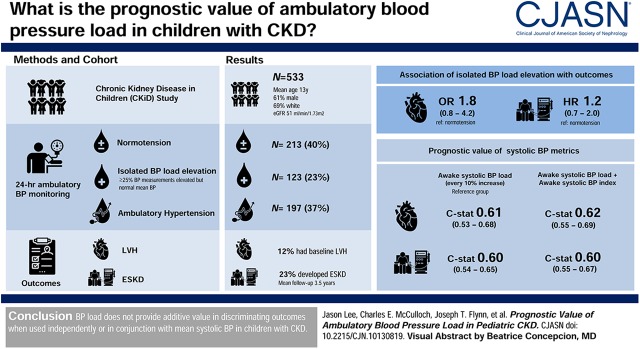
Visual Abstract
Keywords: hypertension, left ventricular hypertrophy, end-stage renal disease, ambulatory blood pressure monitoring, pediatric nephrology, blood pressure, chronic kidney failure, cohort studies
Abstract
Background and objectives
Elevated BP load is part of the criteria for ambulatory hypertension in pediatric but not adult guidelines. Our objectives were to determine the prevalence of isolated BP load elevation and associated risk with adverse outcomes in children with CKD, and to ascertain whether BP load offers risk discrimination independently or in conjunction with mean ambulatory BPs.
Design, setting, participants, & measurements
We studied 533 children in the CKD in Children (CKiD) Study to determine the prevalence of normotension, isolated BP load elevation (≥25% of all readings elevated but mean BP normal), and ambulatory hypertension. We examined the association between these categories of BP control and adverse outcomes (left ventricular hypertrophy [LVH] or ESKD). We used c-statistics to determine risk discrimination for outcomes by BP load used either independently or in conjunction with other BP parameters.
Results
Overall, 23% of the cohort had isolated BP load elevation, but isolated BP load elevation was not statistically significantly associated with LVH in cross-section (odds ratio, 1.8; 95% CI, 0.8 to 4.2) or time to ESKD (hazard ratio, 1.2; 95% CI, 0.7 to 2.0). In unadjusted cross-sectional analysis, every 10% higher systolic BP load was associated with 1.1-times higher odds of LVH (95% CI, 1.0 to 1.3), but discrimination for LVH was poor (c=0.61). In unadjusted longitudinal analysis, every 10% higher systolic BP load was associated with a 1.2-times higher risk of ESKD (95% CI, 1.1 to 1.2), but discrimination for ESKD was also poor (c=0.60). After accounting for mean systolic BP, systolic BP load was not statistically significantly associated with either LVH or ESKD. Findings were similar with diastolic BP load.
Conclusions
BP load does not provide additive value in discriminating outcomes when used independently or in conjunction with mean systolic BP in children with CKD.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2020_03_11_CPOD10130819.mp3
Introduction
Ambulatory BP monitoring is regarded as the gold standard for the evaluation, diagnosis, and confirmation of hypertension in children (1,2). The major advantage of ambulatory BP monitoring is its ability to provide repeated BP measurements over 24 hours outside of a provider’s office (1–3). Ambulatory BP monitoring also provides an assessment of BP load (percentage of readings above the upper limit of normal in a 24-hour period) (4,5). However, the inclusion of BP load as a criterion for the diagnosis of ambulatory hypertension is not uniform across all existing ambulatory BP monitoring guidelines. For example, BP load is part of the definition for ambulatory hypertension in the 2014 American Heart Association (AHA) pediatric ambulatory BP monitoring guidelines and mentioned in the American Academy of Pediatrics BP guidelines for children (2,3,6). In contrast, BP load is not included in the European guidelines for children or in the adult AHA 2017 guidelines as a criterion for the diagnosis of ambulatory hypertension (1,6–8).
Clinically, incorporating BP load into the routine assessment of hypertension can lead to confusion for practitioners, because patients can have a normal mean ambulatory BP but an elevated BP load (defined by guidelines as having ≥25% of ambulatory readings during a 24-hour period above the 95th percentile for sex and height for children) (1,6,9). When these situations occur, it is unclear whether to classify the patient as hypertensive (elevated ambulatory BP and BP load) or not (normal ambulatory BP and BP load). The prevalence of children with an “uncategorized” BP status (e.g., isolated elevations in BP load) is unknown given the lack of large centralized ambulatory BP monitoring registries. It is also unclear whether BP load is prognostically important: many observational studies in both the adult and pediatric literature have found elevated BP load to be independently associated with target organ damage, but not all of these studies address whether BP load is prognostic of target organ damage after accounting for other ambulatory BP parameters (e.g., absolute mean BPs) (10–15).
Our objective was to determine the prevalence of normotension, isolated BP load elevation (defined as elevated systolic BP or diastolic BP load but normal mean systolic BP and diastolic BPs), and hypertension, and their association with left ventricular hypertrophy (LVH) or ESKD in children with CKD. We also investigated whether elevated BP load provides either independent or additive prognostic value (when used in conjunction with mean awake or sleep ambulatory BPs) for risk of LVH (in cross-sectional and longitudinal analysis) or ESKD (in longitudinal analysis) in the CKD in Children (CKiD) Study.
Materials and Methods
Study Population
Details of the CKiD Study have been previously described (16). Briefly, CKiD is an ongoing prospective multicenter cohort study of children between the ages of 1 and 16 years with an eGFR of between 30 and 90 ml/min per 1.73 m2 (16,17). Outcomes of interest in the CKiD Study include the progression of kidney disease, cardiovascular disease, growth, and cognition. We included 533 participants who had a baseline visit with echocardiogram and ambulatory BP monitoring, and excluded a total of 247 children who had either missing echocardiogram (n=69) or ambulatory BP monitoring (n=178), a subset of whom were under the age of 5 when ambulatory BP monitoring would not be routinely performed (9). Informed consent was obtained from study participants across all CKiD sites. De-identified data were derived from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository and administratively censored as of July 2014. The University of California San Francisco Institutional Review Board considers this study not human subjects research.
Predictors of Interest
Ambulatory Blood Pressure Monitoring.
Ambulatory BP monitoring was performed during the CKiD Study using a SpaceLabs 90217 monitor (SpaceLabs Healthcare, Snoqualmie, WA), with BPs taken every 20 minutes over 24 hours and centrally analyzed as described previously (9,18). All ambulatory BP monitoring performed as part of the baseline assessment (and typically on the same day as echocardiogram) were included for analysis. We used awake or asleep ambulatory BP readings as separate predictors of interest. We converted all ambulatory BP measurements into BP indices as in prior CKiD studies using a 95th percentile threshold to define ambulatory hypertension, such that an index of 1 would represent a BP that was exactly at the 95th percentile for sex and height (9,19–21). Similar to prior CKiD studies, we used European normative data from Soergel and colleagues to determine thresholds for ambulatory BP (18,20,21).
Blood Pressure Parameters of Interest.
We were interested in comparing the strength of the association between systolic BP and diastolic BP load with outcomes of interest, both as independent and additive predictors (e.g., used jointly with other ambulatory BP monitoring parameters such as awake and asleep mean BP index). Systolic BP and diastolic BP load were treated as continuous predictors of interest ranging from 0% to 100% and assessed separately during awake and sleep periods as in prior CKiD studies (9,18). We considered a BP load ≥25% to be elevated (as defined in pediatric ambulatory BP monitoring guidelines and in prior CKiD studies) (1,9). In sensitivity analysis, we also defined systolic BP load as a categorical predictor ranging from 0% to <25%, 25% to <50%, and 50% to 100%. Of note, we focused primarily on systolic BP and its association with adverse outcomes throughout this study (except when classifying patients as hypertensive or normotensive, in which case both systolic BP and diastolic BP were considered), given that prior studies have demonstrated the greater prognostic importance of systolic BPs, as opposed to diastolic BPs in their association with outcomes in children with CKD (18,19,22).
Outcomes of Interest
Left Ventricular Hypertrophy.
Echocardiograms and 24-hour ambulatory BP monitoring were performed every 2 years in the CKiD Study after baseline determination and were included in our primary analysis. M-mode and Doppler echocardiograms were performed by trained technologists using a standardized protocol at CKiD sites (18). We defined LVH using the same definitions used in prior CKiD studies, which is a left ventricular mass index ≥95th percentile for children and adolescents (18). The median time difference between 24-hour ambulatory BP monitoring and echocardiogram performance was 0 days (interquartile range [IQR], 0–0) at baseline. Our primary analysis of LVH focused on data collected at baseline; however, in secondary analysis, we examined the association between various BP parameters and the presence or absence of LVH at the baseline and the 2-year follow-up visit.
Long-Term ESKD Ascertainment.
Ascertainment of ESKD onset (defined as the first date of dialysis or transplantation) was performed at annual CKiD visits, by phone follow-up, or by the provision of data from providers. Patients were administratively censored if they were alive as of July 2014 and had not yet developed ESKD, or if they were lost to follow-up at the time of the last known study visit.
Statistical Analyses
Isolated Blood Pressure Load Elevation and Its Association with Outcomes of Interest.
First, we categorized the BP status of each individual in the cohort by ambulatory BP monitoring as either normotensive (both normal systolic BP and diastolic BP awake and asleep indices [i.e., index <1.0] and normal awake and asleep BP load, respectively), hypertensive (elevated systolic BP or diastolic BP awake or asleep indices [i.e., index ≥1.0], regardless of BP load), versus isolated BP load elevation (normal systolic BP and diastolic BP awake and asleep indices [i.e., index <1.0], but either elevated awake or asleep systolic BP or diastolic BP load ≥25%). We then compared the characteristics of these subgroups using chi-squared or Kruskal–Wallis tests as appropriate. Next, we examined the association between BP status (normotensive, isolated BP load elevation, or hypertensive) and LVH at baseline using logistic models (cross-sectional analysis) and ESKD in Cox models (longitudinal analysis) using both unadjusted and adjusted analyses. For longitudinal ESKD analyses, time to event was determined by starting from the date of the visit when the echocardiogram was performed (which was also typically the date of performance of ambulatory BP monitoring) (23). In adjusted analyses, we accounted for age, sex, race (white, black, or other), cause of CKD (glomerular or nonglomerular), body mass index z-score, eGFR by the bedside Schwartz equation, urine protein-creatinine ratio, and use of antihypertensive medications (all ascertained at the visit where echocardiogram was obtained) as covariates.
Prognostic Value of Blood Pressure Load.
We first included awake or asleep systolic BP or diastolic BP load as independent predictors of baseline LVH (using logistic models) and ESKD (using Cox models) in unadjusted analyses.
In secondary analysis, we examined whether systolic BP load and ambulatory systolic BPs predicted LVH longitudinally (at baseline and at the 2-year follow-up visit) using mixed-effects logistic regression models incorporating repeated measures of BP parameters and LVH.
We considered these unadjusted models our primary analysis because we were interested in comparisons of different BP parameters in the same CKiD participant as predictors of outcomes. To provide formal tests of the ability of each BP metric to discriminate risk of outcomes, c-statistics were determined for each logistic or Cox model. In logistic models, the c-statistics were determined as the area under the receiver operator curve. In Cox models, Harrell's c-statistics were used. C-statistics, or concordance statistics, provide a measure of risk discrimination (i.e., the probability that a person with the event has a higher predicted probability than a person without the event). Confidence intervals for c-statistics and their differences were determined via a bootstrapping technique (using 500 repetitions) to evaluate the fit of each Cox model. We repeated our models in adjusted analyses, accounting for the same covariates as described above and determined the c-statistics for these adjusted models.
We then performed separate analyses using awake or asleep mean BP indices as independent predictors of the outcomes of interest in unadjusted and adjusted analyses, and determined the c-statistics for these models.
Finally, we used nested models to determine whether BP load provided additive prognostic value when used in conjunction with awake or asleep BP indices. In nested models, we included both awake BP indices and awake BP load in the same logistic or Cox model and repeated our unadjusted and adjusted analyses. We repeated these models for sleep parameters separately. We used the c-statistic for systolic BP (or diastolic BP) load as the reference group when determining whether differences in c-statistic between unadjusted or nested models (e.g., awake systolic BP load versus awake mean systolic BP index) were statistically significant.
In sensitivity analysis, we repeated all analyses above using awake or asleep systolic BP load as a categorical (rather than continuous) predictor for both outcomes of interest, and determined model discrimination.
Stata 14 (StataCorp LLC, College Station, TX) was used for the performance of all statistical analyses and verified by a separate analyst. P<0.05 was considered statistically significant for all analyses.
Results
Baseline characteristics of the 533 CKiD Study participants included for analysis are shown in Table 1. The median age was 13 (IQR, 9–16) years, 61% were boys, and the median eGFR was 51 (IQR, 34–66) ml/min per 1.73 m2. We found that approximately one-quarter of the cohort had isolated BP (either systolic or diastolic) load elevation. Approximately one-third of the participants had elevated mean systolic BP or diastolic BP indices that met the criteria for ambulatory hypertension, and 40% were normotensive (Table 1). The prevalence of LVH was statistically significantly higher in children with hypertension compared with those with normotension (17% versus 7%, P=0.001) but did not differ between those with isolated BP load elevation and normotension (12% versus 7%, P=0.08). The mean awake systolic BP index (and SD) in this cohort was 0.91 (SD=0.08) and mean asleep systolic BP index was 0.92 (SD=0.10).
Table 1.
Characteristics of the participants in CKiD by ambulatory BP status
| Characteristic | Overall Cohort | Normotensiona | Isolated BP Load Elevationb | Hypertensionc |
|---|---|---|---|---|
| N (%) | 533 (100) | 213 (40) | 123 (23) | 197 (37) |
| Median age, yr (IQR) | 13 (9, 16) | 13 (10, 15) | 13 (9, 16) | 12 (9, 16) |
| Maled | 326 (61) | 123 (58) | 85 (69) | 118 (60) |
| Raced | ||||
| White | 366 (69) | 167 (78) | 84 (69) | 115 (58) |
| Black | 76 (14) | 15 (7) | 20 (16) | 41 (21) |
| Other | 91 (17) | 31 (15) | 19 (15) | 41 (21) |
| Cause of CKD | ||||
| Glomerular | 142 (27) | 63 (30) | 30 (24) | 49 (25) |
| Nonglomerular | 391 (73) | 150 (70) | 93 (76) | 148 (75) |
| Age- and sex-adjusted BMI z-score (IQR)d | 0.3 (−0.4, 1.3) | 0.3 (−0.5, 1.4) | 0.6 (−0.1, 1.5) | 0.2 (−0.5, 0.9) |
| Median eGFR in ml/min per 1.73 m2 by bedside Schwartz (IQR) | 51 (34, 66) | 54 (34, 68) | 51 (39, 64) | 49 (31, 64) |
| Hemoglobin, g/dl (IQR) | 13 (12, 14) | 13 (12, 14) | 13 (12, 14) | 13 (12, 14) |
| Serum albumin, g/dl (IQR) | 4.3 (4.1, 4.6) | 4.4 (4.2, 4.6) | 4.3 (4.2, 4.6) | 4.3 (4.1, 4.6) |
| Left ventricular hypertrophy at baselined | 62 (12) | 14 (7) | 15 (12) | 33 (17) |
| Clinic systolic BP indexd,e | 0.88±0.10 | 0.84±0.08 | 0.88±0.08 | 0.93±0.10 |
| Clinic diastolic BP indexd,e | 0.82±0.14 | 0.77±0.12 | 0.80±0.12 | 0.89±0.15 |
| Awake systolic BP indexd | 0.91±0.08 | 0.86±0.06 | 0.91±0.05 | 0.98±0.07 |
| Awake diastolic BP indexd | 0.88±0.10 | 0.81±0.07 | 0.88±0.06 | 0.96±0.10 |
| Awake systolic BP loadd | 13 (3, 33) | 3 (0, 9) | 14 (3, 26) | 40 (19, 59) |
| Awake diastolic BP loadd | 13 (5, 31) | 6 (2, 10) | 16 (7, 27) | 36 (21, 55) |
| Asleep systolic BP indexd | 0.92±0.10 | 0.85±0.06 | 0.91±0.04 | 1.00±0.07 |
| Asleep diastolic BP indexd | 0.92±0.13 | 0.81±0.07 | 0.92±0.06 | 1.03±0.11 |
| Asleep systolic BP loadd | 14 (0, 39) | 0 (0, 6) | 15 (5, 28) | 50 (28, 75) |
| Asleep diastolic BP loadd | 21 (6, 47) | 5 (0, 12) | 28 (19, 36) | 56 (33, 75) |
| Antihypertensive medication use | 362 (68) | 152 (71) | 77 (63) | 133 (68) |
| Awake systolic BP loadd | ||||
| 0% to <25% | 366 (68) | 213 (100) | 90 (73) | 63 (32) |
| 25% to <50% | 89 (17) | 0 (0) | 33 (27) | 56 (28) |
| 50%–100% | 78 (15) | 0 (0) | 0 (0) | 78 (40) |
| Asleep systolic BP loadd | ||||
| 0% to <25% | 340 (64) | 213 (100) | 86 (70) | 41 (21) |
| 25% to <50% | 89 (17) | 0 (0) | 35 (28) | 54 (27) |
| 50%%–100% | 104 (19) | 0 (0) | 2 (2) | 102 (52) |
| Awake diastolic BP loadd | ||||
| 0% to <25% | 365 (69) | 213 (100) | 86 (70) | 66 (34) |
| 25% to <50% | 102 (19) | 0 (0) | 35 (28) | 67 (34) |
| 50%–100% | 66 (12) | 0 (0) | 2 (2) | 64 (32) |
| Asleep diastolic BP loadd | ||||
| 0% to <25% | 284 (54) | 213 (0) | 44 (36) | 27 (14) |
| 25% to <50% | 126 (24) | 0 (0) | 78 (63) | 48 (24) |
| 50%–100% | 123 (23) | 0 (0) | 1 (1) | 122 (62) |
Values are means±SD or N (%), unless otherwise specified. IQR, interquartile range; BMI, body mass index.
Normotension was defined as awake and asleep systolic BP and diastolic BP index <1.0 (e.g., mean ambulatory BP <95th percentile for sex and height), as well as awake and asleep systolic BP or diastolic BP load <25%.
Isolated BP load elevation was defined as awake and asleep systolic BP or diastolic BP index <1.0, with awake or asleep systolic BP or diastolic BP load ≥25%.
Hypertension was defined as ambulatory awake or sleep systolic BP or diastolic BP index ≥1.0, regardless of BP load.
Statistically significantly different by BP control category (P<0.05).
Clinic BP readings were indexed to the 95th percentile thresholds for age, sex, and height; an index of 1 would represent a clinic BP that was exactly at the 95th percentile for age, sex, and height derived from the National High BP Education Program Fourth Report as in prior CKiD studies.
Ambulatory Blood Pressure Status and Risk of Adverse Outcomes
Approximately 12% of the cohort (n=62) had LVH on their baseline echocardiogram (Table 1). Among the 224 participants with available echocardiogram and ambulatory BP data at the 2-year visit, 11% (n=25) had LVH at follow-up. During a mean follow-up of 3.5 years (SD=2.5), 23% (n=125) of participants developed ESKD. In both unadjusted and adjusted analysis, isolated BP load elevation was not statistically significantly associated with the odds of LVH at baseline or time to ESKD (Table 2) compared with normotension. In contrast, hypertension was strongly associated with LVH and ESKD (Table 2), although the association with ESKD was attenuated in adjusted models.
Table 2.
Risk of LVH or ESKD by ambulatory BP status in unadjusted and adjusted analyses
| Ambulatory BP Classification (n=533) | LVH | ESKD | ||||
|---|---|---|---|---|---|---|
| Number of Events (n=62) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | Number of Events (n=125) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a | |
| Normotension (n=213)b | 14 | Reference | Reference | 41 | Reference | Reference |
| Isolated BP load elevation (n=123)c | 15 | 2.0 (0.9 to 4.2) | 1.8 (0.8 to 4.2) | 26 | 1.1 (0.7 to 1.8) | 1.2 (0.7 to 2.0) |
| Ambulatory Hypertension (n=197)d | 33 | 2.9 (1.5 to 5.5) | 2.8 (1.4 to 5.8) | 58 | 1.8 (1.2 to 2.7) | 1.3 (0.8 to 1.9) |
LVH, left ventricular hypertrophy; OR, odds ratio; HR, hazard ratio.
Adjusted for age, sex, race, cause of CKD, body mass index z-score, urine protein-creatinine ratio, antihypertensive use, and baseline eGFR (by bedside Schwartz), n=515 included due to missing covariates.
Normotension was defined as awake and asleep systolic BP or diastolic BP index <1.0 (e.g., mean ambulatory BP <95th percentile for sex and height), with awake and asleep systolic BP or diastolic BP load <25%.
Isolated BP load elevation was defined as awake and asleep systolic BP or diastolic BP index <1.0, with awake or sleep BP load ≥25%.
Ambulatory hypertension was defined as awake or asleep systolic BP or diastolic BP index ≥1.0, regardless of BP load.
Blood Pressure Load and Left Ventricular Hypertrophy
When we examined awake and asleep systolic BP load as a continuous variable, neither awake nor asleep systolic BP load were statistically significantly associated with the odds of LVH in unadjusted analyses (Table 3). Similar findings were noted in fully adjusted models and nested models (incorporating other ambulatory BP parameters), as shown in Table 3.
Table 3.
Association between systolic BP parameters and LVH (in cross-section) in unadjusted and adjusted analyses along with model discrimination
| Systolic BP Metrics (n=533) | Unadjusted OR (95% CI)a | Unadjusted c-Statistic (95% CI) | Adjusted OR (95% CI)a,b | Adjusted c-Statistic (95% CI)b |
|---|---|---|---|---|
| Awake systolic BP load (every 10% higher) | 1.1 (1.0 to 1.3) | 0.61 (0.53 to 0.68)c | 1.1 (1.0 to 1.3) | 0.77 (0.72 to 0.83)c |
| Awake systolic BP index (every 0.1 higher) | 1.7 (1.2 to 2.3) | 0.62 (0.55 to 0.69) | 1.6 (1.1 to 2.3) | 0.78 (0.73 to 0.83) |
| Awake systolic BP load plus awake systolic BP index | 0.9 (0.7 to 1.2) 2.4 (1.0 to 5.6) |
0.62 (0.55 to 0.69) | 0.8 (0.6 to 1.1) 2.7 (1.0 to 7.2) |
0.78 (0.73 to 0.84) |
| Asleep systolic BP load (every 10% higher) | 1.1 (1.0 to 1.2) | 0.62 (0.55 to 0.69)c | 1.1 (1.0 to 1.2) | 0.77 (0.72 to 0.83)c |
| Asleep systolic BP index (every 0.1 higher) | 1.5 (1.1 to 2.0) | 0.63 (0.56 to 0.70) | 1.5 (1.1 to 2.0) | 0.78 (0.72 to 0.83) |
| Asleep systolic BP load plus asleep systolic BP index | 0.9 (0.8 to 1.2) 1.8 (0.9 to 3.6) |
0.63 (0.56 to 0.71) | 0.9 (0.7 to 1.2) 1.8 (0.8 to 3.9) |
0.78 (0.72 to 0.83) |
LVH, left ventricular hypertrophy; OR, odds ratio.
OR for every 0.1 higher systolic BP index or 10% higher systolic BP load.
Adjusted for age, sex, race, cause of CKD, body mass index z-score, urine protein-creatinine ratio, antihypertensive use, and baseline eGFR (by bedside Schwartz), n=515 included due to missing covariates.
Reference group for awake or asleep c-statistic comparisons.
The c-statistics for models including awake or asleep systolic BP load as the sole predictor of LVH were low (c=0.61 and 0.62, respectively; Table 3). The c-statistic for models including both awake systolic BP load and awake systolic BP index was not improved in comparison with the model with awake systolic BP load as a sole predictor (c=0.62). Similar findings were noted with nested models of asleep systolic BP load and asleep systolic BP index. In fully adjusted models, the c-statistics for all models were improved, but systolic BP load did not improve risk discrimination when used in conjunction with mean BP parameters (Table 3).
When we performed analyses by diastolic BP, we found that awake and asleep diastolic BP load were associated with the odds of LVH as sole predictors, although model discrimination remained low (Supplemental Table 1A). When awake or asleep diastolic BP indices were added to diastolic BP load, diastolic BP load was no longer statistically significantly associated with the odds of LVH.
When we repeated our models using repeated measures of BP load and other BP parameters to predict LVH during the longitudinal follow-up at 2 years using mixed logistic models, we found that systolic BP load did not consistently provide better risk discrimination compared with systolic BP index (Supplemental Table 2). Overall, the c-statistic remained low for all BP parameters. In adjusted models, systolic BP load also did not provide statistically significantly better risk discrimination, compared with the systolic BP index, consistently for risk of LVH.
In sensitivity analysis, when we repeated our models using awake or asleep systolic BP load as a categorical predictor, we found that results were similar to our primary analysis for the outcome of LVH (Supplemental Table 3A).
Longitudinal Analysis of ESKD Risk
Awake and asleep systolic BP load were both independently associated with the risk of ESKD in unadjusted analysis (Table 4). However, after accounting for awake or asleep mean systolic BP indices by ambulatory BP monitoring, awake and asleep systolic BP load were no longer statistically significantly associated with the risk of ESKD. In fully adjusted models, neither awake nor asleep systolic BP load were statistically significantly associated with risk of ESKD independently or as an additive prognostic risk factor (Table 4).
Table 4.
Association between systolic BP parameters and ESKD in unadjusted and adjusted analyses along with model discrimination
| Systolic BP Metrics (n=533) | Unadjusted HR (95% CI)a | Unadjusted c-Statistic (95% CI) | Adjusted HR (95% CI)a,b | Adjusted c-Statistic (95% CI)b |
|---|---|---|---|---|
| Awake systolic BP load (every 10% higher) | 1.2 (1.1 to 1.2) | 0.60 (0.54 to 0.65)c | 1.1 (1.0 to 1.1) | 0.89 (0.86 to 0.91)c |
| Awake systolic BP index (every 0.1 higher) | 1.6 (1.3 to 1.9) | 0.60 (0.55 to 0.65) | 1.2 (1.0 to 1.5) | 0.89 (0.86 to 0.91) |
| Awake systolic BP load plus awake systolic BP index | 1.1 (1.0 to 1.3) 1.2 (0.7 to 1.9) |
0.60 (0.55 to 0.67) | 0.9 (0.7 to 1.0) 1.8 (1.1 to 3.2) |
0.89 (0.85 to 0.91) |
| Asleep systolic BP load (every 10% higher) | 1.1 (1.0 to 1.2) | 0.58 (0.52 to 0.64)c | 1.0 (1.0 to 1.0) | 0.88 (0.85 to 0.91)c |
| Asleep systolic BP index (every 0.1 higher) | 1.4 (1.1 to 1.6) | 0.57 (0.52 to 0.63) | 1.2 (1.0 to 1.4) | 0.89 (0.85 to 0.91) |
| Asleep systolic BP load plus asleep systolic BP index | 1.1 (0.9 to 1.2) 1.1(0.7 to 1.7) |
0.58 (0.52 to 0.64) | 1.0 (0.8 to 1.1) 1.3 (0.9 to 2.1) |
0.88 (0.85 to 0.91) |
HR, hazard ratio.
HR reported for every 0.1 higher in systolic BP index or 10% higher systolic BP load.
Adjusted for age, sex, race, cause of CKD, body mass index z-score, duration of CKD, urine protein-creatinine ratio, serum albumin, hemoglobin, antihypertensive use, and baseline eGFR (by bedside Schwartz), n=515 included due to missing covariates.
Reference group for awake or asleep c-statistic comparisons.
Overall, the c-statistic for models including awake or asleep systolic BP load for the risk of ESKD was also low (Table 4). In fully adjusted models, the c-statistics were improved and similar across all models (c=0.88, Table 4), but awake or asleep systolic BP load did not appear to provide additive prognostic value to either awake or asleep mean BP indices, respectively.
When we performed analyses by diastolic BP, similar findings were noted for the outcome of ESKD (Supplemental Table 1B). Awake and asleep diastolic BP load were associated with the risk of ESKD as sole predictors, although model discrimination remained low. When awake or asleep diastolic BP indices were added to diastolic BP load, diastolic BP load was no longer statistically significantly associated with the risk of ESKD.
In sensitivity analysis, when we repeated our models using awake or asleep systolic BP load as a categorical predictor, results were similar in both unadjusted and adjusted analyses for hazard of ESKD (Supplemental Table 3B).
Discussion
BP load has been considered a predictor of target organ damage due to its reflection of the overall burden of elevated BPs during 24-hour ambulatory BP monitoring (4,5). However, whether BP load offers prognostic information regarding the risk of target organ damage in a manner that is independent of mean ambulatory BP in children with CKD is uncertain (10,12–14,24,25). The association between isolated BP load elevation and risk of adverse outcomes has also been unclear in children with CKD. Yet, to date, BP load remains a part of the criteria for the diagnosis of ambulatory hypertension in most pediatric guidelines (1,6). In this study, we found that one-quarter of a cohort of children with CKD had an elevated BP load in the setting of normal mean ambulatory BP, although the majority of these children did not have a BP load of ≥50%. Although both awake and asleep BP loads were independently associated with the odds of LVH in unadjusted analyses, we found that after accounting for mean BP, BP load (as a continuous or categorical predictor) was no longer statistically significantly associated with outcomes and did not improve discrimination of the risk for LVH in cross-section or ESKD. We also did not find BP load to be associated with LVH in longitudinal follow-up at the 2-year visit after considering BP indices. Our study is one of the first to demonstrate that BP load was not independently associated with important clinical outcomes in a population of children with CKD.
Prior studies in the adult literature have evaluated the relationship between elevated BP load and target organ damage, but there is no clear consensus as to whether BP load offers additional information beyond that of mean ambulatory BPs (12,26–28). For example, Wang et al. (25) found a strong association between nighttime BP load and target organ damage independent of mean ambulatory BP values in a cohort of 1219 Chinese patients with CKD, but this analysis included mostly patients who were hospitalized. More recent literature has suggested that BP load may not provide additive information in predicting the risk of cardiovascular outcomes compared with mean absolute ambulatory BPs (13,14). The largest analysis to date to investigate whether BP load is independently prognostic of adverse outcomes was performed using data from the International Database of Ambulatory BP in relation to Cardiovascular Outcome (13). Although this study found that BP load and mean ambulatory BP were equally predictive of nonfatal and fatal CV events in a cohort of adults without significant CKD, BP load did not improve the discrimination of the risk for these cardiovascular outcomes. Another study that evaluated 869 adults with untreated hypertension also found that higher tertiles of BP load were more predictive of target organ damage; however, when the investigators additionally adjusted for mean ambulatory BPs, these findings were no longer statistically significant (14).
Although there is consensus across pediatric ambulatory BP guidelines that ambulatory BP monitoring should be used for the confirmation of a diagnosis of hypertension, recommendations regarding the incorporation of BP load in the diagnostic approach for hypertension are inconsistent across different guidelines (6,7,29). Several pediatric studies have evaluated the association between BP load and target organ damage in children without CKD (10,11,15). One small study previously reported that BP load >50% in the presence of a 24-hour systolic BP index >1.0 was associated with LVH in a cohort of 37 children with untreated hypertension without CKD (15). Richey and colleagues found that every 25% higher systolic BP load was associated with higher odds of LVH in children, but these models did not account for mean ambulatory BP (15,30). Another study of children without CKD found that systolic BP load >25% was associated with higher carotid-intima media thickness and higher pulse-wave velocity, but not microalbuminuria or abnormal left ventricular mass index (11). Although our data are consistent with prior literature showing that BP load is associated with cardiovascular and kidney outcomes when used independently (and not accounting for mean ambulatory BPs), our study shows that BP load does not improve discrimination of these outcomes overall after accounting for mean ambulatory BPs. Isolated BP load elevation was associated with a tendency toward higher risk of LVH and ESKD compared with normotension, but this finding did not achieve statistical significance and it is unclear whether isolated BP load elevation would warrant treatment.
The strengths of our study include the availability of research-grade ambulatory BP monitoring data obtained from a well characterized cohort of children with CKD from large pediatric academic centers. We were able to examine the association between BP load and “hard outcomes” representative of target organ damage, such as ESKD onset. We note a high number of ESKD events over a relatively short follow-up duration, which is a strength in CKiD. These rates are comparable to other pediatric cohorts such as the ESCAPE trial in European children with CKD (31). Both pediatric CKD cohorts demonstrate a higher event rate compared with outcomes from adult cohorts that may reflect differences in the etiology of CKD, of which glomerular disease may be more common in the pediatric population (32). Limitations to our study include the use of normative ambulatory BP monitoring data derived from a homogenous European population which may or may not apply to children of greater racial and ethnic diversity; however, no other ambulatory BP monitoring norms are currently available.
In conclusion, BP load does not appear to provide additional risk discrimination for cardiovascular or kidney outcomes above and beyond mean ambulatory BP indices obtained during ambulatory BP monitoring. Future studies are needed to confirm our findings in other pediatric populations with CKD and to determine whether BP load should still be incorporated into routine assessments of cardiovascular or kidney risk in children.
Disclosures
Dr. Ku reports a position on the pediatric advisory board at Reata Pharmaceuticals and received consultant fees from Tricida, outside of the submitted work. Dr. Flynn reports personal fees from Silvergate Pharmaceuticals, Ultragenyx, Vertex Pharmaceuticals, and royalties from Up To Date, all outside the published work. Dr. Furth, Dr. Mitsnefes, Dr. Grimes, Dr. Lee, Dr. McCulloch, Dr. Samuels, Ms. Seth, and Dr. Warady have nothing to disclose.
Funding
Dr. Lee is supported by T32 Ruth L. Kirschtein Institutional National Service award 2T32DK007219. Dr. Ku is supported by National Heart, Lung, and Blood Institute grant HL131023. Dr. Mitsnefes is supported by NIDDK grant DK090070. The CKiD Study was conducted by the CKiD Investigators and supported by NIDDK, with additional funding from the National Institute of Child Health and Human Development, the National Heart, Lung, and Blood Institute and NIDDK (U01-DK-66143, U01-DK-66174, U01DK-082194, and U01-DK-66116). This publication was also supported by the National Center for Advanced Translational Sciences, NIH, through Clinical and Translational Science Institute, University of California San Francisco grant UL1 TR001872.
Supplementary Material
Acknowledgments
The data and samples from the CKiD Study reported here were supplied by the NIDDK Central Repositories. This manuscript does not necessarily reflect the opinions or views of the CKiD Study, the NIDDK Central Repositories, or the NIDDK. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
All authors have read and approved the submission of the manuscript; the manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an abstract.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10130819/-/DCSupplemental.
Supplemental Table 1A. Association between diastolic BP parameters and LVH (in cross-section) in unadjusted and adjusted analyses, along with model discrimination.
Supplemental Table 1B. Association between diastolic BP parameters and ESKD and in unadjusted and adjusted analyses, along with model discrimination.
Supplemental Table 2. Association between systolic BP parameters and risk of LVH during 2-year follow-up, along with model discrimination.
Supplemental Table 3A. Association between systolic BP parameters and LVH in unadjusted and adjusted analyses using BP load as a categorical predictor along with model discrimination.
Supplemental Table 3B. Association between systolic BP parameters and ESKD in unadjusted and adjusted analyses using BP load as a categorical predictor along with model discrimination.
References
- 1.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young: Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the American Heart Association. Hypertension 63: 1116–1135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee: Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 52: 433–451, 2008 [DOI] [PubMed] [Google Scholar]
- 3.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring: European Society of Hypertension position paper on ambulatory blood pressure monitoring [published correction appears in J Hypertens 31: 2467, 2013]. J Hypertens 31: 1731–1768, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Zachariah PK, Sheps SG, Bailey KR, Wiltgen CM, Moore AG: Age-related characteristics of ambulatory blood pressure load and mean blood pressure in normotensive subjects. JAMA 265: 1414–1417, 1991 [PubMed] [Google Scholar]
- 5.White WB: Blood pressure load and target organ effects in patients with essential hypertension. J Hypertens Suppl 9: S39–S41, 1991 [PubMed] [Google Scholar]
- 6.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM; Subcommittee on Screening and Management of High Blood Pressure in Children: Clinical practice guideline for screening and management of high blood pressure in children and adolescents [published correction appears in Pediatrics 140: e20171904, 2017]. Pediatrics 140: e20171904, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wühl E, Zanchetti A: 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34: 1887–1920, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D; American Heart Association; American Society of Hypertension; Preventive Cardiovascular Nurses Association: Call to action on use and reimbursement for home blood pressure monitoring: A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs 23: 299–323, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S; Chronic Kidney Disease in Children Study Group: Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60: 43–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri A, Sutherland SM, Begin B, Salsbery K, McCabe L, Potter D, Alexander SR, Wong CJ: Role of twenty-four-hour ambulatory blood pressure monitoring in children on dialysis. Clin J Am Soc Nephrol 6: 870–876, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conkar S, Yılmaz E, Hacıkara Ş, Bozabalı S, Mir S: Is daytime systolic load an important risk factor for target organ damage in pediatric hypertension? J Clin Hypertens (Greenwich) 17: 760–766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Deng Q, Li H, Ma X, Zhang J, Peng H, Wang C, Lou T: Prognostic value of nighttime blood pressure load in Chinese patients with nondialysis chronic kidney disease. J Clin Hypertens (Greenwich) 19: 890–898, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovsky J, Imai Y, Ibsen H, O’Brien E, Wang J, Staessen JA; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators: Blood pressure load does not add to ambulatory blood pressure level for cardiovascular risk stratification [published correction appears in Hypertension 64: e1, 2014]. Hypertension 63: 925–933, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Li Y, Wei FF, Zhang L, Han JL, Wang JG: Is blood pressure load associated, independently of blood pressure level, with target organ damage? J Hypertens 31: 1812–1818, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Sorof JM, Cardwell G, Franco K, Portman RJ: Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension 39: 903–908, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL: CKiD (CKD in children) prospective cohort study: A review of current findings. Am J Kidney Dis 60: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B; CKiD Study Group: Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 21: 137–144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M; CKiD Study Group: BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol 25: 167–174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W: Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: A multicenter trial including 1141 subjects. J Pediatr 130: 178–184, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Ku E, McCulloch CE, Warady BA, Furth SL, Grimes BA, Mitsnefes MM: Twenty-four-hour ambulatory blood pressure versus clinic blood pressure measurements and risk of adverse outcomes in children with CKD. Clin J Am Soc Nephrol 13: 422–428, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S: Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB Sr.: Evaluating discrimination of risk prediction models: The C statistic. JAMA 314: 1063–1064, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Andrade SS, Serro-Azul JB, Nussbacher A, Giorgi D, Pierri H, Gebara O, Wajngarten M: Daytime systolic blood pressure load and previous stroke predict cardiovascular events in treated octogenarians with hypertension. J Am Geriatr Soc 58: 2232–2234, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Zhang J, Deng W, Gong W, Liu X, Ye Z, Peng H, Lou T: Nighttime systolic blood-pressure load is correlated with target-organ damage independent of ambulatory blood-pressure level in patients with non-diabetic chronic kidney disease. PLoS One 10: e0131546, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauwens F, Duprez D, De Buyzere M, Clement DL: Blood pressure load determines left ventricular mass in essential hypertension. Int J Cardiol 34: 335–338, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Mulè G, Nardi E, Andronico G, Cottone S, Raspanti F, Piazza G, Volpe V, Ferrara D, Cerasola G: Relationships between 24 h blood pressure load and target organ damage in patients with mild-to-moderate essential hypertension. Blood Press Monit 6: 115–123, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Viazzi F, Leoncini G, Ratto E, Vaccaro V, Tomolillo C, Falqui V, Parodi A, Conti N, Deferrari G, Pontremoli R: Microalbuminuria, blood pressure load, and systemic vascular permeability in primary hypertension. Am J Hypertens 19: 1183–1189, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr.: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American Heart association task force on clinical practice guidelines. Hypertension 71: 1269–1328, 2018. [DOI] [PubMed] [Google Scholar]
- 30.Richey PA, Disessa TG, Hastings MC, Somes GW, Alpert BS, Jones DP: Ambulatory blood pressure and increased left ventricular mass in children at risk for hypertension. J Pediatr 152: 343–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F; ESCAPE Trial Group: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW, Feldman HI; CRIC Study Investigators: Association of kidney disease outcomes with risk factors for CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 63: 236–243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.