Short abstract
Aim
To assess the glycaemic profile and glycaemic variation in the second and third trimesters of normal pregnancies.
Methodology
Healthy pregnant women aged 19–35 years between 24 and 36 weeks of gestation were recruited for ambulatory glucose profile monitoring. A total of 18 women in the second trimester, 15 women in the third trimester and 9 healthy non-pregnant women were recruited providing, respectively, 205 days (19,680 data points), 147 days (14,112 data points) and 100 days (9,600 data points) for analysis.
Results
Mean blood glucose level was 20.2% lower in the second trimester and 10.6% lower in the third trimester than non-pregnant women (p < 0.001). In pregnancy, it took 15 to 20 minutes more to reach peak postprandial blood glucose levels compared to non-pregnant women (p = 0.003). Glycaemic variability was more in the third trimester (p < 0.001).
Conclusion
There is tight blood sugar control along with lower mean blood glucose in healthy pregnant women compared to non-pregnant women. Despite this tight glycaemic control, glycaemic variability is higher during pregnancy.
Keywords: Gestational diabetes mellitus, glycaemic variability
Introduction
The complexities of fetal growth has been thoroughly investigated over the past decades but the intricacies still remain to be completely resolved. Maternal normoglycaemia is one of the most important factors for ensuring optimal fetal growth, as it influences the levels of other body nutrients such as amino acids and lipids. This is why blood glucose remains the single maternal metabolic parameter routinely assessed in all pregnancies to diagnose gestational diabetes mellitus (GDM). Indeed, the criteria for metabolic control and therapeutic strategies of diabetes in pregnancy are based almost exclusively on maternal glucose levels. It is well documented that good perinatal outcomes can be achieved in pregnancies in women with diabetes with the normalization of maternal glucose values, but achieving this can be challenging. A better understanding of the pattern of blood glucose fluctuations during normal pregnancy could make it easier to optimize glycaemic control in pregnant women with diabetes. Only a few studies have focused on the importance of glucose fluctuations during pregnancy.1,2 One example was a small series in hospitalized subjects and considered only glucose values collected during a single day in the third trimester of pregnancy.
The present study employed the ambulatory glucose profile (AGP) to determine the blood glucose profile and glycaemic variation in the second and third trimesters of normal pregnancies.
Methodology
This pilot study was conducted from October 2016 to May 2017. Subjects were recruited from the antenatal outpatient department of the Hamdard Institute of Medical Sciences and Research and associated HAH Centenary Hospital, New Delhi. Written informed consent was obtained from all subjects before their participation in the study. The study was approved by the Institutional ethics committee.
Pregnant women aged between 19 and 35 years, who were between 24 and 36 weeks of gestation were screened for the study. A 2 hour 75 g oral glucose tolerance test was performed for each subject to exclude gestational diabetes mellitus (GDM) according to Indian guidelines.3 Pregnant women with a 2 hour blood glucose less than 140 mg/dl were considered as normal and included. Women with type 2 diabetes mellitus or GDM (in present or past pregnancy), or pre-gestational body mass index (BMI) greater than 35 kg/m2, subsequent development of GDM later in pregnancy or an intrauterine death, or if they developed GDM later on, or had intrauterine death, were excluded from the study. Demographic, anthropometric and clinical data were recorded for all the participants.
The AGP monitor (Abbot Freestyle Libre Pro Flash Glucose Monitoring System) was applied to the back of the non-dominant upper arm for a period of 14 days. Subjects were advised to note down the timing of their main breakfast, lunch and dinner and continue their diet and lifestyle unhindered, as before.
AGP data
The AGP monitor is approved by the Food and Drug Administration, USA and monitors the glucose level for 14 days.4 The glucose level is automatically measured and recorded every 15 minutes in the interstitial fluid via a small (5 mm long, 0.4 mm wide) filament that is inserted just under the skin (Figure 1). There is no requirement for fingerstick calibration as required with other continuous glucose monitoring (CGM) devices.5,6 Data from the AGP monitor were downloaded on to a computer at the end of 14 days for analysis. The AGP monitor records glucose every 15 minutes giving 96 data points every 24 hours. It was observed that the AGP monitor required 24 to 48 hours to adjust and stabilize. Hence the first 48 hours of readings were excluded from data analysis. Therefore, a maximum of 12 days’ data was available for any subject, i.e. a maximum of 1152 data points.
Figure 1.
(a) AGP monitor applied on the upper part of the arm, (b) AGP sensor apparatus with small thin filament which goes subcutaneously and (c) AGP reader.
After the monitoring period was complete, all women were followed until delivery and the outcome recorded. If any complication occurred (development of GDM, intrauterine death or stillbirth), the data of these women were excluded from the analysis.
Twenty subjects were enrolled in the second trimester of pregnancy and an equal number in the third trimester. However, after excluding dropouts, inadvertent premature removal of the AGP sensors and patients with adverse pregnancy outcomes, finally 18 patients in the second trimester (24–28 weeks) with 205 days of glucose monitoring (19,680 data points) and 15 patients in the third trimester (28–36 weeks) with total 147 days (14,112 data points) were available for analysis. Similarly, nine healthy non-pregnant women volunteers were also included and had a glucose profile of 100 days (9,600 data points). None of our subjects developed GDM or had an intrauterine death.
Statistical analysis
The collected data were tabulated on SPSS version 20. Data were evaluated descriptively and arranged graphically for a better understanding of the variation in blood glucose profile in 24 hour time intervals in each trimester. Fasting blood glucose values were taken as the mean of the measure of the two values before and two values after 6 a.m., whereas nocturnal glucose values were the average of the values between 12 midnight to 6 a.m. These data points were also organized into frequency percentiles so that each woman will have one value representing 25th percentile, 50th percentile and 75th percentile for each hour out of 24 hour. As described in Carreiro et al.,7 the glucose variability was estimated by interquartile range (IQR) (the difference between 75th and 25th percentiles). Comparisons were drawn between groups using ANOVA, wherever appropriate. Level of significance was taken as P value <0.05.
Results
Subject characteristics are shown in Table 1. The age, pre-pregnancy body mass index, HbA1c levels and 2 hour blood glucose value after a 75 g glucose load were similar in the three groups. Table 2 describes the comparison of the glucose profile in different trimesters of pregnancy. The mean blood glucose (MBG) levels of non-pregnant women were significantly higher than pregnant women, and the values were higher in the third trimester compared to the second trimester. Day and night values of MBG in the second trimester were also lower than the respective values in the third trimester. Values during the second trimester were 20.2% lower and in the third trimester were 10.6% lower than the non-pregnant women.
Table 1.
Demographic profile of second and third trimesters of healthy pregnant women and non-pregnant women.
| Non-pregnant (N = 9) | Second trimester (N = 18) | Third trimester (N = 15) | |
|---|---|---|---|
| Age (years) | 24.8 ± 3.22 | 27.1 ± 3.92 | 26.2 ± 3.52 |
| Pre-pregnancy body mass index (kg/m2) | 20.7 ± 2.4 | 21.4 ± 4.01 | 22.06 ± 3.21 |
| Gestational age (weeks) | NA | 20.7 ± 4.02 | 32.5 ± 1.01 |
| Women in their first pregnancy (Primigravida) | 70% | 72% | 60% |
| 2 h 75 g GTT value (mg/dl) | 100.8 ± 13.06 | 101.8 ± 17.9 | 109.2 ± 17.8 |
| HbA1c (%) | 4.95 ± 0.38 | 4.28 ± 0.37 | 4.53 ± 0.42 |
GTT: glucose tolerance test.
Value are given as Mean ± SD.
Table 2.
Comparison of glucose profile of second and third trimesters of healthy pregnant women and non-pregnant women.
| CGM variables | Healthy non-pregnant women (N = 9) | Second trimester healthy pregnant women (N = 18) | Third trimester healthy pregnant women (N = 15) | F-value | P value |
|---|---|---|---|---|---|
| Total number of subject days | 100 | 205 | 147 | ||
| Total number of data points | 9600 | 19680 | 14112 | ||
| MBG (mg/dl) | 93.98 | 75.09 | 82.70 | 2838.662 | <0.001 |
| MBG day time (mg/dl) | 95.99 | 77.25 | 84.37 | 1999.763 | <0.001 |
| MBG night time (mg/dl) | 88.07 | 68.62 | 77.73 | 999.561 | <0.001 |
| Maximum day time glucose value (mg/dl) | 201 | 171 | 183 | ||
| Max night time glucose value (mg/dl) | 168 | 155 | 170 | ||
| Mean fasting glucose value around 6 a.m. (mg/dl) | 82.26 | 62.10 | 67.16 | 166.989 | <0.001 |
| Number of days maximum value reached 140–160 mg/dl | 31 (31%) | 17 (8.29%) | 37 (25.17%) | ||
| Number of days maximum value crossed 160 mg/dl | 22 (22%) | 2 (0.97%) | 17 (11.56%) | ||
| Breakfast | |||||
| Preprandial (average of previous three values) (mg/dl) | 86.18 | 64.50 | 68.57 | 88.379 | <0.001 |
| 1 h postprandial (mg/dl) | 100.83 | 82.50 | 88.30 | 92.851 | <0.001 |
| 2 h postprandial (mg/dl) | 96.54 | 77.87 | 87.86 | 99.917 | <0.001 |
| 3 h postprandial (mg/dl) | 92.07 | 75.84 | 83.31 | 87.060 | <0.001 |
| Peak value with 3 h | 116.51 | 98.48 | 111.69 | 26.933 | <0.001 |
| Time to peak (min) | 66 | 73 | 86 | 5.835 | 0.003 |
| Mean glucose between breakfast and lunch (mg/dl) | 92.56 | 76.02 | 84.27 | 43.051 | <0.001 |
| Lunch | |||||
| Preprandial (average of previous three values) (mg/dl) | 86.24 | 70.11 | 77.99 | 24.142 | <0.001 |
| 1 h postprandial (mg/dl) | 108.81 | 87.06 | 97.92 | 125.255 | <0.001 |
| 2 h postprandial (mg/dl) | 106.24 | 85.54 | 97.73 | 123.216 | <0.001 |
| 3 h postprandial (mg/dl) | 98.25 | 82.36 | 92.71 | 90.201 | <0.001 |
| Peak value within 3 h | 126.62 | 101.16 | 118.06 | 48.044 | <0.001 |
| Time to peak (min) | 66 | 71 | 80 | 3.376 | 0.035 |
| Mean glucose between lunch and dinner (mg/dl) | 101.58 | 79.29 | 88.33 | 54.362 | <0.001 |
| Dinner | |||||
| Preprandial (average of previous three values) (mg/dl) | 91.41 | 72.55 | 73.41 | 42.311 | <0.001 |
| 1 h postprandial (mg/dl) | 103.33 | 87.67 | 94.57 | 79.479 | <0.001 |
| 2 h postprandial (mg/dl) | 105.73 | 86.21 | 99.70 | 125.378 | <0.001 |
| 3 h postprandial (mg/dl) | 100.57 | 82.09 | 91.25 | 112.793 | <0.001 |
| Peak value with 3 h (mg/dl) | 120.15 | 101.68 | 116.72 | 32.484 | <0.001 |
| Time to peak (min) | 78 | 74 | 84 | 1.971 | 0.141 |
CGM: continuous glucose monitoring; MBG: mean blood glucose.
Values are mean. Time to peak is given in minutes and other variables in mg/dl; ANOVA is applied for comparing the groups. P value <.05 is significant. Time to peak is time from meal start to peak value within 3 h. Peak value is highest glucose value within 3 h of meal start time. Nocturnal value is the measure of the values between 0 and 6 a.m.; fasting value is the measure of the two values before and two values after 6 a.m.
Table 2 also describes the glucose measurements with regard to meals, overnight and fasting. Each meal was analysed for the first 3 hours for peak and average values. Further analysis was done to assess the difference between the three meals. It has been observed that during pregnancy it took 20 more minutes to reach peak levels after breakfast and 15 more minutes after lunch as compared to non-pregnant women, although there was no difference in the time to reach peak levels after dinner (Table 2). On average, the postprandial peak of glucose level occurred after 80--86 minutes and most of the time this level did not exceed 120 mg/dl during pregnancy (Table 2). Table 2 highlights the proportion of days when the values crossed 140 mg/dl. However, the excursions were primarily in the postprandial state and were short-lived.
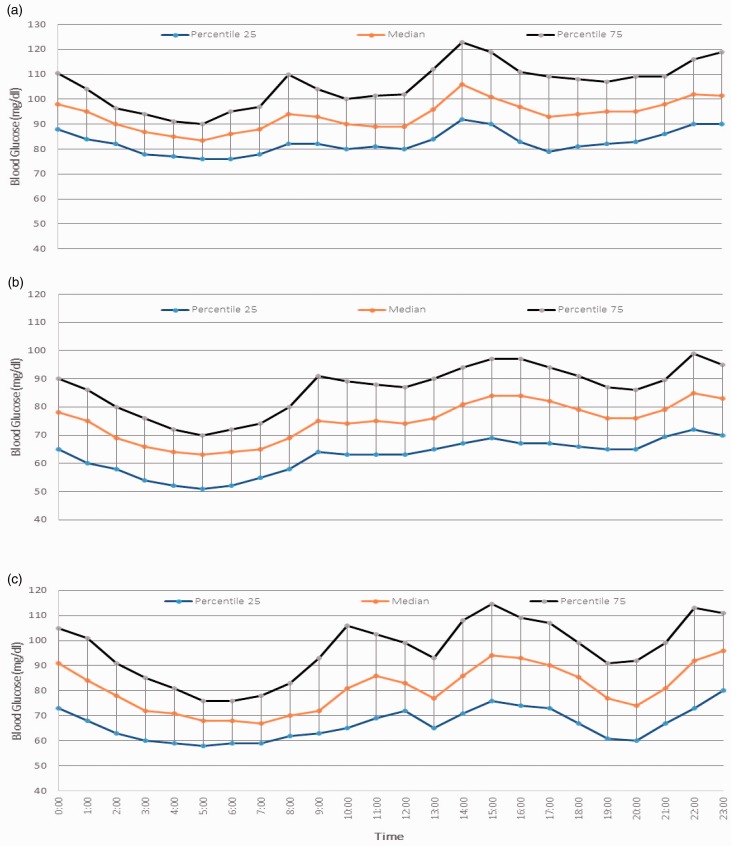
Figure 2 shows the compiled data in each group representing the 25th, 50th (median) and 75th frequency percentiles. The lowest blood sugar levels were recorded at around 5:00 a.m. followed by gradual rise in blood sugar levels in all the three groups. As evident from the graph there was an immediate rise in the MBG after the morning dip in non-pregnant women but during pregnancy the dipping in blood sugar levels was in hypoglycaemic range (≤70 mg/dl) and was sustained for 45 minutes to 1 hour.
Figure 2.
Percentile curves of blood glucose in the three groups: (a) non-pregnant (b) second trimester and (c) third trimester.
The interquartile range during the second trimester was 19–31 mg/dl, during the third trimester it was 17–41 mg/dl and in non-pregnant women it was 14–31 mg/dl. On comparing the combined observations of both the trimesters and non-pregnant women employing contrast in ANOVA, it has been observed that glycaemic variability (GV), as represented by the mean of IQR, was significantly higher in the third trimester as compared to non-pregnant and second trimester healthy pregnant females (p value <0.001, F test= 11.823).
Discussion
Glucose dynamics in pregnancy is altered and physiologic adaptation occurs throughout gestation to ensure adequate transfer of glucose to the fetus for proper development. The aim of the management of pregnancies with gestational diabetes is to maintain a normal glucose profile, as in a healthy pregnant female. However, there is a paucity of data regarding the detailed glucose profile in normal pregnancies. The recent availability of AGP monitoring equipment provides an opportunity to gain an insight into the trend of these changes. This study was conducted in the Asian Indian population, which is considered to be an ethnically susceptible population to diabetes and cardiovascular diseases, with the average height and weight less than that of the western population, but with greater visceral adiposity and insulin resistance. This is the first study of this type from this region.
In the current study the Diabetes in Pregnancy Study Group of India criteria was used for making a diagnosis of GDM, which states that all the antenatal women should be given 75 g glucose orally in 300 ml of water irrespective of the last meal and if the blood glucose value at 2 hours exceeds 140 mg/dl the women should be considered as having GDM. The above has been found to be more feasible in the Indian scenario, whereas the International Association of Diabetes in Pregnancy Study Group criteria, which is more stringent, uses 75 g glucose tolerance test with fasting, 1 and 2 hour values of 92, 180 and 153 mg/dl, respectively. If any single value is abnormal, the patient is labelled as having GDM.
Previous studies have evaluated 72 hours CGM systems during pregnancy to understand the glucose profile and as an educational tool in GDM.8,9 These studies compared the CGM glycaemic patterns in pregnant women with and without GDM, which corroborated well with self-monitoring of blood glucose values and gave better insight into periods of hypo- and hyperglycaemia. However, the 10 to 14 days of AGP data provided by the present study provide hourly as well as daily variations in glucose profile in the pregnant and the non-pregnant states.
The MBG level in pregnant women in the present study was found to be 15–20% lower than that of non-pregnant women, which is similar to other studies.10,11 The MBG levels in normal pregnancy are reported in various studies, albeit in the third trimester (83.7, 87.2 and 79.38 mg/dl)12,13 are comparable to the results in our study. Hernandez et al.14 in their meta-analysis also reported lower glucose concentrations in normal pregnancy compared to non-pregnant women. They report MBG and mean fasting and postprandial (1 and 2 hour) blood glucose values in pregnancy were 88 ± 10, 71 ± 8, 109 ± 13 and 99 ± 10 mg/dl, respectively. Fasting MBG value in pregnancy in our study was also comparable to other studies,12,15,16 the value being lower than non-pregnant women. Increased glucose utilization by the fetal–placental unit throughout the pregnancy and removing glucose from the maternal circulation contribute to this decline.17 Another reason suggested for the lowered fasting blood glucose levels is the dilutional effect caused by the increase in maternal blood volume.17,18 The lowered renal threshold for blood sugar excretion could also be one of the plausible factors which, despite the diabetogenic state of pregnancy, helps keep blood sugar values lower than the non-pregnant state.
This study has shown that there is a decrease in the MBG in the second trimester which gradually rises in the third trimester (Figure 2). The rise in blood sugar levels was also shown by Siegmund et al.13 who had compared the levels at 16, 22, 30 and 36 weeks of pregnancy, but no comparison was done with non-pregnant healthy women. It is important to note that MBG is significantly higher in the third trimester of pregnancy compared to second trimester however its clinical significance cannot be surmised at present.
The current study shows that morning dip in blood glucose levels occurs in both pregnant and non-pregnant women at around 5:00 a.m. but this dip is in hypoglycaemic range (≤70 mg/dl)19 and is sustained for 45 minutes to an hour in the pregnant females, although in the non-pregnant state it is very brief. This sustained hypoglycaemia can be due to the decreased sensitivity of body to the counter-regulatory hormones in pregnancy.20
The nocturnal glucose levels were found to be lower during pregnancy, with the lowest values in the second trimester. Nocturnal hypoglycaemia has been reported by other studies also.20 The mechanism of relatively lower glucose level during pregnancy could be lower area under the curve for 24 hour glucose.21 Another reason for the nocturnal hypoglycaemia could be due to increased insulin resistance causing increased transplacental transfer of glucose from the mother to the fetus.22 It has been documented that a mild fasting hypoglycaemia, postprandial hyperglycaemia and hyperinsulinaemia can occur in a normal pregnancy.23
In the present study it took 80 to 85 minutes to reach the peak value after meals which is comparable to other studies (82 ± 18 minutes).15 It was also demonstrated that hyperglycaemia after the peak level is reached can persist for around 2 hours compared to the non-pregnant women (Figure 2). This study demonstrated that there is tight blood glucose control during pregnancy, and the majority of the time blood glucose levels remained below 140 mg/dl even after food. The peak postprandial glucose level was 183 mg/dl, which supports the highest value (1 hour value 180 mg/dl) of glucose tolerance test given by most of the professional associations.24
One of the objectives of the study was to describe the blood glucose variation (GV) in different trimesters of pregnancy and compare with non-pregnant females. GV is important to assess as it is associated with oxidative stress, cell damage and side effects in the mother and the fetus.25 GV refers to swings in blood glucose levels that occur throughout the day, including hypoglycaemic periods and postprandial increases, as well as blood glucose fluctuations that occur at the same time on different days.26 GV in the current study shows that it is increased during pregnancy compared to non-pregnant women. Monnier and Colette27 proposed that the target level of GV should not be more than 40 mg/dl, which is in agreement with our results in which the highest figure for IQR was 41 mg/dl. GV is a physiological consequence of circadian rhythm of hormones involved in the control of glucose metabolism and also is a result of carbohydrate intake.26 A certain degree of GV is always observed even in a healthy individual but more importantly it is crucial to identify the limit beyond which it becomes pathological.
The limitation of the study was that AGP is a measurement of interstitial fluid glucose and needs to be translated as a marker of blood glucose with lag time. The lag of 5 to 10 minutes is maximum during the times of rapidly changing plasma glucose levels, e.g. after meals.28 The interstitial fluid sugar values are 8–12% higher than venous plasma glucose values. It was proposed to convert the values into blood glucose values, but other studies employing CGM systems had employed the term blood glucose itself, and hence to maintain uniformity, the term ‘blood glucose’ was used here. In this study we did not have simultaneous venous blood glucose values to compare to the CGM glucose readings. Another potential confounder was the physiological change in the interstitial fluid that occurs with pregnancy states; however, one study reported it to be irrelevant.29 Sensor accuracy may be questionable in the event of intake of pharmacological agents, such as acetaminophen, aspirin and vitamin C which can affect the readings. None of these substances were used by the subjects during this period of monitoring.30 This device has the advantage that it is inserted at the back of the arm, this differs to earlier three-day CGM monitors which are usually inserted on the abdomen which is a hindrance during pregnancy and is less socially acceptable.
It would indeed be very interesting to see the rates of common maternal blood glucose-related outcomes, including macrosomic babies, caesarean delivery and neonatal hypoglycaemia, but a larger sample size and a different study design would be required, with blood glucose measurements being performed in both trimesters. Further studies including pregnant women with diabetes would also be of use.
Conclusion
Employing the AGP monitor, 24 hour glycaemic excursions including fasting and postprandial excursions determined in healthy non-pregnant and pregnant (second and third trimesters) women indicate that MBG values as well as mean fasting and postprandial values during pregnancy are lower than non-pregnant adult females. Despite tight glycaemic control observed during pregnancy, the postprandial peak in pregnant women occurred at 80–85 minutes and there is sustained fasting hypoglycaemia for approx. 45 minutes. GV (as represented by the IQR) is significantly more in the third trimester as compared to second trimester healthy pregnant women and non-pregnant women.
Acknowledgements
We are thankful to Professor CP Gupta, Department of Financial Studies, South Campus University of Delhi who helped us in statistical analysis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We are thankful to Delhi Diabetic Forum for providing partial financial assistance in procuring the AGP sensors and supporting this study.
Ethical approval
The study is approved by Institutional ethics committee. Informed written consent was taken from all the study participants.
Guarantor
AN.
Contributorship
AN has contributed in the design, conception, conduct, analysis and writing of the article.NV has contributed in the conduct, analysis and writing of the article.SS has contributed in the conduct and analysis of the article.YPM has contributed in the design and analysis of the article.AP has contributed in the design, analysis and writing of the article.
References
- 1.Cousins L, Rigg L, Hollingsworth D, et al. The 24-hour excursion and diurnal rhythm of glucose, insulin, and C-peptide in normal pregnancy. Am J Obstet Gynecol 1980; 136: 483–488. [DOI] [PubMed] [Google Scholar]
- 2.Phelps RL, Metzger BE, Freinkel N. Carbohydrate metabolism in pregnancy: diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am J Obstet Gynecol 1981; 140: 730–736. [PubMed] [Google Scholar]
- 3.Seshiah V, Sahay BK, Das AK, et al. Gestational diabetes mellitus – Indian guidelines. J Indian Med Assoc 2009; 107: 799–802. [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration (2018) Premarket Approval (PMA). Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma_template.cfm?id=p150021 (accessed 23 July 2017).
- 5.Assardo V (2016) Abbott Receives FDA Approval for the Freestyle Libre Pro System, A Revolutionary Diabetes Sensing Technology for Healthcare Professionals to use with Their Patients. Available at: http://abbott.mediaroom.com/2016-09-28-Abbott-Receives-FDA-Approval-for-the-FreeStyle-Libre-Pro-System-a-Revolutionary-Diabetes-Sensing-Technology-for-Healthcare-Professionals-to-Use-with-Their-Patients (accessed 20 July 2017).
- 6.Pustovalova O (2016) Difference between Freestyle Libre and Libre Pro. Available at: http://diabetesviews.com/2016/11/difference-between-freestyle-libre-and-libre-pro/ (accessed 19 July 2017).
- 7.Carreiro MP, Lauria MW, Naves GN, et al. Seventy two-hour glucose monitoring profiles in mild gestational diabetes mellitus: differences from healthy pregnancies and influence of diet counseling. Eur J Endocrinol 2016; 175: 201–209. [DOI] [PubMed] [Google Scholar]
- 8.Alfadhli E, Osman E, Basri T. Use of a real time continuous glucose monitoring system as an educational tool for patients with gestational diabetes. Diabetol Metab Syndr 2016; 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polsky S, Garcetti R. CGM, pregnancy and remote monitoring. Diabetes Technol Ther 2017; 19: S-49–S-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yogev Y, Ben-Haroush A, Chen R, et al. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol 2004; 191: 949–953. [DOI] [PubMed] [Google Scholar]
- 11.Murphy HR, Rayman G, Duffeield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomized clinical trial. BMJ 2008; 337: a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yogev Y, Ben-Haroush A, Chen R, et al. Continuous glucose monitoring for treatment adjustment in diabetic pregnancies – a pilot study. Diabet Med 2003; 20: 558–562. [DOI] [PubMed] [Google Scholar]
- 13.Siegmund T, Rad NT, Ritterath C, et al. Longitudinal changes in the continuous glucose profile measured by CGMS in healthy pregnant women and determination of cut off values. EJOG 2008; 139: 46–52. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez TL, Friedman JE, Van Pelt RE, et al. Pattern of glycaemia in normal pregnancy: should the current therapeutic targets be challenged. Diabetes Care 2011; 34: 1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerssen A, de Valk HW, Visser GH. The continuous glucose monitoring system during pregnancy of women with type 1 diabetes mellitus: accuracy assessment. Diabetes Technol Ther 2004; 6: 645–651. [DOI] [PubMed] [Google Scholar]
- 16.Secher AL, Stage E, Ringholm L, et al. Real-time continuous glucose monitoring as a tool to prevent severe hypoglycaemia in selected pregnant women with Type 1 diabetes – an observational study. Diabet Med 2014; 31: 352–356. [DOI] [PubMed] [Google Scholar]
- 17.Di Cianni G, Miccoli R, Volpe L, et al. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab Res Rev 2003; 19: 259–227. [DOI] [PubMed] [Google Scholar]
- 18.Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med 2009; 14: 66–71. [DOI] [PubMed] [Google Scholar]
- 19.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes association and the endocrine society. Diab Care 2013; 36: 1384--1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik D, Shyamsundef AH, Mruthyunjaya MD, et al. Masked hypoglycaemia in pregnancy. J Diabetes 2016; 9: 778–786. [DOI] [PubMed] [Google Scholar]
- 21.Cousin L, Rigg L, Hollingsworth D, et al. The 24 hour excursion and diurnal rhythm of glucose, insulin and C peptide in normal pregnancy. Am J Obstet Gynaecol 1980; 136: 483–488. [DOI] [PubMed] [Google Scholar]
- 22.Leonce J, Brockton N, Robinson S, et al. Glucose production in human placenta. Placenta 2006; 27: S103–S108. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham GF, Leveno KJ, Bloom SL, et al. Maternal physiology. In: Cunningham GF, Leveno KJ, Bloom SL (eds) William obstetrics 23rd ed. McGraw Hill, pp.107–135.
- 24.Duran A, Sáenz S, Torrejón MJ, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos gestational diabetes study. Diabetes Care 2014; 37: 2442–2450. [DOI] [PubMed] [Google Scholar]
- 25.Buhling KJ, Winkel T, Wolf C, et al. Optimal timing for blood glucose measurement in pregnant women with diabetes and non-diabetic pregnant population evaluated by continuous glucose monitoring system (CGMS). J Perinat Med 2005; 33: 125–131. [DOI] [PubMed] [Google Scholar]
- 26.Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J 2015; 39: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monnier L, Colette C. Glycemic variability. Should we and can we prevent it? Diabetes Care 2008; 31: S150–S154. [DOI] [PubMed] [Google Scholar]
- 28.Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther 2009; 11: S11–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy HR, Elleri D, Allen JM, et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011; 34: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther 2016; 18: S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]