Short abstract
Background
To investigate the prognostic value of pre-treatment serum alpha-fetoprotein (AFP) levels in patients with gastric cancer (GC).
Methods
PubMed, EMBASE, Medline and Web of Science databases were systematically searched for studies published between January 01, 1998 and December 31, 2018 that investigated the relationship between pre-treatment serum AFP levels and prognosis of patients with GC. Hazard ratios (HR) for overall survival (OS), disease-free survival (DFS), and their corresponding 95% confidence intervals (CIs) were evaluated.
Results
13 studies involving 9099 patients with GC were included in the meta-analysis. High pre-treatment serum AFP levels were significantly associated with poor outcome in patients with GC. Although there was significant heterogeneity between studies, sub-group analyses found that studies of ‘non-China’ countries, sample size <500, mixed treatment, or AFP cut-off value ≥20 ng/ml, had low heterogeneity.
Conclusions
The pooled analysis suggests that pre-treatment serum AFP levels can be used as a prognostic indicator in patients with GC. Further research is required to confirm these results.
Keywords: Alpha-fetoprotein, AFP, gastric cancer, prognosis, survival, meta-analysis
Introduction
Although there has been a sharp decline in the incidence of gastric cancer (GC) over the past century, it remains the second leading cause of cancer-related deaths worldwide,1 and one of the four most common cancers in China.2 Surgical resection is the primary method of treatment for non-metastatic GC and is curative in many cases but for some patients recurrence and metastases may occur which can be fatal.3 Therefore, it is important to identify biomarkers which can assist in the identification of patients who are at risk of relapse.3
Currently, the screening methods commonly used for GC include gastroscopy, barium meal imaging and computed tomography (CT). However, these methods have limited sensitivity, are not prognostic, tend to be invasive and can cause severe discomfort for the patient. Identification of tumour markers is becoming increasingly popular in clinical oncology as a non-invasive method for cancer diagnosis and for monitoring response to treatment; their use is simple and easily accepted by patients.4
Alpha-fetoprotein (AFP) is a glycoprotein synthesized by the foetal yolk sac and liver during pregnancy,5 and is a common marker of tumours of the digestive system. 6 The diagnostic and prognostic value of AFP for hepatocellular carcinoma and yolk sac tumours has been determined, but serum levels may also be elevated in other cancers including primary GC.6,7 Indeed, high AFP levels in GC accompanied by liver metastases were first reported in 1970.8 Over subsequent decades, several studies have emphasised that patients with AFP-producing GC were at increased risks of lympho-vascular invasion and liver metastases, as well as a poor prognosis and aggressive cancer.9–11 However, differences among studies in methodology and sample size has meant that the exact association between serum AFP levels and GC remains controversial.12Therefore, we decided to conduct a meta-analysis of relevant studies to evaluate the relationship between pre-treatment serum AFP levels and clinical outcome of patients with GC.
Methods
PubMed, EMBASE, Medline and Web of Science databases were systematically searched for studies published between January 01, 1998 and December 31, 2018 that investigated AFP levels in patients with gastric cancer. Key words/terms in both AND and OR combinations included: alpha fetoprotein; AFP; gastric cancer; gastric carcinoma; gastric tumour; gastric neoplasm; survival; prognosis; outcome. For a published report to be included in the meta-analysis, it had to fulfil the following criteria: (1) be a clinical study of patients with GC; (2) report serum AFP levels for overall survival (OS), disease-free survival (DFS) and prognostic indicators; (3) provide hazard ratios (HR) and 95% confidence interval (CI) values. Duplicate publications, reviews, editorials, abstracts, comments, case reports, meetings and animal studies were excluded.
Two reviewers independently selected the published papers and any discrepancy was resolved by consultation with a third reviewer. The following information was extracted from each article: name of the first author; publication year; country of origin; sample size; sample year; survival analysis method; patient information (i.e., region, age, sex, tumour stage, cut-off value for AFP levels, and treatment); prognostic outcomes. If results from both univariate and multivariate analyses were provided, only multivariate analysis data were extracted. An email was also sent to authors requesting any missing relevant data. The quality of each of the studies was assessed according to the Newcastle–Ottawa Scale,13 where a score of ≥6 was defined as a high-quality study.
The study was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University and because this was a meta-analysis of previously published articles, ethical approval was not required.
Statistical analyses
All statistical analyses were conducted using the soft-ware package Stata version12 (StataCorp, College Station, Texas). Cochran's Q test and Higgins' I2 statistical test were used to assess the statistical heterogeneity of the pooled results. If I2 statistic ≥50% and P < 0.05, a random effects model (DerSimonian–Laird method) was applied. If no heterogeneity was observed, a fixed effect model (Mantel–Haenszel method) was used. To assess possible sources of heterogeneity, subgroup analyses were performed based on country, sample size, tumour stage, treatment and cut-off values. A sensitivity analysis was applied to assess the robustness of the results. Begg’s funnel plot and Egger's linear regression test were used to assess potential publication bias.
Results
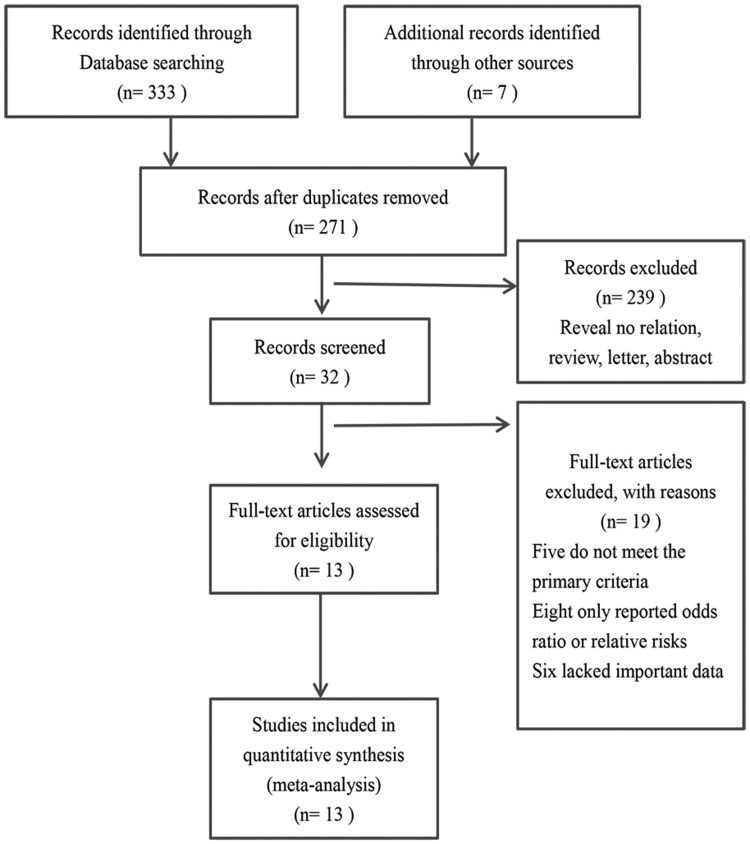
The literature search identified 340 articles from which 13 articles ultimately met the eligibility criteria (Figure 1).12,14–25 All 13 studies scored ≥6 (i.e., high-quality study) on the Newcastle–Ottawa Scale.13 The main features of the 13 studies are summarized in Table 1. Eight studies were performed in China, two in Turkey, two in Japan and one in South Korea. Apart from the Korean study, all were in English. Twelve articles contained data on OS and three had data on DFS. Treatments included surgery, chemotherapy and mixed therapy. All studies used multivariate analysis to determine the HRs.
Figure 1.
Flow diagram of included and excluded studies.
Table 1.
Characteristics of the 13 studies included in the meta-analysis of the relationship between pre-treatment serum AFP levels and outcome in patients with gastric cancer.
| First author (year) | Country, Region | N | Age (y) | Men/women | Study period | Stage | Serum AFP cut-off value ng/ml | Treatment | Outcome | Follow-up duration (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bozkaya et al. (2017)14 | Turkey, West Asia | 362 | median 58 | 267/95 | 2009–2015 | I–III | 9 | Mixed | OS/DFS | 48 |
| Chen et al. (2015)12 | China, East Asia | 1286 | 942/344(≤65/>65y) | 982/304 | 2004–2008 | I–III | 20 | Surgery | OS/DFS | 60 |
| Chun and Kwon (2011)15 | China, East Asia | 694 | median 59 | 477/217 | 2001–2008 | I–III | 7 | Surgery | OS | 38 |
| Dang et al. (2016)16 | China, East Asia | 400 | 208/192(≥60/<60y) | 295/105 | 2010–2013 | I–IV | 20 | Mixed | OS | 40 |
| Kochi et al. (2004)17 | Japan, East Asia | 57 | median 62 | 47/10 | 1989–2002 | IV | 20 | Chemotherapy | OS | NR |
| Lew et al. (2013)18 | Korea, East Asia | 771 | mean 62 | 532/239 | 2005–2013 | I–IV | 10 | Mixed | OS | 60 |
| Lin et al. (2014)19 | China, East Asia | 1294 | mean 66 | 1027/267 | 1988–2011 | I–IV | 20 | Surgery | OS | 43 |
| Liu et al. (2016)20 | China, East Asia | 1294 | median 58 | 1010/284 | 2008–2014 | I–III | 8.1 | Surgery | OS | 91 |
| Tachibana et al. (1998)21 | Japan, East Asia | 196 | 108/88 (≤69/≥70y) | 136/60 | 1986–1996 | I–IV | NR | Surgery | OS | 120 |
| Ucar et al. (2008)22 | Turkey, West Asia | 95 | mean 58 | 63/32 | 2001–2005 | I–IV | 10 | Mixed | DFS | 18 |
| Wang et al. (2015)23 | China, East Asia | 634 | 287/347 (≥60/<60y) | 449/185 | 2009–2012 | I–IV | 7 | Surgery | OS | NR |
| Wang et al. (2018)24 | China, East Asia | 105 | median 59 | 82/23 | 2006–2016 | I–IV | 500 | Chemotherapy | OS | 60 |
| Xu et al. (2018)25 | China, East Asia | 1911 | 815/1096(≥65/<65y) | 1549/362 | 2008–2014 | I–III | 8.1 | Mixed | OS | 91 |
Abbreviations: AFP, alpha-fetoprotein; OS, overall survival; DFS, disease-free survival; HR, hazard ratio; NR, not reported.
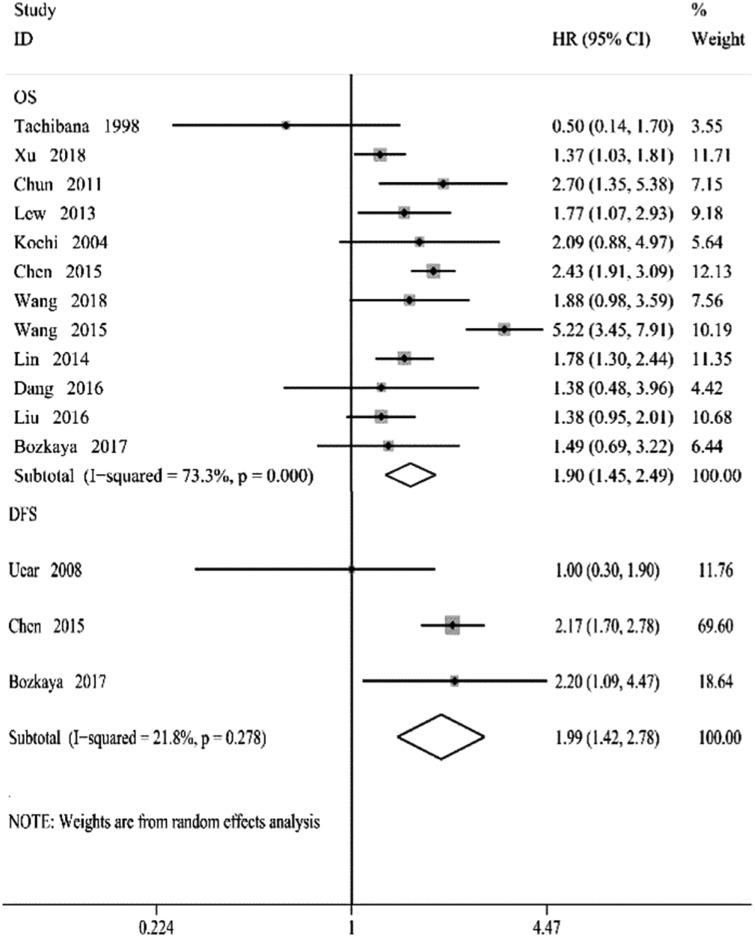
Twelve 12 studies involving 9004 patients assessed the association between pre-treatment serum AFP levels and OS. Pooled analysis showed that high serum AFP levels were associated with poor OS, compared with low pre-treatment serum AFP levels (HR = 1.90, 95% CI = 1.45, 2.49, P < 0.001). However, heterogeneity between the studies was statistically significant (I2 = 73.30%, P < 0.001) (Figure 2).
Figure 2.
Forest plots of studies evaluating the association between pre-treatment serum AFP levels and gastric cancer. The squares and horizontal lines correspond to the study specific hazard ratios (HR) and 95% confidence intervals (CIs), respectively. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary HR and 95% CI.
Three studies involving 1743 patients assessed DFS. The combined results showed that high serum AFP levels were associated with poor DFS (HR = 2.08, 95% CI = 1.66–2.60, P < 0.001) and heterogeneity between the studies was minimal (Figure 2).
A series of subgroup analyses was performed based on country, sample size, tumour stage, treatment and cut-off values and assessed possible sources of heterogeneity (Table 2). Results showed that studies of OS rates using ‘non-China’ countries (n = 4), sample size < 500 (n = 5), mixed treatment (n = 4), or AFP cut-off value ≥20 ng/ml (n = 5), all had low heterogeneity. All the other sub-groups had significant heterogeneity (Table 2).
Table 2.
Summary of the subgroup analysis.
| Subgroup | Outcome | No. studies | Number of patients | HR (95% CI) | Statistical significance | Model |
Heterogeneity |
|
|---|---|---|---|---|---|---|---|---|
| I2 | Statistical significance | |||||||
| All variables | OS | 12 | 9004 | 1.90 (1.45, 2.49) | P < 0.001 | random | 73.3% | P < 0.001 |
| DFS | 3 | 1743 | 2.08 (1.66, 2.60) | P < 0.001 | fixed | 21.8% | ns | |
| Country | ||||||||
| China | OS | 8 | 7618 | 2.07 (1.49, 2.86) | P < 0.001 | random | 80.3% | P < 0.001 |
| DFS | 1 | 1286 | 2.17 (1.70, 2.78) | |||||
| Other countries | OS | 4 | 1386 | 1.57 (1.10, 2.26) | P = 0.014 | fixed | 25.9% | ns |
| DFS | 2 | 457 | 1.65 (0.94, 2.89) | ns | fixed | 43.7% | ns | |
| Sample size | ||||||||
| >500 | OS | 7 | 7884 | 2.10 (1.50, 2.93) | P < 0.001 | random | 83.0% | P < 0.001 |
| DFS | 1 | 1286 | 2.17 (1.70, 2.78) | |||||
| < 500 | OS | 5 | 1120 | 1.53 (1.05, 2.24) | P = 0.027 | fixed | 3.7% | ns |
| DFS | 2 | 457 | 1.65 (0.94, 2.89) | ns | fixed | 43.7% | ns | |
| Stage | ||||||||
| I–III | OS | 5 | 5547 | 1.77 (1.28, 2.44) | 0.001 | random | 69.5% | P = 0.011 |
| DFS | 2 | 1648 | 2.17 (1.72, 2.74) | P < 0.001 | fixed | 0 | ns | |
| I–IV | OS | 7 | 3457 | 1.94 (1.21, 3.10) | P = 0.006 | random | 76.7% | P < 0.001 |
| DFS | 1 | 95 | 1.00 (0.30, 1.90) | |||||
| Treatment | ||||||||
| Surgery | OS | 6 | 5398 | 2.12 (1.38, 3.24) | P = 0.001 | random | 83.8% | P < 0.001 |
| DFS | 1 | 1286 | 2.17 (1.70, 2.78) | |||||
| Mixed treatments | OS | 4 | 3444 | 1.45 (1.16, 1.83) | P = 0.001 | fixed | 0 | ns |
| DFS | 2 | 457 | 1.65 (0.94, 2.89) | ns | fixed | 43.7% | ns | |
| Serum AFP cut-off value | ||||||||
| ≥20 ng/ml | OS | 5 | 3142 | 2.11 (1.77, 2.52) | P < 0.001 | fixed | 0 | ns |
| DFS | 1 | 1286 | 2.17 (1.70, 2.78) | |||||
| < 20 ng/ml | OS | 6 | 5666 | 2.03 (1.26, 3.25) | P = 0.003 | random | 84.5% | P < 0.001 |
| DFS | 2 | 457 | 1.65 (0.94, 2.89) | ns | fixed | 43.7% | ns | |
Abbreviations: OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; AFP, alpha-fetoprotein; ns, not statistically significant.
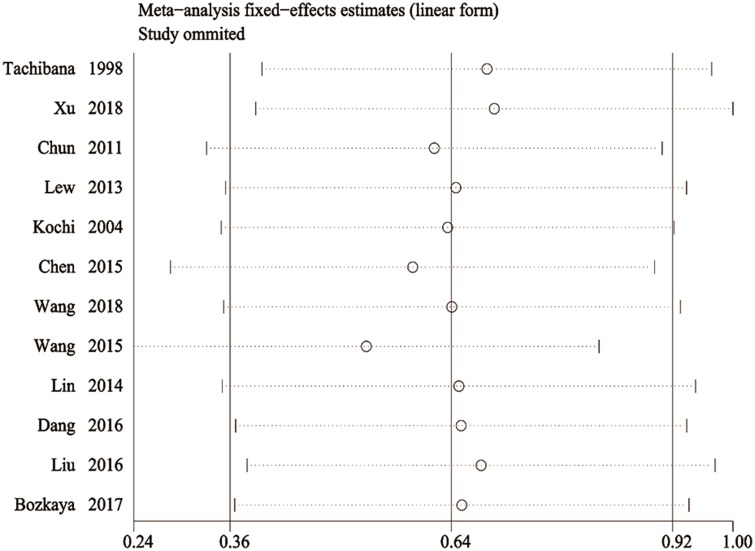
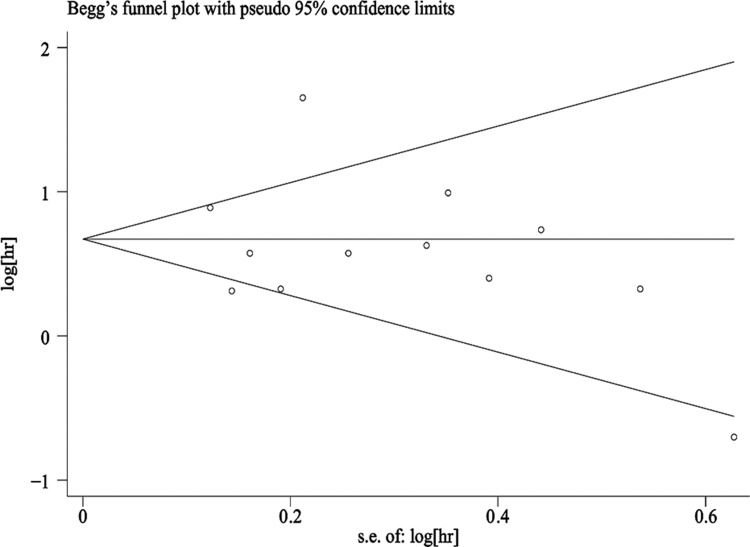
Sensitivity analysis of the OS rates was performed to examine the effects of each individual study on the combined HR. The results of this analysis showed that the omission of each study did not significantly alter the overall results indicating that our analysis result was robust (Figure 3). In addition, the results from Begg’s funnel plot and Egger’s test indicated that there was no significant publication bias in this meta-analysis (Figure 4).
Figure 3.
Sensitivity analysis of the relationship between pre-treatment serum AFP levels and overall survival (OS) rates to confirm the robustness of the results by removing one study at a time.
Figure 4.
Funnel plot for evaluating potential publication bias.
Discussion
This meta-analysis included data from 13 studies involving 9099 patients with GC. With regard to the quality of the evidence, the risk of bias was minimal as indicated by Begg’s funnel plot and Egger's test. In addition, a sensitivity analysis showed that omission of each study did not significantly alter the overall results indicating that the analysis was robust. Pooled analysis of the 12 studies that assessed OS showed a significant correlation between elevated pre-treatment serum AFP levels and poor prognosis in patients with GC. However, there was substantial heterogeneity among the studies. Nevertheless, a subgroup analysis found that studies using ‘non-China’ countries, sample size < 500, mixed treatment or AFP cut-off values ≥20 ng/ml had low, heterogeneity (i.e., I2 < 50%).
In spite of many treatment options for GC, the 5-year survival rate is low and poor prognosis is mainly due to local recurrence, lymphatic invasion and distant metastasis.26 Therefore, it is important to identify new reliable biomarkers to improve early detection of the cancer and assess its prognosis. In clinical practice, AFP is considered to be a useful tumour marker for hepatocellular carcinoma and yolk sac tumors,6,27 and some studies have shown that serum AFP levels are also elevated in a variety of extrahepatic tumours, including those of the stomach, lung, pancreas, colon, bladder and ovary.6 Of these cancers, GC is the most common type that is accompanied by high serum AFP levels.6Although AFP is a useful biomarker for predicting survival and detecting and/or monitoring hepatocellular carcinoma, its correlation with GC remains to be clarified.12
Since both the stomach and liver were derived from the original foregut of the embryo, it has been suggested that GCs may produce large amounts of AFP in the same manner as liver cancer when there is an abnormality during differentiation. 28Indeed, the concept of a hepatoid adenocarcinoma of the stomach has been proposed for primary GC that is characterized by hepatoid differentiation and production of large amounts of AFP. 28 However, other authors have suggested that AFP-producing GCs are not always derived from hepatocyte differentiation and AFP in primary GC may be a gastrointestinal-specific foetal protein.29 Another study proposed that AFP has immunosuppressive functions and inhibits the production of cytokines, interferons, and tumour necrosis factor by natural killer cells and macrophages. 30 The authors suggested that in the presence of AFP, cancer cells grow rapidly and can cause distant metastases following blood vessel invasion.30 In addition, it has been reported that the prognosis for AFP-producing GC is worse than that for AFP-negative GC, because the former is characterized by aggressive biological behaviour and high potential for liver metastasis. 6,15 Corroborating this observation, other authors have found that high levels of serum AFP were associated with shorter survival times and that patients with high serum AFP had high frequencies of liver and lymph node metastasis with poor prognosis.19 Accordingly, the treatment of these patients may require multimodal therapy (i.e., chemotherapy, radiotherapy, biotherapy).12,15
The study had some limitations. For example, there were only 13 studies and eight were performed in China which may have introduced some bias. In addition, the numbers of studies in the sub-group analyses were low which may have influenced heterogeneity. Furthermore, the serum AFP cut-off values differed among studies which may also have led to imbalance. Therefore, future research is required to clarify accurately the association between pre-treatment serum AFP levels and the prognosis of patients with GC. We will continue to search for high quality articles and update our meta-analysis accordingly.
The results of this meta-analysis suggest that pre-treatment serum AFP levels are an independent prognostic factor for assessing the outcome of patients with GC. High levels of pre-treatment serum AFP were associated with a poor prognosis. Therefore, patients with high serum AFP levels should be closely monitored and have frequent follow-up visits using tests such as computed tomography (CT), magnetic resonance imaging, positron emission tomography/CT as deemed necessary.
Author contributions
H.C., Z.G., G.Q. and Q.L. conducted the database search and collected the data. X.X., Q.W. and Y.W. analysed the data and wrote the manuscript.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
The present study was supported by the Changzhou Municipal Scientific Research grant (grant no. CE20125020).
ORCID iD
Yugang Wu https://orcid.org/0000-0001-8447-8252
References
- 1.Terry MB, Gaudet MM, Gammon MD. The epidemiology of gastric cancer. Semin Radiat Oncol 2002; 12: 111–127. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 3.Marrelli D, De Stefano A, de Manzoni G, et al. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg 2005; 241:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong W, Yu Z, Zhan J, et al. Association of serum levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and disease characteristics in colorectal cancer. Pathol Oncol Res 2015; 21: 83–95. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest 1956; 8: 174. [DOI] [PubMed] [Google Scholar]
- 6.Tatli AM, Urakci Z, Kalender ME, et al. Alpha-fetoprotein (AFP) elevation gastric adenocarcinoma and importance of AFP change in tumor response evaluation. Asian Pac J Cancer Prev 2015; 16:2003–2007. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Takamine Y, Nakamura K, et al. Des-gamma-carboxy prothrombin (PIVKA-II) and alpha-fetoprotein-producing IIc-type early gastric cancer. Am J Gastroenterol 1992; 87: 1859–1862. [PubMed] [Google Scholar]
- 8.Bourreille J, Metayer P, Sauger F, et al. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med 1970; 78: 1277–1278. [PubMed] [Google Scholar]
- 9.Chang YC, Nagasue N, Abe S, et al. The characters of AFP-producing early gastric cancer. Nihon Geka Gakkai Zasshi 1990; 91: 1574–1580. [PubMed] [Google Scholar]
- 10.Chang YC, Nagasue N, Abe S, et al. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol 1992; 87: 321–325. [PubMed] [Google Scholar]
- 11.Motoyama T, Aizawa K, Watanabe H, et al. alpha-Fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn 1993; 43: 654–661. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Qu H, Jian M, et al. High level of serum AFP is an independent negative prognostic factor in gastric cancer. Int J Biol Markers 2015; 30 (4): e387–e393. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 14.Bozkaya Y, Demirci NS, Kurtipek A, et al. Clinicopathological and prognostic characteristics in patients with AFP-secreting gastric carcinoma. Mol Clin Oncol 2017; 7: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun H, Kwon SJ. Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J Gastric Cancer 2011; 11: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang Y, Ouyang X, Wang K, et al. Evaluation of the clinical significance of serum carcinoembryonic antigen in patients with resectable gastric adenocarcinoma. Arch Med Res 2016; 47: 196–199. [DOI] [PubMed] [Google Scholar]
- 17.Kochi M, Fujii M, Kaiga T, et al. FLEP chemotherapy for alpha-fetoprotein-producing gastric cancer. Oncology 2004; 66: 445–449. [DOI] [PubMed] [Google Scholar]
- 18.Lew DH, Jung WT, Kim HJ, et al. Clinicopathological characteristics and prognosis of alpha-fetoprotein producing gastric cancer. Korean J Gastroenterol 2013; 62: 327–335. [DOI] [PubMed] [Google Scholar]
- 19.Lin HJ, Hsieh YH, Fang WL, et al. Clinical manifestations in patients with alpha-fetoprotein-producing gastric cancer. Curr Oncol 2014; 21: e394–e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Feng F, Xu G, et al. Clinicopathological features and prognosis of gastric cancer in young patients. BMC Cancer 2016; 16: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana M, Takemoto Y, Nakashima Y, et al. Serum carcinoembryonic antigen as a prognostic factor in resectable gastric cancer. J Am Coll Surg 1998; 187: 64–68. [DOI] [PubMed] [Google Scholar]
- 22.Ucar E, Semerci E, Ustun H, et al. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther 2008; 25: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Li C, Xu Y, et al. Clinicopathological characteristics and prognosis of alpha-fetoprotein positive gastric cancer in Chinese patients. Int J Clin Exp Pathol 2015; 8: 6345–6355. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YK, Shen L, Jiao X, et al. Predictive and prognostic value of serum AFP level and its dynamic changes in advanced gastric cancer patients with elevated serum AFP. World J Gastroenterol 2018; 24: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu G, Feng F, Liu S, et al. Clinicopathological features and prognosis in elderly gastric cancer patients: a retrospective cohort study. Onco Targets Ther 2018; 11: 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng L, Poon RT, Pang R. Biomarkers for predicting future metastasis of human gastrointestinal tumors. Cell Mol Life Sci 2013; 70: 3631–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Bahrawy M. Alpha-fetoprotein-producing non-germ cell tumours of the female genital tract. Eur J Cancer 2010; 46: 1317–1322. [DOI] [PubMed] [Google Scholar]
- 28.Ishikura H, Kirimoto K, Shamoto M, et al. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer 1986; 58: 119–126. [DOI] [PubMed] [Google Scholar]
- 29.Ooi A, Nakanishi I, Sakamoto N, et al. Alpha-fetoprotein (AFP)-producing gastric carcinoma. Is it hepatoid differentiation? Cancer 1990; 65: 1741–1747. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita T, Nakane A, Watanabe T, et al. Evidence that alpha-fetoprotein suppresses the immunological function in transgenic mice. Biochem Biophys Res Commun 1994; 201: 1154–1159. [DOI] [PubMed] [Google Scholar]