Introduction
In general, nephrologists give little thought to contraception, leaving this task to other practitioners, but the significant risks associated with drugs routinely prescribed to treat kidney disease; the potential for pregnancy-associated adverse outcomes in women with CKD; and the effect that hormones may have on underlying hypertension and proteinuria along with the potential associated side effects merit some basic knowledge of various contraceptive options. However, in a recent survey of Canadian and American nephrologists, the vast majority of participants provided reproductive counseling to fewer than one woman a month, with 65% citing lack of confidence in the subject matter as the primary barrier due to lack of training and personal knowledge (1).
Rationale for Contraception in CKD
Nephrologists frequently prescribe potentially teratogenic medications. All prescriptions for inhibitors of the renin-angiotensin-aldosterone system (RAAS; mycophenolate mofetil, cyclophosphamide, and rituximab) should be preceded by confirmation of a negative pregnancy test and a discussion of the potential for associated teratogenicity along with a review of effective contraceptive options. Teratogenicity associated with exposure to RAAS inhibition includes cardiac and kidney defects, whereas exposure to mycophenolate mofetil is associated with increased rates of pregnancy loss as well as characteristic anomalies, including cleft lip and palate, absent auditory canals, hypertelorism, and microtia. Cyclophosphamide is also associated with an increased risk of fetal loss, and it is highly teratogenic, causing multiple anomalies along with growth issues. Rituximab is associated with neonatal B cell depletion, the long-term effects of which are unclear.
In women with CKD, pregnancy is associated with significant maternal and fetal risks, including the risk for a flare of the underlying kidney disease (e.g., lupus nephritis), progression of kidney dysfunction, and the significant potential for adverse pregnancy complications (e.g., preeclampsia, prematurity, and poor fetal growth). Overall rates of preeclampsia are tenfold higher than in the general population, whereas the risk for prematurity is increased sixfold (2). Furthermore, the pregnancy-associated risk for poor maternal and fetal outcomes increases with the severity of preconception CKD, with woman who attempt pregnancy at the most advanced stages of CKD potentially requiring dialysis in 20% of pregnancies or delivering a small baby prior to 37 weeks of gestation in excess of 50% of pregnancies (3). These adverse outcomes in turn increase both the mother’s and baby’s risks for future vascular disease, contributing to future morbidity and mortality in both generations. As such, pregnancies must be planned, and effective methods of contraception are needed to ensure that pregnancies do not occur prior to maternal optimization.
Sex Hormones and Their Effects on the Kidneys
CKD is well known to affect sex hormones with progressive hypothalamic pituitary gonadal axis dysfunction causing loss of the cyclical release of gonadotropin-releasing hormone, leading to a loss of the pulsatile release of luteinizing hormone and follicle-stimulating hormone and a decrease in the release of estradiol as kidney function worsens. The reverse is also true, with many animal studies demonstrating the effects of sex hormones on the kidney. Conflicting evidence exists, but CKD may progress slower in women than men, and certainly, the incidence and prevalence of KRT are higher in men than women, suggesting that sex hormones may have a role in the susceptibility of progressive kidney disease.
Although endogenous estradiol may be renoprotective in women, the same may not hold true for exogenous estradiol. Estrogen/progesterone oral combination pills have been shown to activate the RAAS, have been found to cause a small increase in BP in the general population (by as much as 8/6 mm Hg) (4), and have been associated with a higher incidence of development of albuminuria in patients with diabetes mellitus (5). This is thought to be related to its first-pass hepatic metabolism. Interestingly, the use of the combined hormonal contraception patch, which does not involve first-pass hepatic metabolism, has a significantly lower activation of the RAAS and also causes a blunting of RAAS response to an orthostatic stress (6). Exogenous progesterone may also have negative consequences on the kidneys, with a small study showing the higher the progestational and androgenic activity of the progesterone used in oral contraceptive, the higher the upregulation of the RAAS (7).
Contraceptive Methods and Their Pros and Cons
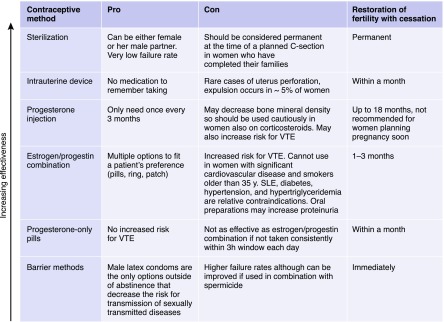
When counseling patients with CKD on their contraception options, it is important to understand the pros and cons of each option. There are currently five main categories of contraception: estrogen/progestin combination, progestin only, barrier methods, intrauterine device (IUD), and sterilization (Box 1). Also, some women, because of their cultural or religious beliefs, opt for natural family planning. The ultimate decision should be guided by patient preference as well as her comorbid conditions.
Box 1.
Contraception pros and cons. Contraceptive options have different pros and cons, vary in effectiveness as well as the time required for full fertility to resume.
Estrogen-containing contraception comes in several options, including pills, patches, and vaginal rings. A key contraindication to these agents is the risk for development of venous thromboembolism (VTE). There is conflicting evidence, but the pills, patches, and vaginal rings all probably increase the risk to similar levels from a baseline of 1–5 events per 10,000 in nonpregnant nonusers to 3–15 events per 10,000 woman years in users (4,8). Women taking these agents are also at an increased risk for myocardial infarction and stroke, and therefore, important relative contraindications to these agents in the setting of kidney disease include diabetes mellitus, SLE, hypertension, and hypertriglyceridemia. Given the potential for increased BP and worsening proteinuria as well as a flare of lupus nephritis with these agents, these conditions should be monitored closely with initiation. Although there are no particular monitoring guidelines, we would typically recommend BP self-monitoring where possible and reassess hypertension and proteinuria within 1–3 months post-initiation depending on the severity at baseline, unless symptoms (e.g., worsening edema or symptoms of systemic lupus) dictate a need to reassess earlier. In women with nephrotic or antiphospholipid antibody syndrome who are already at high risk for VTE, we would recommend avoiding these agents.
Progestin-only options do not significantly affect the BP, and they do not worsen proteinuria. Progestin-only pills and progestin-impregnated IUDs do not seem to increase the risk of VTE. Limited evidence suggests that long-acting injectable progestin may increase the risk of VTE twofold (4). The pills, however, must be taken within the same 3-hour window each day for best effectiveness. The progestin, Drospirenone, has antimineralocorticoid activity, and therefore, it could theoretically cause hyperkalemia in at-risk women, but there are no studies in women with CKD. Long-acting injectable formulations are associated with bone loss that has been documented to be reversible after cessation (9), but alternative options should be considered in women on or who may require prednisone therapy.
In women who cannot tolerate hormonal contraception, barrier method, IUD, and sterilization are alternatives. The barrier method does have higher rates of failure than the other methods, but it decreases the risk of transmission of sexually transmitted disease. New versions of hormone-impregnated IUDs are highly effective and can be used without concern even in those women on immunosuppression (10), but screening for sexually transmitted diseases prior to insertion is recommended to avoid pelvic inflammatory disease. Sterilization of the woman or her male partner should be considered permanent.
Natural family planning methods allow women to estimate peak fertility, and therefore, intercourse can be avoided. Various degrees of effectiveness are reported in the literature, with a high degree of reported failures being related to improper application of these methods (up to 20%) (11). There are no studies of the effectiveness of natural family planning methods in women with CKD, but given the irregularity of menstrual cycles often present in this population, the failure rate of natural family planning methods may be even higher, exposing women and their babies to the harm associated with unplanned pregnancies.
It is the basic right of every woman to decide freely and responsibly the number, timing, and spacing of her children and to be able to attain the highest level of reproductive health. As such, nephrologists need to enter the conversation to promote shared decision making with young women with CKD along with their other health care providers who will look to nephrology for guidance.
Disclosures
Dr. Burgner and Dr. Hladunewich have nothing to disclose.
Acknowledgments
The content of this article does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed therein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hendren EM, Reynolds ML, Mariani LH, Zee J, O’Shaughnessy MM, Oliverio AL, Moore NW, Hill-Callahan P, Rizk DV, Almanni S, Twombley KE, Herreshoff E, Nester CM, Hladunewich MA: Confidence in women’s health: A cross border survey of adult nephrologists. J Clin Med 8: E176, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H: A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 10: 1964–1978, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccoli GB, Cabiddu G, Attini R, Vigotti FN, Maxia S, Lepori N, Tuveri M, Massidda M, Marchi C, Mura S, Coscia A, Biolcati M, Gaglioti P, Nichelatti M, Pibiri L, Chessa G, Pani A, Todros T: Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 26: 2011–2022, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG: ACOG practice bulletin No. 206: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 133: e128–e150, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed SB, Hovind P, Parving HH, Rossing P, Price DA, Laffel LM, Lansang MC, Stevanovic R, Fisher ND, Hollenberg NK: Oral contraceptives, angiotensin-dependent renal vasoconstriction, and risk of diabetic nephropathy. Diabetes Care 28: 1988–1994, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Odutayo A, Cherney D, Miller J, Ahmed SB, Lai V, Dunn S, Pun N, Moineddin R, Hladunewich MA: Transdermal contraception and the renin-angiotensin-aldosterone system in premenopausal women. Am J Physiol Renal Physiol 308: F535–F540, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Sarna MA, Hollenberg NK, Seely EW, Ahmed SB: Oral contraceptive progestins and angiotensin-dependent control of the renal circulation in humans. J Hum Hypertens 23: 407–414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tepper NK, Dragoman MV, Gaffield ME, Curtis KM: Nonoral combined hormonal contraceptives and thromboembolism: A systematic review. Contraception 95: 130–139, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harel Z, Johnson CC, Gold MA, Cromer B, Peterson E, Burkman R, Stager M, Brown R, Bruner A, Coupey S, Hertweck P, Bone H, Wolter K, Nelson A, Marshall S, Bachrach LK: Recovery of bone mineral density in adolescents following the use of depot medroxyprogesterone acetate contraceptive injections. Contraception 81: 281–291, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Ramhendar T, Byrne P: Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: A retrospective case review. Contraception 86: 288–289, 2012 [DOI] [PubMed] [Google Scholar]
- 11.A prospective multicentre trial of the ovulation method of natural family planning. II. The effectiveness phase. Fertil Steril 36: 591–598, 1981 [DOI] [PubMed] [Google Scholar]