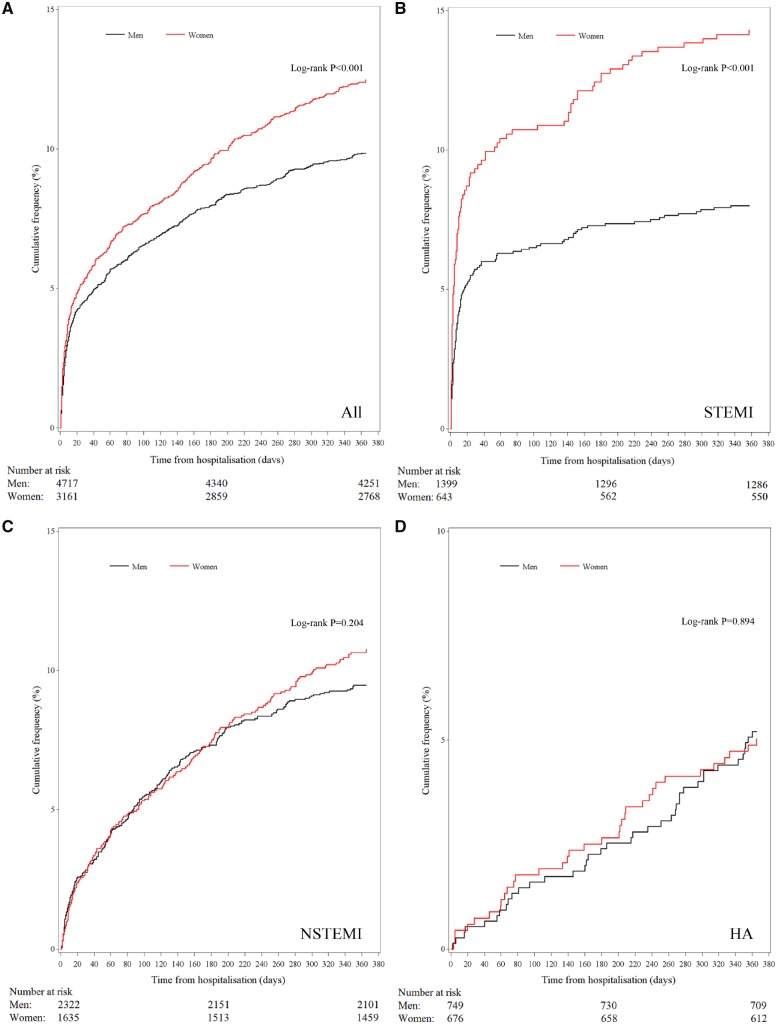
Case-fatality at 30 days was 4.9% in all patients, 6.9% in STEMI patients, and 2.9% in NSTEMI patients (Table 3). Case-fatality at 1 year was 10.9% in all patients, 10% in STEMI and NSTEMI patients, and 5.1% in patients hospitalized for angina. Survival was worse for women than for men, driven by marked differences in outcomes in STEMI (Figure 3); in this group, 6.3% more women than men had died by 1 year (14.3% vs. 8.0%, P < 0.001). However, after adjustment for baseline demographics, comorbidities, and PCI, the association between sex and mortality after STEMI was not significant and male sex emerged as an independent predictor of death in patients with NSTEMI (1-year hazard ratio 1.38, CI 1.12–1.69) (Table 3). A subgroup analysis of patients treated with PCI showed similar results (data not shown). Analysis of age-stratified groups (<65 years and ≥65 years) did not consistently show significant differences by sex, but this may be due to small numbers of events in the subgroups (Supplementary material online, Figure S2).
Table 3.
All-cause death at 30 days and 1 year according to sex and diagnosis (adjusted hazard ratioa and 95% confidence interval shown for men vs. women)
| All | Men | Women | P-value | HR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| All | n = 7878 | n = 4717 | n = 3161 | |||
| All-cause death, n (%) | ||||||
| 30 days | 386 (4.9) | 216 (4.6) | 170 (5.4) | 0.107 | 1.28 (1.04–1.57) | 0.022 |
| 1 year | 861 (10.9) | 465 (9.9) | 396 (12.5) | <0.001 | 1.21 (1.06–1.40) | 0.006 |
| STEMI | n = 2042 | n = 1399 | n = 643 | |||
| All-cause death, n (%) | ||||||
| 30 days | 140 (6.9) | 80 (5.7) | 60 (9.3) | 0.003 | 1.00 (0.70–1.43) | 0.985 |
| 1 year | 204 (10.0) | 112 (8.0) | 92 (14.3) | <0.001 | 0.95 (0.71–1.27) | 0.713 |
| NSTEMI | n = 3957 | n = 2322 | n = 1635 | |||
| All-cause death, n (%) | ||||||
| 30 days | 111 (2.8) | 66 (2.8) | 45 (2.8) | 0.866 | 1.72 (1.16–2.56) | 0.007 |
| 1 year | 396 (10.0) | 220 (9.5) | 176 (10.8) | 0.183 | 1.38 (1.12–1.69) | 0.002 |
| HA | n = 1425 | n = 749 | n = 676 | |||
| All-cause death, n (%) | ||||||
| 30 days | 9 (0.6) | <9 | <9 | 0.625 | 0.55 (0.13–2.31) | 0.416 |
| 1 year | 73 (5.1) | 39 (5.2) | 34 (5.0) | 0.880 | 1.19 (0.74–1.91) | 0.486 |
HA, hospitalized angina; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Adjusted for age, Scottish Index of Multiple Deprivation quintile, hypertension, diabetes, atrial fibrillation, renal failure, respiratory disease, cerebrovascular disease, peripheral vascular disease, heart failure, previous myocardial infarction, dementia, depression, and percutaneous coronary intervention.
Figure 3.
Kaplan–Meier curves for all-cause death according to sex and diagnosis.
Discussion
In this study of 7878 patients with hospitalized MI or angina from 2013 to 2016, we found that women had a higher crude rate of death but, after accounting for baseline risk factors, men were more likely to die following NSTEMI, with no difference for patients with STEMI or hospitalized angina. After taking account of baseline risk factors, there remain sex disparities for patients with MI related to treatment times, invasive management and use of secondary prevention therapies. Our findings highlight the need for renewed focus on achieving health and healthcare equity for women and men through prioritization of guideline-directed management.
Our analysis serves evidence of the persistently high crude mortality event rate in women, particularly with STEMI. We found that death from any cause was 2.6% more common amongst women than men at 1 year, driven predominantly by deaths in the STEMI population, for whom the crude difference was in excess of 6%. The survival curves for men and women with STEMI separate almost immediately, and this is reflected in the 3.6% mortality difference as early as 30 days. In this study, the crude differences were explained by the older age of women compared to men, greater burden of comorbidity, higher relative degree of deprivation, and reduced access to coronary angiography and PCI.
We have included a comprehensive indicator of social deprivation, which measures deprivation across seven weighted domains. In our study, women were more often from deprived socioeconomic groups. Socioeconomic deprivation is strongly linked with poorer outcomes in MI and in women the effect is more prominent.17 In Scotland, rates of coronary revascularization have increased across all deprivation categories over the past 10 years with the exception of the least deprived.2
Important sex differences in cardiovascular risk factors are evident; diabetes and hypertension are more common in women (particularly younger women), and they may increase risk more in women than men.18 There are a number of other risk factors specific to women, including hypertensive disorders of pregnancy and pregnancy-related diabetes mellitus, which are associated with a higher later cardiovascular risk.19 We evaluated additional important comorbidities, notably dementia and depression. Although we must interpret the results with caution due to small numbers of patients identified with each condition, the presence of dementia was associated with a lower likelihood of coronary angiography. Dementia likely serves as a disincentive for clinicians and the families of affected patients to adopt invasive management. It’s rising prevalence and emergence as a leading cause of death in women in several countries will increase the magnitude of this disparity.20,21 Large trials to investigate the appropriate treatment strategy for older patients with MI, including those with dementia, are underway.22,23
We found that an invasive strategy was used less often in the management of women with MI than it was for men, and this mirrors existing literature.5,7,24,25 Women were less likely to undergo coronary angiography and PCI. Our analyses suggest that this factor may, in part, explain why crude survival is worse for women than it is for men. There are several reasons why this discrepancy may exist. There were notable differences in route of admission to hospital, with fewer women than men taken directly to the catheterization laboratory irrespective of MI type. This will incur delays to revascularization and may reduce the likelihood of coronary angiography altogether. Differences in admission route may be explained by greater diagnostic uncertainty amongst women, who report non-specific or atypical symptoms more often than men.26 Data on the time between symptom-onset and first contact with medical services would highlight delays in presentation, when the benefits of emergent coronary revascularization are less certain. Finally, emergency care decisions regarding coronary angiography and PCI in women may be influenced by smaller coronary anatomy, more technically challenging vascular access (the excess door-to-balloon time seen in older women in this study may also reflect this), and greater risk of procedure-related complications and post-procedural mortality.25 Although bleeding complications remain more prevalent in women despite accounting for age, comorbidity and medication use, major adverse cardiac events are largely explained by baseline factors such as these.25,27
A further important finding of our study is that male sex was independently associated with a higher risk of death in patients with NSTEMI. This association has been recognized previously and highlights the importance of evaluating subtypes of MI separately.28,29 The reason for this is likely multifactorial. One possible explanation is that women have less obstructive coronary artery disease than men and, in post-menopausal women, more efficient vascular tissue repair.30 Differences in provision of primary preventative medical therapy may also contribute towards the findings. Finally, we lack data on cigarette smoking. In MI, smoking is not only more prevalent in men than in women5,24 but is also thought to be associated with different pathologic mechanisms—predominantly plaque rupture and acute thrombosis in men, and plaque erosion with superimposed thrombosis in women.31
Our study has a number of limitations. In addition to those that are inherent to the retrospective design, we were unable to include several important prognostic variables, including haematological and biochemical bloods tests, biomarkers, haemodynamics, left ventricular systolic function, coronary anatomy and extent of disease. We lack information regarding rates of prior PCI, subsequent coronary artery bypass grafting and symptom-burden after the event. However, women are less likely than men to undergo coronary artery bypass grafting and, even in the absence of adjusting for this, the crude association between female sex and death was removed. A further confounder is lack of data on sex of the treating physician; female patients with MI treated by male physicians are less likely to survive than if treated by female physicians, and greater male physician-experience in treating female patients is linked to better outcomes.32
Conclusion
Survival at 30 days and 1 year following STEMI is worse for women than for men. However, this is explained by relative differences in baseline characteristics such as older age, greater deprivation, more prevalent comorbidity, and lower rates of coronary angiography and PCI. Differences in the use of evidence-based drug therapy following MI also exist, with women at a disadvantage. Amongst patients with NSTEMI, male sex is an independent predictor of mortality. Efforts to address these sex disparities should be directed towards better understanding the differences in baseline risk and care pathways in order to highlight areas that would benefit from target, sex-specific intervention.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the following members of the project team for their support: Roma Armstrong, Jim Christie, Karen Fairbrother, Alan Foster, Stewart Hatrick, Neil Hillen, Brian Lawson, and Karen Ross. This work uses data provided by patients and collected by the NHS as part of their care.
Funding
This work was supported by a Joint Working Agreement with AstraZeneca UK Ltd, NHS Greater Glasgow and Clyde, the Golden Jubilee Foundation and the British Heart Foundation (Centre of Research Excellence Award RE/186134217, Clinical Training Fellowship FS/15/54/31639 to K.M. and Clinical Training Fellowship FS/18/14/33330 to A.M.J.).
Conflict of interest: B.F., S.S., and T.M. are employed by AstraZeneca UK Ltd, a biopharmaceutical company that manufactures drugs for the treatment of cardiovascular disease. C.B., A.M., C.M., A.M.J., K.M. and P.S.J. are/were employed by the University of Glasgow which received grants from AstraZeneca in support of this project. R.Z. is a PhD student at the University of Glasgow on an Industrial Studentship funded by AstraZeneca UK Ltd Iain Findlay reports receiving research funding from AstraZeneca UK Ltd. Based on a contract with the University of Glasgow, C.B. has acted as a consultant and speaker for AstraZeneca UK Ltd. M.L. and K.R. report receiving speaker fees and/or travel support from AstraZeneca UK Ltd.
References
- 1.GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2017;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Information Services Division. Scottish Heart Disease statistics year ending 31 March 2017. https://www.isdscotland.org/Health-Topics/Heart-Disease/Publications/2018-01-30/2018-01-30-Heart-Disease-Report.pdf (27 November 2018).
- 3. Dudas K, Lappas G, Rosengren A.. Long-term prognosis after hospital admission for acute myocardial infarction from 1987 to 2006. Int J Cardiol 2012;155:400–405. [DOI] [PubMed] [Google Scholar]
- 4. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS.. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 5. Wilkinson C, Bebb O, Dondo TB, Munyombwe T, Casadei B, Clarke S.. Sex differences in quality indicator attainment for myocardial infarction: a nationwide cohort study. Heart 2019;105:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah ASV, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV.. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350:g7873.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hvelplund A, Galatius S, Madsen M, Rasmussen JN, Rasmussen S, Madsen JK. et al. Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur Heart J 2010;31:684–690. [DOI] [PubMed] [Google Scholar]
- 8. Bugiardini R, Yan AT, Yan RT, Fitchett D, Langer A, Manfrini O. et al. Factors influencing underutilization of evidence-based therapies in women. Eur Heart J 2011;32:1313–1315. [DOI] [PubMed] [Google Scholar]
- 9. Jacobs AK, Johnston JM, Haviland A, Brooks MM, Kelsey SF, Holmes DR. et al. Improved outcomes for women undergoing contemporary percutaneous coronary intervention: a report from the National Heart, Lung, and Blood Institute Dynamic registry. J Am Coll Cardiol 2002;39:1608–1614. [DOI] [PubMed] [Google Scholar]
- 10. Velders MA, Boden H, van Boven AJ, van der Hoeven BL, Heestermans AACM, Cannegieter SC. et al. Influence of gender on ischemic times and outcomes after ST-elevation myocardial infarction. Am J Cardiol 2013;111:312–318. [DOI] [PubMed] [Google Scholar]
- 11. Lam CSP, McEntegart M, Claggett B, Liu J, Skali H, Lewis E. et al. Sex differences in clinical characteristics and outcomes after myocardial infarction: insights from the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur J Heart Fail 2015;17:301–312. [DOI] [PubMed] [Google Scholar]
- 12. Findlay I, Morris T, Zhang R, Mccowan C, Shield S, Forbes B. et al. Linking hospital patient records for suspected or established acute coronary syndrome in a complex secondary care system: a proof-of-concept e-registry in National Health Service Scotland. Eur Heart J Qual Care Clin Outcomes 2018;4:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHS Greater Glasgow and Clyde. About the safe haven. https://www.nhsggc.org.uk/about-us/professional-support-sites/nhsggc-safe-haven/about-the-safe-haven/ (27 November 2018).
- 14.The Scottish Government. Scottish Index of Multiple Deprivation. A National Statistics Publication for Scotland 18 December 2012. Executive Summary. http://simd.scotland.gov.uk/publication-2012/introduction-to-simd-2012/overview-of-the-simd/what-is-the-simd/ (27 November 2018).
- 15.The Scottish Government. Local Authority Summary—SIMD 2012 Glasgow City. http://simd.scotland.gov.uk/publication-2012 (27 November 2018).
- 16. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA.. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288–1294. [DOI] [PubMed] [Google Scholar]
- 17. Macintyre K, Stewart S, Chalmers J, Pell J, Finlayson A, Boyd J. et al. Relation between socioeconomic deprivation and death from a first myocardial infarction in Scotland: population based analysis. BMJ 2001;322:1152–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yusuf PS, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 19. Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N. et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 2012;125:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Records of Scotland. Vital Events Reference Tables 2017. Section 6: Deaths—Causes. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/general-publications/vital-events-reference-tables/2017/section-6-death-causes (27 November 2018).
- 21.Public Health England. Research and Analysis. Chapter 2: major causes of death and how they have changed. 2017. https://www.gov.uk/national-curriculum/key-stage-3-and-4 (27 November 2018).
- 22.The British Heart Foundation SENIOR-RITA Trial (SENIOR-RITA). ClinicalTrials.gov Identifier: NCT03052036.
- 23.Revascularisation or medical therapy in elderly patients with acute anginal syndromes (RINCAL). ClinicalTrials.gov Identifier: NCT02086019. [DOI] [PMC free article] [PubMed]
- 24. Anand SS, Xie CC, Mehta S, Franzosi MG, Joyner C, Chrolavicius S. et al. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J Am Coll Cardiol 2005;46:1845–1851. [DOI] [PubMed] [Google Scholar]
- 25. Lansky AJ, Hochman JS, Ward PA, Mintz GS, Fabunmi R, Berger PB. et al. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation 2005;111:940–953. [DOI] [PubMed] [Google Scholar]
- 26. Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V. et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012;307:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hess CN, McCoy LA, Duggirala HJ, Tavris DR, O'Callaghan K, Douglas PS. et al. Sex-based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE-ACS. J Am Heart Assoc 2014;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW. et al. Sex differences in mortality following acute coronary syndromes. J Am Med Assoc 2009;302:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Champney KP, Frederick PD, Bueno H, Parashar S, Foody J, Merz CNB. et al. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart 2009;95:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A. et al. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors?: position Paper from the Working Group on Coronary Pathophysiology and Microcirculation of the European Society of Cardiology. Cardiovasc Res 2011;90:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ambrose JA, Barua RS.. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43:1731–1737. [DOI] [PubMed] [Google Scholar]
- 32. Greenwood BN, Carnahan S, Huang L.. Patient-physician gender concordance and increased mortality among female heart attack patients. Proc Natl Acad Sci USA 2017;91:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.