Abstract
Judgments of learning (JOL) pertain to introspective metamemory processes evaluating how well information is learned. Using a functional magnetic resonance imaging (fMRI) task, we investigated the neural substrates of JOL predictions in a group of 105 cognitively unimpaired older adults from the Harvard Aging Brain Study. Associations of JOL performance and its neural correlates with amyloid-β (Aβ) and tau pathology, two proteinopathies associated with Alzheimer’s disease (AD) and aging, were also examined. We found that trials judged as learned well relative to trials judged as learned less well (high JOL > low JOL) engaged the ventromedial prefrontal cortex and precuneus, among other midline regions, in addition to bilateral hippocampi. In this cohort of older adults, greater levels of entorhinal tau deposition were associated with overestimation of memory performance and with lower fMRI signal in midline regions during predicted memory success. No associations with Aβ were found. The findings suggest that tau pathology in unimpaired older adults may play a role in altered metamemory processes. We discuss our findings in light of the hypothesis that JOLs are partially dependent on a process involving attempts to retrieve a correct answer from memory, as well as implications for clinical research investigating unawareness of memory performance (i.e., anosognosia) in patients with AD dementia.
Keywords: amyloid, fMRI, judgments of learning, metamemory, tau
Introduction
Metamemory refers to introspective knowledge of one’s own memory capabilities. It includes self-monitoring of memory performance, and it plays a crucial role in how people learn and use memories. For example, metamemory helps one decide whether or not information is learned well enough for successful recall at a later time. Active regulation of learning strategies (e.g., mnemonic techniques) and successful retrieval of information rely on this type of self-monitoring, a process overall associated with greater memory performance (Schneider and Pressley 1989). As people age and experience memory decline, accurate metamemory becomes increasingly important. A better understanding of the influence of normal aging on metamemory processes would help clarify under which conditions older adults do or do not accurately use introspective monitoring processes to regulate learning.
Accuracy of self-monitoring processes can be ascertained by using an often used method that involves asking an individual to judge how well they will remember a set of particular items on a future examination (Nelson and Narens 1990). This approach is known as “judgments of learning” (JOL). While some studies have suggested that older individuals judge their learning equally well as young adults (Rebok and Balcerak 1989; McDonald-Miszczak et al. 1994; Connor et al. 1997; Emanuel Robinson et al. 2006; Rast and Zimprich 2009), other studies report age-related differences (Bruce et al. 1982; Connor et al. 1997; Tauber and Dunlosky 2012). In particular, older adults may overestimate their memory performance (Murphy et al. 1981; Bruce et al. 1982; Coyne 1985; Rebok and Balcerak 1989; Devolder et al. 1990; Connor et al. 1997). More specifically, global predictions in which people judge the total amount of items they will subsequently be able to recall appear to be more affected by age than are item-by-item predictions of the likelihood of subsequent recall (Connor et al. 1997). Despite this growing body of work, the neural and pathological correlates underlying JOL in older adults remain unknown.
The neural substrates of JOL in healthy young individuals comprise a network of regions (for a review see Fleming and Dolan 2012) that includes the anterior cingulate cortex (ACC), insula, precuneus, angular gyri, posterior cingulate cortex (PCC), and ventromedial prefrontal cortex (vmPFC) (Kao et al. 2005; Do Lam et al. 2012; Yang et al. 2015; Hu et al. 2017). Activity in the medial PFC and posteromedial cortices has been shown to relate to JOL functional magnetic resonance imaging (fMRI) task trials of high predictions of memory success (i.e., higher JOL trials than low JOL trials) (Kao et al. 2005; Do Lam et al. 2012; Yang et al. 2015). These findings indicate a potential role for these brain regions in metacognitive judgments during learning. To date, however, no fMRI study has yet measured the neural patterns of activity related to JOL in cognitively intact older individuals.
Frontal and posterior midline regions are known to atrophy with greater age (Resnick et al. 2003; Raz et al. 2005) and are considered hubs of accumulation for brain pathology such as amyloid-β (Aβ) plaque deposits, a hallmark pathology in Alzheimer’s disease (AD) (Braak and Braak 1991; Dickerson et al. 2009). Altered memory self-awareness, when assessed by calculating the discrepancy between a subjective questionnaire and objective memory task(s), has been previously shown in cognitively normal older adults with increased Aβ pathology in the association cortex, including posteromedial brain regions (Vannini et al. 2017). Neurofibrillary tangles of tau, the other major hallmark proteinopathy in AD, are more frequent in the medial temporal lobe (e.g., hippocampus, parahippocampus, and entorhinal cortex [EC]) in unimpaired older adults. The EC, in particular, has been shown to be an early site of tau accumulation in older adults with or without amyloid burden (Scholl et al. 2016), with deposition extending to the hippocampus in later stages (Braak and Braak 1996; Braak and Del Tredici 2015). As accumulation of abnormal proteinopathies has been shown to begin decades prior to overt cognitive impairment along the AD trajectory (Sperling et al. 2011) and previous work has shown early altered metamemory function even in cognitively intact older adults (Vannini et al. 2017), a convergence between change in metacognitive accuracy and pathology in midline frontal and posterior regions is proposed. Yet, the explicit links between AD-related pathological deposits (Aβ and tau) and function of brain systems underlying metacognitive monitoring have not been explored.
In this study, we sought to address these questions by conducting an fMRI JOL task in a group of cognitively unimpaired older adults. We focused on global JOL predictions for subsequent retrieval of items encoded during a face–name association fMRI task. Based on the previous literature, we hypothesized that while most older adults would overall accurately predict their memory performance, some individuals would overestimate their memory. We then sought to investigate the pattern of brain activation during fMRI task trials of high JOL relative to low JOL. Based on the previous findings in young individuals, we hypothesized that older adults would engage a network of regions during high JOL relative to low JOL that would include the medial prefrontal and posteromedial cortex. Lastly, we sought to examine the association between global JOL and JOL-related neural activity with tau deposition in the EC and Aβ deposition in the neocortex. Based on anatomic patterns of pathology and regions associated with the JOL paradigm, we hypothesized that overestimation of memory performance would be related to increased levels of Aβ and tau pathology.
Materials and Methods
Participants and Behavioral Measures
This study included 105 healthy adults (66% females; Mage = 76.04, range: 65–92.75 years old) from the Harvard Aging Brain Study (HABS) (Table 1). Written informed consent was obtained prior to experimental procedures. Study protocols were approved and conducted in accordance with the Partners Human Research Committee at the Massachusetts General Hospital, Boston, MA.
Table 1.
Demographic and neuroimaging characteristics of study sample
| Characteristic | Valuea | Range |
|---|---|---|
| Age (years) | 76.04 (6.37) | 65–92.75 |
| Female, no. (%) | 69 (66) | NA |
| Hollingshead score | 27.15 (15.29) | 11–65 |
| Race (%) (Asian/Black/White) | 1/13/86 | NA |
| Mini-Mental State Examination | 29.29 (1.05) | 25–30 |
| APOEε4 carrier status, positive, no. (%) (n = 97) | 28 (29) | NA |
| Total percent of high JOL (JOL1) | 39.86 (22.56) | 0–100 |
| Total percent of hits | 41.08 (21.32) | 0–84.30 |
| Absolute Accuracy memory self-appraisal (%) | −1.22 (26.75) | −52.00– 89.33 |
| Entorhinal cortical FTP binding (n = 90) | 1.33 (0.34) | 0.86–2.57 |
| Amyloid status, high Aβ, no. (%) (n = 99) | 32 (32) | NA |
| Neocortical PiB binding (n = 99) | 1.42 (0.44) | 1.05–2.69 |
| FTP to MRI absolute time (days) (n = 91) | 63.57 (57.90) | 1.0–334.0 |
| PiB to MRI absolute time (days) (n = 91) | 83.85 (137.90) | 1.0–762.0 |
Note: APOEε4, apolipoprotein E ε4 allele.
aUnless otherwise indicated, mean (SD) is reported for 105 participants.
Participants completed neurocognitive testing including the Mini-Mental State Examination [MMSE (Folstein et al. 1975)], the Clinical Dementia Rating [CDR (Morris 1993)], and the Logical Memory Story—a 30-min delayed recall subtest of the Wechsler Memory Scale (Wechsler 1987). All participants in the present study were cognitively unimpaired as defined by a CDR of 0 and free of any active psychiatric or physical conditions per exclusionary criteria previously adjudicated in the HABS cohort (Dagley et al. 2017).
MRI Data Acquisition
Data were collected using a Siemens 3-Tesla Tim/Trio MR system with a 12-channel whole-head phased-array coil at the Athinoula A. Martinos Center for Biomedical Imaging, in Charlestown, Boston, MA. T1-weighted MPRAGE (magnetization-prepared rapid gradient-echo) structural images were acquired using a high-resolution sequence: 5.12 min acquisition time (TA), 176 sagittal slices, 2300 ms repetition time (TR), 2.95 ms echo time (TE), 900 ms inversion time (TI), 9° flip angle (FA), 270 mm field of view (FoV), 1.1 × 1.1 × 1.2 mm resolution, and integrated parallel imaging (iPAT GRAPPA) acceleration factor of 2. Task-related blood oxygen level–dependent (BOLD) functional images were acquired using a T2*-weighted gradient-echo planar imaging (EPI) sequence: 159 volumes, 5.18 min TA, 2000 ms TR, 30 ms TE, 90° FA, 192 mm FoV, 3 × 3 × 3 mm resolution.
fMRI JOL Task Procedure
Participants underwent a fMRI task that involved encoding of face–name associations with subsequent JOL ratings. A total of 75 face–name pairs consisted of face photographs paired with a random fictional name in white font written underneath. The common American names for each decade from 1910 to 1990 were derived from public lists on the Internet, similarly done as in the previous work from our group (Sperling et al. 2001; Sperling et al. 2003). Names varied such that not all names began with the same letter, and there were approximately equal amounts of female to male (38/37) gender stimuli. Each name was randomly assigned to each face using in-house scripts.
Face–name pairs were presented in a pseudorandom order for 2.75 s over 3 runs (5.7 min per run) in groups of 5, such that 25 faces were presented per run, and each face–name pair was repeated twice within each run (second presentation displayed within 15 s from the first presentation). Face–name pairs were interleaved with fixation trials that consisted of a white cross-hair presented in the middle of a black background. Halfway through each run, participants were asked to indicate how well they learned the name for each face by pressing either the button under their index finger if they thought they “learned the name well” (JOL1), the button under their middle finger if they “did not learn the name well” (JOL2), or the button under their ring finger if they “learned the name poorly” (JOL3). Accuracy was ascertained under no time restriction outside the scanner. During the multiple choice retrieval assessment performed outside of the scanner, participants had three name choices to pick from, along with a “don’t know” option—incorrect name options were unique and not used in other parts of the task. Participants completed a practice session prior to entering the scanner and again inside the scanner immediately preceding the task. In summary, in each run, participants viewed 25 face–name pairs of stimuli presented in a pseudorandom order in groups of 5 face–name pairs (see caption of Supplementary Fig. 1 for additional details of the design). Each of the 25 face–name pairs was presented a total of 3 times during the run. The first 2 times they saw the stimuli, participants were asked to press a button indicating a purely subjective decision about whether the name was a good “fit” for the face or not. The run ended with the presentation of all 25 face–name stimuli again while participants indicated how well they encoded the stimuli (JOL). The run ended with the presentation of all 25 faces (without the names) while participants indicated how well they encoded the stimuli (JOL).
An estimate of overall memory predictions known as Absolute Accuracy was calculated by subtracting the percentage of correct responses from the percentage of JOL1 predictions (score of 0 indicating overall accurate memory self-appraisal, >0 indicating overall memory overestimation, and <0 indicating overall memory under-estimation). Absolute Accuracy allows for an assessment of the directionality of a metacognitive deficit, specifically over- or underestimation of predictions of memory success (Dunlosky and Lipko 2007; Sunderaraman and Cosentino 2017), and has previously been demonstrated to be sensitive to age-related differences (Connor et al. 1997).
fMRI Data Preprocessing
Functional data were preprocessed and analyzed using SPM12 (Wellcome Trust Centre for Neuroimaging, UCL, London: https://www.fil.ion.ucl.ac.uk/spm/). The first 3 volumes of each run were dropped to enable the analysis of only images collected after magnetization equilibrium. The following preprocessing steps were performed: 1) slice-time correction; 2) motion correction using INRIA realignment (Freire and Mangin 2001; Freire et al. 2002) without reslicing; 3) spatial normalization to the Montreal Neurological Institute (MNI) EPI template image; 4) resampling to 3 mm isotropic voxels; and 5) smoothing with a 6 mm3 full-width at half-maximum Gaussian kernel. Bad volumes were defined as any of the following: 1) spikes in the data > 2.5 standard deviation (SD) of the average variation, 2) movement > 0.75 mm between TRs, or 3) rotation > 1.5° between TRs. Fixation periods were not explicitly modeled.
Flortaucipir Positron Emission Tomography
Tau-positron emission tomography (PET) imaging data were acquired on average 63.57 days [median = 47, interquartile range (IQR) = 16–95, minimum = 1, maximum = 334, days] from the MRI visit. Tau pathology was measured using fluorine 18-flortaucipir (FTP) according to previously described methods (Johnson et al. 2016; Jacobs et al. 2018). FTP was acquired from 80 to 100 min after a 9.0–11.0 mCi bolus injection in 4 × 5-min frames. PET data were reconstructed and attenuation-corrected, and each frame was evaluated to verify adequate count statistics and absence of head motion. To evaluate the anatomy of cortical FTP binding, each individual PET dataset was rigidly coregistered to the participant’s MPRAGE data using SPM12.
FreeSurfer (FS, v6.0) regions of interest (ROIs) were defined by MRI and transformed into the PET native space. PET data were partial volume-corrected (PVC) using the geometrical transfer matrix (GTM) method as implemented in FreeSurfer (Greve et al. 2014). FTP-specific binding was expressed in FS ROIs in the EC, combining left and right hemispheres, as the standardized uptake value ratio (SUVr) using FS’s cerebellar gray ROI as reference.
Pittsburgh Compound-B Positron Emission Tomography
Pittsburgh Compound-B (PiB)-PET imaging data were acquired on average 83.85 days (median = 41, IQR = 14–84, minimum = 1, maximum = 762, days) from the MRI visit. Aβ-deposition was measured using PiB according to previously described methods (Johnson et al. 2007). Using Logan’s graphical analysis method (Logan 2000), PiB was calculated as retention expressed as a distribution volume ratio (DVR) (Price et al. 2005) using a gray matter cerebellum reference region and applying the GTM-PVC (Greve et al. 2014). Neocortical PiB binding was quantified using an aggregate PVC DVR from a set of FS ROIs that comprise association cortices, including the frontal, lateral parietal, lateral temporal, and retrosplenial (FLR) cortices (Hanseeuw et al. 2018; Jacobs et al. 2018). Dichotomous high and low Aβ retention was determined using a threshold value >1.32 to indicate high Aβ as previously defined (Hanseeuw et al. 2017) using Gaussian mixture modeling. A continuous measure of PiB binding was used in the main analyses.
Data Analysis
The association of Absolute Accuracy with EC-FTP binding or PiB binding was assessed in separate hierarchical multiple regression models controlling for age and sex. For the task-based fMRI analysis, we used a general linear model as implemented in SPM12. Face–name stimuli were categorized based on participants’ responses during JOL trials, and individual statistical models were constructed for JOL trials where the participant responded they had learned the name well (JOL = 1), and JOL trials where they had learned the name poorly (JOL = 3). Onsets for these events were convolved with the SPM12 canonical hemodynamic response function over trial durations. A fixed-effects model was used to estimate subject-specific effects, and linear contrasts were computed to generate subject-specific SPM(t) contrasts representing statistical differences in brain activation between JOL1 and JOL3 conditions (JOL1 > JOL3). Models included regressors for motion parameters, bad volumes, and a high-pass filter (1/128 Hz). Automated quality control excluded any imaging run that had > 10 bad volumes, overall motion > 5 mm, or overall rotation > 5°.
A JOL1 > JOL3 fMRI contrast was conducted to identify regions related to predicted learning success (higher JOL trials relative to low JOL trials). ROI analyses were conducted using mean BOLD signal from the max peak of clusters in the anterior cingulate, medial prefrontal cortex, hippocampus, precuneus, posterior cingulate, pallidum, and insula, extracted using 3 mm spheres from a JOL1 > JOL3 one-sided positive t-map. We used the False Discovery Rate (FDR) and its analog, the q-value, to identify as many significant comparisons while still maintaining a low false positive rate (qFDR < 0.05, uncorrected P = 0.002, t > 2.9, with an extent threshold > 30 contiguous voxels). These regions were included due to their previous implication in MRI studies of high JOL (Kao et al. 2005; Do Lam et al. 2012; Yang et al. 2015). ROI BOLD signal percent change was used in multiple regression analyses with the BOLD signal from each 3 mm sphere entered as a dependent variable and EC-FTP entered as a continuous independent variable. Models were controlled for age and sex. ROI models were corrected for multiple comparisons (α = 0.05, 9 models, P-threshold = 0.0056).
Results
Behavioral Results and Unadjusted Relationships
On average across all participants, 27.1 items were judged on average as “learned well” (JOL1) during the JOL phase, 23.1 were judged on average as “not learned well” (JOL2), and 17.17 were judged on average as “learned poorly” (JOL3). It was necessary for JOL ratings to be selected prior to the end of the MRI task trial duration, and some responses were not captured during this time window. There was an average 7.64 (SD = 9.82) missing JOL ratings across participants. There was a notable spread in values in the Absolute Accuracy data, with an average Absolute Accuracy of −1.22% (SD = 26.75) and a range of −52% to 89.33%. Based on how Absolute Accuracy was operationalized, the below zero mean average suggested a minor underestimation of memory performance across the sample. Importantly, Absolute Accuracy did not statistically differ from zero on a one-sample t-test (t = −0.47, P = 0.642), suggesting that metamemory was overall accurate. Furthermore, greater percent of high JOL ratings (JOL1) corresponded with greater percent of total hits (r = 0.26, P = 0.008).
There were no unadjusted associations between Absolute Accuracy and age (r = 0.09, P = 0.350), years of education (r = −0.04, P = 0.650), Hollingshead score (r = −0.03, P = 0.757), or Logical Memory Delayed Recall scores (r = −0.04, P = 0.6734). In an unadjusted linear model, sex was significantly associated with Absolute Accuracy such that males exhibited greater overestimation of memory than females (β = 17.05%, P = 0.002). EC-FTP binding was correlated with Absolute Accuracy (r = 0.22, P = 0.042) in an unadjusted model, such that greater EC-FTP tau was related to greater overestimation of memory. There was a trend-level unadjusted correlation between Absolute Accuracy and PiB binding (r = 0.17, P = 0.086). EC-FTP tau was not associated with frequency of JOL1 (P = 0.246), JOL2 (P = 0.519), or JOL3 ratings (P = 0.536).
fMRI Correlates of JOL
A JOL1 > JOL3 second-level contrast revealed greater activity in frontal and temporoparietal regions for JOL1 trials relative to JOL3 trials. Corrected results (qFDR < 0.05, uncorrected P = 0.002) are rendered in Figure 1 using SPM.
Figure 1.
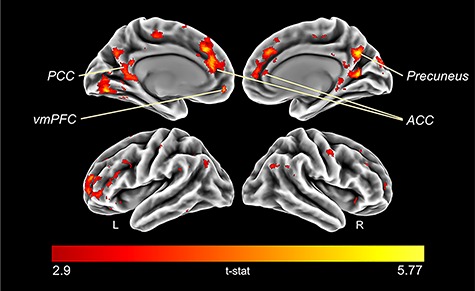
Brain regions activated during greater predicted learning success. BOLD signal percent change for JOL1 relative to JOL3 (JOL1 > JOL3, qFDR < 0.05, uncorrected P = 0.002, t > 2.9). L, left; R, right.
Absolute Accuracy and AD Pathology
Controlling for the effect of age and sex, EC-FTP binding was related to Absolute Accuracy (β = 18.42 ± 8.25%/FTP-SUVr, P = 0.028, Cohen’s f = 0.241), such that greater tau deposition in this region was associated with overestimation of memory performance (Table 2, Fig. 2). In this fully nested model, there was also an independent contribution of sex as a predictor of Absolute Accuracy (β = 17.74 ± 5.52%, P = 0.002, Cohen’s f = 0.344) such that overestimation was related to the male group.
Table 2.
Entorhinal cortex tau and Absolute Accuracy memory self-appraisal
| Dependent variable: Absolute Accuracy (%) | ||||
|---|---|---|---|---|
| A | B | C | ||
| Age | β | −0.19 | −0.29 | |
| SE | (0.46) | (0.44) | ||
| P value | 0.688 | 0.507 | ||
| Sex | β | 17.74 | ||
| SE | (5.52) | |||
| P value | 0.002 ** | |||
| EC-FTP binding | β | 17.15 | 18.13 | 18.42 |
| SE | (8.29) | (8.68) | (8.25) | |
| P value | 0.042 * | 0.040 * | 0.028 * | |
| Cohen’s f | 0.220 | 0.224 | 0.241 | |
| Model adjusted R2 | 0.036 | 0.026 | 0.120 | |
Note: (A) Unadjusted linear regression estimates for a model examining the association of EC-FTP binding with Absolute Accuracy. (B) Estimates for an EC-FTP model predicting Absolute Accuracy while controlling for the effect of age. (C) Estimates for an EC-FTP model predicting Absolute Accuracy while controlling for age and sex effects. Statistics are reported as the estimate coefficient (β), standard error (SE), and P value.
* P < 0.05.
** P < 0.01.
Figure 2.
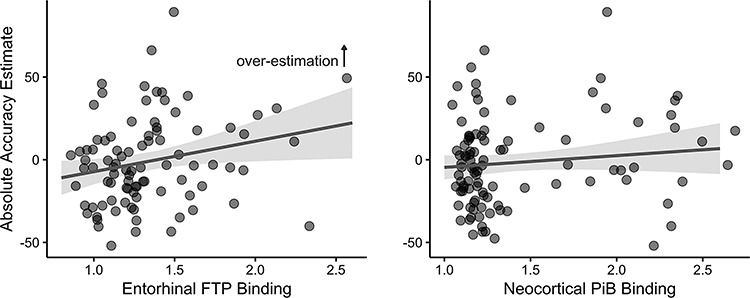
Main effect of bilateral EC-FTP binding (left) or neocortical PiB binding (right) as independent continuous variables in models predicting Absolute Accuracy, controlling for age and sex effects. EC-FTP tau significantly predicted Absolute Accuracy scores (β = 18.42 ± 8.25%/FTP SUVr, P = 0.028, Cohen’s f = 0.241) such that higher EC-FTP was related with overestimation of memory. There was no association with PiB FLR binding (P = 0.253). Shaded regions indicate the 95% confidence interval.
There was no association between neocortical PiB binding and Absolute Accuracy (Table 3). When adding the nonsignificant effect of PiB binding to our entorhinal FTP binding model predicting Absolute Accuracy, we found that the effect of entorhinal FTP binding was not significant when both tau and PiB were in the same model predicting Absolute Accuracy (entorhinal tau: β = 13.49 ± 9.77%, P = 0.171, PiB: β = 5.11 ± 7.28%, P = 0.484). There were no interactions between EC-FTP and PiB in predicting Absolute Accuracy (not shown).
Table 3.
Neocortical amyloid-β and Absolute Accuracy memory self-appraisal
| Dependent variable: Absolute Accuracy (%) | ||||
|---|---|---|---|---|
| A | B | C | ||
| Age | β | 0.34 | 0.28 | |
| SE | (0.44) | (0.42) | ||
| P value | 0.438 | 0.506 | ||
| Sex | β | 17.53 | ||
| SE | (5.51) | |||
| P value | 0.002 ** | |||
| PiB FLR binding | β | 10.75 | 9.53 | 7.10 |
| SE | (6.20) | (6.41) | (6.17) | |
| P value | 0.086 | 0.140 | 0.253 | |
| Cohen’s f | 0.176 | 0.152 | 0.118 | |
| Model Adjusted R2 | 0.020 | 0.016 | 0.101 | |
Note: (A) Unadjusted linear regression estimates for a model examining the association of PiB FLR binding with Absolute Accuracy. (B) Estimates for a PiB FLR model predicting Absolute Accuracy while controlling for the effect of age. (C) Estimates for a PiB FLR model predicting Absolute Accuracy while controlling for age and sex effects. Statistics are reported as the estimate coefficient (β), SE, and P value.
**Significant P values are shown in bold. P < 0.01.
ROI Signal Change and AD Pathology
Previous reports in cognitively normal older adults suggest that elevated levels of tau are related with decreased connectivity (Sepulcre et al. 2017), an effect prominent in those with high PiB FLR binding (Schultz et al. 2017). We conducted models using the levels of entorhinal tau as the independent variable and BOLD signal from each ROI during trials of high JOL as dependent variables in separate models. Max peak MNI coordinates per ROI can be found in Table 4.
Table 4.
Functional MRI activity in relevant ROIs during memory self-appraisal
| Max peak MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast | x | y | z | T/F | Hemi | Region |
| JOL1 > JOL3 | −9 | 38 | 23 | 5.31 | L | Anterior cingulate |
| 12 | 44 | 14 | 4.20 | R | Anterior cingulate | |
| 3 | −64 | 32 | 5.27 | R | Precuneus | |
| −3 | −44 | 17 | 4.63 | L | Posterior cingulate | |
| −30 | −22 | −7 | 4.92 | L | Hippocampus | |
| 21 | −13 | −7 | 4.37 | R | Hippocampus | |
| −6 | 53 | −10 | 4.62 | L | vmPFC | |
| 24 | −4 | −1 | 4.20 | R | Pallidum | |
| 27 | 26 | −4 | 4.34 | R | Insula | |
Note: Clusters of >30 contiguous voxels and qFDR < 0.05 (uncorrected-P = 0.002, t > 2.9) are reported.
Controlling for the effect of age and sex, associations were found between EC-FTP binding and BOLD signal percent change (contrast: JOL1 > JOL3) in the left ACC (β = −0.84 ± 0.38%/FTP-SUVr, P = 0.029, Cohen’s f = 0.261), precuneus (β = −1.50 ± 0.44%/FTP-SUVr, P = 0.001, Cohen’s f = 0.395), vmPFC (β = −1.46 ± 0.48%/FTP-SUVr, P = 0.003, Cohen’s f = 0.354), and right pallidum (β = −0.58 ± 0.27%/FTP-SUVr, P = 0.038, Cohen’s f = 0.248), such that higher levels of tau were related to lower neural activity in these regions during predicted learning success (Table 5). After correcting for multiple comparisons, EC-FTP showed an association only with BOLD signal percent change in the vmPFC and precuneus ROIs (Fig. 3).
Table 5.
Entorhinal cortex tau and brain activity in ROIs during memory self-appraisal
| Dependent variable: ROI BOLD % change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L ACC | R ACC | R Prec | L PCC | L Hipp | R Hipp | L vmPFC | R Pall | R Insula | ||
| Age | β | 0.01 | 0.01 | 0.01 | −0.001 | 0.005 | −0.01 | 0.003 | −0.01 | −0.01 |
| SE | (0.02) | (0.01) | (0.02) | (0.03) | (0.01) | (0.01) | (0.03) | (0.01) | (0.01) | |
| P value | 0.594 | 0.687 | 0.631 | 0.960 | 0.677 | 0.676 | 0.915 | 0.426 | 0.256 | |
| Sex | β | 0.04 | 0.11 | −0.11 | −0.29 | −0.01 | −0.15 | −0.19 | −0.15 | 0.02 |
| SE | (0.25) | (0.19) | (0.30) | (0.36) | (0.15) | (0.18) | (0.32) | (0.18) | (0.15) | |
| P value | 0.872 | 0.550 | 0.704 | 0.419 | 0.957 | 0.397 | 0.560 | 0.427 | 0.867 | |
| EC FTP | β | −0.84 | −0.52 | −1.50 | −0.569 | −0.30 | −0.13 | −1.46 | −0.58 | −0.41 |
| Binding | SE | (0.38) | (0.28) | (0.44) | (0.55) | (0.22) | (0.26) | (0.48) | (0.27) | (0.22) |
| P value | 0.029* | 0.071 | 0.001 ** | 0.301 | 0.182 | 0.623 | 0.003 ** | 0.038* | 0.069 | |
| Cohen’s f | 0.261 | 0.214 | 0.395 | 0.122 | 0.158 | 0.06 | 0.354 | 0.248 | 0.216 | |
| Model adjusted R2 | 0.026 | 0.010 | 0.104 | −0.015 | −0.016 | −0.022 | 0.086 | 0.055 | 0.045 | |
Note: The linear regression estimates for models examining the association of EC-FTP binding with BOLD signal from each separate ROI, controlling for age and sex effects are shown. Prec, precuneus; Hipp, hippocampus; Pall, pallidum; L, left; R, right. Statistics are reported as the estimate coefficient (β), SE, and P value. *P < 0.05, **P < 0.01 in bold text, represents significance after Bonferroni correction for multiple comparisons (α = 0.05, 9 models, P-threshold = 0.0056).
Figure 3.
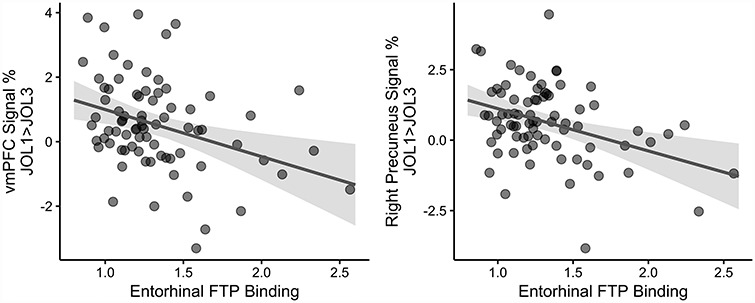
Mean signal extracted using 3 mm spheres around peaks of clusters of BOLD signal percent change for a JOL1 > JOL3 contrast (qFDR < 0.05, uncorrected P = 0.002) was entered into linear regression models with signal modeled as the dependent variable and bilateral entorhinal cortex FTP binding as an independent variable, controlling for age and sex. After correction for multiple comparisons (α = 0.05, 9 models, P-threshold = 0.0056), higher EC-FTP was related to % BOLD signal change in the vmPFC (β = −1.46 ± 0.48%/FTP SUVr, P = 0.003, Cohen’s f = 0.354) and right precuneus (β = −1.50 ± 0.44%/FTP SUVr, P = 0.001, Cohen’s f = 0.395) for fMRI trials of predicted learning success.
Neocortical PiB did not relate to functional activity in any ROI controlling for the same set of covariates (Supplementary Table 1). Investigating the potential additive effect of PiB FLR binding on the Bonferroni-corrected ROI BOLD signal versus entorhinal tau models (i.e., BOLD signal predicted by entorhinal tau, controlling for age, sex, and PiB FLR binding), namely the vmPFC and precuneus models, we found that while the effect of PiB was not a significant predictor of either vmPFC or precuneus BOLD signal during trials of high JOL relative to low JOL, entorhinal tau remained a significant predictor of vmPFC and precuneus BOLD signal above and beyond PiB (entorhinal tau vs. vmPFC BOLD signal model: β = −1.74 ± 0.54%, P = 0.002; entorhinal tau vs. precuneus BOLD signal model: β = −1.66 ± 0.51%, P = 0.002) (Supplementary Table 2). There were no interactions between EC-FTP and PiB on neural activity during memory self-appraisal in any ROI (not shown).
Supplementary Models Examining Local Tau Effects on JOL Activity
Lastly, we sought to explore local effects of tau on BOLD signal during trials of high JOL relative to low JOL in the two BOLD ROIs that survived Bonferroni-correction, the vmPFC and precuneus. To that end, we conducted two post hoc linear regression models controlling for the effect of age and sex, one using FTP binding from the FreeSurfer precuneus ROI as the independent variable and precuneus ROI BOLD signal from the JOL1 > JOL3 fMRI contrast as the dependent variable, and a second model using FTP binding from the FreeSurfer medial orbitofrontal ROI as the independent variable and vmPFC ROI BOLD signal from the JOL1 > JOL3 fMRI contrast as the dependent variable. We found that precuneus BOLD signal during high JOL trials relative to low JOL trials was not related to precuneus FTP binding (P = 0.218). vmPFC BOLD signal during high JOL trials was also not related to medial orbitofrontal FTP binding (P = 0.067).
Discussion
This study investigated the neural correlates of JOL in a group of cognitively unimpaired older adults and assessed functional and behavioral relationships to entorhinal tau and Aβ deposition. We found that older adults engaged a similar network of brain regions during predicted memory success as previously shown in young cohorts (Kao et al. 2005; Do Lam et al. 2012; Yang et al. 2015; Hu et al. 2017). In line with previous findings, we found that while older adults overall accurately predicted their memory performance, some individuals overestimated their memory. This overestimation of memory performance was related to greater tau pathology, suggesting a link between alterations in metamemory processes and markers of AD, an effect not entirely explained by age or sex. Moreover, increased pathology was also related to decreased activation during trials of high JOL, suggesting functional alterations in the network underlying predicted memory success.
Spellman and Bjork (1992) described the JOL process as partly based on the retrieval attempt of a to-be-judged item (Spellman and Bjork 1992). According to this hypothesis, the attempt of trying to retrieve the correct answer during the judgment ensures a high level of accuracy (Spellman and Bjork 1992). Indeed, in our group of cognitively unimpaired older adults, we found that a greater number of high judgments of learning (JOL1) responses was related to overall increased accuracy on the task. Furthermore, while entorhinal tau overall did not influence the number of times individuals rated an item as low JOL or high JOL, this pathology was associated with lower neural BOLD signal during high JOL trials (Fig. 3). Moreover, tau was also related to overestimation of memory performance (Fig. 2). We note that JOL Absolute Accuracy is not fully interchangeable with our fMRI BOLD contrast. Specifically, while the fMRI contrast of high JOL trials is relative to activity in low JOL trials (JOL1 > JOL3), Absolute Accuracy only considers high JOL responses in relation to overall accuracy. Our findings may nonetheless be interpreted in the context of Spellman and Bjork (1992), such that increased entorhinal tau deposition may influence the retrieval attempts during JOL, which in turn may influence a downstream effect leading to discordance in memory accuracy. The latter aligns well with previous reports describing a relationship between greater AD pathology and anosognosia (Perrotin et al. 2015; Vannini et al. 2017; Mondragon et al. 2019) along the AD pathophysiologic process, suggesting that altered metacognition processes might underlie this debilitating syndrome.
These results corroborate that activity in the medial prefrontal and posteromedial cortex is implicated in memory self-appraisal in older adults. Medial prefrontal regions have long been considered important for accurate self-appraisal (Luria 1972, 1973), and recent neuroimaging work using fMRI in young adults has contributed to this growing body of evidence. For example, in one of the first neuroimaging studies of JOL (Kao et al. 2005), activity in the vmPFC in young healthy adults was shown to relate to higher predicted memory success (i.e., higher JOL trials than low JOL trials). In a separate study, items judged as “will be remembered” (high JOL) relative to “will be forgotten” (low JOL) were also related to stronger activity in the vmPFC (Yang et al. 2015).
In a group of young adults, Do Lam et al. (2012) identified the ACC and medial PFC as related to high JOL fMRI trials. In the current study, the ACC region was also involved in predicted memory success, and we found associations between entorhinal cortical tau deposition and BOLD signal in the ACC region. Those associations, however, did not survive correction for multiple comparisons. Other regions implicated in metamemory include the right insula (Shany-Ur et al. 2014; Cosentino et al. 2015). In the present study, we identified the right insula as a region also related to JOL. A previous analysis also leveraging data from the HABS found an association between entorhinal tau deposition and right insula metabolism as measured with fludeoxyglucose-PET imaging, an association not dependent on the levels of amyloid pathology (Hanseeuw et al. 2017). We also found activation in the right pallidum. The pallidum has been implicated in several studies investigating self-awareness (Fleming and Dolan 2012; Shany-Ur et al. 2014; Cosentino et al. 2015), and right lateralization of effects related to metamemory has been noted in the AD literature (Sollberger et al. 2014; Cosentino et al. 2015). Finally, we found that the left and right hippocampi were implicated in trials of predicted memory success.
While tau deposition in the EC did not have an impact on the judgment of learning itself (tau was not related to frequency of high JOL or low JOL ratings), tau pathology in the EC had a large effect size on precuneus BOLD signal during memory prediction and a medium-to-large effect size on vmPFC activity. The effect of tau deposition on high JOL-related neural activity was distal, such that tau in the EC predicted lower BOLD signal in the precuneus or vmPFC during trials of high JOL, while tau in the precuneus did not have an effect on BOLD signal in the precuneus and tau in the medial prefrontal cortex did not have an effect on BOLD signal in the medial prefrontal cortex either. We speculate that studies in individuals with more diffused tauopathy may find more clear associations between local tauopathy and activity in these two metamemory hubs, the precuneus and vmPFC.
Our findings of an association of entorhinal tau with activity across medial PFC and precuneus ROIs during predicted memory success are noteworthy given how densely connected these medial frontal/parietal areas are to other cortical and subcortical regions. For example, clinical studies have demonstrated that reduced memory self-awareness is associated with reduced metabolism (Hanseeuw et al. 2017) and reduced intrinsic connectivity between the PCC and orbitofrontal cortex in patients with MCI (Vannini et al. 2017) and AD dementia (Perrotin et al. 2015). Functional connectivity between the PCC and medial PFC has in fact been shown to be disrupted in cognitively normal older individuals harboring greater levels of amyloid burden (Hedden et al. 2009). In a study by Bertrand et al. (2018) investigating the feeling-of-knowing process, another metamemory process that changes with age (Souchay et al. 2007; Morson et al. 2015), reduced item level, and global FOK accuracy were related to lower cortical thickness in the mPFC and posteromedial cortex in a heterogeneous sample of healthy and mildly impaired individuals (Bertrand et al. 2018), suggesting that compromised metamemory may be related to functional connectivity between these two regions within the context of default mode network integrity. In the current study, we investigated global metamemory predictions in a sample of cognitively unimpaired individuals, and it may be that deficits in both item level and global metamemory may be more apparent in an enriched study sample like the one found in the study by Bertrand et al. (2018). Taken together with previous findings, we speculate that the distal effect that entorhinal tau had in the current study with the functional network underlying the high JOL process may be related to compromised cortical thickness and functional connectivity between areas implicated in reduced self-awareness.
In an adjudicated healthy sample of older adults from the HABS, we found that males were more likely to overestimate their memory than females, despite more females having participated in the study. In a study of personality and metacognition, Colvin et al. (2018) found that men were also more likely to be overconfident than women. While in the current study we used Absolute Accuracy as our measure of metacognitive accuracy within an fMRI task, Colvin et al. (2018) compared two separate neuropsychological battery scores with one another to determine degree of over, under, or accurate self-assessment. Despite differences in our sample characteristics and way of operationalizing metamemory accuracy, our study aligns well with the finding from Colvin et al. Interestingly, the effect of sex in the current study was only significant in the model predicting Absolute Accuracy, and it was not a significant predictor of neural activity in the vmPFC or precuneus during trials of high JOL. This suggests varying degrees of influence between sex and neural signal during trials of high JOL versus sex and overall Absolute Accuracy on the fMRI task.
Our findings in this sample of cognitively unimpaired older adults highlight a possible association of memory self-appraisal and tau deposition in an early site of tauopathy in aging and AD. This finding is intriguing, as deficits in memory self-appraisal have been found across the AD continuum (Zanetti et al. 1999; Wilson et al. 2015; Turro-Garriga et al. 2016), and evidence of these kinds of deficits have been shown to predict progression onset of dementia (Munro et al. 2018). Future studies in older adults with and without memory impairment in whom amyloid and tau propagation are assessed over time in relation to JOL are encouraged to help ascertain how propagation of pathology across the cortical mantle tracks with measurements of predicted memory success.
Limitations
While the Goodman–Kruskal gamma coefficient has been used in numerous studies of metamemory as a measure of item-by-item (relative) predictions, here we opted to use Absolute (global) Accuracy as our measure of choice. This was based on previous findings in older adults who overestimate their memory performance (Murphy et al. 1981; Bruce et al. 1982; Coyne 1985; Rebok and Balcerak 1989; Devolder et al. 1990; Connor et al. 1997). Additionally, the direction of deviation of predictions is not provided by the Goodman–Kruskal gamma statistic, and it has been postulated that the gamma statistic may be prone to response bias and may lead to erroneous interpretations of JOL accuracy (Nelson 1996; Masson and Rotello 2009; Sunderaraman and Cosentino 2017). Connor et al. (1997) also presented findings to suggest that age is more related to absolute than relative metamemory accuracy.
Conclusions
To our knowledge, this is the first study to investigate the functional and pathological correlates of JOL in unimpaired older adults. Older adults engaged a network during JOL predictions that included the medial prefrontal and posteromedial cortex, and overestimation of memory was related to greater entorhinal tau deposition but not neocortical Aβ pathology. These observations link deposition of tau pathology with alterations in metamemory processes. In doing so, these findings add to the discussion of metamemory in older age by demonstrating a possible pathological basis for overestimation of memory performance in older individuals.
Funding
National Institutes of Health (NIA P01 AG036694 to R.A.S. and K.A.J., K24AG035007 to R.A.S., and K01AG048287 to P.V.); Alzheimer’s Association (IIRG-06-27374 to R.A.S.); European Union’s Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie Grant agreement (IF-2015-GF, 706714 to H.I.L.J.); Center for Functional Neuroimaging Techniques (P41EB015896). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Notes
Conflict of Interest: F. d’Oleire Uquillas, H.I.L. Jacobs, A.P. Schultz, B.J. Hanseeuw, R.F. Buckley, J. Sepulcre, A. Pascual-Leone, and P. Vannini and have no disclosures to report. K.A. Johnson has served as a paid consultant for Abbvie, AZtherapies, Biogen, GE Healthcare, Genetech, Genzyme, Isis, Janssen, Lundbeck, Merck, Novartis, Piramal, Roche, Eli Lilly, and Merck and Siemens Medical Solutions. He has served as a site principal investigator or co-investigator for Biogen, Eisai, Eli Lilly/Avid, Janssen, Lundbeck and Pfizer. R.A. Sperling has served as a paid consultant for Abbvie, Biogen, Bracket, GE, Genentech, Lundbeck, Merck, Otsuka, Pfizer, Roche and Sanofi. She has served as a co-investigator for Avid, Eli Lilly, and Janssen. She has spoken at symposia sponsored by Biogen, Eli Lilly, and Janssen. N.J. Donovan has received salary support from Eisai and Eli Lilly. She has served as a paid consultant to Avanir. None of these disclosures are related to the content of this manuscript.
Supplementary Material
References
- Bertrand E, Azar M, Rizvi B, Brickman AM, Huey ED, Habeck C, Landeira-Fernandez J, Mograbi DC, Cosentino S. 2018. Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology. 32:700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. 1991. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1:213–216. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. 1996. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 92:197–201. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. 2015. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 138:2814–2833. [DOI] [PubMed] [Google Scholar]
- Bruce PR, Coyne AC, Botwinick J. 1982. Adult age differences in metamemory. J Gerontol. 37:354–357. [DOI] [PubMed] [Google Scholar]
- Colvin LE, Malgaroli M, Chapman S, MacKay-Brandt A, Cosentino S. 2018. Mood and personality characteristics are associated with metamemory knowledge accuracy in a community-based cohort of older adults. J Int Neuropsychol Soc. 24:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor LT, Dunlosky J, Hertzog C. 1997. Age-related differences in absolute but not relative metamemory accuracy. Psychol Aging. 12:50–71. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Brickman AM, Griffith E, Habeck C, Cines S, Farrell M, Shaked D, Huey ED, Briner T, Stern Y. 2015. The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia. 75:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne AC. 1985. Adult age, presentation time, and memory performance. Exp Aging Res. 11:147–149. [DOI] [PubMed] [Google Scholar]
- Dagley A, LaPoint M, Huijbers W, Hedden T, McLaren DG, Chatwal JP, Papp KV, Amariglio RE, Blacker D, Rentz DM et al. 2017. Harvard Aging Brain Study: dataset and accessibility. NeuroImage. 144:255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devolder PA, Brigham MC, Pressley M. 1990. Memory performance awareness in younger and older adults. Psychol Aging. 5:291–303. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD et al. 2009. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 19:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Lam AT, Axmacher N, Fell J, Staresina BP, Gauggel S, Wagner T, Olligs J, Weis S. 2012. Monitoring the mind: the neurocognitive correlates of metamemory. PLoS One. 7:e30009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlosky J, Lipko AR. 2007. Metacomprehension: a brief history and how to improve its accuracy. Curr Dir Psychol Sci. 16:228–232. [Google Scholar]
- Emanuel Robinson A, Hertzog C, Dunlosky J. 2006. Aging, encoding fluency, and metacognitive monitoring. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 13:458–478. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Dolan RJ. 2012. The neural basis of metacognitive ability. Philos Trans R Soc Lond Ser B Biol Sci. 367:1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12:189–198. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. 2001. Motion correction algorithms may create spurious brain activations in the absence of subject motion. NeuroImage. 14:709–722. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. 2002. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 21:470–484. [DOI] [PubMed] [Google Scholar]
- Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, Rosen B, Fischl B, Knudsen GM. 2014. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. NeuroImage. 92:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Mormino EC, Schultz AP, Sepulcre J, Becker JA, Jacobs HIL, Buckley RF, LaPoint MR, Vannini P et al. 2018. PET staging of amyloidosis using striatum. Alzheimers Dement. 14:1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Schultz AP, Papp KV, Mormino EC, Sepulcre J, Bark JS, Cosio DM, LaPoint M, Chhatwal JP et al. 2017. Fluorodeoxyglucose metabolism associated with tau-amyloid interaction predicts memory decline. Ann Neurol. 81:583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. 2009. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 29:12686–12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Liu Z, Chen W, Zheng J, Su N, Wang W, Lin C, Luo L. 2017. Individual differences in the accuracy of judgments of learning are related to the Gray matter volume and functional connectivity of the left mid-insula. Front Hum Neurosci. 11:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Hedden T, Schultz AP, Sepulcre J, Perea RD, Amariglio RE, Papp KV, Rentz DM, Sperling RA, Johnson KA. 2018. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nat Neurosci. 21:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE et al. 2007. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 62:229–234. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K et al. 2016. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Davis ES, Gabrieli JD. 2005. Neural correlates of actual and predicted memory formation. Nat Neurosci. 8:1776–1783. [DOI] [PubMed] [Google Scholar]
- Logan J. 2000. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. New York: Basic Books, Inc. 27:661–670. [DOI] [PubMed] [Google Scholar]
- Luria AR. 1972. The man with a shattered world: the history of a brain wound: eighth printing. New York (NY): Basic Books, Inc. [Google Scholar]
- Luria AR. 1973. The working brain. An introduction to neuropsychology. New York (NY): Basic Books. [Google Scholar]
- Masson ME, Rotello CM. 2009. Sources of bias in the Goodman-Kruskal gamma coefficient measure of association: implications for studies of metacognitive processes. J Exp Psychol Learn Mem Cogn. 35:509–527. [DOI] [PubMed] [Google Scholar]
- McDonald-Miszczak L, Hunter MA, Hultsch DF. 1994. Adult age differences in predicting memory performance: the effects of normative information and task experience. Can J Exp Psychol. 48:95–118. [DOI] [PubMed] [Google Scholar]
- Mondragon JD, Maurits NM, De Deyn PP. 2019. Functional neural correlates of anosognosia in mild cognitive impairment and Alzheimer’s disease: a systematic review. Neuropsychol Rev. 29:139–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. 1993. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- Morson SM, Moulin CJ, Souchay C. 2015. Selective deficits in episodic feeling of knowing in ageing: a novel use of the general knowledge task. Acta Psychol. 157:85–92. [DOI] [PubMed] [Google Scholar]
- Munro CE, Donovan NJ, Amariglio RE, Papp KV, Marshall GA, Rentz DM, Pascual-Leone A, Sperling RA, Locascio JJ, Vannini P. 2018. The impact of awareness of and concern about memory performance on the prediction of progression from mild cognitive impairment to Alzheimer disease dementia. Am J Geriatr Psychiatry. 26:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MD, Sanders RE, Gabriesheski AS, Schmitt FA. 1981. Metamemory in the aged. J Gerontol. 36:185–193. [DOI] [PubMed] [Google Scholar]
- Nelson TO. 1996. Gamma is a measure of the accuracy of predicting performance on one item relative to another item, not of the absolute performance on an individual item comments on Schraw (1995). Appl Cogn Psychol. 10:257–260. [Google Scholar]
- Nelson TO, Narens L. 1990. Metamemory: a theoretical framework and new findings In: The psychology of learning and motivation. Cambridge (MA): Academic Press, pp. 125–173. [Google Scholar]
- Perrotin A, Desgranges B, Landeau B, Mezenge F, La Joie R, Egret S, Pelerin A, de la Sayette V, Eustache F, Chetelat G. 2015. Anosognosia in Alzheimer disease: disconnection between memory and self-related brain networks. Ann Neurol. 78:477–486. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. 2005. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 25:1528–1547. [DOI] [PubMed] [Google Scholar]
- Rast P, Zimprich D. 2009. Individual differences and reliability of paired associates learning in younger and older adults. Psychol Aging. 24:1001–1006. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Rebok GW, Balcerak LJ. 1989. Memory self-efficacy and performance differences in young and old adults: the effect of mnemonic training. Dev Psychol. 25:714–721. [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. 2003. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 23:3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Pressley M. 1989. Memory development between 2 and 20. New York (NY): Springer-Verlag Publishing. [Google Scholar]
- Scholl M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD et al. 2016. PET imaging of tau deposition in the aging human brain. Neuron. 89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, Huijbers W, LaPoint M, Buckley RF, Johnson KA et al. 2017. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and tau in clinically normal individuals. J Neurosci. 37:4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu MR, Li Q, El Fakhri G, Sperling R, Johnson KA. 2017. Tau and amyloid beta proteins distinctively associate to functional network changes in the aging brain. Alzheimers Dement. 13:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. 2014. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain. 137:2368–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger M, Rosen HJ, Shany-Ur T, Ullah J, Stanley CM, Laluz V, Weiner MW, Wilson SM, Miller BL, Rankin KP. 2014. Neural substrates of socioemotional self-awareness in neurodegenerative disease. Brain Behav. 4:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchay C, Moulin CJ, Clarys D, Taconnat L, Isingrini M. 2007. Diminished episodic memory awareness in older adults: evidence from feeling-of-knowing and recollection. Conscious Cogn. 16:769–784. [DOI] [PubMed] [Google Scholar]
- Spellman BA, Bjork RA. 1992. When predictions create reality: judgments of learning may alter what they are intended to assess. Psychol Sci. 3:315–317. [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ et al. 2011. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. 2003. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 74:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. 2001. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 14:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderaraman P, Cosentino S. 2017. Integrating the constructs of anosognosia and metacognition: a review of recent findings in dementia. Curr Neurol Neurosci Rep. 17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber SK, Dunlosky J. 2012. Can older adults accurately judge their learning of emotional information? Psychol Aging. 27:924–933. [DOI] [PubMed] [Google Scholar]
- Turro-Garriga O, Garre-Olmo J, Calvo-Perxas L, Rene-Ramirez R, Gascon-Bayarri J, Conde-Sala JL. 2016. Course and determinants of anosognosia in Alzheimer’s disease: a 12-month follow-up. J Alzheimers Dis. 51:357–366. [DOI] [PubMed] [Google Scholar]
- Vannini P, Hanseeuw B, Munro CE, Amariglio RE, Marshall GA, Rentz DM, Pascual-Leone A, Johnson KA, Sperling RA. 2017. Anosognosia for memory deficits in mild cognitive impairment: insight into the neural mechanism using functional and molecular imaging. NeuroImage Clin. 15:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1987. WMS-R: Wechsler Memory Scale-revised: manual. San Antonio (TX): Psychological Corp. [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Sytsma J, Buchman AS, Bennett DA, Schneider JA. 2015. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 85:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Cai Y, Liu Q, Zhao X, Wang Q, Chen C, Xue G. 2015. Differential neural correlates underlie judgment of learning and subsequent memory performance. Front Psychol. 6:1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti O, Vallotti B, Frisoni GB, Geroldi C, Bianchetti A, Pasqualetti P, Trabucchi M. 1999. Insight in dementia: when does it occur? Evidence for a nonlinear relationship between insight and cognitive status. J Gerontol B Psychol Sci Soc Sci. 54:P100–P106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


