Abstract
Objective:
Delayed puberty is a common condition, and typical management includes “watchful waiting” and/or sex-steroid therapy. We sought to characterize treatment practices and to assess provider comfort with the management of delayed puberty in girls and boys.
Methods:
A national survey of pediatric endocrine providers assessed definitions of delayed puberty, practices around sex-steroid therapy, reasons for treatment, and comfort in managing delayed puberty in girls and boys.
Results:
Of 184 respondents (12% participation rate), 64% and 71% used the traditional age cutoffs for defining delayed puberty of 13 years for girls and 14 years for boys, respectively. Nearly half (45%) of providers would treat boys relatively earlier than girls, compared to 18% who would treat girls relatively earlier (p<0.0001). Providers were more likely to cite bone density as a reason to treat girls and alleviating patient and parental distress, accelerating growth, and “jump starting” puberty as reasons to treat boys. Greater experience in endocrine practice was associated with greater comfort managing delayed puberty in both boys and girls. Approximately 80% of providers agreed that clinical guidelines are needed for management of delayed puberty.
Conclusion:
There is a high degree of variability in the clinical management of delayed puberty, and our results suggest that providers are more hesitant to treat girls compared to boys and have different reasons for treating each. It remains to be determined if these discrepancies in treatment are justified by biological differences between girls and boys or represent non-evidence-based disparities in care.
Keywords: Delayed puberty, Clinical practice, Sex steroids, Survey
Introduction
Delayed puberty is a common condition defined as the absence of secondary sexual characteristics at an age two or more standard deviations beyond the population mean (1). Based on historical data (2, 3), delayed puberty is frequently defined as an absence of breast budding by 13 years for girls and absence of testicular growth by 14 years for boys. However, recent trends toward earlier pubertal timing in the U.S. (4–8) and other developed countries (9–13) and increasingly recognized variation in pubertal timing between different racial and ethnic groups (14, 15) challenge these traditional age cutoffs. It is unclear whether these trends have changed the age cutoffs used by pediatricians and endocrinologists to define delayed puberty.
Initial evaluation for delayed puberty includes excluding primary gonadal insufficiency and functional etiologies of delayed puberty, such as chronic illness or undernutrition (1). In the absence of an identifiable cause of delayed puberty, constitutional delay or self-limited delayed puberty is presumed. Management approaches for delayed puberty can be highly variable and often begin with a “watchful waiting” approach (16–18). However, a delay in pubertal onset can lead to psychosocial distress from concerns regarding height and physical appearance (16, 17, 19) and potential long-term health effects (20, 21). Induction of puberty with sex steroids is a well-established and safe practice to mitigate these concerns by advancing growth and sexual maturation (22–24). Based primarily on anecdotal experience, sex steroids have also been suggested to accelerate or “jump start” endogenous pubertal onset (25). There are no clinical guidelines for management of delayed puberty, and it is unknown whether continued observation (“watchful waiting”) or treatment with sex-steroids is better, especially in girls. Current practices, including when to initiate treatment, are based on expert clinical opinion and provider preferences (17).
In this study, we sought to characterize current practices of pediatric endocrine providers in the definition and treatment of delayed puberty in girls and boys. We also assessed providers’ comfort level in the management of delayed puberty.
Patients and Methods
Survey design and administration
We developed a survey to assess the comfort level and practice variability in managing self-limited delayed puberty among pediatric endocrine providers (Supplemental Material). This survey study was considered exempt from review by the Boston Children’s Hospital (BCH) Institutional Review Board.
The 29-item survey targeted pediatric endocrine providers in the U.S. and Canada who have cared for children with delayed puberty. Two parallel clinical vignettes were provided, one girl and one boy, to illustrate typical clinical scenarios of delayed puberty. Providers were asked 8 questions about their clinical practice, including the minimum age at which providers would define delayed puberty for girls and for boys and the minimum age at which providers would initiate sex-steroid treatment. This was followed by 3 questions for each sex addressing clinical opinions about management of self-limited delayed puberty. One question assessed comfort in the management of delayed puberty. The second assessed the belief that treatment with sex-steroids can accelerate the onset of puberty using a five-point Likert scale ranging from “strongly disagree” to “strongly agree.” The third question assessed the need for clinical guidelines for management of delayed puberty. The last section consisted of 15 questions on provider characteristics.
Prior to distribution, the survey was piloted in a group of thirteen pediatric endocrine providers at BCH to ensure face validity, and the survey was revised based on their feedback as well as feedback from the Pediatric Endocrine Society (PES) Research Affairs Committee. PES members were then invited by EMail to participate in the survey. Results were collected anonymously throughout the study period, and no incentive was provided for survey completion. A data quality check did not identify any duplicate responses by a single provider.
Statistical analyses
Statistical analyses were performed using SAS software (version 9.2; SAS Institute, Inc, Cary, NC). Provider characteristics and survey data are presented as mean ± SD (standard deviation) or percentages. The survey assessed providers’ use of a variety of treatment options (for example, conjugated estrogens, oral estradiol, estradiol patch, oral contraceptive pills, and oxandrolone for girls and testosterone and oxandrolone for boys); because few respondents used treatments other than testosterone for boys (7 providers) and estradiol for girls (2 providers), any form of hormonal treatment was considered “treat” and no treatment or watchful waiting was considered “not treat.”
Survey responses were used to determine the provider’s definition of (1) the age cutoff for considering puberty to be delayed in boys and in girls; (2) the youngest age at which the provider would consider treatment of girls with an estrogen and boys with an androgen. A “difference between cutoffs” variable was defined as the difference between the age cutoff for considering treatment and the age cutoff for defining delayed puberty.
Independent-sample Student’s t-tests and one-way analyses of variance were used to assess differences between provider groups by gender, number of years of practice, and clinical practice type for continuous variables, and the Fisher exact test was used to assess differences for categorical variables. Pearson correlation was used to assess associations between continuous variables. The McNemar’s test for paired dichotomous variables was used to assess within-provider differences in management of girls versus boys. Three hierarchical linear regression models were used to assess the joint influences of provider gender, number of years of practice, and clinical practice type on clinical opinion statements. In regression and correlation analyses, we treated the five-point Likert opinion scales as quasi-continuous variables. We applied alternative methods (Spearman correlation, robust regression) to confirm that the results were not sensitive to the distributional assumptions for linear correlation and regression analysis. All tests were two-sided, and a p-value less than 0.05 was considered statistically significant.
Results
Respondent characteristics
A total of 184 surveys were completed by providers, representing a response rate of 12% (Fig. 1). Characteristics of survey respondents are summarized in Table 1. The majority of respondents were female (59%), and all major geographic regions of the US were represented. Most providers practiced in university-affiliated hospital practices (78%), and providers represented all experience levels (length in medical practice 0 to > 20 years).
Fig. 1.
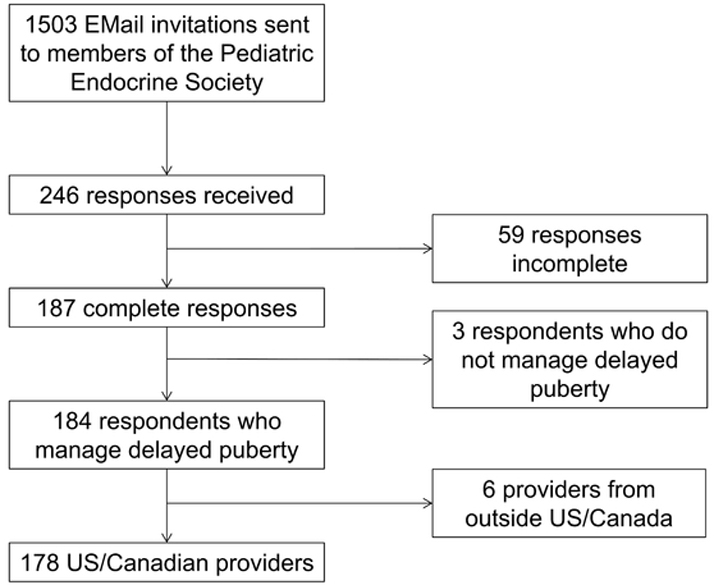
Recruitment of eligible providers. Providers from outside the US and Canada were excluded due to potential differences in availability of medications and formulations.
Table 1.
Provider Characteristics
| n=178 | n (%) |
|---|---|
| Gender (n=175) | |
| Male | 71 (41) |
| Female | 104 (59) |
| Race and Ethnicity (n=161) | |
| Asian | 20 (12) |
| Black, African American | |
| Hispanic | 0 (0) |
| Non-Hispanic | 3 (2) |
| Native Hawaiian, Pacific Islander | 1 (1) |
| White, Caucasian | |
| Hispanic | 5 (3) |
| Non-Hispanic | 132 (82) |
| Region of Medical Practice (n=178) | |
| United States | |
| Northeast | 36 (20) |
| South | 51 (29) |
| Midwest | 37 (21) |
| West | 39 (22) |
| Canada | 15 (8) |
| Medical Practice Type (n=175) | |
| Private Practice | 16 (9) |
| Non-Academic Practice | 14 (8) |
| University-affiliated Practice | 137 (78) |
| Other* | 8 (5) |
| Practice Size (n=178) | |
| Single Provider | 12 (7) |
| Small Group Practice (2–5 physicians) | 55 (31) |
| Medium Group Practice (6–10 physicians) | 66 (37) |
| Large Group Practice (≥ 10 physicians) | 45 (25) |
| Practice Setting (n=178) | |
| Urban | 132 (74) |
| Suburban | 40 (23) |
| Rural | 6 (3) |
| Length of Pediatric Endocrine Practice (n=176) | |
| 0–5 years | 38 (22) |
| 6–10 years | 37 (21) |
| 11–15 years | 27 (15) |
| 16–20 years | 13 (7) |
| > 20 years | 61 (35) |
Responses include “Military Medical Center,” “Managed Care Organization,” “Nonprofit Children’s Hospital,” and “Federally Qualified Health Center”
Age cutoffs for defining delayed puberty and for treatment
Mirroring historical clinical conventions, 64% of providers used an age cutoff of 13 years for defining delayed puberty in girls, and 71% used an age cutoff of 14 years for boys. The remaining providers were approximately equally split between using earlier or later age cutoffs (Fig. 2); for girls, 18% of providers specified a cutoff below 13 years and 18% specified a cutoff above 13 years, whereas for boys, 14% of providers specified a cutoff below 14 years and 15% specified a cutoff above 14 years. There were no associations between the age cutoff used to define delayed puberty and provider characteristics, such as experience level.
Fig. 2.
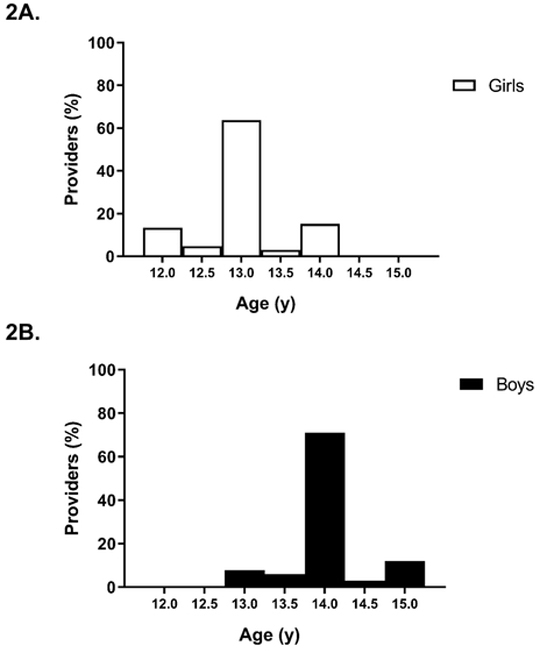
Age cutoffs used by providers to define delayed puberty. Providers were provided a range of ages and asked what they would consider the minimum chronological age at which a child can be classified as having delayed puberty. Fig. 2A shows provider responses for girls, and 2B for boys.
Providers reported that they would initiate treatment with sex steroids at a mean ± SD age of 14.3 ± 0.7 y in boys and 13.6 ± 0.9 y in girls (Fig. 3). For boys, 55% of providers would start treatment by age 14 years, and 99% would start treatment by 16 years; for girls, 42% would start treatment by age 13 years and 94% by 15 years. Because the age at which a provider would initiate sex-steroid treatment is dependent on the age that the provider uses to define delayed puberty, we assessed the difference between these two age cutoffs used by each provider.
Fig. 3.
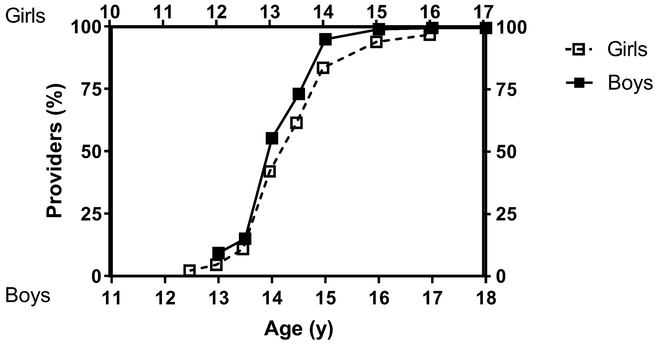
Ages at which providers would treat constitutional delay with sex steroids. Providers were given a range of ages and asked whether they would typically treat a child at each age with sex steroids. Upper x-axis, white squares, and dashed line represent girls, and lower x-axis, black squares, and solid line represent boys.
Difference between treatment and diagnosis age cutoffs
Because the age at which a provider would initiate treatment for delayed puberty could be greater or less than the age cutoff for defining delayed puberty, we examined the difference between these age cutoffs. The distribution of the difference between providers’ cutoffs for considering treatment and for defining delayed puberty (“difference between cutoffs”) is shown in Fig. 4A.
Fig. 4.
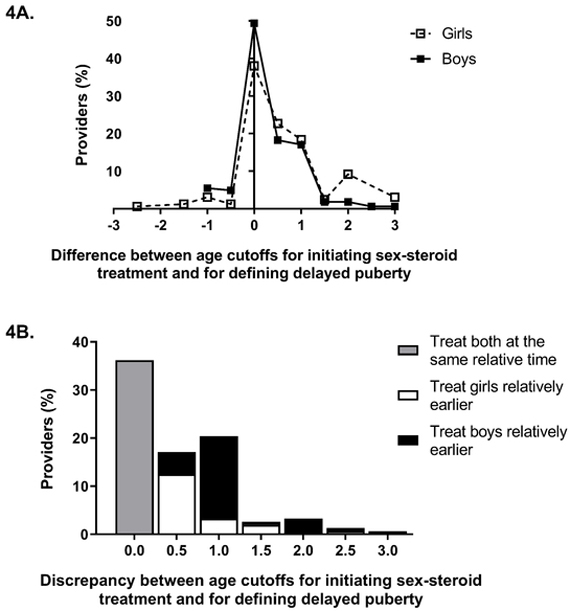
Difference between age cutoffs for initiating sex-steroid treatment and for defining delayed puberty. Fig. 4A shows the difference for girls and boys; white squares with dotted line represent girls, and black squares with solid line represent boys. Both distributions extend slightly into the negative region, illustrating that some providers specified an earlier age cutoff for treatment than for diagnosis. Fig. 4B shows the difference between the age cutoff for considering treatment and the age cutoff for defining delayed puberty in girls compared to boys. The gray bar represents providers who would treat girls and boys at the same relative time (total 36%). White bars represent providers who would treat girls relatively earlier compared to boys (18%), and black bars represent the significantly greater number of providers who would treat boys relatively earlier (45%, p<0.0001 by McNemar test).
Overall, providers tend to treat girls relatively later than boys (Fig. 4A). For boys, 60% of providers would start treatment by the cutoff age they used to define delayed puberty (that is, the “difference between cutoffs” was no greater than zero years), and 95% would start treatment by 1 year or less after the age cutoff (that is, the “difference between cutoffs” was no greater than 1 year). In contrast, for girls only 44% of providers would start treatment by the age cutoff used to defined delayed puberty and 85% by 1 year or less after the age cutoff. While 36% of providers would consider treating both boys and girls at the same age relative to cutoffs, a significantly greater number of providers (45%) would treat boys relatively sooner than girls (that is, had a “difference between cutoffs” that was smaller for boys than for girls), compared to only 18% who would treat girls relatively sooner than boys (p<0.0001 by the McNemar test, Fig. 4B).
Associations with provider characteristics
Providers with more experience tended to use lower age cutoffs for considering treatment with sex steroids for boys (r =−0.17, p=0.03), but there was no such relationship for girls. There was no association detected between other provider characteristics and age cutoffs for sex-steroid treatment or for the difference between the age cutoffs for treatment and diagnosis of delayed puberty.
Reasons to initiate treatment
Reasons to initiate sex-steroid therapy in boys and girls are summarized in Fig. 5. Though the majority of providers would initiate treatment in both boys and girls to relieve psychosocial distress of the patient and accelerate onset of endogenous puberty, providers were slightly more likely to cite these as reasons to treat for boys than for girls (p=0.005 and <0.0001 by the McNemar test, respectively). Conversely, providers were more likely to cite “increase bone-mineral density” as a reason to treat girls than to treat boys (p<0.0001 by the McNemar test, Fig. 5). There were no associations detected between provider characteristics and reasons to treat in boys or girls.
Fig. 5.
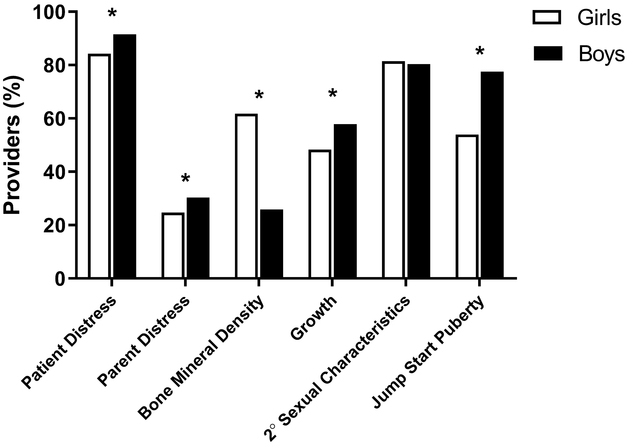
Reasons for providers to treat constitutional delay with sex steroids. Providers chose from a list of reasons for starting sex-steroid therapy for self-limited delayed puberty in boys and in girls. Reasons to initiate medical management of delayed puberty were compared between boys and girls by McNemar’s test. The white bars represent girls and the black bars boys. Asterisks denote statistically significant within-provider differences (p<0.05) between reasons given for girls and for boys. 2°: Secondary
Providers who cited “increase muscle mass” as a reason to treat would treat boys earlier relative to their age cutoffs for diagnosis of delayed puberty compared to those who did not cite this as a reason to treat. Specifically, an independent-sample t-test indicated that the “difference between cutoffs” was lower by 0.28 years (95% CI 0.03–0.52 years, p=0.03). Similarly, providers who cited “accelerate growth” as a reason to treat would also treat boys earlier relative to their age cutoffs for diagnosis of delayed puberty (earlier by 0.21 years [95% CI 0.01–0.41 years, p=0.04]). There was no such association for girls.
Comfort level in management of delayed puberty
Over 80% of providers reported being either very comfortable or comfortable managing delayed puberty in both boys and girls, and providers with more experience reported greater comfort (Supplemental Table 1). Male providers reported greater comfort managing delayed puberty than female providers in a bivariate regression model (Supplemental Table 1). This appeared to be mediated by greater experience, as the association between provider gender and comfort managing delayed puberty was no longer significant after adjusting for experience level in a covariate-adjusted regression model (Supplemental Table 2). There was no difference detected between other provider characteristics and comfort level with management.
Providers who were more comfortable managing delayed puberty would treat girls earlier relative to the age cutoff for diagnosis of delayed puberty (r=−0.20, p=0.01), but this relationship was not seen for boys (r=−0.13, p=0.1). Similar results held when the analysis was restricted to providers of one gender.
Opinion about whether sex-steroids “jump start” puberty
We assessed whether providers believe that sex-steroid therapy can accelerate or “jump start” endogenous pubertal onset in children with delayed puberty. Of 176 providers, 146 (83%) agreed or strongly agreed that treatment with sex steroids can accelerate the onset of puberty in boys, but only 99 (56%) agreed or strongly agreed with the statement in girls. The majority of providers (125, 71%) gave the same response regarding boys and girls (97 positive, 28 negative). Of the 51 providers responding differently for boys than for girls, a significantly greater number agreed that sex-steroids can accelerate the onset of puberty for boys but not for girls than the opposite (49 agree for boys, not for girls vs 2 agree for girls, not for boys, p<0.0001 by the McNemar test). Male providers were more likely than female providers to agree that sex-steroids can accelerate the onset of puberty in girls, but this difference was not seen for boys in a bivariate regression model (Supplemental Table 1). The association seen in girls was partially attenuated after adjusting for experience level in a covariate-adjusted regression model, suggesting that experience level mediates the relationship between male provider gender and belief in jump-starting, at least in part (Supplemental Table 2). There was no difference detected between other provider characteristics and opinions on whether sex steroids can accelerate the onset of puberty (Supplemental Table 1).
Providers who agreed more strongly that sex steroids can accelerate onset of puberty indicated they would treat girls earlier relative to the age cutoff for diagnosis of delayed puberty (r=−0.19, p=0.02), but this relationship was absent for boys (r=0.01, p=0.90). Similar results held when the analysis was restricted to providers of one gender.
Opinions concerning need for clinical guidelines
Approximately 80% of providers agreed or strongly agreed that clinical guidelines for the management of delayed puberty are needed for boys (79%) and girls (81%). More experienced providers were less likely to agree with the need for clinical guidelines in both boys and girls (Supplemental Table 1). Female providers were more likely to agree with the need for clinical guidelines in boys, but not in girls in a bivariate regression model (Supplemental Table 1); however, this relationship appeared to be mediated by experience level, as the association between female provider gender and agreement with need for clinical guidelines was no longer seen after adjusting for experience level in a covariate-adjusted regression model (Supplemental Table 2). There was no difference detected between the other provider characteristics and opinion about need for clinical guidelines.
Discussion
We assessed the management practices of self-limited delayed puberty in boys and girls among pediatric endocrine providers using an electronic survey. Although most providers used the traditional age cutoffs for defining delayed puberty of 13 years for girls and 14 years for boys, about one-third of providers used either older or younger ages as cutoffs. Even after adjusting for differences in age cutoffs used to define delayed puberty, our results indicate that providers are more hesitant to treat girls with sex steroids compared to boys. Furthermore, providers reported different reasons for treating girls versus boys. Greater experience was associated with greater comfort managing delayed puberty, and the majority of providers agreed that clinical guidelines are needed for management of delayed puberty.
Discrepancies in treatment between boys and girls could relate to the greater proportion of boys presenting for delayed puberty compared to girls (which likely reflects referral bias)(26–29), resulting in greater experience and comfort with managing boys compared to girls. In addition, the hesitancy to treat girls versus boys and the different reasons for treating each revealed in our study may reflect differences in providers’ internalization of patient and family preferences between the sexes. For example, boys may be more distressed or perceived to be more distressed about delayed puberty than girls due to concerns about body image, self-esteem, and/or socialization with peers(30), and thus, more eager to start sex-steroid therapy. Indeed, providers were more likely to cite psychosocial concerns as reasons to treat boys versus girls. However, the clinical vignettes in our survey provided parallel scenarios of delayed puberty in girls and boys to attempt to standardize any potential psychosocial concerns.
Variability in management between boys and girls may also be due to differences in etiologies of delayed puberty among boys and girls. Although our survey questions specifically inquired about delayed puberty due to presumed constitutional delay, it is possible that providers’ answers were influenced by the fact that functional hypogonadotropic hypogonadism can be difficult to distinguish from self-limited delayed puberty in girls at initial presentation (1, 25). Functional hypogonadotropic hypogonadism occurs secondary to physiological and/or psychological stress (31), and for children presenting for delayed puberty, functional hypogonadotropic hypogonadism is more common in girls than boys (32). Although sex steroids may “jump start” self-limited delayed puberty, sex steroids would not be expected to induce endogenous reproductive endocrine function in children with functional hypogonadotropic hypogonadism (25). As a result, a “watch and wait” approach may be deemed to be more appropriate initial management in girls (32).
Not surprisingly, greater length in medical practice was associated with greater comfort with management of self-limited delayed puberty. Greater comfort with management was in turn associated with less time between the age cutoffs for initiating treatment and defining delayed puberty. While over 80% of providers reporting being comfortable or very comfortable managing delayed puberty, an approximately equal percentage still agreed or strongly agreed that clinical guidelines are needed for management of delayed puberty.
Strengths of our study include a broad geographic distribution of pediatric endocrine providers across the U.S. and Canada with varied length of medical practices. Limitations of our study include the relatively low survey response rate of 12%; however, this is comparable to response rates in recent physician electronic surveys (33, 34), particularly those targeting sub-specialists (35, 36). In addition, it is unknown if survey respondents differ from the general population of practicing endocrinologists. Notably, a high proportion of responders practice at university-affiliated hospitals; while this likely reflects practice locations for most pediatric endocrine providers, this may limit generalizability of our findings to community practices. Furthermore, the sample may not be adequately powered to detect differences in management practices of delayed puberty by practice type, setting, or geographic region. Our survey also did not explore providers’ reasons not to treat with sex-steroids or reasons for comfort in management of delayed puberty.
Conclusion
Our understanding of delayed puberty is an evolving entity, and ongoing studies are underway to decipher the genetic and physiological underpinnings of the condition and to develop methods to optimize diagnosis. For now, there is no clear “right” or “wrong” approach to evaluation and management of delayed puberty, and clinical management decisions are based on providers’ opinions and practice patterns and the preferences of the patient and family. Emerging evidence suggests potential short- and long-term adverse consequences of delayed puberty, and more data on the effects of sex-steroid treatment and timing of treatment are needed to inform whether and when to treat (20, 21).
Given the incomplete understanding of delayed puberty, variability in clinical practice can be expected. However, our results show a notable discrepancy between management of girls versus boys. Even though we surveyed only pediatric endocrine providers, there is evidence that these are pervasive attitudes. Referral bias in delayed puberty suggests that these discrepancies exist even prior to evaluation by a pediatric endocrinologist and may stem from differences in perceptions and attitudes of the patient, family, and/or primary care providers (26–29). It remains to be determined if these discrepancies in treatment are justified by biological differences between girls and boys or represent bias and non-evidence-based disparities in care. Further studies, such as analysis of large longitudinal cohorts and electronic health record databases and potentially clinical trials, are needed to investigate the impact of delayed puberty, to determine the possible modifying role of sex-steroid therapy, and potentially to inform future clinical practice guidelines.
Supplementary Material
References
- 1.Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366(5):443–53. [DOI] [PubMed] [Google Scholar]
- 2.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51(3):170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317–29. [DOI] [PubMed] [Google Scholar]
- 4.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505–12. [DOI] [PubMed] [Google Scholar]
- 5.Anderson SE, Must A. Interpreting the continued decline in the average age at menarche: results from two nationally representative surveys of U.S. girls studied 10 years apart. J Pediatr. 2005;147(6):753–60. [DOI] [PubMed] [Google Scholar]
- 6.Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9½ and 15½ years. Arch Pediatr Adolesc Med. 2010;164(2):166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman-Giddens ME, Steffes J, Harris D, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130(5):e1058–68. [DOI] [PubMed] [Google Scholar]
- 8.Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YX, Wikland KA, Karlberg J. New reference for the age at childhood onset of growth and secular trend in the timing of puberty in Swedish. Acta Paediatr. 2000;89(6):637–43. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen K, Aksglaede L, Petersen JH, et al. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metab. 2010;95(1):263–70. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JR. A secular trend toward earlier male sexual maturity: evidence from shifting ages of male young adult mortality. PLoS One. 2011;6(8):e14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma HM, Chen SK, Chen RM, et al. Pubertal development timing in urban Chinese boys. Int J Androl. 2011;34(5 Pt 2):e435–45. [DOI] [PubMed] [Google Scholar]
- 13.Monteilh C, Kieszak S, Flanders WD, et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2011;25(1):75–87. [DOI] [PubMed] [Google Scholar]
- 14.Sun SS, Schubert CM, Chumlea WC, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110(5):911–9. [DOI] [PubMed] [Google Scholar]
- 15.Wu T, Mendola P, Buck GM. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2002;110(4):752–7. [DOI] [PubMed] [Google Scholar]
- 16.Dunkel L, Quinton R. Transition in endocrinology: induction of puberty. Eur J Endocrinol. 2014;170(6):R229–39. [DOI] [PubMed] [Google Scholar]
- 17.Wei C, Crowne EC. Recent advances in the understanding and management of delayed puberty. Arch Dis Child. 2016;101(5):481–8. [DOI] [PubMed] [Google Scholar]
- 18.Lucas-Herald AK, Mason E, Beaumont P, et al. Single-Centre Experience of Testosterone Therapy for Boys with Hypogonadism. Horm Res Paediatr. 2018;90(2):123–7. [DOI] [PubMed] [Google Scholar]
- 19.Trotman GE. Delayed puberty in the female patient. Curr Opin Obstet Gynecol. 2016;28(5):366–72. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Chan YM. Adult Consequences of Self-Limited Delayed Puberty. Pediatrics. 2017;139(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan YM, Feld A, Lewis EJ. Effects of the Timing of Sex-Steroid Exposure in Adolescence on Adult Health Outcomes. J Clin Endocrinol Metab. 2019. June 13. pii: jc.2019–00569. doi: 10.1210/jc.2019-00569. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld RG, Northcraft GB, Hintz RL. A prospective, randomized study of testosterone treatment of constitutional delay of growth and development in male adolescents. Pediatrics. 1982;69(6):681–7. [PubMed] [Google Scholar]
- 23.Richman RA, Kirsch LR. Testosterone treatment in adolescent boys with constitutional delay in growth and development. N Engl J Med. 1988;319(24):1563–7. [DOI] [PubMed] [Google Scholar]
- 24.Soliman AT, Khadir MM, Asfour M. Testosterone treatment in adolescent boys with constitutional delay of growth and development. Metabolism. 1995;44(8):1013–5. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer AA, Phan-Hug F, Hauschild M, et al. TRANSITION IN ENDOCRINOLOGY: Hypogonadism in adolescence. Eur J Endocrinol. 2015;173(1):R15–24. [DOI] [PubMed] [Google Scholar]
- 26.Crowne EC, Shalet SM, Wallace WH, et al. Final height in girls with untreated constitutional delay in growth and puberty. Eur J Pediatr. 1991;150(10):708–12. [DOI] [PubMed] [Google Scholar]
- 27.Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87(4):1613–20. [DOI] [PubMed] [Google Scholar]
- 28.Sedlmeyer IL, Hirschhorn JN, Palmert MR. Pedigree analysis of constitutional delay of growth and maturation: determination of familial aggregation and inheritance patterns. J Clin Endocrinol Metab. 2002;87(12):5581–6. [DOI] [PubMed] [Google Scholar]
- 29.Wehkalampi K, Widen E, Laine T, et al. Patterns of inheritance of constitutional delay of growth and puberty in families of adolescent girls and boys referred to specialist pediatric care. J Clin Endocrinol Metab. 2008;93(3):723–8. [DOI] [PubMed] [Google Scholar]
- 30.Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: interacting contributions of puberty and peer stress. Dev Psychopathol. 2009;21(2):593–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon CM, Ackerman KE, Berga SL, et al. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(5):1413–39. [DOI] [PubMed] [Google Scholar]
- 32.Varimo T, Miettinen PJ, Kansakoski J, et al. Congenital hypogonadotropic hypogonadism, functional hypogonadotropism or constitutional delay of growth and puberty? An analysis of a large patient series from a single tertiary center. Hum Reprod. 2017;32(1):147–53. [DOI] [PubMed] [Google Scholar]
- 33.Cook DA, Wittich CM, Daniels WL, et al. Incentive and Reminder Strategies to Improve Response Rate for Internet-Based Physician Surveys: A Randomized Experiment. J Med Internet Res. 2016;18(9):e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marlin R, Kadakia A, Ethridge B, et al. Physician Attitudes Toward Homosexuality and HIV: The PATHH-III Survey. LGBT Health. 2018;5(7):431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glenn D, Ocegueda S, Nazareth M, et al. The global pediatric nephrology workforce: a survey of the International Pediatric Nephrology Association. BMC Nephrol. 2016;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernier R, Raj SR, Tran D, et al. Assessing physician knowledge regarding indications for a primary prevention implantable defibrillator and potential barriers for referral. J Cardiovasc Electrophysiol. 2017;28(11):1334–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


