Introduction
Fabry disease is a rare X-linked lysosomal storage disorder due to mutations in the GLA gene causing complete or partial deficiency of the enzyme α-galactosidase A (α-Gal A), and subsequent slow accumulation of mainly globotriaocylceramide (Gb3 or GL3) and its deacylated derivative globotriaosylsphingosine (lyso-GL3, also called Lyso-Gb3) in several cell types and body fluids. Early and often asymptomatic cellular damage typically precedes various degrees of organ affection and late organ failure. Clinical symptoms are highly variable and mostly nonspecific (1). Kidney cells, cardiomyocytes, and vascular endothelium are target cells of particular interest in a usually slowly progressive disease. Major complications are secondary to kidney, cardiac, and/or central nervous system affection, usually from the fourth decade onward (2). The disease has had a long journey from the original description of cutaneous angiokeratoma in 1898 (3) to the current recognition of a treatable, highly complex, and heterogenous multisystem disease carrying a high rate of morbidity and mortality (4). The grim natural disease course in the era before enzyme replacement therapy should not be forgotten and was described in 2002 by Branton et al. (5) in 105 hemizygous classic male patients; only 25 of 105 patients survived until the age of 50 and no patients survived past age 60 years. Before the advent of dialysis and kidney transplantation, the most common cause of death was uremia, at a mean age of 41 years (6). Overall, before enzyme replacement was available, a reduced life span of about 25 years in males and 10 years in females was expected compared with the general population (7). Although the pathophysiology is still only partly understood (Figure 1), increasing knowledge of the complexity of mutations and disease manifestations has been fueled by a surge in clinical research and numerous publications after the introduction of enzyme replacement therapy nearly 20 years ago. Agalsidase α and agalsidase β were approved in Europe and the United States (agalsidase β only) in 2001; licensed doses are 0.2 mg/kg per every other week and 1.0 mg/kg per every other week, respectively (8,9). Ten-year follow-up registry data clearly demonstrate a modifying effect of enzyme replacement therapy on serious organ complications and mortality (10,11), and there has been a change in all-cause mortality from predominantly kidney to cardiac deaths (12,13).
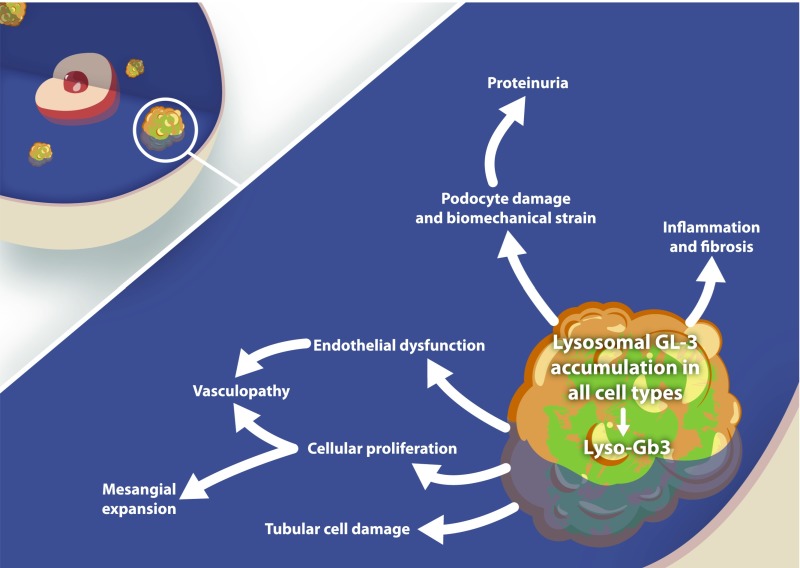
Figure 1.
Pathomechanisms of Fabry nephropathy [Eikrem et al. (81)]. GL3, globotriaocylceramide; Lyso-Gb3, globotriaosylsphingosine.
Historically, the disease has been hampered by diagnostic delays and subsequent delays of therapeutic intervention until irreversible organ damage prevails. Hence, the median age at diagnosis was 24 years in males and 31 years in females in a survey of >2200 patients, and the median time between onset of symptoms (usually neurologic pain and gastrointestinal dysfunction) and diagnosis was about 11 years in both sexes (14). The rationale for the increasing focus on early therapy has been clearly demonstrated by several reports highlighting the serious prognostic effect of diagnostic and therapeutic delays (15,16).
Epidemiology, Genetics, and Characterization of Phenotypes
Over the last two decades, general knowledge of Fabry disease and therapeutic challenges have changed dramatically, and prognosis has improved for several reasons. Enormous progress in genetic sequencing technology has led to a paradigm change in the understanding of the complexity of genotype–phenotype interactions. Recently, a general description of clinically relevant categories of variants in Mendelian disorders using the terminology “pathogenic,” “likely pathogenic,” “uncertain significance,” “likely benign,” or “benign” has been suggested (17,18). This is especially relevant for the classification of clinical phenotypes harboring GLA missense mutations accounting for about 60% of >900 known mutations (Human Gene Mutation Database, www.hgmd.org). Importantly, the increasing incidence and prevalence of late-onset, nonclassic mutations, and genetic variants of unclear significance, with milder disease phenotypes have been acknowledged (17,19), with symptoms often confined to a single organ (especially cardiac) and some of these presumably accompanied by no clinical disease at all (17,20). The wide phenotypic spectrum of disease severity, even within the same family, and the fact that not all mutations are causing disease (e.g., polymorphisms) highlight the necessity of careful individual diagnostic and prognostic assessment, to allow correct and timely intervention, as well as avoidance or postponement of unnecessary high-cost treatment in patients with non–disease-causing mutations or very mild disease (19–22). The increasing birth prevalence of GLA mutations, from previous estimates of 1:40,000–170,000 (23) up to 1:1250 in newborn screening studies (19,24,25), reflects the existence of a majority of nonclassic mutations and variants of unknown significance where natural history and effectiveness of enzyme replacement are unknown (19). Currently, research on clinically relevant genotype–phenotype relationships is increasingly prioritized. Clear criteria exist for the diagnosis of classic early-onset Fabry disease (also called Type 1) with absent or low levels of enzyme activity, and typical symptoms (4). However, precise diagnosis may be difficult in late-onset, nonclassic (also called Type 2) male and female patients harboring any variant in the GLA gene with residual enzyme activity, and variable X-chromosome inactivation patterns (females), often presenting with cardiac, minimal, or unclear symptoms (26,27) in early or late adulthood (17,28,29). A widely used algorithm for diagnosing Fabry disease in these latter categories has been published by van der Tol et al. (19,30,31).
New Insight into the Effectiveness of Enzyme Replacement Therapy
The introduction of intravenous enzyme replacement has fueled a tremendous amount of research, opening many new avenues for collaborative work, involving experts from key medical specialties. The way forward has served as a model for learning and organizing clinical research, defining challenges and caveats in the field of rare metabolic diseases (15,32). Although enzyme replacement therapy has undoubtedly turned Fabry disease into a treatable disease, it has become obvious that intervention should be regarded as a disease modifier rather than a cure, and persistent risk of serious complications and increased mortality raises major concerns over current therapeutic strategies (32,33). Furthermore, numerous observational studies and case series published the last decade have disclosed a conundrum of Fabry disease heterogeneity, highlighting the necessity of individual assessment and targeting of therapy, even in patients within the same family (15,34,35). Given a slowly progressive heterogenous disease, new light has been shed on the validity of biases and limitations of few small randomized controlled trials of short duration in comparison with numerous long-term follow-up studies with a large number of patients (36–38). The value of analysis of unpooled data from systematic comprehensive literature searches of observational studies and case series/reports through January 2017 has recently been reported (38). These much larger patient cohorts and attempts to separate data among relevant clinical phenotypes (e.g., children, females, males, classic, and nonclassic), some of them treated for >15 years, have provided new insight that helps the treating physician better define individual patient risk and adequate therapeutic goals. Importantly, this knowledge has strengthened the need for an individual comprehensive multidisciplinary approach, carefully addressing genotype, phenotype, family history, and biomarkers including kidney histology when possible (34,35,39,40).
Individual Therapeutic Goals and Risk Profiles
Organ-specific therapeutic goal recommendations, covering altogether 249 publications (67% male patients including 36 clinical trials), suggest a significant slowing of decline of eGFR and reduction/stabilization of cardiac mass (adult males). The kidney and cardiac therapeutic benefits provide new information expanding the knowledge reported in a previous meta-analysis (33). Although a cardiac benefit was suggested in both sexes, recent data have been generally less robust in females, likely because of a wider disease spectrum ranging from asymptomatic to severely (rare) affected individuals (40). Interestingly, quality of life outcomes were improved in both sexes (34,40). The prognostic importance of younger age and an absence of organ damage when enzyme replacement therapy is initiated have been shown in several studies (10,41). Germain et al. (10) defined “low renal involvement” as urine protein creatinine ratio <0.5 g/g and <50% sclerotic glomeruli in a well-defined observational 10-year follow-up study of 52 classic patients (2 females), mean age 30 years and normal eGFR at start of agalsidase β 1.0 mg/kg every other week. The low renal involvement group (n=32) was younger (mean 25 years at treatment initiation) than the “high renal involvement” group and showed less deterioration of eGFR (mean slope −1.89 versus −6.82 ml/min per 1.73 m2 per year). Of note, 94% of the patients were alive at the end of the study and 81% did not experience any events. In patients on enzyme replacement therapy, lower eGFR and higher levels of proteinuria strongly predict faster disease progression (16). Supplemental therapy with renin angiotensin system inhibition to lower proteinuria to ≤0.5 g/d and potentially stabilize GFR should be considered in classic patients with reduced GFR and severe proteinuria (15,42). The effects of enzyme replacement therapy on cerebrovascular events remains unknown, although a recent meta-analysis suggested a potential benefit on stroke prevention (43). Although no clear consensus exists, recommendations for considering withdrawal of enzyme replacement in patients with advanced disease have also been published (44).
Is Higher Agalsidase Dose Beneficial?
Although in vitro milligram per milligram equipotency of agalsidase α and β has been demonstrated (45), the discussion about clinical equipotency of licensed drug regimens remains unsettled. The beneficial effect of higher cumulative agalsidase dose on kidney histology has been reported by Skrunes et al. (41) in serial kidney biopsies in 20 classic patients (median age 21 years, 12 males) with stable microalbuminuria and normal measured GFR followed for 10 years. A clear dose-dependent effect on clearance of podocyte GL3 deposits was found in this cohort, and residual lyso-GL3 correlated with the cumulative enzyme dosage in male patients. The clinical benefits of higher enzyme doses have been corroborated and likely underscored in a larger observational multicenter study with systematic follow-up of a high number of patients of both sexes from three European Fabry centers. The compulsory switch from agalsidase β 1.0 mg/kg every other week to agalsidase α 0.2 mg/kg every other week in many patients (due to the worldwide shortage of agalsidase β supply from June 2009 to January 2012) and subsequent reswitch to agalsidase β 1.0 mg/kg every other week in a number of patients showed conspicuous dose-dependent benefits regarding GFR slopes, lyso-GL3 levels, and gastrointestinal symptoms (46). Moreover, an overview of available evidence indicates that higher doses of agalsidase are beneficial given optimal timing of therapy and selection of patients with classic phenotypes (47,48). Importantly, the majority of literature-based observations of dose-dependent clinical effects so far are confined to classic male patients, and further long-term studies in expanded cohorts of high-risk patients are warranted. Long-term data on therapy outcomes in general are insufficient in female patients and sex-mixed study populations, likely because of variations in X-chromosome inactivation, which are usually not reported in clinical studies (34,40). This may also in part be the reason why no differences were found in clinical events in a recent European multicenter study including a mixture of classic and nonclassic patients [n=387 (192 females), mean age 46±15 years at therapy initiation] comparing licensed doses of agalsidase α and β (49). However, a more robust decrease of lyso-GL3 and better reduction in left ventricular mass were reported in patients receiving a higher enzyme dose (49). There is one study reporting kidney benefit of increasing the dose of agalsidase α to 0.2 mg/kg every week in a limited number of patients (50), but no such evidence is reported for agalsidase β. Although current enzyme substitution regimens fail to normalize elevated lyso-GL3, a clear dose- and age-dependent decrease of lyso-GL3 has been observed after therapy (41,49,51), and the recent therapeutic goal initiative recommends to strive at the lowest possible level of lyso-GL3 (35). More importantly, enzyme replacement therapy has limited effect when started late in the course (15,49,52).
Neutralizing Anti-Agalsidase Antibodies
A major reason for treatment failure is the formation of neutralizing antidrug antibodies (ADAs), which is reported to affect 40% of male patients treated with agalsidase β or α, and leads to subsequent decline of GFR and increase in lyso-GL3 (53). Lenders et al. (53) elegantly demonstrated that agalsidase dose escalation may overcome the detrimental inhibitory effects of these antibodies. A potential therapeutic approach has recently been published, highlighting the need to standardize assays and methods for individual dose escalations to obtain a saturated ADA status (54). Furthermore, future prospective studies are warranted to elucidate the clinical effect of ADAs (54). The role of immunosuppressive therapy is unknown.
Initiation of Pharmacologic Therapy: How Early Is Early Enough?
The strategy of “early treatment” of Fabry disease has been a major focus and is based on the experience in classic patients that progressive disease is more frequent when therapy is delayed until irreversible organ damage is manifest (16,17,52). Our experience in a youngish classic symptomatic patient cohort with normal heart, normal measured GFR, and normo/microalbuminuria suggests that enzyme replacement should be initiated within the teens (before the age of 18 years) (41,51,55), with the goal to prevent or delay the progression to irreversible kidney and heart damage. This “window of opportunity” approach is supported by several authors (15,39,44). Earlier start of therapy in childhood has to be decided on individual basis in patients with severe symptoms and signs. Systematic follow-up to define the individual appropriate window for “early therapy,” often at higher age, is mandatory, especially in slowly progressive nonclassic, late-onset patients (usually females) (34,35,40).
New Therapies
The need for more effective therapies for Fabry disease has stimulated research addressing alternative mechanisms to enhance the efficacy of endogenous or infused enzymes. Substrate reduction therapy (56) and gene therapy trials as listed by ClinicalTrials.gov (57) are currently recruiting patients for phase 1–3 studies.
Migalastat, a small-molecule pharmacological chaperone first approved in Europe in 2016 and the US in 2018, was developed as a stabilizer of specific mutant (amenable) forms of α-Gal to facilitate its normal lysosomal trafficking (58,59). In an 18-month phase 3 trial in predominantly female patients, this agent was well tolerated and was associated with a decrease in left ventricular mass index. Migalastat and enzyme replacement therapy had similar effects on kidney function (59). Another phase 3 study showed modest reduction of GL3 in interstitial capillaries and glomerular cells after 6 and 12 months of therapy (58,60). Thus, migalastat could represent an oral monotherapy alternative to enzyme replacement in such patients (59). Notably, the concept of in vitro and in vivo amenability is under scrutiny, especially in patients with lower range (<10%) enzymatic activity (61). Pegunigalsidase α, a novel polyethylene glycol incubated (PEGylated) enzyme replacement agent, has a prolonged half-life and potential benefits regarding immunogenicity compared with agalsidase. Phase 1 and 2 studies, as well as switch (from agalsidase α) and comparative (agalsidase β) studies, have recently been launched (62).
Plasma and Tissue-Specific Markers
There is no single ideal biomarker in Fabry disease. Elevated plasma lyso-GL3 has been designated a hallmark of Fabry disease (63). Currently, measurement of plasma lyso-GL3 has increasingly replaced plasma and urine GL3 as the most significant, and technically more easily measured, noninvasive diagnostic biomarker (64), supplementing standard measurements of leukocyte GLA enzyme activity and genetic analysis. Lyso-GL3 allows better discrimination between patients with classic and nonclassic disease and subjects without Fabry disease. Furthermore, elevated lyso-GL3 has been linked to clinical events and a higher disease burden (21,29,65). Because skewed X-chromosome inactivation may differ between cells and organs in females, a normal plasma lyso-GL3 value does not rule out the existence of Fabry disease (29,64,65). After initiation of enzyme replacement therapy, a rapid reduction in lyso-GL3 levels is seen in classic males, whereas a slower decline or stabilization typically follows in most nonclassic patients and females (49,66). Beyond being a marker of disease, lyso-GL3 is likely also directly involved in disease pathogenesis via the stimulation of inflammatory and fibrotic mechanisms that are upregulated in Fabry disease (67,68). A novel experimental finding suggesting persistent dysregulated inflammatory signaling in spite of agalsidase-induced podocyte GL3 elimination was recently published (69), shedding new light on potential contributions of glycolipid-stimulated inflammatory markers to vascular remodeling and progressive vasculopathy. Standardization of laboratory assays for lyso-GL3 and limited availability of the analysis in many centers are still a challenge. Further elucidation of the specificity of urinary lyso-GL3 analogs is a matter of ongoing research, especially in late-onset variants with cardiac disease (70). Markers of chronic inflammation in Fabry disease are not yet implemented in clinical practice (67).
In tissue biopsies, abundant lysosomal deposits of glycosphingolipids, mainly GL3, are hallmarks of Fabry disease; these are especially conspicuous in podocytes (71,72) and can easily be diagnosed by bedside stereomicroscopy immediately after a kidney biopsy (73). Recently, measurement of abnormal podocyturia has been tested as a potential early diagnostic and prognostic noninvasive tool in ascertainment of progressive disease and disease burden in patients with classic disease (74). Increased podocyturia has even been reported in very young classically affected children (75). Larger-scale validation of the future role of this parameter is needed. General cardiac biomarkers may also be useful in establishing a full assessment and risk profile for the patient (76).
Kidney Biopsies
Routine clinical laboratory tests (eGFR and albuminuria) are insensitive markers of early progressive Fabry nephropathy. On the other hand, the assessment of specific and nonspecific reversible or irreversible histologic changes provides early information on kidney damage, even in patients with normal GFR and normoalbuminuria. These are crucial diagnostic findings for choosing optimal therapeutic strategies and follow-up of high-risk patients (41,55,77–80). In general, a kidney biopsy is recommended in Fabry patients with atypical symptoms and unclear diagnosis, often with normal kidney function, as well as in patients with unexpected disease course and suspected concomitant diseases (15,30,31). A conspicuous finding in recent case series is the discrepant beneficial effect of enzyme replacement therapy on removal of GL3 inclusion from podocytes, and glomerular capillary endothelial and mesangial cells, contrasting with a worrisome lack of vascular protection. In the study of Tøndel et al. (51) in 12 young classic patients [median age 16.5 (range 7–33) years at treatment initiation, 1 female] treated for 5 years, total clearance of podocyte GL3 was obtained in the youngest patient and microalbuminuria normalized in nearly one-half of the patients. These findings were further expanded by Skrunes et al. (41), who reported paired serial kidney biopsies after 10 years of treatment in an expanded cohort [20 classic patients, 12 males, median age 21 (range 7–61) years], confirming a dose-dependent effect on the elimination of podocyte GL3 deposits (Figure 2) contrasting with a persistent failure to protect smooth muscle cells in media layers of kidney arterioles/arteries (41). This finding suggests that current therapy is insufficient to prevent long-term vascular complications. The underscoring of serious vasculopathy, even in young patients, was further highlighted by the randomized multicenter study of 31 classic pediatric males with minimal disease symptoms and normal measured GFR [median age 12 (range 5–18) years] receiving enzyme replacement (agalsidase beta) for 5 years comparing two treatment regimens [0.5 mg/kg every 2 weeks (n=16) or 1.0 mg/kg every 4 weeks (n=15)] (80). Six patients [mean baseline age 15.5 (range 14–17) years] had repeated paired kidney biopsies; arteriopathy (replacement of arterial/arteriolar muscle cells with hyaline-like material) was evident in all baseline biopsies and, surprisingly, showed progression in all but one patient despite a mean reduction of lyso-GL3 of 71%.
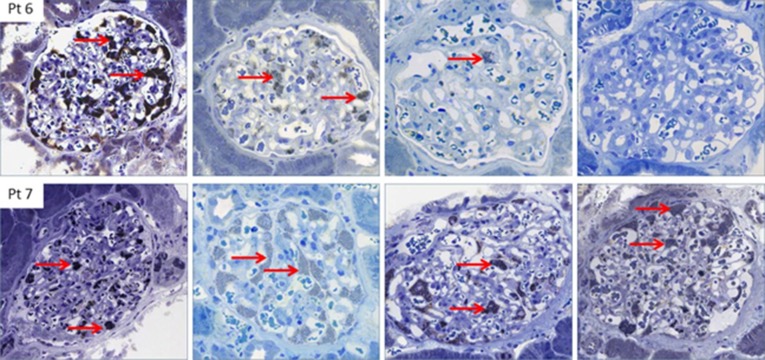
Figure 2.
Histology of Fabry nephropathy. Dose-dependent clearance of podocyte GL3 (red arrows) in two brothers with classic Fabry disease, aged 13 and 15 years at initiation of enzyme replacement therapy. Biopsies are baseline (upper and lower left column, respectively), and after 3, 7, and 13 years of agalsidase therapy. After 6 years of enzyme replacement therapy, the younger brother (upper row) was switched to agalsidase β 1.0 mg/kg every other week. After the switch, the podocytes were virtually completely cleared of GL3, whereas his older brother (lower row), who received a lower cumulative agalsidase dose, continued to have a full podocyte score after a total of 13 years [Skrunes et al. (41)]. Pt, patient.
Systematic kidney biopsies are underused in Fabry disease, and further prospective studies are warranted to help clarify disease mechanisms and potential correlation with clinical phenotypes. Histologic examinations may allow early differentiation between patients with high, low, or no risk of progressive tissue damage, and sometimes unnecessary treatment can be avoided when normal or only minimally affected tissue, or superimposed disease, is identified (20,30,73). Kidney biopsies provide the earliest and most sensitive insights into important cellular and vascular involvement, which are surrogate markers of disease activity. New unbiased stereological histologic methods (79) have shown capacity for assessment of therapeutic response after 1 year of therapy (58,79). To detect and expand potentially relevant pathophysiologic mechanisms, kidney biopsies can be used beyond routine diagnostics by the application of omics-related technologies. A preliminary study from our institution exploited next-generation mRNA sequencing of mRNA from microdissected nephron compartments of serial long-term kidney biopsies with Fabry nephropathy. First analyses pointed toward increased expression of genes, e.g., related to the extracellular matrix, immune response, and inflammation, compared with baseline tissues. Thus, RNA sequencing is feasible in archival Fabry disease kidney biopsies, and may help to delineate potential novel disease markers and therapeutic targets.
Disclosures
Prof. Svarstad reports receiving a grant from Sanofi Genzyme; positions on the advisory boards of Amicus and Sanofi Genzyme; and speaker fees and travel support from Amicus, Sanofi Genzyme, and Shire-Takeda, all outside of the submitted work. Dr. Marti reports receiving grants from Alexion, Amicus, Sanofi Genzyme, and Shire outside of the submitted work.
Funding
This work is supported by the Western Norway Regional Health Authority Helse Vest grant 912233 (to Dr. Marti).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Germain DP: Fabry disease. Orphanet J Rare Dis 5: 30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L: Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med 276: 1163–1167, 1967 [DOI] [PubMed] [Google Scholar]
- 3.Anderson W: A case of “angeiokeratoma”. Br J Dermatol 10: 113–117, 1898 [Google Scholar]
- 4.Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR: Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 138: 338–346, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin Iii HA, Kopp JB: Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81: 122–138, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Colombi A, Kostyal A, Bracher R, Gloor F, Mazzi R, Thölen H: Angiokeratoma corporis diffusum--Fabry’s disease. Helv Med Acta 34: 67–83, 1967 [PubMed] [Google Scholar]
- 7.Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, Sorensen SA, Wilcox WR, Desnick RJ: Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant 24: 2102–2111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ; International Collaborative Fabry Disease Study Group: Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR: Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 52: 353–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck M, Hughes D, Kampmann C, Larroque S, Mehta A, Pintos-Morell G, Ramaswami U, West M, Wijatyk A, Giugliani R; Fabry Outcome Survey Study Group: Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A fabry outcome survey analysis. Mol Genet Metab Rep 3: 21–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, Sunder-Plassmann G; FOS Investigators: Natural course of Fabry disease: Changing pattern of causes of death in FOS - Fabry Outcome Survey. J Med Genet 46: 548–552, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P: Life expectancy and cause of death in males and females with Fabry disease: Findings from the Fabry Registry. Genet Med 11: 790–796, 2009. [DOI] [PubMed]
- 14.Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, Sims K, Waldek S, Pastores GM, Lee P, Eng CM, Marodi L, Stanford KE, Breunig F, Wanner C, Warnock DG, Lemay RM, Germain DP; Fabry Registry: Females with Fabry disease frequently have major organ involvement: Lessons from the Fabry Registry. Mol Genet Metab 93: 112–128, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Schiffmann R, Hughes DA, Linthorst GE, Ortiz A, Svarstad E, Warnock DG, West ML, Wanner C; Conference Participants: Screening, diagnosis, and management of patients with Fabry disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 91: 284–293, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Arends M, Biegstraaten M, Hughes DA, Mehta A, Elliott PM, Oder D, Watkinson OT, Vaz FM, van Kuilenburg ABP, Wanner C, Hollak CEM: Retrospective study of long-term outcomes of enzyme replacement therapy in Fabry disease: Analysis of prognostic factors. PLoS One 12: e0182379, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz A, Germain DP, Desnick RJ, Politei J, Mauer M, Burlina A, Eng C, Hopkin RJ, Laney D, Linhart A, Waldek S, Wallace E, Weidemann F, Wilcox WR: Fabry disease revisited: Management and treatment recommendations for adult patients. Mol Genet Metab 123: 416–427, 2018 [DOI] [PubMed] [Google Scholar]
- 18. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee: Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–424, 2015. [DOI] [PMC free article] [PubMed]
- 19.van der Tol L, Smid BE, Poorthuis BJ, Biegstraaten M, Deprez RH, Linthorst GE, Hollak CE: A systematic review on screening for Fabry disease: Prevalence of individuals with genetic variants of unknown significance. J Med Genet 51: 1–9, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Houge G, Tøndel C, Kaarbøe Ø, Hirth A, Bostad L, Svarstad E: Fabry or not Fabry–a question of ascertainment. Eur J Hum Genet 19: 1111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arends M, Wanner C, Hughes D, Mehta A, Oder D, Watkinson OT, Elliott PM, Linthorst GE, Wijburg FA, Biegstraaten M, Hollak CE: Characterization of classical and nonclassical Fabry disease: A multicenter study. J Am Soc Nephrol 28: 1631–1641, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira S, Ortiz A, Germain DP, Viana-Baptista M, Caldeira-Gomes A, Camprecios M, Fenollar-Cortés M, Gallegos-Villalobos Á, Garcia D, García-Robles JA, Egido J, Gutiérrez-Rivas E, Herrero JA, Mas S, Oancea R, Péres P, Salazar-Martín LM, Solera-Garcia J, Alves H, Garman SC, Oliveira JP: The alpha-galactosidase A p.Arg118Cys variant does not cause a Fabry disease phenotype: Data from individual patients and family studies. Mol Genet Metab 114: 248–258, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desnick RJ, Ioannu YA, Eng CM: α-Galactosidase a deficiency: Fabry disease. In: The Online Metabolic and Molecular Bases of Inherited Disease, edited by Valle D, 8th Ed., Pennsylvania, NY, McGraw Hill Medical, 2007, pp 3733–3774 [Google Scholar]
- 24.Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, Ponzone A, Desnick RJ: High incidence of later-onset Fabry disease revealed by newborn screening. Am J Hum Genet 79: 31–40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu TR, Niu DM: Fabry disease: Review and experience during newborn screening. Trends Cardiovasc Med 28: 274–281, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Pan X, Ouyang Y, Wang Z, Ren H, Shen P, Wang W, Xu Y, Ni L, Yu X, Chen X, Zhang W, Yang L, Li X, Xu J, Chen N: Genotype: A crucial but not unique factor affecting the clinical phenotypes in Fabry disease. PLoS One 11: e0161330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juchniewicz P, Kloska A, Tylki-Szymańska A, Jakóbkiewicz-Banecka J, Węgrzyn G, Moskot M, Gabig-Cimińska M, Piotrowska E: Female Fabry disease patients and X-chromosome inactivation. Gene 641: 259–264, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Doheny D, Srinivasan R, Pagant S, Chen B, Yasuda M, Desnick RJ: Fabry Disease: Prevalence of affected males and heterozygotes with pathogenic GLA mutations identified by screening renal, cardiac and stroke clinics, 1995-2017. J Med Genet 55: 261–268, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Smid BE, van der Tol L, Biegstraaten M, Linthorst GE, Hollak CE, Poorthuis BJ: Plasma globotriaosylsphingosine in relation to phenotypes of Fabry disease. J Med Genet 52: 262–268, 2015 [DOI] [PubMed] [Google Scholar]
- 30.van der Tol L, Svarstad E, Ortiz A, Tøndel C, Oliveira JP, Vogt L, Waldek S, Hughes DA, Lachmann RH, Terryn W, Hollak CE, Florquin S, van den Bergh Weerman MA, Wanner C, West ML, Biegstraaten M, Linthorst GE: Chronic kidney disease and an uncertain diagnosis of Fabry disease: Approach to a correct diagnosis. Mol Genet Metab 114: 242–247, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Smid BE, van der Tol L, Cecchi F, Elliott PM, Hughes DA, Linthorst GE, Timmermans J, Weidemann F, West ML, Biegstraaten M, Lekanne Deprez RH, Florquin S, Postema PG, Tomberli B, van der Wal AC, van den Bergh Weerman MA, Hollak CE: Uncertain diagnosis of Fabry disease: Consensus recommendation on diagnosis in adults with left ventricular hypertrophy and genetic variants of unknown significance. Int J Cardiol 177: 400–408, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Hollak CE, Wijburg FA: Treatment of lysosomal storage disorders: Successes and challenges. J Inherit Metab Dis 37: 587–598, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Rombach SM, Smid BE, Linthorst GE, Dijkgraaf MG, Hollak CE: Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: A systematic review and meta-analysis: Effectiveness of ERT in different disease stages. J Inherit Metab Dis 37: 341–352, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Germain DP, Elliott PM, Falissard B, Fomin VV, Hilz MJ, Jovanovic A, Kantola I, Linhart A, Mignani R, Namdar M, Nowak A, Oliveira JP, Pieroni M, Viana-Baptista M, Wanner C, Spada M: The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts. Mol Genet Metab Rep 19: 100454, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanner C, Arad M, Baron R, Burlina A, Elliott PM, Feldt-Rasmussen U, Fomin VV, Germain DP, Hughes DA, Jovanovic A, Kantola I, Linhart A, Mignani R, Monserrat L, Namdar M, Nowak A, Oliveira JP, Ortiz A, Pieroni M, Spada M, Tylki-Szymańska A, Tøndel C, Viana-Baptista M, Weidemann F, Hilz MJ: European expert consensus statement on therapeutic goals in Fabry disease. Mol Genet Metab 124: 189–203, 2018 [DOI] [PubMed] [Google Scholar]
- 36.El Dib R, Gomaa H, Carvalho RP, Camargo SE, Bazan R, Barretti P, Barreto FC: Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev 7: CD006663, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Dib R, Gomaa H, Ortiz A, Politei J, Kapoor A, Barreto F: Enzyme replacement therapy for Anderson-Fabry disease: A complementary overview of a Cochrane publication through a linear regression and a pooled analysis of proportions from cohort studies. PLoS One 12: e0173358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott PM, Germain DP, Hilz MJ, Spada M, Wanner C, Falissard B: Why systematic literature reviews in Fabry disease should include all published evidence. Eur J Med Genet 62: 103702, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Spada M, Baron R, Elliott PM, Falissard B, Hilz MJ, Monserrat L, Tøndel C, Tylki-Szymańska A, Wanner C, Germain DP: The effect of enzyme replacement therapy on clinical outcomes in paediatric patients with Fabry disease - A systematic literature review by a European panel of experts. Mol Genet Metab 126: 212–223, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Germain DP, Arad M, Burlina A, Elliott PM, Falissard B, Feldt-Rasmussen U, Hilz MJ, Hughes DA, Ortiz A, Wanner C, Weidemann F, Spada M: The effect of enzyme replacement therapy on clinical outcomes in female patients with Fabry disease - A systematic literature review by a European panel of experts. Mol Genet Metab 126: 224–235, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Skrunes R, Tøndel C, Leh S, Larsen KK, Houge G, Davidsen ES, Hollak C, van Kuilenburg ABP, Vaz FM, Svarstad E: Long-term dose-dependent agalsidase effects on kidney histology in Fabry disease. Clin J Am Soc Nephrol 12: 1470–1479, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warnock DG, Thomas CP, Vujkovac B, Campbell RC, Charrow J, Laney DA, Jackson LL, Wilcox WR, Wanner C: Antiproteinuric therapy and Fabry nephropathy: Factors associated with preserved kidney function during agalsidase-beta therapy. J Med Genet 52: 860–866, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng S, Wu L, Nalleballe K, Sharma R, Brown A, Ranabothu S, Kapoor N, Onteddu S: Fabry’s disease and stroke: Effectiveness of enzyme replacement therapy (ERT) in stroke prevention, a review with meta-analysis. J Clin Neurosci 65: 83–86, 2019 [DOI] [PubMed] [Google Scholar]
- 44.Biegstraaten M, Arngrímsson R, Barbey F, Boks L, Cecchi F, Deegan PB, Feldt-Rasmussen U, Geberhiwot T, Germain DP, Hendriksz C, Hughes DA, Kantola I, Karabul N, Lavery C, Linthorst GE, Mehta A, van de Mheen E, Oliveira JP, Parini R, Ramaswami U, Rudnicki M, Serra A, Sommer C, Sunder-Plassmann G, Svarstad E, Sweeb A, Terryn W, Tylki-Szymanska A, Tøndel C, Vujkovac B, Weidemann F, Wijburg FA, Woolfson P, Hollak CE: Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: The European Fabry Working Group consensus document. Orphanet J Rare Dis 10: 36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blom D, Speijer D, Linthorst GE, Donker-Koopman WG, Strijland A, Aerts JM: Recombinant enzyme therapy for Fabry disease: Absence of editing of human alpha-galactosidase A mRNA. Am J Hum Genet 72: 23–31, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krämer J, Lenders M, Canaan-Kühl S, Nordbeck P, Üçeyler N, Blaschke D, Duning T, Reiermann S, Stypmann J, Brand SM, Gottschling T, Störk S, Wanner C, Sommer C, Brand E, Weidemann F: Fabry disease under enzyme replacement therapy-new insights in efficacy of different dosages. Nephrol Dial Transplant 33: 1362–1372, 2018 [DOI] [PubMed] [Google Scholar]
- 47.Ortiz A, Sanchez-Niño MD: Enzyme replacement therapy dose and Fabry nephropathy. Nephrol Dial Transplant 33: 1284–1289, 2018 [DOI] [PubMed] [Google Scholar]
- 48.Warnock DG, Mauer M: Fabry disease: Dose matters. J Am Soc Nephrol 25: 653–655, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arends M, Biegstraaten M, Wanner C, Sirrs S, Mehta A, Elliott PM, Oder D, Watkinson OT, Bichet DG, Khan A, Iwanochko M, Vaz FM, van Kuilenburg ABP, West ML, Hughes DA, Hollak CEM: Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: An international cohort study. J Med Genet 55: 351–358, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiffmann R, Askari H, Timmons M, Robinson C, Benko W, Brady RO, Ries M: Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol 18: 1576–1583, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tøndel C, Bostad L, Larsen KK, Hirth A, Vikse BE, Houge G, Svarstad E: Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol 24: 137–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hopkin RJ, Cabrera G, Charrow J, Lemay R, Martins AM, Mauer M, Ortiz A, Patel MR, Sims K, Waldek S, Warnock DG, Wilcox WR: Risk factors for severe clinical events in male and female patients with Fabry disease treated with agalsidase beta enzyme replacement therapy: Data from the Fabry Registry. Mol Genet Metab 119: 151–159, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Lenders M, Stypmann J, Duning T, Schmitz B, Brand SM, Brand E: Serum-mediated inhibition of enzyme replacement therapy in Fabry disease. J Am Soc Nephrol 27: 256–264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lenders M, Neußer LP, Rudnicki M, Nordbeck P, Canaan-Kühl S, Nowak A, Cybulla M, Schmitz B, Lukas J, Wanner C, Brand SM, Brand E: Dose-dependent effect of enzyme replacement therapy on neutralizing antidrug antibody titers and clinical outcome in patients with Fabry disease. J Am Soc Nephrol 29: 2879–2889, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tøndel C, Kanai T, Larsen KK, Ito S, Politei JM, Warnock DG, Svarstad E: Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron 129: 16–21, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Guérard N, Oder D, Nordbeck P, Zwingelstein C, Morand O, Welford RWD, Dingemanse J, Wanner C: Lucerastat, an iminosugar for substrate reduction therapy: Tolerability, pharmacodynamics, and pharmacokinetics in patients with Fabry disease on enzyme replacement. Clin Pharmacol Ther 103: 703–711, 2018 [DOI] [PubMed] [Google Scholar]
- 57. Autologous Stem Cell Transplantation of Cells Engineered to Express Alpha-Galactosidase A in Patients With Fabry Disease. Sponsor: University Health Network, Toronto. ClinicalTrials.gov Identifier: NCT02800070. Available at: https://clinicaltrials.gov/ct2/show/NCT02800070. Accessed February 6, 2020.
- 58.Mauer M, Sokolovskiy A, Barth JA, Castelli JP, Williams HN, Benjamin ER, Najafian B: Reduction of podocyte globotriaosylceramide content in adult male patients with Fabry disease with amenable GLA mutations following 6 months of migalastat treatment. J Med Genet 54: 781–786, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes DA, Nicholls K, Shankar SP, Sunder-Plassmann G, Koeller D, Nedd K, Vockley G, Hamazaki T, Lachmann R, Ohashi T, Olivotto I, Sakai N, Deegan P, Dimmock D, Eyskens F, Germain DP, Goker-Alpan O, Hachulla E, Jovanovic A, Lourenco CM, Narita I, Thomas M, Wilcox WR, Bichet DG, Schiffmann R, Ludington E, Viereck C, Kirk J, Yu J, Johnson F, Boudes P, Benjamin ER, Lockhart DJ, Barlow C, Skuban N, Castelli JP, Barth J, Feldt-Rasmussen U: Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet 54: 288–296, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, Feliciani C, Shankar SP, Ezgu F, Amartino H, Bratkovic D, Feldt-Rasmussen U, Nedd K, Sharaf El Din U, Lourenco CM, Banikazemi M, Charrow J, Dasouki M, Finegold D, Giraldo P, Goker-Alpan O, Longo N, Scott CR, Torra R, Tuffaha A, Jovanovic A, Waldek S, Packman S, Ludington E, Viereck C, Kirk J, Yu J, Benjamin ER, Johnson F, Lockhart DJ, Skuban N, Castelli J, Barth J, Barlow C, Schiffmann R: Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med 375: 545–555, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Lenders M, Stappers F, Niemietz C, Schmitz B, Boutin M, Ballmaier PJ, Zibert A, Schmidt H, Brand SM, Auray-Blais C, Brand E: Mutation-specific Fabry disease patient-derived cell model to evaluate the amenability to chaperone therapy. J Med Genet 56: 548–556, 2019 [DOI] [PubMed] [Google Scholar]
- 62.Schiffmann R, Goker-Alpan O, Holida M, Giraldo P, Barisoni L, Colvin RB, Jennette CJ, Maegawa G, Boyadjiev SA, Gonzalez D, Nicholls K, Tuffaha A, Atta MG, Rup B, Charney MR, Paz A, Szlaifer M, Alon S, Brill-Almon E, Chertkoff R, Hughes D: Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. J Inherit Metab Dis 42: 534–544, 2019 [DOI] [PubMed] [Google Scholar]
- 63.Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ: Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A 105: 2812–2817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nowak A, Mechtler TP, Desnick RJ, Kasper DC: Plasma LysoGb3: A useful biomarker for the diagnosis and treatment of Fabry disease heterozygotes. Mol Genet Metab 120: 57–61, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Nowak A, Mechtler T, Kasper DC, Desnick RJ: Correlation of Lyso-Gb3 levels in dried blood spots and sera from patients with classic and Later-Onset Fabry disease. Mol Genet Metab 121: 320–324, 2017 [DOI] [PubMed] [Google Scholar]
- 66.Sakuraba H, Togawa T, Tsukimura T, Kato H: Plasma lyso-Gb3: A biomarker for monitoring Fabry patients during enzyme replacement therapy. Clin Exp Nephrol 22: 843–849, 2018 [DOI] [PubMed] [Google Scholar]
- 67.Rozenfeld P, Feriozzi S: Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab 122: 19–27, 2017 [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Niño MD, Sanz AB, Carrasco S, Saleem MA, Mathieson PW, Valdivielso JM, Ruiz-Ortega M, Egido J, Ortiz A: Globotriaosylsphingosine actions on human glomerular podocytes: Implications for Fabry nephropathy. Nephrol Dial Transplant 26: 1797–1802, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Braun F, Blomberg L, Brodesser S, Liebau MC, Schermer B, Benzing T, Kurschat CE: Enzyme replacement therapy clears Gb3 deposits from a podocyte cell culture model of Fabry disease but fails to restore altered cellular signaling. Cell Physiol Biochem 52: 1139–1150, 2019 [DOI] [PubMed] [Google Scholar]
- 70. Auray-Blais C, Lavoie P, Boutin M, Ntwari A, Hsu TR, Huang CK, Niu DM: Biomarkers associated with clinical manifestations in Fabry disease patients with a late-onset cardiac variant mutation. Clin Chim Acta 466: 185–193, 2017. [DOI] [PubMed]
- 71.Gubler MC, Lenoir G, Grünfeld JP, Ulmann A, Droz D, Habib R: Early renal changes in hemizygous and heterozygous patients with Fabry’s disease. Kidney Int 13: 223–235, 1978 [DOI] [PubMed] [Google Scholar]
- 72.Alroy J, Sabnis S, Kopp JB: Renal pathology in Fabry disease. J Am Soc Nephrol 13[Suppl 2]: S134–S138, 2002 [PubMed] [Google Scholar]
- 73.Svarstad E, Leh S, Skrunes R, Kampevold Larsen K, Eikrem Ø, Tøndel C: Bedside stereomicroscopy of Fabry kidney biopsies: An easily available method for diagnosis and assessment of sphingolipid deposits. Nephron 138: 13–21, 2018 [DOI] [PubMed] [Google Scholar]
- 74.Fall B, Scott CR, Mauer M, Shankland S, Pippin J, Jefferson JA, Wallace E, Warnock D, Najafian B: Urinary podocyte loss is increased in patients with Fabry disease and correlates with clinical severity of Fabry nephropathy. PLoS One 11: e0168346, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Politei J, Alberton V, Amoreo O, Antongiovanni N, Arán MN, Barán M, Cabrera G, Di Pietrantonio S, Durand C, Fainboim A, Frabasil J, Pizarro FG, Iotti R, Liern M, Perretta F, Ripeau D, Toniolo F, Trimarchi H, Rivas DV, Wallace E, Schenone AB: Clinical parameters, LysoGb3, podocyturia, and kidney biopsy in children with Fabry disease: Is a correlation possible? Pediatr Nephrol 33: 2095–2101, 2018 [DOI] [PubMed] [Google Scholar]
- 76.Weidemann F, Beer M, Kralewski M, Siwy J, Kampmann C: Early detection of organ involvement in Fabry disease by biomarker assessment in conjunction with LGE cardiac MRI: Results from the SOPHIA study. Mol Genet Metab 126: 169–182, 2019 [DOI] [PubMed] [Google Scholar]
- 77.Fogo AB, Bostad L, Svarstad E, Cook WJ, Moll S, Barbey F, Geldenhuys L, West M, Ferluga D, Vujkovac B, Howie AJ, Burns A, Reeve R, Waldek S, Noël LH, Grünfeld JP, Valbuena C, Oliveira JP, Müller J, Breunig F, Zhang X, Warnock DG; all members of the International Study Group of Fabry Nephropathy (ISGFN): Scoring system for renal pathology in Fabry disease: Report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant 25: 2168–2177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skrunes R, Svarstad E, Kampevold Larsen K, Leh S, Tøndel C: Reaccumulation of globotriaosylceramide in podocytes after agalsidase dose reduction in young Fabry patients. Nephrol Dial Transplant 32: 807–813, 2017 [DOI] [PubMed] [Google Scholar]
- 79.Najafian B, Svarstad E, Bostad L, Gubler MC, Tøndel C, Whitley C, Mauer M: Progressive podocyte injury and globotriaosylceramide (GL-3) accumulation in young patients with Fabry disease. Kidney Int 79: 663–670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramaswami U, Bichet DG, Clarke LA, Dostalova G, Fainboim A, Fellgiebel A, Forcelini CM, An Haack K, Hopkin RJ, Mauer M, Najafian B, Scott CR, Shankar SP, Thurberg BL, Tøndel C, Tylki-Szymanska A, Bénichou B, Wijburg FA: Low-dose agalsidase beta treatment in male pediatric patients with Fabry disease: A 5-year randomized controlled trial. Mol Genet Metab 127: 86–94, 2019 [DOI] [PubMed] [Google Scholar]
- 81.Eikrem Ø, Skrunes R, Tøndel C, Leh S, Houge G, Svarstad E, Marti HP: Pathomechanisms of renal Fabry disease. Cell Tissue Res 369: 53–62, 2017 [DOI] [PubMed] [Google Scholar]