Abstract
Significant improvements in cognitive control occur from childhood through adolescence, supported by the maturation of prefrontal systems. However, less is known about the neural basis of refinements in cognitive control proceeding from adolescence to adulthood. Accumulating evidence indicates that integration between hippocampus (HPC) and prefrontal cortex (PFC) supports flexible cognition and has a protracted neural maturation. Using a longitudinal design (487 scans), we characterized developmental changes from 8 to 32 years of age in HPC-PFC functional connectivity at rest and its associations with cognitive development. Results indicated significant increases in functional connectivity between HPC and ventromedial PFC (vmPFC), but not dorsolateral PFC. Importantly, HPC-vmPFC connectivity exclusively predicted performance on the Stockings of Cambridge task, which probes problem solving and future planning. These data provide evidence that maturation of high-level cognition into adulthood is supported by increased functional integration across the HPC and vmPFC through adolescence.
Keywords: adolescence, executive function, hippocampus, prefrontal cortex, resting state
Introduction
Executive functions (EF), including the ability to generate actions that are planned and goal directed, necessitate integration of function across circuitries supporting core processes of cognitive function. Developmentally, core EF functions such as working memory and cognitive control are present in infancy (Diamond 1989; Johnson 1995), show significant growth through childhood (Jones et al. 2003; Garon et al. 2008), and refinement toward reliable engagement in their ability to support higher-order cognition in adolescence (Luna et al. 2004; Ordaz et al. 2013; Simmonds et al. 2017). For example, by early adolescence, individuals can perform simple stimulus–response learning, working memory maintenance, and response inhibition, while precision, speed of processing, and reliability of responses continues to improve into adulthood supported by increased engagement of specialized brain regions (Ordaz et al. 2013; Simmonds et al. 2017). Higher-order EF, which we operationalize as processes that require the simultaneous engagement and integration of multiple core executive processes, continue to mature throughout adolescence. Thus, as requirements on EFs become more complex necessitating proactive response planning and flexibility, adolescent immaturities in problem solving become evident. For example, future planning that requires the simultaneous maintenance of concurrent task goals and prior related experiences shows protracted development throughout adolescence into early adulthood (Albert and Steinberg 2011; Decker et al. 2016). This refinement in higher-order cognitive processes (reviewed in detail in Murty et al. 2016) suggests that mechanisms linked to these processes may be uniquely coming on-line through adolescence.
The hippocampus (HPC) has been increasingly seen to be involved in EF tasks through its connectivity with the prefrontal cortex (PFC), and there is growing evidence for protracted maturation of HPC-PFC circuits through adolescence. While PFC has long been associated with cognitive control tasks, accumulating research suggests that there is a role for the HPC in supporting both working memory and EF during delayed match-to-sample, including those encompassing memory-guided saccades (Ryan and Cohen 2004; Olsen et al. 2009, 2013; Dudukovic et al. 2011; Warren et al. 2011; Nauer et al. 2015), as well as spatial span (SSP) (Spellman et al. 2015) tasks, although at least some of these functions remain intact following hippocampal damage (Cave and Squire 1992). HPC, and in particular its connectivity with medial PFC, has also been linked to EFs related to future planning and problem solving (Buckner 2010; Shohamy and Turk-Browne 2013), suggesting that it may play a more general role across different EFs.
Developmental changes in HPC-PFC physiology provide support for the hypothesis that cognitive development may be linked to changes in this circuitry through adolescence. Several white matter tracts, including the uncinate fasciculus, fornix, and cingulum bundle provide pathways for hippocampal–PFC integration. In particular, the uncinate fasciculus and ventral cingulum bundle show protracted maturation through adolescence and are among the last white matter tracts to mature (Lebel et al. 2012; Simmonds et al. 2014; Olson et al. 2015; Tamnes et al. 2018). Age-related changes in these structural pathways have been linked to the development of a diverse array of EFs, including mnemonic control (Wendelken et al. 2015), cognitive control (Simmonds et al. 2014), emotion regulation (d’Arbeloff et al. 2018), and more. Relatedly, task-based functional magnetic resonance imaging (fMRI) has shown that interactions between the HPC and PFC continue to strengthen into adolescence during working memory maintenance and episodic memory retrieval (Menon et al. 2005; Ofen et al. 2007, 2012; Finn et al. 2010; Demaster and Ghetti 2013; Tang et al. 2017). Further, preliminary research, particularly in animal models, has begun to unpack how HPC-PFC integration during adolescence occurs. Increased dopaminergic neurotransmission has been shown to enhance plasticity and circuit integration across the HPC and PFC (Gurden et al. 1999, 2000; Goto and Grace 2008; Fujisawa and Buzsáki 2011). Significant aspects of this modulation become available during the periadolescent period (Tseng and O’Donnell 2005, 2007; Caballero et al. 2016), leading to enhanced HPC-PFC functional integration through puberty. We have recently proposed that the functional consequences of these developmental increases in integration across the HPC and PFC is an enhancement of the ability to use prior experience to guide decision-making (Murty et al. 2016). However, direct evidence for increased integration across the HPC-PFC and its consequences on cognitive processes is lacking.
In this study, we investigated developmental changes in HPC-PFC functional connectivity throughout adolescence into adulthood and its contributions to cognitive maturation, using a large, extended longitudinal cohort design fMRI study. Given evidence for HPC-PFC circuits supporting refinement in higher-order cognition and protracted maturation of this circuitry, we hypothesize that this system may be playing a unique role in supporting refinements in cognitive maturation into adulthood (Murty et al. 2016). Thus, the goal of the current study was to characterize developmental changes in interactions between the HPC and PFC through adolescence into early adulthood, as well as their associations with the development of simple and higher-order EF. We assayed multiple HPC-PFC circuits at rest during an fMRI acquisition in a sample of individuals ranging in age from 8 to 32 sampled with up to 12 yearly visits in an accelerated longitudinal cohort design. We hypothesized that we would observe protracted maturation of HPC-PFC connectivity, which would be associated with different aspects of cognitive development. Given that anterior and posterior HPC have different developmental trajectories and underlie unique processes, we probed these separately. We first characterized neurodevelopmental trajectories of connectivity across the entire HPC with the dorsolateral PFC (dlPFC), ventrolateral PFC (vlPFC), ventromedial PFC (vmPFC), and replicated these findings in a large, independent, cross-sectional sample. Given that the HPC has an anterior and posterior portion that differ in their structure and connectivity and develop at different rates (Gogtay et al. 2006; DeMaster et al. 2014), which may have unique impact on adolescent cognitive development (Poppenk et al. 2013), and that treatment of the HPC as a unitary region (rather than considering its subregions) may obscure developmental trajectories (DeMaster et al. 2014), we further investigated whether these developmental trajectories depended on HPC subregion. Next, we characterized how connectivity across HPC-PFC circuits related to a battery of cognitive tasks reflecting simple and higher-order EF. Finally, we have previously suggested based on animal models (Caballero et al. 2016) that the development of dopaminergic signaling may provide the impetus for increased integration between HPC and PFC (Murty et al. 2016). As such, we investigated whether development of mesolimbic circuitry contributed to HPC-PFC integration based on functional connectivity between the ventral tegmental area (VTA), where dopamine is produced, and PFC hypothesizing that it may account for the timing of developmental changes in HPC-PFC functional connectivity in adolescence.
Materials and Methods
Subjects
Data from 143 subjects were analyzed (78 females, ages 8.1–32.6, mean age 18.7), with subjects completing 1–12 yearly visits. Each visit comprised a neurocognitive battery, and MRI scan session completed on different days. In total, participants completed 487 scans (mean 3.1 scans/subject, ranging from 1 to 10 visits), with 91 subjects returning for at least one follow-up visit, 74 with at least 3 visits, and 54 with at least 4 visits (see Supplementary Fig. 1). We have previously published the results of separate developmental research inquiries on subsets of this data set (Velanova et al. 2008; Hwang et al. 2013; Ordaz et al. 2013). Participants were recruited from the local population and were screened for psychiatric and neurological problems, medication history, first-degree relatives with major psychiatric illness, and MRI contraindications (e.g., metal in the body). Subjects returning for follow-up visits that had been diagnosed with psychiatric illness were excluded from further study, and previous data were eliminated from the data set. The University of Pittsburgh’s Institutional Review Board approved the study. Participants and their parents (for subjects under 18) gave informed consent, and participants were compensated for their participation.
Behavioral Assessments
A behavioral visit was completed prior to each scan session, typically within 2 weeks of the scan. Subjects completed a number of neurocognitive assessments, including a battery of CANTAB tasks (Luciana 2003) and eye movement tasks. Subjects performed tasks assessing core EF, including working memory (delayed match to sample [DMS], Spatial Span [SSP], memory-guided saccade) and response inhibition (antisaccade task), as well as higher-order EF that relies on integrating multiple core executive processes (Stockings of Cambridge [SOC] task) (Asato et al. 2006). We describe each of these briefly.
DMS was administered as part of the CANTAB battery. DMS provides a measure of spatial working memory, in which subjects must view and retain a complex visual scene, then after a delay select the matching pattern. Performance was assessed based on total proportion correct and latency of correct trials.
SSP was administered as part of the CANTAB battery. SSP provides a measure of spatial working memory capacity, in which subjects view a pattern of dots sequentially changing color, after which they must reproduce the pattern in the correct order. For SSP, our dependent variables were total errors and maximum span length successfully reproduced.
Memory-Guided Saccade (MGS) was performed as an eye movement task while subjects were in the scanner, which has previously been reported by our group (Montez et al. 2017; Simmonds et al. 2017). MGS provided an additional assessment of spatial working memory. In this task, subjects were presented and instructed to fixate a peripheral dot, then returned their gaze to a central fixation mark. After a variable delay period, subjects were prompted to return their fixation to the location of the dot in the absence of any stimulus. We measured both the mean accuracy error in the location of the MGS returning to the initially fixated location, as well as latency to perform the MGS.
Anti-saccade (AS) was administered as an eye-movement task during behavioral assessments in the lab (Luna et al. 2004). In this task, subjects fixated a central mark, which disappeared at an unknown time while a new mark was displayed in the periphery. Subjects had to suppress an eye movement toward the peripheral mark and instead make an eye movement to the opposite side of the screen. Performance was assessed as the percent of successful trials, and the latency to eye movement onset, both derived from eye tracking data.
SOC was administered as part of the CANTAB battery. In the SOC, subjects are presented a set of objects stacked in adjacent columns, as well as a “goal” configuration of the objects. Participants must move objects one at a time to produce the goal configuration in as few moves as possible. We used the total number of problems solved in minimum moves (i.e., the proportion of trials in which subjects were able to generate an optimal solution) as the dependent variable.
SOC provides four summary measures (initial and subsequent thinking time, number of moves per trial, and proportion of trials solved in the optimal number of moves). We chose not to use the “thinking time” metrics since subjects were not given any specific instruction regarding how fast to respond, and as such have extremely high intersubject variances. The two performance metrics (mean moves and number of optimally performed trials) are extremely highly correlated (r = 0.91). We choose to report performance based on the number of optimally performed trials, since it better accounts for the fact that the optimal number of moves varies across trials (between 2 and 5). See Supplemental Material for associations of performance on these measures with age (see Supplementary Table 1 for all measures and Supplementary Fig. 2 for the SOC task in particular), and each other (see Supplementary Fig. 3).
MR Acquisition Parameters
Data were acquired on a Siemens 3 tesla MAGNETOM Allegra fitted with a standard circularity-polarized head coil. Importantly, the scanner was unchanged other than standard maintenance through the duration of the 10-year project. Subjects’ heads were immobilized using pillows placed inside the head coils, and subjects were fitted with earplugs to minimize scanner noise. Structural images were acquired using a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence (repetition time [TR] = 1570 ms; echo time [TE] = 3.04 ms; flip angle, 8°; inversion time [TI] = 800 ms, voxel size = 0.78125 × 0.78125 × 1 mm). Functional images were acquired using a blood oxygen-level dependent (BOLD) signal from an echoplanar sequence (TR, 1500 ms; TE, 25 ms; flip angle, 70°; voxel size, 3.125 × 3.125 mm in-plane resolution) with contiguous 4-mm-thick slices aligned to the subject’s anterior–posterior commissure plane.
Region of Interest Selection
To avoid functionally defined regions stemming from studies of adult functional connectivity, we used a priori anatomical definitions of the PFC in each hemisphere to determine the center of mass for three PFC subregions we expected could reflect these changes (see Fig. 1): vmPFC (conjunction of all regions from a population vmPFC atlas (Mackey and Petrides 2014), excluding the subgenual ACC), vlPFC (conjunction of BAs 44, 45, and 47) and dlPFC (the middle frontal gyrus bordered by the superior frontal and precentral sulci including BAs 9 and 46). For each region, we placed a sphere on the defined center of mass, applied gray matter, and hemispheric masks. Spheres were grown to include approximately 200 voxels (resulting sphere radii 8.7–10.6 mm, surviving voxels ranged between 189 and 210). Hippocampal seed regions were defined anatomically in each hemisphere. To investigate the relative contributions of anterior and posterior HPC, two regions per hemisphere were derived from the Harvard-Oxford by dividing the HPC into thirds (Staresina et al. 2011; Murty et al. 2017), while matching the total number of voxels in each region (136–147 voxels per HPC region; see Fig. 1). This produced a central gap between the seeds, preventing signal spread between the two regions and allowing us to functionally isolate anterior and posterior time courses. This approach is limited in its ability to fully characterize connectivity of the hippocampal body per se, but rather is defined to focus on the anterior and posterior extents, based on the functional properties that have been associated with each. For our post-hoc analysis investigating connectivity between the VTA and vmPFC, the VTA region of interest (ROI) was characterized using a probabilistic atlas of the VTA (Murty et al. 2014), thresholded at a level of 50% overlap across individuals.
Figure 1.
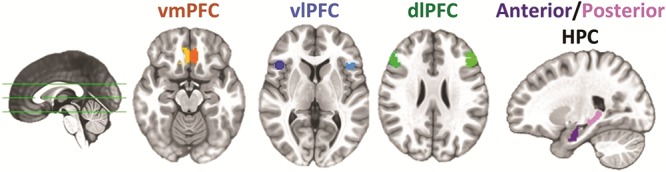
Regions of interest for connectivity analyses. Correlations were computed between anterior and hippocampal seeds and three anatomically defined regions of the PFC: ventromedial (vmPFC), ventrolateral (vlPFC), and dorsolateral (dlPFC) separately for each hemisphere.
Data quality for ROIs was assessed by visual inspection of each scan. We visualized the nonlinearly warped MPRAGE in MNI space with the HPC seeds overlaid. We found a high degree of alignment between the anatomical boundaries of the HPC and the edges of the atlas derived seed regions. A small number of scans (n = 8) were excluded due to registration errors, which caused a significant misalignment between the ROIs and individual anatomy.
Data Preprocessing
Structural MRI data were preprocessed to extract the brain from the skull and warped to the MNI standard brain using both linear (FLIRT) and nonlinear (FNIRT) transformations. Functional images were processed using a pipeline designed to minimize the effects of head motion (Hallquist et al. 2013). This included 4D slice-timing and head motion correction, wavelet despiking (Patel et al. 2014), coregistration to the structural image and nonlinear warping to MNI space, local spatial smoothing with a 5-mm Gaussian kernel based on the SUSAN algorithm, intensity normalization, nuisance regression based on head motion (6 DoF motion estimates and their derivatives) and nongray matter signal (white matter and CSF, and their derivatives), and bandpass filtering between 0.009 and 0.08 Hz. Frame-wise motion estimates were computed, and volumes containing frame-wise displacement (FD) > 0.3 mm were censored from connectivity computations. Subjects with more than 25% of TRs censored, or a mean FD > 0.5 mm (before censoring), were excluded from analyses. In total, 50 sessions were excluded based on these criteria (10.2%), leaving 437 sessions in the final analyses. To ensure that any residual motion effects were not driving age-related findings, all analyses included mean FD as a covariate.
Functional data were extracted from the OFF periods of a mixed block-event–related design (Ordaz et al. 2013), based on a previously described method (Fair et al. 2007). Subjects performed four block design runs, each of which followed an OFF-ON-OFF-ON-OFF design, where ON periods consisted of either a pro- or antisaccade task. Data from each OFF period were extracted, excluding the 15 s following the preceding ON period. This resulted in blocks of 27, 25, and 16 TRs respectively (40.5, 39.0 and 22.5 s). Across the four runs, this produced a total of 272 TRs, or 6:48 min of data.
Connectivity Analysis
For each ROI, time courses were extracted from each subject’s data by taking the first principal component across all voxels within the ROI from the preprocessed and head motion scrubbed voxel-wise time courses. Pearson correlation coefficients were computed between the four hippocampal seeds (left and right hemispheres, anterior and posterior) and the six PFC seeds (left and right vmPFC, vlPFC, dlPFC) and normalized using Fisher’s z transformation, resulting in 24 separate connections. Age effects were assessed using a generalized additive model (GAM; Wood 2017) applied to each ROI pair, including a smoothed fixed effects of age and head motion (mean FD), a fixed effect of gender, and a random effect of subject. This allows for assessing age effects in a semiparametric manner, without assuming the shape of developmental trajectories (Wierenga et al. 2019). We performed separate GAM models for anterior and posterior HPC, in each case including an age × ROI interaction to model smoothed effects of age separately for connectivity to vmPFC, vlPFC, and dlPFC. Age effects were corrected based on six comparisons (Bonferroni significant at P < 0.0083). The smoothed head motion term controls for nonlinear relationships between mean head motion and connectivity strength, estimated simultaneously to any age effects.
For connections showing significant age-dependent changes, we performed an analysis to identify age intervals of significant change based on the GAM model fit. In order to define specific periods (age ranges) of age-related change, a posterior simulation was performed based on the first derivative of the GAM fit. Following established guidelines (Wood 2017) and related previous work from our group (Simmonds et al. 2014), 10 000 simulated GAM fits and their derivatives (generated at 0.1 year age intervals) were computed from a multivariate normal distribution, whose vector of means and covariance corresponded to the fitted GAM parameters. Confidence intervals (95%) were generated from the resulting simulated derivatives. Significant periods of age-related change were identified as those ages when this confidence interval did not include zero (P < 0.05). We note the utilized posterior simulation method is more appropriate for GAM models, compared with case-resampling approaches (e.g., bootstrap), which are likely to bias smoothing estimates (Wood 2017).
Replication Data
To confirm our results characterize resting-state fMRI, we replicate our age-related changes in connectivity strength in a parallel, cross-sectional data set spanning a similar age range, collected on a separate set of subjects at a separate site. We used resting-state fMRI data from the Philadelphia Neurodevelopmental Cohort (PNC) (Calkins et al. 2015; Satterthwaite et al. 2016). Of the 9498 subjects who participated in the PNC study, 807 participated in a resting state fMRI scan and passed motion and quality checking standards. Of these, we identified a subset of 321 subjects (ages 10–22 years old, mean = 15.9, 156 female) that we considered to be typically developing according to criteria similar to the inclusion criteria for our study. This was based on the computerized structured interview data (GOASSESS), which we used to compute DSM-IV diagnosis rankings. Similar to a previous publication (Calkins et al. 2015), subjects were considered to have significant psychopathology if they endorsed symptoms with frequency and duration of a DSM-IV psychiatric disorder, along with significant distress or impairment (a rating of >5 on a scale of 0–10) and were excluded from our sample. PNC resting state data was acquired with a 3 × 3 × 3 mm voxel resolution, with TR = 3000 ms, TE = 32 ms, and flip angle = 90°. Acquisitions lasted 6:15 min, producing 125 TRs per run. We repeated our preprocessing pipeline, ROI definitions, time course extraction, and correlation computations on the replication data as described above. As in our main sample, we assessed significance of age-related change using a GAM with a flexible effect of age assessed for anterior and posterior HPC seeds separately.
Brain Behavior Relationship
To assess the relationship of connectivity to cognitive assessments, we performed an additional mixed effects model for each ROI pair (n = 2), and behavioral outcome (n = 10, see above) showing age-related changes. Given our interest in characterizing developmental effects, we limited this analysis to tasks and connections showing age-related change within our sample. In each case, we used the residuals from GAM models (described above) fit separately to the connectivity and behavioral data, effectively regressing out effects of age and gender, and in the case of connectivity values, head motion. We then performed a linear regression between connectivity and behavioral residuals to determine if there was a significant relationship that could not be accounted for by concurrent age-related change.
Results
Age-Related Changes in HPC-PFC Connectivity
Results of generalized additive mixed model fits to connectivity data indicated that both anterior and posterior HPC connectivity to the vmPFC showed age-related increases (aHPC: F = 9.79, P = 0.0018; pHPC: F = 5.88, P = 0.0026). No significant age-related change in connectivity strength was observed for either the aHPC or pHPC with the vlPFC or dlPFC (all P > 0.5 uncorrected). Models including both ipsi- and contra-lateral connectivity separately confirmed these age effects with vmPFC (aHPC: F = 28.6, P = 5.9E-7, pHPC: F = 15.8, P = 2.3E-8), indicating that age effects were present for both left and right HPC, as well as both ipsi- and contra-lateral connections. Results were consistent when we regressed out the global signal and implemented ICA-AROMA as additional preprocessing steps (aHPC: F = 4.87, P = 0.027; pHPC: F = 5.38, P = 0.038, see Supplementary Fig. 6). There was no significant effect of gender on connectivity values for either aHPC or pHPC (P > 0.4). We note that an exploratory, voxel-wise analysis of HPC connectivity performed across the entire PFC confirmed our findings of age-related increases in HPC-vmPFC connectivity and revealed that while age-related changes were not unique to vmPFC, there were relatively few regions showing age-related increases, and none of these were directly adjacent to our dlPFC or vlPFC regions, suggesting that the null results observed for those regions cannot be accounted for by specific placement of the ROIs (see Supplementary text and Supplementary Fig. 5).
Inspection of the age-related connectivity changes in the HPC-vmPFC data suggested that increases were not linear and instead were concentrated in specific periods of development. To test this assertion, we computed the derivative of the GAM model fit to identify time periods in which there was significant age-related change (confidence interval of the derivative did not include zero; see Methods). Based on this, age-related change in pHPC-vmPFC connectivity was identified between 12.3 and 18.6 years old, while aHPC-vmPFC occurred later, from 17.6 to 20.7 years old (see Fig. 2).
Figure 2.
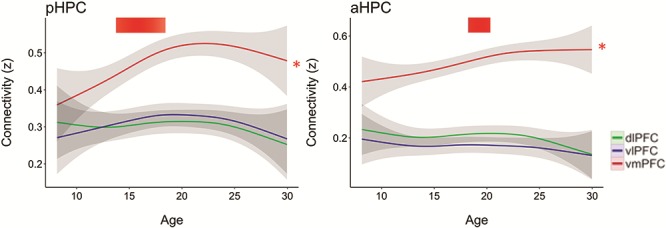
Age-related differences in connectivity of the posterior (left) and anterior (right) HPC with the vmPFC (red), vlPFC (blue), and dlPFC (green) using our longitudinal neuroimaging data set. Significant age-related changes were only seen between the HPC and vmPFC. Shaded bars indicate ±1 SEM (* = P < 0.05). Red bars indicate regions of significant change in HPC-vmPFC connectivity based on the derivative of the GAM model fit. For individual data, see Supplementary Figure 4.
Given that our results are based on off-period connectivity, we were interested in the extent to which these patterns of age-related changes in HPC-vmPFC connectivity generalized to a more typical resting state scan. Thus, we performed a replication analysis using a normative subsampling of cross-sectional data from the PNC cohort (Satterthwaite et al. 2014). Replicating the above findings, connectivity to vmPFC from both anterior (aHPC-vmPFC: F = 8.34, P = 0.004) and posterior (pHPC-vmPFC: F = 9.79, P = 0.001) HPC showed significant age-related increases (see Supplementary Fig. 7). These effects also remained when global signal regression and ICA-AROMA were included in the preprocessing pipeline (aHPC: corrected F = 5.24, P = 0.022, pHPC: corrected F = 6.13, P = 0.014). As in our longitudinal data, no significant age-related changes were found for anterior or posterior HPC to either dlPFC or vlPFC in the replication data set, either with or without GSR and ICA-AROMA (all P > 0.08 uncorrected).
Brain–Behavior Relationship
For each of five tasks included in our behavioral battery, we assessed whether there were significant age effects for its associated measures based on GAM model fits (see Supplemental Material, Table S1). We found significant age-related change for DMS accuracy (P < 0.0001) and latency (P < 0.0001), SSP span length (P < 0.0001), error rate (P = 0.001) and latency (P < 0.0001), SOC performance (% optimal moves, P < 0.0001, see Supplementary Fig. 2), AS % correct (P < 0.0001) and latency (P < 0.0001), and MGS % correct (P < 0.0001) and latency (P < 0.0001) after correcting for multiple comparisons. Only SOC initiation latency did not show a significant effect after Bonferroni correction (corrected P = 0.2).
For each connection that showed significant age effects (anterior and posterior HPC to vmPFC), we assessed associations between connection strength and behavioral performance for each measurement in our cognitive battery. We found a significant positive relationship between the pHPC-vmPFC connectivity and performance on the SOC task (T = 4.12, P = 4.7e-5, Bonferroni corrected significant based on 11 task comparisons), a measure of higher-order EF (Fig. 3). To ensure correlations were not present due to concurrent change with age, we correlated residuals from the GAM models performed independently for both connectivity and behavioral data. The relationship between pHPC-vmPFC connectivity and SOC performance remained significant when residualizing for age, gender, and head motion (T = 2.77, P = 0.0059). No such relationship was present for aHPC-vmPFC in SOC performance (T = 1.84, P > 0.05 uncorrected), and no other associations between either posterior or anterior HPC-vmPFC connectivity and any other behavioral measure in our study survived multiple comparison correction, with or without controlling for age (P > 0.05, see Supplemental Material, Table S2).
Figure 3.
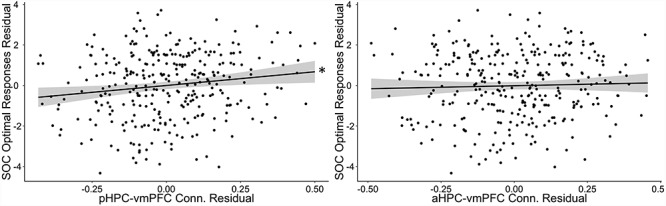
Connectivity of the pHPC (left) and aHPC (right) with vmPFC (residualized to remove age, gender and head motion) to predict individual differences in performance on the SOC task (residualized to remove age and gender). Only pHPC-vmPFC showed a significant relationship with task performance. Shaded bars indicate ±1 SEM.
Mediation of Age-Related Connectivity by VTA Functional Coupling
Rodent models show that VTA neuromodulation enhances PFC-HPC plasticity as well as circuit integration (Gurden et al. 1999, 2000; Goto and Grace 2008; Fujisawa and Buzsáki 2011) through a processes that emerges in the periadolescent period (Tseng and O’Donnell 2007). In order to assess the contribution of putatively dopaminergic systems on developmental changes in HPC-PFC connectivity, we investigated developmental changes in connectivity to the VTA, where dopaminergic cells originate. To test the hypothesis that signaling from dopaminergic regions such as the VTA contributes to the enhancement of HPC-PFC coupling, we assessed whether VTA connectivity with the vmPFC was associated with the age-related changes in PFC-hippocampal integration. Given that typical mediation analyses based on the product of coefficients (“a*b indirect effect”) are not readily available for GAM models (Cerin et al, 2018), we assessed mediation based on a joint significance test (MacKinnon and Luecken, 2008). Consistent with a mediation of age-related changes in HPC-vmPFC connectivity by VTA projections, there was a significant association between age and VTA-vmPFC (F = 4.09, P = 0.0088; see Supplementary Fig. 8) and a significant relationship between VTA-vmPFC and HPC-vmPFC when controlling for age (aHPC: F = 37.4, P < 0.0001; pHPC: T = 57.4, P < 0.0001). Furthermore, when separately partialling out age, gender, and head motion from both VTA-vmPFC and HPC-vmPFC connectivity separately via GAM models, these connections remained significantly correlated (aHPC: T = 2.48, P = 0.013; pHPC: T = 4.56, P < 0.0001). There was no significant interaction with age for either model (P > 0.05). Finally, a formal mediation analysis (based on the “a*b indirect effect”) performed by approximating age-related change as a linear effect, showed that for both anterior and posterior HPC, VTA-vmPFC connectivity was a significant partial mediator of the age-related changes (aHPC: unmediated age effect t = 2.8, mediated age effect t = 1.8, P = 0.0022; pHPC: unmediated age effect t = 3.7, mediated age effect t = 2.5, P = 0.0026; see Supplementary Fig. 9).
Discussion
In the current study, we characterized the neurodevelopmental trajectories of functional connectivity at rest between the anterior and posterior HPC with multiple targets throughout the PFC. Synthesis of prior work suggests that adolescence represents a unique period of development in which information processing across these regions is refined (Murty et al. 2016). We found increased functional connectivity of both the anterior and posterior HPC that was specific to connections with the vmPFC during rest throughout adolescence and into early adulthood. We then replicated these findings in an independent rs-fMRI data set to confirm that similar effects were seen in an extended, continuous resting state as what we observed in the off-periods of our block design data. Next, we found that age-related changes in this circuit predicted individual differences in performance on the SOC task. Finally, we found that age-related functional connectivity of the VTA with the vmPFC, a putative marker of dopamine signaling, partially mediated the age-related changes in HPC-vmPFC connectivity. Together, these findings support a model of enhanced information processing across the HPC and vmPFC during adolescence, which underlies a refinement of complex EF.
Results from our data and the PNC indicated a protracted maturation in functional connectivity of both the anterior and posterior HPC with vmPFC, such that there was increased functional connectivity of these regions as individuals approached adulthood. Prior models of hippocampal function have suggested a functional gradient across the long axis of the HPC, such that more flexible integrative memory encoding is localized to anterior portions (Preston and Eichenbaum 2013; Strange et al. 2014). Within this framework, posterior HPC would support the stable representation of task contexts, whereas, anterior HPC may support effective updating of contextual information to guide higher-order cognition. Developmental studies of hippocampal structure have shown protracted maturation, which varies significantly along the anterior–posterior long axis (Gogtay et al. 2006; DeMaster et al. 2014). Our large, longitudinal sample coupled with the GAM modeling approach allowed us to characterize nonlinear patterns of development across HPC subregions. Based on an analysis of age intervals showing developmental change, we found that both anterior and posterior HPC connectivity to vmPFC exhibited increases in connectivity in early to mid adolescence, before reaching a plateau in early adulthood. Interestingly, this analysis indicated sequential maturation, with posterior HPC connectivity maturing earlier than anterior. These connectivity findings are interesting in light of structural neuroimaging studies within the HPC that show differential maturation along the HPC long axis (Gogtay et al. 2006; DeMaster et al. 2014; Schlichting et al. 2017). These studies find that the head and tail of the HPC show similar patterns of development through adolescence, in contrast to the HPC body, which dovetails with the similarity in age intervals of change we observed between the anterior and posterior portions of the HPC.
These findings are, on the surface, in contrast to prior studies showing that the development of associative memory is associated with a greater reliance on the dlPFC in adulthood, as well as increased HPC-dlPFC coupling (Menon et al. 2005; Ofen et al. 2007, 2012; Demaster and Ghetti 2013). For example, a recent study showed that adolescence was associated with increased coupling between lateral PFC and medial temporal lobe during memory retrieval, without any changes in coupling between mPFC and medial temporal lobe (Tang et al. 2017). Rather than contradict, however, we believe we extend these findings by showing that HPC-vmPFC connectivity, which is highly sensitive to task context (Huijbers et al. 2011), continues to increase in task-free contexts. However, future work is needed to understand in what contexts HPC connectivity with dlPFC vs vmPFC supports behavior, and how these two networks may interact.
Importantly, we found that greater HPC-vmPFC connectivity supported better SOC performance but not improvements in other cognitive tasks that target core processes of working memory and inhibitory control. The SOC task is a well-validated, highly reliable neuropsychological test that probes individual’s ability to plan future sequences of actions and their consequences as they relate to complex problem solving (Luciana 2003). What may differentiate the SOC from these other tasks is that the SOC engages multiple core processes to support a proactive multistep response that requires sequential updating (Asato et al. 2006), further supported by an association with fluid intelligence generally beyond individual cognitive processes (Unterrainer et al. 2004; Zook et al. 2004). This interpretation falls in line with previous research in adult samples, which have shown increased HPC-vmPFC coupling predicting schema formation (van Kesteren et al. 2010, 2014; Bein et al. 2014; Spalding et al. 2015), memory-guided decision-making (Gluth et al. 2015; Voss and Cohen 2017), sequence learning (Davachi and DuBrow 2015), the emergence of conceptual knowledge (Kumaran et al. 2009), and future planning (Campbell et al. 2018). Thus, the HPC’s role in storing prior experiences (Davachi and DuBrow 2015; Gruber et al. 2016; Eichenbaum 2017), promoting working memory maintenance (Leszczynski 2011) and simulating future experiences (Addis et al. 2011; Mullally and Maguire 2014) may make it particularly well suited for supporting SOC performance. Although our battery of cognitive tasks rule out associations with core cognitive components such as working memory and response inhibition, further work with more extensive batteries of cognitive tests are required to fully understand whether the SOC associations we observe relate to specific cognitive functions such as future planning (which itself likely requires the integration of multiple cognitive functions) or to the processes governing integration across core cognitive components generally. While connectivity of the vmPFC with both the anterior and posterior HPC showed age effects and relationships with SOC performance, it was only in the posterior HPC in which vmPFC connectivity mediated the relationship between age and SOC performance. In line with prior literatures detailing a functional topography across the HPC (Eichenbaum 2017), this finding suggests that maintenance of a stabile context representation could be especially beneficial for SOC performance.
Finally, we investigated the role of engagement of dopaminergic systems on the specificity of adolescent period development of HPC-PFC FC. We have previously proposed that dopaminergic signaling originating in the VTA and projecting to the PFC plays a primary role in the developmental timing of HPC-PFC maturation. Animal and human studies both show a peak in dopaminergic signaling during adolescence, and rodent models have shown that pubertal DAergic processing enhances HPC-PFC integration (Caballero et al. 2016). To test this facet of our model, we characterized functional coupling of the VTA, a source of mesolimbic dopamine neurons, with the vmPFC, and characterized how this circuit was related to HPC-vmPFC neurodevelopment. We found that VTA-vmPFC connectivity partially mediated the relationship between age and HPC-vmPFC connectivity. Prior work using PET and drug manipulations in humans have shown that BOLD signal in the VTA is sensitive to dopaminergic signaling (Diaconescu et al. 2010; Chowdhury et al. 2013; Jabbi et al. 2013; Hadley et al. 2014; Rieckmann et al. 2015; Dubol et al. 2018), and a recent optogenetic fMRI paper in rodents supports the relationship between these two measures (Ferenczi et al. 2016; Lohani et al. 2017). Thus, our findings are consistent with our predictions that dopaminergic neuromodulation may underlie adolescent maturation of HPC-vmPFC circuits. Notably, our fMRI measure of mesolimbic engagement did not measure dopamine signaling directly, but rather functional properties of this dopamine-relevant circuit. Thus, future work using direct measures of dopaminergic signaling (i.e., PET, pharmacological challenges) is needed to fully understand the role of mesolimbic engagement in stabilizing vmPFC-HPC circuitry throughout adolescence.
While the current findings provide support that HPC-vmPFC may underlie developmental gains in higher-order cognition, future research is necessary to incorporate our findings into the broader literature. First, future planning and problem solving have mainly been associated with engagement of lateral PFC, rather than vmPFC (Goldman-Rakic 1995; Miller and Cohen 2001; Badre 2008). Although a few studies have implicated the vmPFC during tasks like the SOC (Nagano-Saito et al. 2009; Spreng et al. 2010), most studies have focused on engagement of fronto-parietal networks (Fincham et al. 2002; Cazalis et al. 2003; Wagner et al. 2006). Similarly, developmental research has also highlighted dlPFC maturation and network integration in relationship to cognitive development (Klingberg et al. 2002; Luna et al. 2015; Cohen et al. 2016; Crone and Steinbeis 2017). This prior work mostly characterized the dlPFC during task-related activation rather than resting state and did not characterize HPC connectivity. We propose that information processing across the HPC-vmPFC may provide foundational support for dlPFC performance-related increases in developmental differences; however, these interactions have yet to been tested. Second, prior functional neuroanatomical studies suggest that functional connections of aHPC with vmPFC are more prominent than pHPC. In our study, however, we found that while both circuits showed significant developmental changes, behavioral changes were more strongly associated with pHPC. This is likely to reflect a multisynaptic pathway, implicating other regions both in and around the HPC, and the implementation of causal effective connectivity techniques and/or animal models will be necessary to disambiguate the precise role of pHPC-vmPFC circuit maturation. Finally, limitations in study design may limit the scope of results we were able to detect. Assessing functional connectivity from single, short duration (<10 min) scan sessions is prone to low reliability (Noble et al. 2017), which makes longitudinal assessment difficult. Furthermore, our accelerated cohort design means that some subjects with a small number of scans (including a single scan session) are included along with the long longitudinal subjects, and sample biases in which subjects were able to stay in the study could affect the statistical inferences. Thus, while the larger sample size included in our study, as well as the independent replication sample, provide increased confidence in these results, validation will be important as methods and approaches continue to improve.
In sum, the present study characterizes neurodevelopmental trajectories of increased functional coupling of the HPC to vmPFC, which support the development of higher-order EF. These findings provide support for our recently proposed theoretical model, the Experience-Driven Adaptive Cognition model (Murty et al. 2016), in which we propose that increased engagement of dopaminergic systems drives information seeking in adolescents, that can subsequently be used to support goal-oriented behavior. This research shows circuit development consistent with this model and provides the foundation for future research characterizing neurodevelopmental models of hippocampal-guided decision-making. These results provide novel insight into the nature of development that is specific to the adolescent transition to adult-level cognitive processing, and further extends previous models to include the HPC as critical developmental node facilitating executing functions.
Funding
National Institute of Mental Health (grant R01 MH067924); Staunton Farm Foundation (to B.L.); National Institute of Mental Health (grant R03 MH113090 to B.L. and F.J.C.); National Alliance for Research on Schizophrenia & Depression Young Investigator Grant from the Brain Behavioral Research Foundation and National Institute of Mental Health (grant K01 MH111991 to V.P.M.); National Institute of Mental Health (grant K01 MH112774 to M.J.).
Notes
We thank Will Foran for helpful discussion on data analysis. We also thank Catherine Wright, Barbara Fritz, Amanda Wright, Heather Jack, Natalie Nawarawong, Jennifer Fedor, Julia Lekht, and Jessica Graves for their assistance with data collection. Finally, we thank the subjects who participated in this study. Raw data used in this project has been made available through the National Institute of Mental Health Data Archive, project #2831, “Longitudinal Profiles of Neurocognitive Development Through Adolescence” (https://ndar.nih.gov/edit_collection.html?id=2831).
Supplementary Material
References
- Addis DR, Cheng T, Roberts RP, Schacter DL. 2011. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. 21:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert D, Steinberg L. 2011. Age differences in strategic planning as indexed by the tower of London. Child Dev. 82:1501–1517. [DOI] [PubMed] [Google Scholar]
- Asato MR, Sweeney JA, Luna B. 2006. Cognitive processes in the development of TOL performance. Neuropsychologia. 44:2259–2269. [DOI] [PubMed] [Google Scholar]
- Badre D. 2008. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 12:193–200. [DOI] [PubMed] [Google Scholar]
- Bein O, Reggev N, Maril A. 2014. Prior knowledge influences on hippocampus and medial prefrontal cortex interactions in subsequent memory. Neuropsychologia. 64:320–330. [DOI] [PubMed] [Google Scholar]
- Buckner RL. 2010. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 61(27-48):C1–C8. [DOI] [PubMed] [Google Scholar]
- Caballero A, Granberg R, Tseng KY. 2016. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev. 70:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, Ruparel K, Wolf DH, Roalf DR, Mentch FD et al. . 2015. The Philadelphia neurodevelopmental cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 56:1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Madore KP, Benoit RG, Thakral PP, Schacter DL. 2018. Increased hippocampus to ventromedial prefrontal connectivity during the construction of episodic future events. Hippocampus. 28:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave CB, Squire LR. 1992. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 2:151–163. [DOI] [PubMed] [Google Scholar]
- Cazalis F, Valabrègue R, Pélégrini-Issac M, Asloun S, Robbins TW, Granon S. 2003. Individual differences in prefrontal cortical activation on the tower of London planning task: implication for effortful processing. Eur J Neurosci. 17:2219–2225. [DOI] [PubMed] [Google Scholar]
- Cerin E, Conway TL, Adams MA, Barnett A, Cain KL, Owen N, Christiansen LB, van Dyck D, Mitáš J, Sarmiento OL, Davey RC, Reis R, Salvo D, Schofield G, Sallis JF. 2018. Objectively-assessed neighbourhood destination accessibility and physical activity in adults from 10 countries: An analysis of moderators and perceptions as mediators. Soc Sci Med 1982. 211:282–293. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Düzel E, Dolan RJ. 2013. Dopamine restores reward prediction errors in old age. Nat Neurosci. 16:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, Rudolph MD, Chein J, Richeson JA, Heller AS et al. . 2016. When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychol Sci. 27:549–562. [DOI] [PubMed] [Google Scholar]
- Crone EA, Steinbeis N. 2017. Neural perspectives on cognitive control development during childhood and adolescence. Trends Cogn Sci. 21:205–215. [DOI] [PubMed] [Google Scholar]
- d’Arbeloff TC, Kim MJ, Knodt AR, Radtke SR, Brigidi BD, Hariri AR. 2018. Microstructural integrity of a pathway connecting the prefrontal cortex and amygdala moderates the association between cognitive reappraisal and negative emotions. Emotion. 18:912–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, DuBrow S. 2015. How the hippocampus preserves order: the role of prediction and context. Trends Cogn Sci. 19:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JH, Otto AR, Daw ND, Hartley CA. 2016. From creatures of habit to goal-directed learners: tracking the developmental emergence of model-based reinforcement learning. Psychol Sci. 27:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, Ghetti S. 2014. Structural development of the hippocampus and episodic memory: developmental differences along the anterior/posterior axis. Cereb Cortex. 1991(24):3036–3045. [DOI] [PubMed] [Google Scholar]
- Demaster DM, Ghetti S. 2013. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex J Devoted Study Nerv Syst Behav. 49:1482–1493. [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Menon M, Jensen J, Kapur S, McIntosh AR. 2010. Dopamine-induced changes in neural network patterns supporting aversive conditioning. Brain Res. 1313:143–161. [DOI] [PubMed] [Google Scholar]
- Diamond A. 1989. Developmental progression in human infants and infant monkeys, and the neural bases of, inhibitory control of reaching In: The development and neural bases of higher cognitive functions. New York: Academy of Science Press. [DOI] [PubMed] [Google Scholar]
- Dubol M, Trichard C, Leroy C, Sandu A-L, Rahim M, Granger B, Tzavara ET, Karila L, Martinot J-L, Artiges E. 2018. Dopamine transporter and reward anticipation in a dimensional perspective: a multimodal brain imaging study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 43:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudukovic NM, Preston AR, Archie JJ, Glover GH, Wagner AD. 2011. High-resolution fMRI reveals match enhancement and attentional modulation in the human medial temporal lobe. J Cogn Neurosci. 23:670–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. 2017. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci. 18:547–558. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. 2007. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 35:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C et al. . 2016. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 351:aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, van Veen V, Stenger VA, Anderson JR. 2002. Neural mechanisms of planning: a computational analysis using event-related fMRI. Proc Natl Acad Sci U S A. 99:3346–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CLH, Hinshaw S, D’Esposito M. 2010. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci Off J Soc Neurosci. 30:11062–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Buzsáki G. 2011. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 72:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM. 2008. Executive function in preschoolers: a review using an integrative framework. Psychol Bull. 134:31–60. [DOI] [PubMed] [Google Scholar]
- Gluth S, Sommer T, Rieskamp J, Büchel C. 2015. Effective connectivity between hippocampus and ventromedial prefrontal cortex controls preferential choices from memory. Neuron. 86:1078–1090. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL et al. . 2006. Dynamic mapping of normal human hippocampal development. Hippocampus. 16:664–672. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. 1995. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 769:71–83. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. 2008. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 1991(18):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MJ, Ritchey M, Wang S-F, Doss MK, Ranganath C. 2016. Post-learning hippocampal dynamics promote preferential retention of rewarding events. Neuron. 89:1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. 2000. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 20:RC106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Tassin JP, Jay TM. 1999. Integrity of the mesocortical dopaminergic system is necessary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience. 94:1019–1027. [DOI] [PubMed] [Google Scholar]
- Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, Visscher KM, Lahti AC. 2014. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 39:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. 2013. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CMA, Cabeza R, Daselaar SM. 2011. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One. 6:e17463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Hallquist MN, Luna B. 2013. The development of hub architecture in the human functional brain network. Cereb Cortex. 23:2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Nash T, Kohn P, Ianni A, Rubinstein D, Holroyd T, Carver FW, Masdeu JC, Kippenhan JS, Robinson SE et al. . 2013. Midbrain presynaptic dopamine tone predicts sustained and transient neural response to emotional salience in humans: fMRI, MEG, and FDOPA PET. Mol Psychiatry. 18:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. 1995. The inhibition of automatic saccades in early infancy. Dev Psychobiol. 28:281–291. [DOI] [PubMed] [Google Scholar]
- Jones LB, Rothbart MK, Posner MI. 2003. Development of executive attention in preschool children. Dev Sci. 6:498–504. [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. 2002. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 14:1–10. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. 2009. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 63:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 60:340–352. [DOI] [PubMed] [Google Scholar]
- Leszczynski M. 2011. How does hippocampus contribute to working memory processing? Front Hum Neurosci. 5:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohani S, Poplawsky AJ, Kim S-G, Moghaddam B. 2017. Unexpected global impact of VTA dopamine neuron activation as measured by opto-fMRI. Mol Psychiatry. 22:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. 2003. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge neuropsychological testing automated battery (CANTAB). J Child Psychol Psychiatry. 44:649–663. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. 2004. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 75:1357–1372. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. 2015. An integrative model of the maturation of cognitive control. Annu Rev Neurosci. 38:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Luecken LJ. 2008. How and for whom? Mediation and moderation in health psychology. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 27:S99–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Petrides M. 2014. Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci. 40:2777–2796. [DOI] [PubMed] [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. 2005. Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Res Cogn Brain Res. 25:379–385. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24:167–202. [DOI] [PubMed] [Google Scholar]
- Montez DF, Calabro FJ, Luna B. 2017. The expression of established cognitive brain states stabilizes with working memory development. eLife. 6:e25606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. 2014. Memory, imagination, and predicting the future: a common brain mechanism? Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 20:220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Calabro F, Luna B. 2016. The role of experience in adolescent cognitive development: integration of executive, memory, and mesolimbic systems. Neurosci Biobehav Rev. 70:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA. 2014. Resting state networks distinguish human ventral tegmental area from substantia nigra. NeuroImage. 100:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Tompary A, Adcock RA, Davachi L. 2017. Selectivity in postencoding connectivity with high-level visual cortex is associated with reward-motivated memory. J Neurosci. 37:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Liu J, Doyon J, Dagher A. 2009. Dopamine modulates default mode network deactivation in elderly individuals during the tower of London task. Neurosci Lett. 458:1–5. [DOI] [PubMed] [Google Scholar]
- Nauer RK, Whiteman AS, Dunne MF, Stern CE, Schon K. 2015. Hippocampal subfield and medial temporal cortical persistent activity during working memory reflects ongoing encoding. Front Syst Neurosci. 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Scheinost D, Finn ES, Shen X, Papademetris X, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS et al. . 2017. Multisite reliability of MR-based functional connectivity. NeuroImage. 146:959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Chai XJ, Schuil KDI, Whitfield-Gabrieli S, Gabrieli JDE. 2012. The development of brain systems associated with successful memory retrieval of scenes. J Neurosci. 32:10012–10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. 2007. Development of the declarative memory system in the human brain. Nat Neurosci. 10:1198–1205. [DOI] [PubMed] [Google Scholar]
- Olsen R, Nichols E, Chen J, Hunt J, Glover G, Gabrieli J, Wagner A. 2009. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J Neurosci. 29(38):11880–11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Rondina R, Riggs L, Meltzer JA, Ryan JD. 2013. Hippocampal and neocortical oscillatory contributions to visuospatial binding and comparison. J Exp Psychol Gen. 142:1335–1345. [DOI] [PubMed] [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, Vyas G. 2015. Development of the uncinate fasciculus: implications for theory and developmental disorders. Dev Cogn Neurosci. 14:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B. 2013. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci Off J Soc Neurosci. 33:18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vértes PE, Ersche KD, Suckling J, Bullmore ET. 2014. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. NeuroImage. 95:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. 2013. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 17:230–240. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 23:R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Gomperts SN, Johnson KA, Growdon JH, Van Dijk KRA. 2015. Putamen-midbrain functional connectivity is related to striatal dopamine transporter availability in patients with Lewy body diseases. NeuroImage Clin. 8:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. 2004. Processing and short-term retention of relational information in amnesia. Neuropsychologia. 42:497–511. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, Roalf DR, Hopsona KPR, Behr M, Qiu H et al. . 2016. The Philadelphia neurodevelopmental cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 124:1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M et al. . 2014. Neuroimaging of the Philadelphia neurodevelopmental cohort. NeuroImage. 86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Guarino KF, Schapiro AC, Turk-Browne NB, Preston AR. 2017. Hippocampal structure predicts statistical learning and associative inference abilities during development. J Cogn Neurosci. 29:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Turk-Browne NB. 2013. Mechanisms for widespread hippocampal involvement in cognition. J Exp Psychol Gen. 142:1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B. 2014. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage. 92:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Luna B. 2017. Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: a longitudinal fMRI study. NeuroImage. 157:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KN, Jones SH, Duff MC, Tranel D, Warren DE. 2015. Investigating the neural correlates of schemas: ventromedial prefrontal cortex is necessary for normal schematic influence on memory. J Neurosci Off J Soc Neurosci. 35:15746–15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. 2015. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 522:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 53:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. 2011. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 31:8739–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. 2014. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 15:655–669. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Roalf DR, Goddings A-L, Lebel C. 2018. Diffusion MRI of white matter microstructure development in childhood and adolescence: methods, challenges and progress. Dev Cogn Neurosci. 33:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Shafer AT, Ofen N. 2017. Prefrontal cortex contributions to the development of memory formation. Cereb Cortex. 1991:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. 2005. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex N Y N. 5(1):49–57. [DOI] [PubMed] [Google Scholar]
- Tseng K-Y, O’Donnell P. 2007. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex N Y N. 17(5):1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterrainer JM, Rahm B, Kaller CP, Ruff CC, Spreer J, Krause BJ, Schwarzwald R, Hautzel H, Halsband U. 2004. When planning fails: individual differences and error-related brain activity in problem solving. Cereb Cortex. 14:1390–1397. [DOI] [PubMed] [Google Scholar]
- van Kesteren MTR, Fernández G, Norris DG, Hermans EJ. 2010. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A. 107:7550–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MTR, Rijpkema M, Ruiter DJ, Morris RGM, Fernández G. 2014. Building on prior knowledge: schema-dependent encoding processes relate to academic performance. J Cogn Neurosci. 26:2250–2261. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. 2008. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 18(11):2505–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Cohen NJ. 2017. Hippocampal-cortical contributions to strategic exploration during perceptual discrimination. Hippocampus. 27:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlösser RGM. 2006. The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the tower of London paradigm. Neuropsychologia. 44:2337–2347. [DOI] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. 2011. Observing degradation of visual representations over short intervals when medial temporal lobe is damaged. J Cogn Neurosci. 23:3862–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Lee JK, Pospisil J, Sastre M, Ross JM, Bunge SA, Ghetti S. 2015. White matter tracts connected to the medial temporal lobe support the development of mnemonic control. Cereb Cortex. 25(9):2574–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, van Rossenberg F, Crone EA. 2019. Sex effects on development of brain structure and executive functions: greater variance than mean effects. J Cogn Neurosci. 31:730–753. [DOI] [PubMed] [Google Scholar]
- Wood SN. 2017. Generalized Additive Models: An Introduction with R. 2nd Ed. Boca Raton, FL: CRC Press. [Google Scholar]
- Zook NA, Davalos DB, Delosh EL, Davis HP. 2004. Working memory, inhibition, and fluid intelligence as predictors of performance on tower of Hanoi and London tasks. Brain Cogn. 56:286–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


