Short abstract
Importance
Increasing evidence indicates that carbamazepine (CBZ) treatment in people with epilepsy (PWE) has adverse effects on bone health.
Objective
We conducted a meta-analysis to investigate the effect of CBZ on the bone health of PWE.
Methods
Two independent reviewers systematically searched PubMed and EMBASE for eligible studies. Standard mean deviations (SMDs) with 95% confidence intervals were used as a measure to investigate the effect of CBZ on bone-related outcomes in PWE.
Results
Levels of vitamin D (standardized mean difference [SMD]: −0.62, 95% CI: −0.89 to −0.35) and calcium (SMD: −0.38, 95% CI: −0.67 to −0.09) were significantly lower in the CBZ group than controls. Additionally, significantly higher alkaline phosphatase (SMD: 0.67, 95% CI: 0.52–0.82) was observed in patients using CBZ than controls. However, no significant difference in BMD was found between the two groups (SMD: −0.06, 95% CI: −0.55 to 0.43).
Conclusion and significance: This study provided evidence that CBZ treatment has a negative effect on bone health in PWE. Clinical implications are that long-term CBZ treatment may not be a good choice for PWE with skeletal diseases or osteoporosis.
Keywords: Carbamazepine, drug effect, bone health, epilepsy, meta-analysis, vitamin D, calcium, alkaline phosphatase
Introduction
Long-term antiepileptic drug (AED) treatment is required for the majority of people with epilepsy (PWE) with the goal of controlling seizures and minimizing side effects. The prevalence of bone abnormalities and bone diseases such as osteopenia, chronic pain, falls and fractures in epileptic patients is at least 50%,1–3 and much attention has been paid to the association between bone health and AED therapy in epilepsy.4,5 Evidence from a recent meta-analysis found that long-term valproate treatment in children with epilepsy leads to vitamin D deficiency.6 Carbamazepine (CBZ), a common AED, has been widely used to control seizures in focal epilepsy over the past two decades.7,8 Previous investigations revealed that CBZ is an enzyme-inducing AED that increases the metabolism of vitamin D and conversion to its inactivated form, which may have a negative effect on bone health.9,10 However, the specific impact of CBZ on vitamin D, calcium, and bone remains uncertain.
Knowledge of the effect of CBZ on the bone health of PWE would have key clinical significance and the potential to reduce adverse bone consequences. However, the results of studies focusing on the effect of CBZ on bone health in epilepsy remain controversial and even conflicting.11,12 These inconsistencies in findings may be explained partly by differences in basic characteristics and populations among studies. Additionally, the results are limited by the small sample size of each individual study. No systematic review or meta-analysis on this topic has yet been performed, although high-level evidence from a meta-analysis would help identify the effect of CBZ on the bone health of PWE. Therefore, the present study aimed to systematically review the current evidence and conduct a meta-analysis to determine the effect of CBZ on the bone health of PWE.
Methods
The present systematic review and meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.13
Search strategy
Two reviewers independently searched PubMed and EMBASE databases from inception to June 1, 2019 for eligible studies. The following search terms were used: ‘Vit D’ OR ‘vitamin D’ OR ‘Calcium’ OR ‘bone mineral density’ OR ‘bone density’ OR ‘alkaline phosphatase’ OR ‘ALP’ AND ‘epilepsy’ OR ‘epileptic’ OR ‘seizure’ AND ‘Carbamazepine’ OR ‘CBZ’. Only studies published in English were included in our study. Reference lists of related articles and reviews were screened by hand to identify additional relevant studies.
Study selection
Studies were included in the present meta-analysis if they investigated the effect of CBZ on the bone health of epileptic patients and met the following inclusion criteria: 1) case–control, cross-sectional, or prospective study designs; 2) participants in the observation group were PWE receiving CBZ; 3) participants in the observation group were healthy controls or PWE not taking AEDs; and 4) bone-related outcomes included vitamin D levels, calcium levels, alkaline phosphatase (ALP) or BMD, and at least one outcome was reported by each study. Duplicate studies were removed. Case reports, reviews, letters, and comments were also excluded. The titles and abstracts of each study were first reviewed by two independent reviewers, then the studies were assessed using the specified inclusion criteria for eligibility by examining the full text. Disagreements in study selection between the two reviewers were discussed and resolved by consensus.
Quality assessment
The quality of each included study was subjectively graded using the Newcastle–Ottawa scale.14 Studies with a score of ≥7 stars were deemed of high quality, those with a score from 4–6 were of intermediate quality, and those with a score <4 were of inferior quality. Both reviewers independently evaluated the quality of the eligible studies and resolved disagreements by discussion.
Data extraction
Both reviewers independently extracted the following data from the included studies: first author, publication year, country, sample size, age group, outcomes, and study design. BMD measurements can be used to evaluate bone health in clinical practice, while the values of calcium, vitamin D, and ALP can also be used to assess disorders in bone and mineral metabolism.15 Therefore, detailed information on vitamin D levels, calcium levels, ALP, and BMD was also extracted from the included studies. Disagreement in extracting data between the two reviewers was resolved through face-to-face discussion.
Statistical analysis
Heterogeneity was assessed by the P values of χ2 and I2 statistics, and was significant if the I2 statistic was greater than 50% or if its P value was less than 0.05. We pooled data by a random effects model if the value of the I2 statistic was more than 50%, otherwise, a fixed effects model was applied. A meta-analysis was conducted if the outcome was reported by at least two studies. Standardized mean differences (SMDs) with 95% confidence intervals (CI) were used as a measure to investigate the effect of CBZ on bone-related outcomes in PWE. The results were presented as forest plots which included the contribution of each study (weight) to the overall effect. To check the stability of the study findings, sensitivity analysis was conducted by removing one study at a time to evaluate how the overall effect size would change. Because childhood and adolescence are the most important periods for bone development, the effect of CBZ on bone metabolism may be associated with age. Thus, subgroup analysis based on age group was conducted. Finally, we used a funnel plot to determine the presence of publication bias. Egger’s test was also performed to assess publication bias. All statistical analyses were conducted with STATA 14.0 software (StataCorp, College Station, TX, USA), and a P value of <0.05 was considered statistically significant.
Results
Study selection
The search strategy yielded 489 articles. The title and abstract of each article were screened after the exclusion of duplicates. A total of 87 articles were assessed as eligible for inclusion using the specified inclusion criteria after examining the full text. Of these, 22 studies were included in the meta-analysis after a detailed evaluation. The process of study selection is shown Figure 1.
Figure 1.
Flowchart of the study selection.
Study characteristics
Twenty-two studies16–37 were included in our review, of which 19 were cross-sectional studies and three were prospective studies. More than half of the included studies were conducted in Asia (n = 12), with the remainder in Europe (n = 7), North America (n = 2), and Oceania (n = 1). Eleven studies focused on the effect of CBZ on the bone health of children with epilepsy, and 11 studies aimed to explore the effect of CBZ on bone health in adult patients. The mean duration of CBZ treatment among the studies ranged from 0.2–6.16 years. The effect of CBZ on bone health was evaluated by four bone-related outcomes: vitamin D levels, calcium levels, ALP, and BMD. Vitamin D levels were reported by 15 studies, calcium levels by 13, ALP by 13, and BMD by 12. The mean score of the Newcastle–Ottawa Scale among the studies was 6.73 (range, 5–8), indicating an intermediate quality. The characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the included studies.
| Author | Year | Country |
Sample size (No.) |
Age group | CBZ duration | Outcomes | Study design | |
|---|---|---|---|---|---|---|---|---|
| CBZ | Controls | |||||||
| Suljic | 2018 | Bosnia | 49 | 30 | Adults | 6.16 | 1, 4 | Cross-sectional |
| Sreedharan | 2018 | India | 28 | 109 | Children | 1.95 | 1, 2, 3 | Cross-sectional |
| Ahmad | 2016 | Australia | 11 | 53 | Adults | 0.3 | 4 | Cross-sectional |
| Serin | 2015 | Turkey | 11 | 20 | Children | 2 | 1, 2, 3, 4 | Cross-sectional |
| Salimipour | 2013 | Iran | 33 | 38 | Adults | 4 | 4 | Cross-sectional |
| Turan | 2013 | Turkey | 45 | 44 | Children | 0.5 | 1 | Cross-sectional |
| Aksoy | 2011 | Turkey | 23 | 50 | Children | 2.93 | 1, 2, 3, 4 | Cross-sectional |
| Heo | 2011 | Seoul Korea | 36 | 36 | Adults | 4.3 | 1, 2, 4 | Cross-sectional |
| Misra | 2010 | India | 47 | 47 | Children | 0.5 | 1, 2, 3 | Cross-sectional |
| Pack | 2008 | USA | 41 | 41 | Adults | 1 | 1, 2, 4 | Longitudinal |
| Kim | 2007 | Seoul Korea | 10 | 10 | Adults | 0.5 | 1, 2, 3, 4 | Longitudinal |
| Babayigit | 2006 | Turkey | 23 | 30 | Children | 3.65 | 1, 2, 3, 4 | Cross-sectional |
| Mintzer | 2006 | USA | 21 | 24 | Adults | NR | 1, 2, 3 | Cross-sectional |
| Voudris | 2005 | Greece | 22 | 22 | Children | 0.5 | 3 | Longitudinal |
| Ecevit | 2004 | Turkey | 17 | 31 | Children | 2.6 | 4 | Cross-sectional |
| Bramswig | 2003 | Germany | 21 | 21 | Adults | 0.2 | 1, 2 | Cross-sectional |
| Verrotti | 2002 | Italy | 20 | 20 | Children | 2 | 1, 3 | Cross-sectional |
| Altay | 2000 | Turkey | 21 | 22 | Children | 3.7 | 4 | Cross-sectional |
| Verrotti | 2000 | Italy | 12 | 15 | Adults | 1 | 1, 2, 3 | Cross-sectional |
| Akın | 1998 | Turkey | 28 | 26 | Children | 2.6 | 2, 3, 4 | Cross-sectional |
| Okesina, | 1991 | UK | 41 | 32 | Adults | NR | 3 | Cross-sectional |
| Gough | 1986 | Ireland | 53 | 73 | Adults | NR | 1, 2, 3 | Cross-sectional |
CBZ, Carbamazepine; NR, Not reported.
1, vitamin D levels; 2, calcium levels; 3, alkaline phosphatase; 4, bone mineral density.
Results of meta-analysis
The effect of CBZ on vitamin D levels
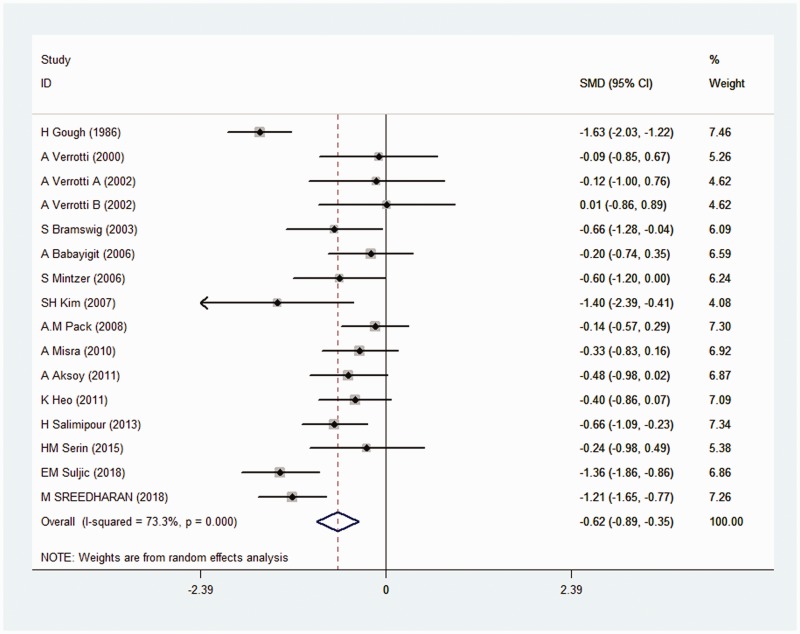
The effect of CBZ on vitamin D levels was reported by 15 studies. Pooled data indicated that vitamin D levels were significantly decreased in the CBZ group compared with the control group (SMD: −0.62, 95% CI: −0.89 to −0.35; I2 = 73.3%) (Figure 2). We used a random effects model because of the high level of heterogeneity.
Figure 2.
Forest plot of the effect of CBZ on vitamin D in PWE.
The effect of CBZ on calcium levels
The effect of CBZ on calcium levels was reported by 13 studies. The combined results revealed that calcium levels were significantly decreased in the CBZ group compared with the control group (SMD: −0.38, 95% CI: −0.67 to −0.09; I2 = 73.8%). We again applied a random effects model because of the high level of heterogeneity.
The effect of CBZ on ALP
The effect of CBZ on ALP was reported by 13 studies. Pooled data indicated that ALP was significantly elevated in the CBZ group compared with the control group (SMD: 0.67, 95% CI: 0.52–0.82; I2 = 14%). We used a fixed effects model because of the low level of heterogeneity.
The effect of CBZ on BMD
The effect of CBZ on BMD was reported by 12 studies. The combined results showed no significant difference between the CBZ group and the control group in terms of BMD (SMD: −0.06, 95% CI: −0.55 to 0.43; I2 = 88.7%). A random effects model was applied because of the high level of heterogeneity.
Results of subgroup analysis
Participants were divided into two age groups: an adult group (≥18 years) and a pediatric group (<18 years). Compared with the controls, low vitamin D levels (SMD: −0.78, 95% CI: −1.23 to −0.32; I2 = 81.5%; n = 8) and high ALP (SMD: 0.47, 95% CI: 0.23–0.70; I2 = 0%; n = 5) were observed in adult patients taking CBZ. However, no significant differences in terms of calcium levels (SMD: −0.46, 95% CI: −0.93 to 0.01; I2 = 79.9%; n = 7) or BMD (SMD: −0.42, 95% CI: −0.87 to 0.03; I2 = 75.5%; n = 6) were observed between adult patients taking CBZ and controls.
We observed low vitamin D levels (SMD: −0.48, 95% CI: −0.77 to −0.19; I2 = 51.8%; n = 7) and high ALP levels (SMD: 0.80, 95% CI: 0.61–1.00; I2 = 0%; n = 8 studies) in pediatric patients taking CBZ compared with controls. However, no significant differences in calcium levels (SMD: −0.29, 95% CI: −0.65 to 0.07; I2 = 63.7%; n = 6) or BMD (SMD: 0.39, 95% CI: −0.54 to 1.32; I2 = 92.6%; n = 6) were found between the two groups.
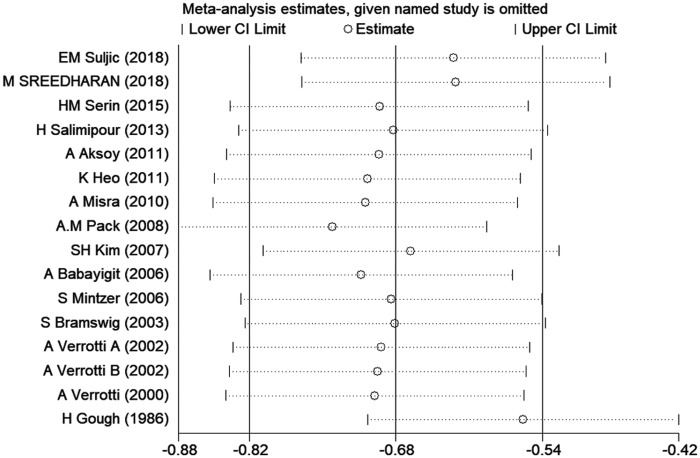
Sensitivity analysis
Sensitivity analysis was performed to investigate the impact of each study on overall SMDs. The stability and reliability of the overall SMDs were examined by removing one study at a time. This had no effect on the SMDs (Figure 3), suggesting that the results were relatively constant and stable.
Figure 3.
Sensitive analysis indicating the robustness of the adverse effects of CBZ on vitamin D.
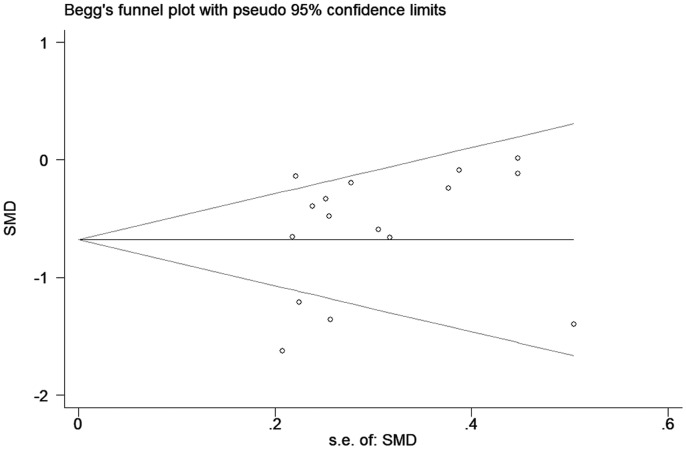
Publication bias
Funnel plots were used to test the reliability of meta-analysis findings. No visually significant publication bias was observed (Figure 4), and this was confirmed by the Egger’s test (data not shown).
Figure 4.
Funnel plot of the selected studies investigating the effect of CBZ on vitamin D.
Discussion
Twenty-two studies were included in this systematic review and meta-analysis, and we established evidence that PWE taking CBZ experienced bone metabolic changes that did not occur in controls. CBZ monotherapy significantly decreased vitamin D and calcium levels, and significantly increased ALP levels in PWE compared with control individuals. However, it had no significant effect on BMD. After subgroup stratification by age, we observed low vitamin D levels and high ALP levels in both adult and pediatric patients taking CBZ compared with controls. The stability and reliability of the results were identified by sensitivity analysis, and no publication bias was found by either funnel plot or Egger’s test.
These findings provide insights into the important association between CBZ treatment in epilepsy and bone metabolic changes. Evidence from a recent meta-analysis revealed that long-term valproate treatment in pediatric patients decreased vitamin D levels.6 In contrast to that meta-analysis, we focused on CBZ treatment in PWE and included both adult and pediatric patients. Importantly, subgroup analysis stratified by age group explored the effect of CBZ on bone health. We also systematically reviewed and summarized four bone-related outcomes: vitamin D, calcium, ALP, and BMD, providing a comprehensive study of the effect of CBZ on bone health in PWE.
We found that PWE taking CBZ had significantly lower calcium levels compared with controls, but this association did not maintain its significance in adult and pediatric patients separately after subgroup analyses. This could be explained by the decreased sample size after subgroup analysis altering SMD values. Salimipour et al. reported that CBZ therapy in epileptic patients was significantly associated with a low lumbar BMD.30 Consistent results were also found in several other studies.16,25 However, our analysis did not support such an association, nor did we find a significant effect of CBZ on BMD in adults or children after subgroup analysis. We also found that the extent of vitamin D reduction was more severe in adult than in pediatric patients, while the extent of ALP elevation was more severe in children than in adult patients.
CYP450 induced by CBZ may lead to bone metabolism abnormalities, reflected by an increased concentration of biochemical markers of bone resorption and degradation in both serum and urine.38–40 Thus, the mechanism underlying CBZ-induced adverse bone consequences can be explained by CYP450 dysregulation, which causes vitamin D deficiency and side effects such as decreased calcitonin, reduced calcium levels, and elevated ALP. The decrease in calcium levels leads to an increased release of parathyroid hormone, which functions to restore normal calcium levels at the cost of eroding bone.41 Elevated ALP also suggests the existence of bone destruction.42 Recent progress has indicated that treatment of animals with CBZ increases RANKL levels, while CBZ also stimulates the synthesis of sex hormone-binding globulin (SHGB).43 Increased SHBG levels are associated with a decreased conversion of estrogen, which elevates interleukin-7 levels in bone that further activate T cells to release tumor necrosis factors, interferons, and RANKL, thus mediating osteoclastogenesis.44
Despite its notable strengths, several limitations should be acknowledged in the present study. First, high heterogeneity existed in the majority of the meta-analyses. Significant heterogeneity persisted after subgroup and sensitivity analysis, and the sources of heterogeneity were not found. Differences in basic characteristics among the studies may have caused this heterogeneity, or it could be explained by differences in measurement methods and units. Second, the effect of CBZ on bone health in epilepsy could be associated with sex, duration of treatment, and duration of disease. However, subgroup analyses based on these factors were not performed, so additional stratification analyses are required in the future. Finally, meta-analyses can be biased if the literature search fails to identify all relevant studies, yet access to unpublished articles remains difficult.
Conclusion
This systematic review and meta-analysis established evidence that CBZ treatment has a negative effect on bone health in PWE. Epileptic patients taking CBZ had significantly lower vitamin D and calcium levels and high ALP compared with controls, suggesting that long-term CBZ treatment may not be a good choice for PWE with skeletal diseases or osteoporosis. Epileptic children with skeletal retardation should also use CBZ with caution. Additionally, bone assessment and monitoring of bone health at intervals should also be conducted during CBZ treatment. Further well-designed studies involving larger sample sizes are required to confirm our findings.
Acknowledgements
None
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Weihong Lin https://orcid.org/0000-0002-6612-3260
References
- 1.Miziak B, Chroscinska-Krawczyk M, Czuczwar SJ. An update on the problem of osteoporosis in people with epilepsy taking antiepileptic drugs. Expert Opin Drug Saf 2019; 18: 679–689. [DOI] [PubMed] [Google Scholar]
- 2.Miziak B, Blaszczyk B, Chroscinska-Krawczyk M, et al. The problem of osteoporosis in epileptic patients taking antiepileptic drugs. Expert Opin Drug Saf 2014; 13: 935–946. [DOI] [PubMed] [Google Scholar]
- 3.Parveen B, Tiwari AK, Jain M, et al. The anti-epileptic drugs valproate, carbamazepine and levetiracetam cause bone loss and modulate Wnt inhibitors in normal and ovariectomised rats. Bone 2018; 113: 57–67. [DOI] [PubMed] [Google Scholar]
- 4.Parveen B, Tripathi M, Vohora D. A cross-sectional study to assess the modulation of Wnt inhibitors following anti-epileptic drug therapy and their correlation with vitamin D and receptor activator of nuclear factor kappa B ligand in Indian women with epilepsy. Basic Clin Pharmacol Toxicol 2018; 123: 271–276. [DOI] [PubMed] [Google Scholar]
- 5.Garip US, Evis Z, Ilbay G, et al. Side-effects of convulsive seizures and anti-seizure therapy on bone in a rat model of epilepsy. Appl Spectrosc 2018; 72: 689–705. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Jing X, Li G, et al. Valproate decreases vitamin D levels in pediatric patients with epilepsy. Seizure 2019; 71: 60–65. [DOI] [PubMed] [Google Scholar]
- 7.Shih JJ, Whitlock JB, Chimato N, et al. Epilepsy treatment in adults and adolescents: expert opinion, 2016. Epilepsy Behav 2017; 69: 186–222. [DOI] [PubMed] [Google Scholar]
- 8.Karceski S, Morrell MJ, Carpenter D. Treatment of epilepsy in adults: expert opinion, 2005. Epilepsy Behav 2005; 7: S1–S64; quiz S65-7. [DOI] [PubMed] [Google Scholar]
- 9.Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav 2004; 5: S24–S29. [DOI] [PubMed] [Google Scholar]
- 10.Rahimdel A, Dehghan A, Moghadam MA, et al. Relationship between bone density and biochemical markers of bone among two groups taking carbamazepine and sodium valproate for epilepsy in comparison with healthy individuals in Yazd. Electron Physician 2016; 8: 3257–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan HC, Lee HS, Chang KP, et al. The impact of anti-epileptic drugs on growth and bone metabolism. Int J Mol Sci 2016; 17: pii: E1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee RH, Lyles KW, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother 2010; 8: 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 15.Svalheim S, Roste LS, Nakken KO, et al. Bone health in adults with epilepsy. Acta Neurol Scand Suppl 2011; 124: 89–95. [DOI] [PubMed] [Google Scholar]
- 16.Suljic EM, Mehicevic A, Mahmutbegovic N. Effect of long-term carbamazepine therapy on bone health. Med Arch 2018; 72: 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sreedharan M, Devadathan K, Mohammed KP, et al. Vitamin D deficiency in ambulant children on carbamazepine or sodium valproate monotherapy. Indian Pediatr 2018; 55: 307–310. [PubMed] [Google Scholar]
- 18.Okesina AB, Donaldson D, Lascelles PT. Isoenzymes of alkaline phosphatase in epileptic patients receiving carbamazepine monotherapy. J Clin Pathol 1991; 44: 480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pack AM, Morrell MJ, Randall A, et al. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology 2008; 70: 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra A, Aggarwal A, Singh O, et al. Effect of carbamazepine therapy on vitamin D and parathormone in epileptic children. Pediatr Neurol 2010; 43: 320–324. [DOI] [PubMed] [Google Scholar]
- 21.Verrotti A, Greco R, Latini G, et al. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia 2002; 43: 1488–1492. [DOI] [PubMed] [Google Scholar]
- 22.Verrotti A, Greco R, Morgese G, et al. Increased bone turnover in epileptic patients treated with carbamazepine. Ann Neurol 2000; 47: 385–388. [PubMed] [Google Scholar]
- 23.Babayigit A, Dirik E, Bober E, et al. Adverse effects of antiepileptic drugs on bone mineral density. Pediatr Neurol 2006; 35: 177–181. [DOI] [PubMed] [Google Scholar]
- 24.Aksoy A, Sonmez FM, Deger O, et al. The effects of antiepileptic drugs on the relationships between leptin levels and bone turnover in prepubertal children with epilepsy. J Pediatr Endocrinol Metab 2011; 24: 703–708. [DOI] [PubMed] [Google Scholar]
- 25.Shiek AB, O’Brien TJ, Gorelik A, et al. Bone mineral changes in epilepsy patients during initial years of antiepileptic drug therapy. J Clin Densitom 2016; 19: 450–456. [DOI] [PubMed] [Google Scholar]
- 26.Ecevit C, Aydogan A, Kavakli T, et al. Effect of carbamazepine and valproate on bone mineral density. Pediatr Neurol 2004; 31: 279–282. [DOI] [PubMed] [Google Scholar]
- 27.Erbayat AE, Serdaroglu A, Tumer L, et al. Evaluation of bone mineral metabolism in children receiving carbamazepine and valproic acid. J Pediatr Endocrinol Metab 2000; 13: 933–939. [DOI] [PubMed] [Google Scholar]
- 28.Gough H, Goggin T, Bissessar A, et al. A comparative study of the relative influence of different anticonvulsant drugs, UV exposure and diet on vitamin D and calcium metabolism in out-patients with epilepsy. Q J Med 1986; 59: 569–577. [PubMed] [Google Scholar]
- 29.Serin HM, Koc ZP, Temelli B, et al. The bone mineral content alterations in pediatric patients medicated with levetiracetam, valproic acid, and carbamazepine. Epilepsy Behav 2015; 51: 221–224. [DOI] [PubMed] [Google Scholar]
- 30.Salimipour H, Kazerooni S, Seyedabadi M, et al. Antiepileptic treatment is associated with bone loss: difference in drug type and region of interest. J Nucl Med Technol 2013; 41: 208–211. [DOI] [PubMed] [Google Scholar]
- 31.Voudris KA, Attilakos A, Katsarou E, et al. Early alteration in bone metabolism in epileptic children receiving carbamazepine monotherapy owing to the induction of hepatic drug-metabolizing enzymes. J Child Neurol 2005; 20: 513–516. [DOI] [PubMed] [Google Scholar]
- 32.Heo K, Rhee Y, Lee HW, et al. The effect of topiramate monotherapy on bone mineral density and markers of bone and mineral metabolism in premenopausal women with epilepsy. Epilepsia 2011; 52: 1884–1889. [DOI] [PubMed] [Google Scholar]
- 33.Turan MI, Cayir A, Ozden O, et al. An examination of the mutual effects of valproic acid, carbamazepine, and phenobarbital on 25-hydroxyvitamin D levels and thyroid function tests. Neuropediatrics 2014; 45: 16–21. [DOI] [PubMed] [Google Scholar]
- 34.Akin R, Okutan V, Sarici U, et al. Evaluation of bone mineral density in children receiving antiepileptic drugs. Pediatr Neurol 1998; 19: 129–131. [DOI] [PubMed] [Google Scholar]
- 35.Bramswig S, Zittermann A, Berthold HK. Carbamazepine does not alter biochemical parameters of bone turnover in healthy male adults. Calcif Tissue Int 2003; 73: 356–360. [DOI] [PubMed] [Google Scholar]
- 36.Mintzer S, Boppana P, Toguri J, et al. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia 2006; 47: 510–515. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Lee JW, Choi KG, et al. A 6-month longitudinal study of bone mineral density with antiepileptic drug monotherapy. Epilepsy Behav 2007; 10: 291–295. [DOI] [PubMed] [Google Scholar]
- 38.Kumandas S, Koklu E, Gumus H, et al. Effect of carbamezapine and valproic acid on bone mineral density, IGF-I and IGFBP-3. J Pediatr Endocrinol Metab 2006; 19: 529–534. [PubMed] [Google Scholar]
- 39.Hamed SA. Markers of bone turnover in patients with epilepsy and their relationship to management of bone diseases induced by antiepileptic drugs. Expert Rev Clin Pharmacol 2016; 9: 267–286. [DOI] [PubMed] [Google Scholar]
- 40.Pratico AD, Pavone P, Scuderi MG, et al. Symptomatic hypocalcemia in an epileptic child treated with valproic acid plus lamotrigine: a case report. Cases J 2009; 2: 7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du C, Norris K, Hanlon Y, et al. Effect of whole blueberry on bone biomarkers in postmenopausal women with mild-to-moderate bone loss (P01-029-19). Curr Dev Nutr 2019; 3: nzz028.P01-029-19. [Google Scholar]
- 42.Isojarvi JI, Tauboll E, Herzog AG. Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS Drugs 2005; 19: 207–223. [DOI] [PubMed] [Google Scholar]
- 43.Khanna S, Pillai KK, Vohora D. Insights into liaison between antiepileptic drugs and bone. Drug Discov Today 2009; 14: 428–435. [DOI] [PubMed] [Google Scholar]