Abstract
Prior studies demonstrated that the irreversible ERBB1/2/4 inhibitor neratinib caused plasma membrane-associated mutant K-RAS to localize in intracellular vesicles, concomitant with its degradation. Herein, we discovered that neratinib interacted with the chemically distinct irreversible ERBB1/2/4 inhibitor afatinib to reduce expression of ERBB1, ERBB2, K-RAS and N-RAS; this was associated with greater-than-additive cell killing of pancreatic tumor cells. Knock down of Beclin1, ATG16L1, Rubicon or cathepsin B significantly lowered the ability of neratinib to reduce ERBB1 and K-RAS expression, and to cause tumor cell death. Knock down of ATM-AMPK suppressed vesicle formation and knock down of cathepsin B-AIF significantly reduced neratinib lethality. PKG phosphorylates K-RAS and HMG CoA reductase inhibitors reduce K-RAS farnesylation both of which remove K-RAS from the plasma membrane, abolishing its activity. Neratinib interacted with the PKG activator sildenafil and the HMG CoA reductase inhibitor atorvastatin to further reduce K-RAS expression, and to further enhance cell killing. Neratinib is also a Ste20 kinase family inhibitor and in carcinoma cells, and hematopoietic cancer cells lacking ERBB1/2/4, it reduced K-RAS expression and the phosphorylation of MST1/3/4/Ezrin by ~30%. Neratinib increased LATS1 phosphorylation as well as that of YAP and TAZ also by ~30%, caused the majority of YAP to translocate into the cytosol and reduced YAP/TAZ protein levels. Neratinib lethality was enhanced by knock down of YAP. Neratinib, in a Rubicon-dependent fashion, reduced PAK1 phosphorylation and that of its substrate Merlin. Our data demonstrate that neratinib coordinately suppresses both mutant K-RAS and YAP function to kill pancreatic tumor cells.
Keywords: RAS, neratinib, sildenafil, HDAC inhibitor, YAP, Hippo, statin
Introduction
Inhibition of mutant RAS signaling has been a “holy grail” in the field of cancer therapeutics for over thirty years. Although attempts are being made to develop agents that directly inhibit mutant RAS function, none have yet entered phase I clinical evaluation. We discovered that the FDA approved drug neratinib is not only an irreversible ERBB1/2/4 inhibitor but is a drug that can act to rapidly down-regulate the expression of other RTKs as well as mutant RAS proteins [1–4].
RAS proteins are small GTPases that regulate cellular signaling cascades downstream of receptor tyrosine kinases to control cell growth, proliferation, and differentiation. The three RAS isoforms, H-, N-, and K-RAS are expressed in mammalian cells; K-RAS is mutated in ~90% of pancreatic tumors. To act as signal transducers, RAS proteins must be localized to the inner leaflet of the plasma membrane (PM) by a COOH-terminal membrane anchor [5]. For K-RAS, the anchor comprises a covalently attached COOH-terminal cysteine farnesyl-methyl ester operating together with a polybasic motif of 6 lysine residues that provide electrostatic membrane affinity. Inhibitors of HMG CoA reductase, i.e. the cholesterol reducing drugs called statins, lower the levels of farnesyl substrate and reduce the amount of K-RAS that can localize in the PM [3]. Many published meta-analyses have shown that statin use enhances the survival time of pancreatic cancer patients, with one study also linking the AMPK activator metformin to prolonged survival [6–10]. Maintenance of K-RAS on the PM additionally requires the chaperone protein PDEδ[11]. PKG directly phosphorylates K-RAS at position S181 [5]. S181 phosphorylation causes dissociation of the K-RAS V12, with a t 1/2 ~ 38 min, from the PM into the endosomal compartment. In these studies, PKG activity was elevated using sildenafil (as a PDE5/6 inhibitor) or by activating the AMPK, which elevated nitric oxide levels – activating guanylyl synthase, and hence PKG. There are as yet no meta-analyses examining the impact of PDE5/6 inhibitors in pancreatic cancer patients [2].
Neratinib was developed to inhibit ERBB1/2/4. It was subsequently postulated via chemical biology techniques that it is also equipotent at inhibiting multiple Ste20 family kinases: MST3, MST4, MAP4K5 and MAP4K3 [12]. In contrast, the chemically distinct irreversible ERBB1/2/4 inhibitor afatinib was stated to not inhibit Ste20 family kinases. The safe C max of neratinib in a patient’s plasma is ~150 nM and the calculated in vitro IC50 inhibitory concentrations of neratinib for kinases below 100 nM also include: GCN2, MAP4K1, MAP3K4, MST2, SLK, YSK1 and YSK4. Thus, the impact of neratinib on tumor cell biology may be multifactorial and likely ERBB family receptor independent. For the kinases neratinib was claimed to inhibit at sub-10 nM concentrations the following information has been published: MST3/4 control the apical brush border of epithelial cells; the major dose limiting toxicity of neratinib is diarrhea, arguing that neratinib is acting in an on-target MST3/4-dependent fashion to cause this event. MST3/4 also coordinate the phosphorylation of cytoskeletal proteins such as Ezrin / Radixin / Moesin (ERM) family to regulate plasma membrane ruffling [13, 14]. MAP4K5 is an apical kinase that interacts with GTP binding proteins downstream of G Protein Coupled Receptors, and links GPCR signaling into MAPK pathways [15]. MAP4K5 and MAP4K3 phosphorylate and activate the LATS1/2 kinases that in turn phosphorylate and inhibit YAP/TAZ, the main effectors of the Hippo Pathway [16] (Supplemental Figure 1). Signaling by MAP4K3 has also been shown to play an important role in amino acid signaling to mTOR/p70 S6K [17, 18]. Thus, neratinib may be able to prevent signaling by mutant K-RAS and by the Hippo Pathway; events that collectively are detrimental to the growth and viability of pancreatic cancer cells.
MST3/MST4 are expressed in both solid and in blood cancer cells. We hypothesized that, via inhibition of MST3/4, neratinib would be able to alter the biology of solid and liquid tumor cells independently of ERBB receptor expression. Our data proves that in solid and liquid tumor cells that neratinib prevented the autophosphorylation of MST3/4; that it reduced the expression of K-RAS and that it inactivated Hippo / YAP Pathway function.
Results
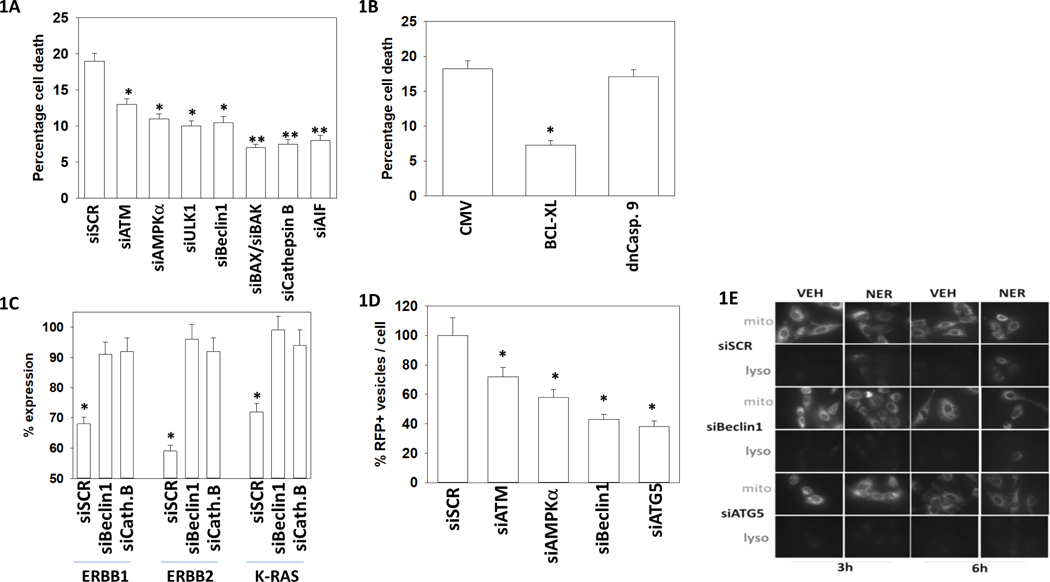
The irreversible ERBB1/2/4 inhibitor neratinib as a single agent, and at low clinically relevant concentrations, causes ~15–20% cell killing of mutant K-RAS expressing tumor cells in 12 hours; this is comparable to the level of killing induced within this time-frame in a BT474 HER2+ mammary carcinoma cell (Figure 1A, not shown). Knock down of ATM, AMPKα, Beclin1, ATG5, [BAX+BAK], cathepsin B and AIF significantly reduced neratinib lethality. Over-expression of BCL-XL, to a significantly greater extent than expression of dominant negative caspase 9, suppressed neratinib lethality (Figure 1B). Knock down of Beclin1 or cathepsin B significantly reduced the ability of neratinib to lower the expression of ERBB1, ERBB2 and of K-RAS (Figure 1C). Cathepsin B is localized in endosomes/lysosomes, and knock down of ATM, AMPKα or Beclin1 significantly reduced the ability of neratinib to cause acidic endosome/lysosome formation as measured using an LC3-GFP-RFP construct (Figure 1D). Neratinib did not alter mitochondrial membrane potential but did increase lysosome acidification in a Beclin1/ATG5-dependent fashion (Figure 1E). Together with data from our prior studies, this information links an ATM-AMPKα-ULK1-autophagosome-autolysosome pathway to the degradation of ERBB1 and mutant K-RAS and to pancreatic tumor cell death [1–4].
Figure 1. Neratinib lethality is mediated by autophagy and mitochondrial dysfunction; lysosome formation and ERBB1/K-RAS digestion requires cathepsin B.
A. PANC1 cells were transfected with a scrambled control siRNA (siSCR) or were transfected to knockdown the expression of the indicated proteins. Twenty-four hours after transfection cells were treated with vehicle control or neratinib [100 nM] for 12 hr. Cells were then isolated, and viability determined by trypan blue exclusion assay; data presented are for neratinib exposure, with vehicle cell death < 3% (n = 3; mean +/− SD) * p < 0.05 less than siSCR value; ** p < 0.01 less than siSCR value. B. PANC1 cells were transfected with an empty vector control plasmid (CMV) or were transfected with plasmids to express the indicated proteins: BCL-XL; dominant negative caspase 9. Twenty-four hours after transfection cells were treated with vehicle control or neratinib (100 nM) for 12 hr. Cells were then isolated, and viability determined by trypan blue exclusion assay (n = 3; mean +/− SD) * p < 0.05 less than CMV value. C. PANC1 cells were transfected with a scrambled siRNA control molecule or siRNA molecules to knockdown the expression of Beclin1 or cathepsin B. Twenty-four hours after transfection cells were treated with vehicle control or with neratinib (100 nM) for 6 hr. Cells were fixed in place after 6h and immunofluorescence staining performed to determine the total expression of ERBB1, ERBB2, and K-RAS (n = 3 +/− SD) *p < 0.05 less than vehicle control. D. PANC1 cells were transfected with a plasmid to express LC3-GFP-RFP and in parallel transfected with a scrambled siRNA molecule or with siRNA molecules to knock down expression of the indicated proteins. Twenty-four h after transfection cells were treated with vehicle control or with neratinib (100 nM) for 8h. Cells were imaged at 60X magnification and the mean number of red punctae per cell determined in at least 40 cells (in in siSCR + vehicle had less than 0.2 punctae per cell; siSCR + neratinib 6.5 punctae per cell were counted). (n = 3 +/− SD) * p < 0.05 less than value in siSCR cells. E. PANC1 cells were transfected with a scrambled siRNA molecule or with siRNA molecules to knock down expression of the indicated proteins. Twenty-four h after transfection cells were treated with mito-tracker green and lyso-tracker red and then treated with vehicle control or with neratinib (100 nM) for 3h. or 6h. Cells were imaged at 60X magnification (a representative of three independent assays).
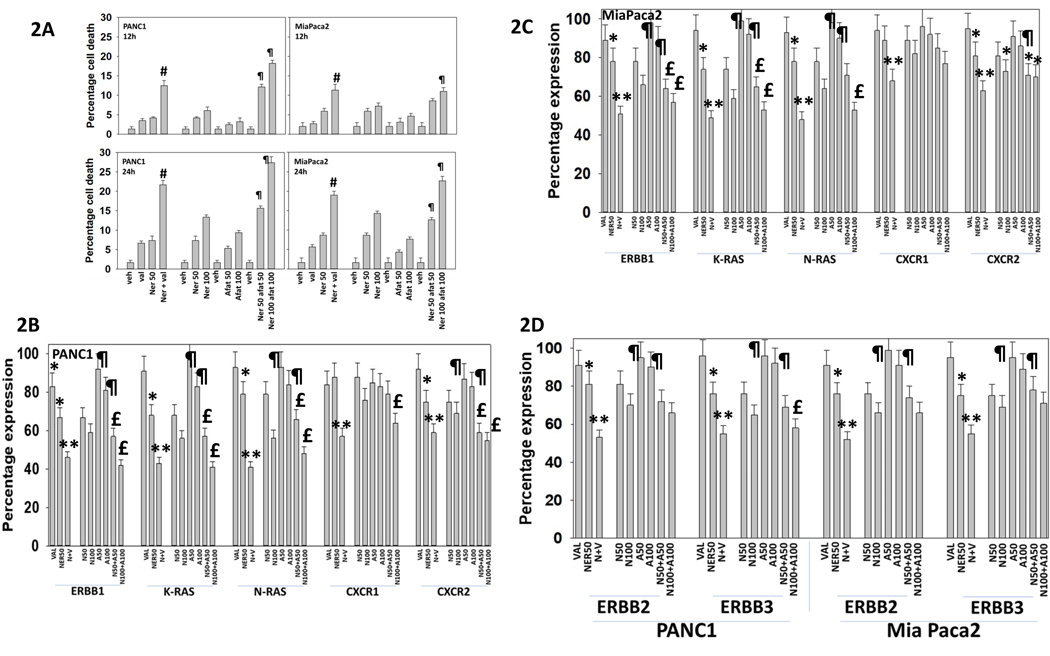
The irreversible ERBB1/2/4 inhibitor afatinib has a restricted specificity for ERBB1/2/4, whereas the specificity of neratinib has been proposed to be less selective, potently inhibiting both the ERBB1/2/4 receptor tyrosine kinase family and well as multiple members of the Ste20 family of serine/threonine kinases [12]. We postulated that due to their potential overlapping but distinct mechanisms of action, neratinib and afatinib may interact to enhance tumor cell killing and further reduce ERBB1, ERBB2, K-RAS and N-RAS levels. In established pancreatic and PDX head and neck squamous carcinoma cells, neratinib and afatinib interacted in a dose-dependent and time-dependent fashion to increase to cell killing (Figure 2A and Supplemental Figure 1A). In all cells tested, neratinib and afatinib interacted to reduce expression of ERBB1/2/3, K-RAS and N-RAS (Figures 2B, 2C and 2D; Supplemental Figures 2B and 2C). Neratinib combined with afatinib was partially capable of reducing expression of the IL-8/CXCL-1 receptors, CXCR1 and CXCR2. The drug combination of neratinib and valproate was included in our analyses as a positive control; [neratinib + valproate] interacted to potently reduce the expression of ERBB1/2/3, K-RAS, N-RAS, CXCR1 and CXCR2. We have shown that neratinib can enhance autophagosome formation that degrades HDAC6 and HSP90; [neratinib + valproate] significantly more so than [neratinib + afatinib] reduced expression of HDAC6 & HSP90 (Supplemental Figure 3).
Figure 2. Neratinib enhances afatinib lethality in vitro.
A. PANC1 and MiaPaca2 tumor cells were treated with vehicle control, neratinib (50, 100 nM), afatinib (50, 100 nM), sodium valproate (250 μM) or the drugs in combination as indicated for 24h. Cells were isolated, and viability determined by trypan blue exclusion assay (n = 3 +/− SD) # p < 0.05 greater than neratinib alone value; p < 0.05 greater than numeric combination of corresponding neratinib alone and afatinib alone values. B.-D. PANC1 and MiaPaca2 cells were treated with vehicle control, neratinib (50, 100 nM), afatinib (50, 100 nM), sodium valproate (250 μM) or the drugs in combination as indicated for 6h. Cells were fixed in place and immunostaining performed to determine the fluorescence intensity for ERBB1, ERBB2, ERBB3, K-RAS, N-RAS, CXCR1 and CXCR2 (n = 3 +/− SD) * p < 0.05 less than vehicle control; ** p < 0.05 less than neratinib alone; ¶ p < 0.05 greater than corresponding neratinib value; £ p < less than corresponding neratinib alone value.
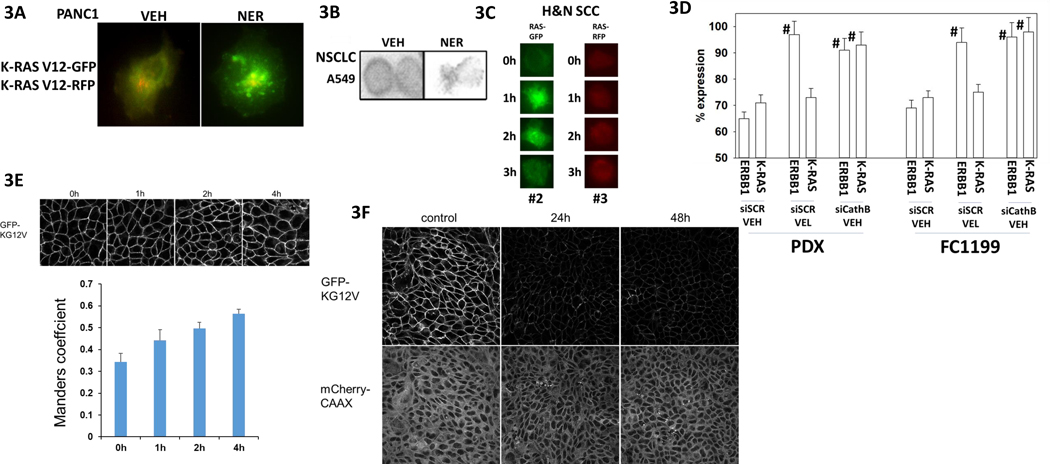
In PANC1 pancreatic adenocarcinoma cells transfected to express K-RAS V12 – GFP and K-RAS V12 – RFP, neratinib caused the proteins to colocalize in small and large intracellular vesicles (Figure 3A). Neratinib down-regulated mutant K-RAS in NSCLC cells (Figure 3B). In head & neck squamous carcinoma cells, neratinib was also competent to cause K-RAS V12 – GFP and K-RAS V12 – RFP to become vesicularized (Figure 3C). As previously reported by us, exposure of a K-RAS V12 / p53 K275H GEMM cell line (FC1199, also known as KPC) and a PDX model of pancreatic cancer (J000077973) that also expresses mutant K-RAS and mutant p53 to the proteasome inhibitor Velcade prevented the neratinib-induced degradation of ERBB1 but did not alter degradation of K-RAS (Figure 3D) [1]. As a positive control, knock down of the lysosomal protease cathepsin B prevented the degradation of both ERBB1 and K-RAS. MDCK cells stably co-expressing the endomembrane marker mCherry-CAAX and K-RAS V12 – GFP were treated with neratinib, cells were fixed at each timepoint and imaged using a confocal microscope [5]. K-RAS mislocalization from the plasma membrane was quantified as the colocalization of K-RAS V12 – GFP and mCherry-CAAX using Manders coefficients. The Manders coefficient provides an estimate of the fraction of K-RAS displaced from the plasma membrane. Neratinib acutely mislocalized K-RAS V12 – GFP from the plasma membrane (Figure 3E). Furthermore, neratinib decreased total K-RAS V12 – GFP expression after 24h and 48h (Figure 3F). The distribution and expression of mCherry CAAX, however, was unchanged.
Figure 3. Neratinib rapidly relocates K-RAS V12 away from the plasma membrane resulting in its degradation.
A. PANC1 cells were transfected with plasmids to express K-RAS V12 – GFP and K-RAS V12 – RFP. Twenty-four h after transfection cells were treated with vehicle control or with neratinib (50 nM). Cells were examined at 60X magnification 120 min after neratinib exposure. Images were taken in the green and red fluorescent channels and merged in Photoshop CS5 (a representative merged image is presented). B. A549 NSCLC cells were treated with vehicle control or with neratinib (50 nM) for 6h. Cells were fixed in place and immunofluorescence staining performed at 60X magnification to detect the expression of K-RAS. C. PDX#2 and PDX#3 H&N SSC cells were transfected with plasmids to express K-RAS V12 – GFP and K-RAS V12 – RFP, respectively. Cells were treated with vehicle control or with neratinib (50 nM) for 0–3h. Representative images are presented (60X magnification). D. FC1199 GEMM cells and PDX human pancreatic cancer cells were transfected with a scrambled siRNA molecules or siRNA molecules to knock down the expression of cathepsin B. After 24h, portions of the cells were treated with vehicle control or with the proteasome inhibitor Velcade (10 nM). Cells were then treated with vehicle control or with neratinib (50 nM) for 4h. Cells were fixed in place and staining performed to detect the fluorescence intensity staining for K-RAS and for ERBB1 (n = 3 +/−SD) # p < 0.05 greater than corresponding value in siSCR + vehicle treated cells. E. MDCK cells stably expressing the endomembrane marker mCherry-CAAX and K-RAS V12 – GFP were treated with vehicle control or neratinib (100 nM), cells were fixed at each timepoint and imaged using a confocal microscope (60X). K-RAS mislocalization from the plasma membrane was quantified as the colocalization of K-RAS V12 – GFP and mCherry-CAAX using Manders coefficients. (n = 3 +/−SD). F. MDCK cells stably expressing the endomembrane marker mCherry-CAAX and K-RAS V12 – GFP were treated with vehicle control or neratinib (100 nM), cells were fixed at each timepoint and imaged using a confocal microscope (60X).
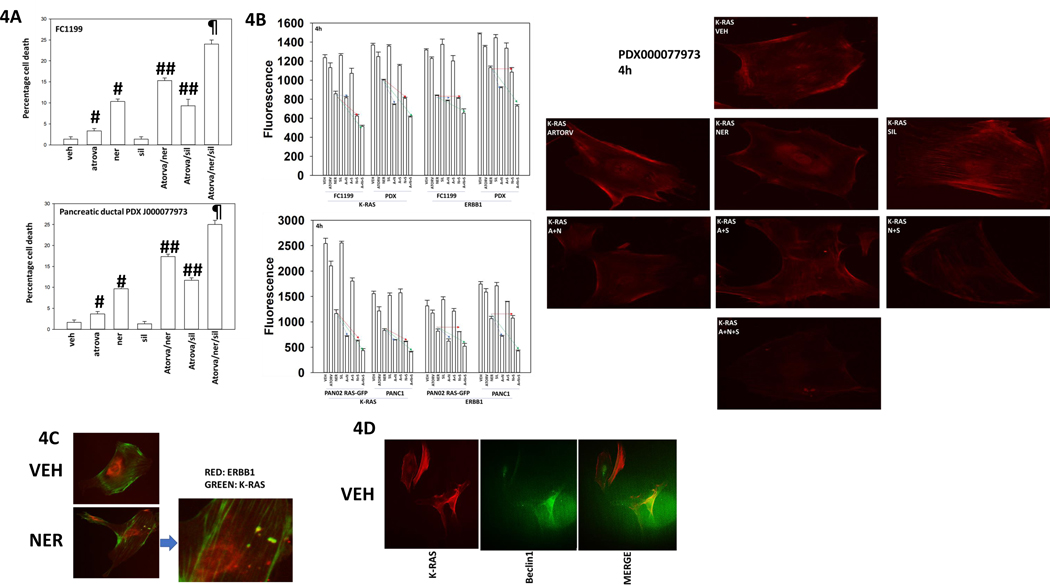
The laboratory of Dr. Hancock demonstrated that sildenafil-stimulated PKG activation could enhance K-RAS phosphorylation and cause it to leave the plasma membrane [5]. Others have shown HMG CoA reductase inhibitors, statins, have anti-pancreatic cancer activity, with reduced K-RAS farnesylation, and thus plasma membrane localization, as one potential mode of action. Neratinib, the statin atorvastatin and the PDE5 inhibitor sildenafil interacted to kill pancreatic cancer cells (Figure 4A). Neratinib reduced the protein levels of K-RAS and of ERBB1 (Figure 4B, p < 0.05). In some pancreatic cancer cell lines, the HMG CoA reductase inhibitor atorvastatin at a clinically achievable concentration also significantly reduced K-RAS levels whereas in other lines the reducing trend did not reach statistical significance. After 4h of drug exposure, no decline in K-RAS or ERBB1 expression was observed in cells exposed to sildenafil. In all lines tested, neratinib and sildenafil interacted to reduce K-RAS expression (red line, p < 0.05). In all lines other than the GEMM FC1199, neratinib and atorvastatin interacted to reduce K-RAS expression (blue line, p < 0.05). In all lines, the three-drug combination caused an additional significant further reduction in K-RAS levels (green line, p < 0.05). In all lines other than FC1199, neratinib and atorvastatin interacted to reduce ERBB1 expression, an effect enhanced in all lines by the addition of sildenafil (p < 0.05). We conclude that neratinib, atorvastatin and sildenafil can interact to cause higher levels of cell killing that correlates with higher levels of K-RAS and ERBB1 down-regulation. K-RAS and ERBB1 appeared to be held in discrete pools in the PDX tumor cells; neratinib caused the formation of intracellular vesicles, some of which stained for either K-RAS or ERBB1, and some that stained for both proteins. Under basal conditions, K-RAS and Beclin1 co-localized in the PDX pancreatic cancer cells (Figures 4C and 4D).
Figure 4. Neratinib, sildenafil and entinostat interact to reduce K-RAS and ERBB1 expression and sub-cellular localization.
A. Pancreatic cancer cells were treated with vehicle control, neratinib (50 nM), atorvastatin (100 nM), sildenafil (1 μM) alone or in combinations as indicated for 12h. Cells were isolated, and viability determined by trypan blue exclusion assay (n = 3 +/−SD). P < 0.05 greater than vehicle control; ## p < 0.05 greater than corresponding single drug exposure value; ¶ p < 0.05 greater than two drug combination. B. Pancreatic cancer cells were treated with vehicle control, neratinib (50 nM), atorvastatin (100 nM), sildenafil (1 μM) alone or in combinations as indicated for 4h. Cells were fixed in place and staining performed to detect the fluorescence intensity staining for K-RAS and for ERBB1 (n = 3 +/−SD). Right: images of drug-treated PDX cells. C. PDX pancreatic cancer cells were treated with vehicle control or with neratinib (50 nM) for 4h. Cells were fixed in place and staining performed to detect K-RAS and ERBB1; images were merged in Adobe Photoshop CS5. D. PDX pancreatic cancer cells were treated with vehicle control or with neratinib (50 nM) for 4h. Cells were fixed in place and staining performed to detect K-RAS and Beclin1; images were merged in Adobe Photoshop CS5.
Neratinib is significantly more capable of down-regulating the levels of plasma membrane proteins than afatinib. Both drugs inhibit ERBB1/2/4 but only neratinib inhibits Ste20 family kinases [12]. The Ste20 family kinases play key roles in maintaining plasma membrane and Golgi integrity, including cell-cell junctions and maintaining the apical brush border in the GI [13–18]. This is of note because the dose-limiting toxicity of neratinib is Grade 3 diarrhea and the drug must be combined with anti-diarrhea medication to reduce this negative sequela in patients; in comparison, the negative GI sequalae of afatinib are lower [19]. If neratinib was acting to reduce the levels of protective RTKs, K-RAS and N-RAS via inhibition of MST3 and/or MST4, the drug should therefore be able to reduce the expression of K-RAS and N-RAS in tumor cells that do not express ERBB family receptors, specifically, hematopoietic cancer cells. Additionally, as the majority of ERBB1 and K-RAS did not co-localize in the PDX isolate, the implication must be that an additional key neratinib target must exist.
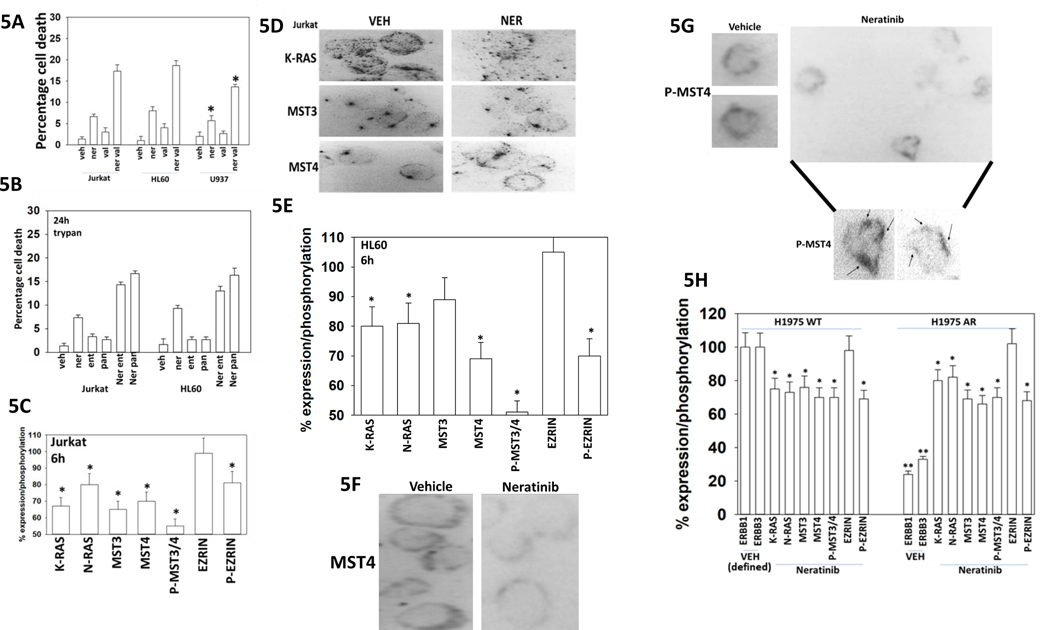
Neratinib combined with the HDAC inhibitor sodium valproate to kill lymphoma, APL and AML blood cancer cells (Figure 5A). Similar cell killing data were obtained using the high-affinity HDAC inhibitors entinostat and panobinostat (Figure 5B). In Jurkat T cells, that in our hands do not stain for ERBB1/2/4, neratinib rapidly reduced the expression of K-RAS, MST3 and MST4 (Figure 5C, not shown). Neratinib reduced MST3/4 autophosphorylation (T174 + T178 + T190), and phosphorylation of their direct substrate Ezrin T567. Neratinib in Jurkat cells also dramatically altered the sub-cellular localization of MST4 (Figure 5D). HL60 leukemia cells also responded to neratinib exposure by reducing K-RAS expression and reducing the phosphorylation of MST3/4 and Ezrin (Figure 5E). As observed for the Jurkat cells, in HL60 cells, neratinib altered the subcellular localization of MST4 (Figures 5F and 5G). Similar expression, phosphorylation and localization effects following neratinib exposure were observed in H1975 NSCLC cells, regardless of whether they were wild type parental of in vivo generated afatinib resistant (Figure 5H) [14].
Figure 5. Neratinib reduces the autophosphorylation of MST3 and MST4 in both solid tumor and in blood cancer cells.
A. Blood cancer cells (HL60, U937, Jurkat) were treated with vehicle control, neratinib (50 nM), valproate (250 μM) or the drugs in combination for 24h. Cells were isolated, and viability determined by trypan blue exclusion assay (n = 3 +/− SD) # p < 0.05 greater than neratinib alone value. B. Blood cancer cells (HL60, Jurkat) were treated with vehicle control, neratinib (50 nM), entinostat / panobinostat (50 nM) or the drugs in combination for 24h. Cells were isolated, and viability determined by trypan blue exclusion assay (n = 3 +/− SD). C. Jurkat T cells were treated with vehicle control or neratinib (50 nM) for 6h. Cells were cytospun onto glass slides, fixed in place, and immunostaining performed to determine the fluorescence intensity for K-RAS, N-RAS, MST3, MST4, phospho-MST3/4, Ezrin, phospho-Ezrin. (n = 3 +/− SD) * p < 0.05 less than vehicle control. D. Representative images of vehicle control and neratinib-treated Jurkat cells at 60X magnification. E. HL60 cells were treated with vehicle control or with neratinib (50 nM). Cells were isolated after 6h and cytospun onto slides, fixed in place, and immunostaining performed to determine the fluorescence intensity for K-RAS, MST3, MST4, phospho-MST3/4, Ezrin, phospho-Ezrin. (n = 3 +/− SD) * p < 0.05 less than vehicle control. F.-G. Representative images of MST4 and phospho-MST4 from vehicle control and neratinib-treated HL60 cells at 60X magnification (images with arrows were expanded and darkened in Photoshop CS5 to visualize P-MST4). H. H1975 NSCLC cells (wild type parental and afatinib-resistant) were treated with vehicle control or neratinib (50 nM) for 6h. Cells were fixed in place and immunostaining performed to determine the fluorescence intensity for K-RAS, ERK2, MST3, MST4, phospho-MST3/4, Ezrin, phospho-Ezrin. (n = 3 +/− SD) * p < 0.05 less than vehicle control.
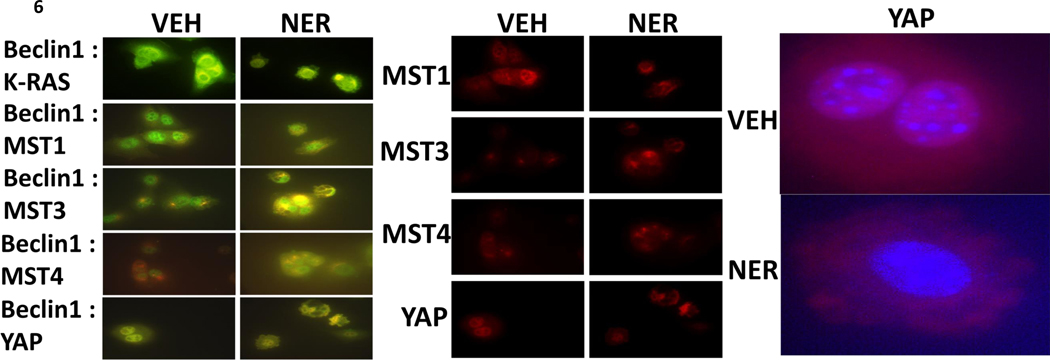
Exposure of Jurkat T cells to neratinib caused both MST4 and MST3 to co-localize with the autophagy regulatory protein Beclin1 (Supplemental Figure 4A). As multiple MAP4K enzymes have been linked to the regulation of LATS1/2 and YAP function, we next determined whether neratinib altered the cellular localization of Hippo pathway proteins (Supplemental Figure 4B). Neratinib increased the expression of MOB1 and its association with MST1 and with MST4. Neratinib also promoted the co-localization of LATS1 with MOB1. Neratinib did not alter the association between MST1 and MOB4, whereas it promoted MST4 localization with the adaptor protein. As was observed in the LATS1/MOB1 images, neratinib altered the sub-cellular localization of LATS1, from being peri-plasma membrane and becoming cytoplasmic. In the cytoplasm, LATS1 and MOB4 co-localized. The adaptor protein MO25 has been reported to localize MST3 and MST4 at the plasma membrane. MST4 localized with MO25 regardless of neratinib whereas the association of MST3 with MO25 was reduced. In contrast to blood cancer cells, in H1975 NSCLC cells MST4 colocalized with Beclin1 under basal conditions around the periphery of the cell; exposure of cells to neratinib resulted in less MST4 expression, still colocalized with Beclin1, that was not associated with the plasma membrane (Supplemental Figure 5). Exposure of the GEMM FC1199 cells to neratinib caused Beclin1 to colocalize with K-RAS in vesicles; for the subcellular localization of MST1 to alter; caused Beclin1 to colocalize with MST3 and with MST4 (Figure 6). Neratinib caused YAP expression to both decline and for YAP to leave the nucleus and become punctate within the cytoplasm.
Figure 6. Neratinib alters the sub-cellular localization of MST1, MST3, MST4 and YAP, and causes these proteins to colocalize with Beclin1.
F1199 pancreatic cancer cells were treated with vehicle control or neratinib (50 nM) for 6h. Cells were fixed in place and the colocalization of Beclin1 with K-RAS, MST1, MST3, MST4 and YAP determined at 60X magnification. Images were merged in Photoshop CS5.
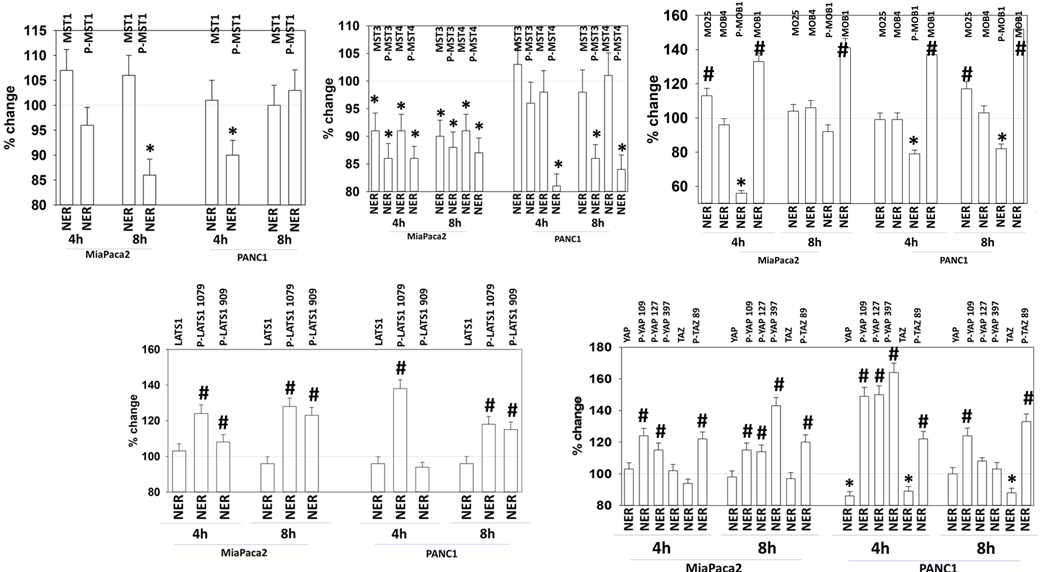
MST4 is known to alter signaling by MST1 through the Hippo – LATS – YAP/TAZ pathway by altering the association of MST1 with MOB1 [34]. In addition to inhibiting MST3/MST4 autophosphorylation, neratinib also reduced MST1/2 phosphorylation (T183/T180) (Figure 7). This observation would not have been predicted based on the published kinome / neratinib screening data but was a possibility based on our altered MST1 localization data. Neratinib modestly enhanced the expression of MO25 and decreased the phosphorylation of MOB1 T35 that was associated with a large increase in MOB1 expression. Dephosphorylation of MOB1 T35 would be predicted to reduce upstream MST kinase signaling into LATS1. Although MST1/2 and MST3/4 phosphorylation were reduced by neratinib, the phosphorylation of their direct substrate, LATS1, at regulatory and auto-phosphorylation sites (S909, T1079), was significantly enhanced by the drug. In agreement with elevated LATS1 phosphorylation, i.e. activity, the phosphorylation of YAP at multiple sites and that of TAZ were increased after neratinib exposure (S109, S127, S397; S89).
Figure 7. Neratinib regulates Hippo pathway signaling.
PANC1 and MiaPaca2 cells were treated with vehicle control or with neratinib (50 nM) for 4h and 8h. At each time point the cells were fixed in place and immunostaining performed to detect the phosphorylation and expression of the indicated proteins (n = 3 +/− SD) * p < 0.05 less than vehicle control; # p < 0.05 greater than vehicle control.
Based on this data, we next determined whether neratinib, atorvastatin and sildenafil interacted to alter phosphorylation of YAP (Supplemental Figure 6). Both atorvastatin and neratinib enhanced YAP phosphorylation, though neratinib more effectively suppressed K-RAS expression; the drugs interacted to further elevate YAP phosphorylation and to reduce K-RAS expression. Sildenafil enhanced the ability of [neratinib + atorvastatin] to further suppress K-RAS expression and to increase YAP phosphorylation. Thus, the three agents in coordination act to block RAS and YAP.
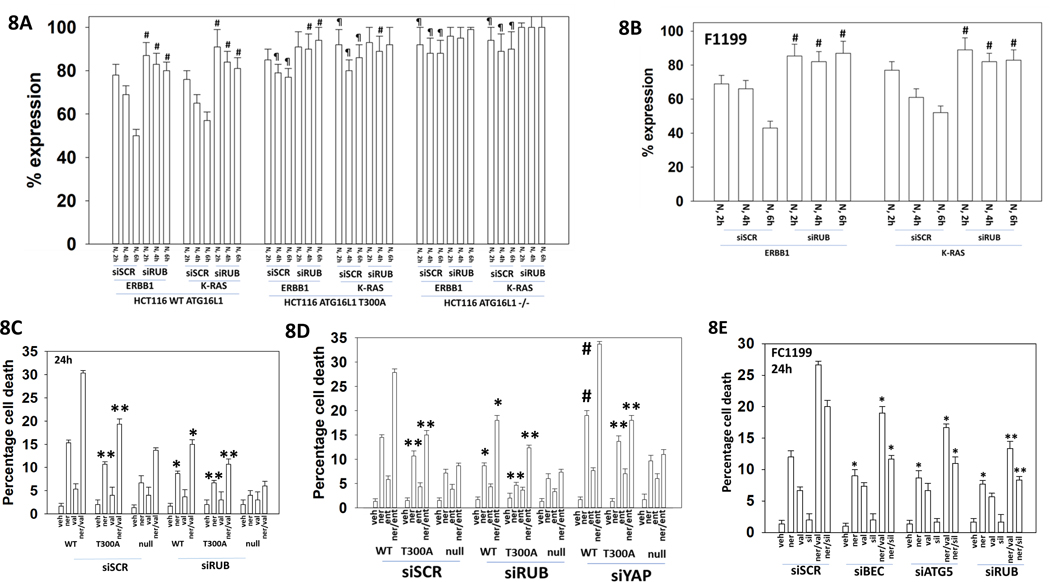
Because neratinib-dependent K-RAS internalization is dependent on ATG5 and Beclin1 and the K-RAS containing vesicles are adjacent to the plasma membrane we considered that K-RAS may in part be internalized and degraded by a process known as LC3-associated phagocytosis (LAP) [1, 21]. Phagocytosed proteins in vesicles are suborned into the autophagic degradation pathway using proteins such as Beclin1, ATG16L1 and Rubicon. We made use of previously described HCT116 colorectal carcinoma cells that lack ATG16L1 or that express the ATG16L1 T300A isoform, and of FC1199 GEMM mouse pancreatic cancer cells that express mutant K-RAS and mutant p53 (Figures 8A and 8B). The ATG16L1 T300A variant, more commonly found in European Americans than in African Americans, predisposes persons to develop Crohn’s Disease [22]. The expression of one ATG16L1 A300 allele significantly increased the risk for Crohn’s disease [23]. Exposure of wild type HCT116 cells that are homozygote ATG16L T300 or of FC1199 cells to neratinib rapidly reduced the expression of ERBB1 and K-RAS. Knock-down of Rubicon significantly reduced the ability of neratinib to lower ERBB1 and K-RAS levels. This data is similar to our prior findings when we knocked down Beclin1 or ATG5 [3]. In cells lacking ATG16L1 expression or expressing the variant ATG16L1 T300A, the ability of neratinib to suppress ERBB1 and K-RAS levels was also blocked. Neratinib caused K-RAS staining to become vesicular, which was absent in cells lacking Rubicon expression. Knock down of Rubicon or expression of ATG16L1 T300A both significantly reduced the ability of [neratinib + valproate] to kill HCT116 colon cancer cells (Figure 8C). Knock down of Rubicon was significantly better at suppressing neratinib and [neratinib + valproate] lethality than expression of ATG16L1 A300.
Figure 8. Loss of ATG16L1 or knock-down of Rubicon prevents neratinib from reducing ERBB1 and K-RAS expression.
A. HCT116 cells (wild type; ATG16L1 null; ATG16L1 null stably transfected to express ATG16L1 T300A) were transfected with a scrambled siRNA or an siRNA to knock down the expression of human Rubicon. Twenty-four h after transfection cells were treated with vehicle control or with neratinib (50 nM). At each time point cells were fixed in place and immunostaining performed to determine the fluorescence intensity levels of ERBB1 and K-RAS from 10X magnification images (120 cells per condition, n = 3 +/−SD). # p < 0.05 greater than corresponding value in siSCR cells; ¶ p < 0.05 great than corresponding value in HCT116 wild type cells. Lower Images: 60X magnification images of drug-exposed cells. B. F1199 GEMM pancreatic cancer cells were transfected with a scrambled siRNA or an siRNA to knock down the expression of mouse Rubicon. Twenty-four h after transfection cells were treated with vehicle control or with neratinib (50 nM). At each time point cells were fixed in place and immunostaining performed to determine the fluorescence intensity levels of ERBB1 and K-RAS from 10X magnification images (120 cells per condition, n = 3 +/−SD). # p < 0.05 greater than corresponding value in siSCR cells. Lower Images: 60X magnification images of drug-exposed cells. C. and D. HCT116 cells were transfected with a scrambled siRNA or siRNA molecules to knock down the expression of human Rubicon or YAP. Twenty-four h after transfection cells were treated with vehicle control, neratinib (50 nM), sodium valproate (250 μM), entinostat (50 nM) or the drugs in combination as indicated for 24h. Cells were isolated, and viability determined by trypan blue exclusion assay (n = 3 +/−SD) * p < 0.05 less than corresponding value in siSCR cells; ** p < 0.05 less than corresponding values ATG16L1 T300 cells; # p < 0.05 greater than corresponding value in siSCR cells. E. FC1199 tumor cells were transfected with a scrambled siRNA, or with siRNA molecules to knock down Rubicon, Beclin1 or ATG5. Twenty-four h after transfection, cells were treated with vehicle control, neratinib (50 nM), sodium valproate (250 μM), sildenafil (2.0 μM) or the drugs in the indicated combinations for 24h. Cells were isolated, and viability determined by trypan blue exclusion assay (n = 3 +/−SD) * p < 0.05 less than corresponding value in siSCR cells; ** p < 0.05 less than corresponding values in siBeclin1 or siATG5 cells.
Similar data were obtained using entinostat instead of valproate (Figure 8D). Knock down of YAP significantly enhanced [neratinib + entinostat] lethality regardless of whether cells expressed ATG16L T300 or ATG16L1 A300. Knock down of Rubicon, Beclin1 or ATG5 suppressed neratinib lethality as a single agent and when it was combined with either the PKG activator sildenafil or the HDAC inhibitor valproate (Figure 8E). Rubicon knock down was more protective than that of either Beclin1 or ATG5.
We next examined the roles of Rubicon in neratinib-induced changes to Hippo Pathway function and cell viability. Our first, unexpected, observation was under control conditions examining the impact of Rubicon knock down on the expression of total YAP, YAP S127 phosphorylation and YAP S397 phosphorylation: knock down of Rubicon in FCC1199 cells increased basal YAP expression by 2.60-fold; basal S127 phosphorylation by 1.20-fold; and basal S397 phosphorylation by 3.10-fold (all p < 0.05 greater than siSCR control values). Rubicon knock down in PDX pancreatic cancer cells elevated basal total YAP expression 4.00-fold; basal S127 phosphorylation by 1.15-fold; and basal S397 phosphorylation by 4.85-fold (all p < 0.05 greater than siSCR control values). Neratinib, and to a greater extent when combined with either the HDAC inhibitor valproate or the PKG activator sildenafil, reduced the phosphorylation of both PAK1 T423 and Merlin S518 (Supplementary Figure 7A). These events were associated with greater levels of LATS1/2 and YAP S127 phosphorylation. In contrast, in cells with their Rubicon expression knocked down, neratinib treatment more modestly enhanced the phosphorylation of PAK1 and Merlin and lowered the phosphorylation of LATS1/2 and YAP S127. Thus: (a) in the absence of Rubicon and LAP, the basal expression levels of YAP increase and the cell tries to counter-act this by increasing S397 phosphorylation which will enhance YAP ubiquitination and degradation; (b) that in the absence of Rubicon, neratinib activated YAP as a co-transcription factor. Phosphorylated Merlin S518 exhibited a punctate staining pattern at 60X magnification, that was abolished, with reduced staining intensity, by neratinib (Supplementary Figure 7A, lower images). Knock-down of Rubicon prevented this localization and expression effect. Knock down of YAP expression enhanced the lethality of neratinib as a single agent and when combined with either sildenafil or valproate (Supplementary Figure 7B).
So far, our studies have been examining cells wherein the mutated GTPase protein has been of the RAS family. We recently demonstrated that neratinib combined with the HDAC inhibitor entinostat could kill uveal melanoma cells; cells that express mutated GTPase inactive forms of G alpha Q or G alpha 11 [4]. In these cells, neratinib could down-regulate expression of the hetero-trimeric mutant GTPase alpha proteins (alpha Q; alpha 11), though not to the same extent as it did to RAS proteins. A PDX uveal melanoma metastatic isolate was treated with neratinib and entinostat in the presence or absence of Rubicon expression. The drugs alone or in combination in a Rubicon-dependent fashion activated MST1 which signals down to YAP and TAZ, causing their phosphorylation (Supplemental Figure 8). This was associated with Merlin dephosphorylation such that Merlin will then act to facilitate the formation of a quaternary protein structure containing MST1 / LATS1/2 / YAP-TAZ. Phosphorylated YAP/TAZ leaves the nucleus and, based on S397 phosphorylation, YAP will then be ubiquitinated and degraded. The expression of both G alpha proteins as well as K-RAS was down-regulated by the drugs in a Rubicon-dependent fashion. We have previously shown that the degradation of both G alpha proteins as well as K-RAS was prevented by knock down of Beclin1 or ATG5 and thus hypothesized that LAP also plays a key role in controlling mutant G alpha protein degradation. However, the mechanisms by which [neratinib + entinostat] down-regulate G alpha proteins and RAS are not identical; regardless of ATG16L1 isoform expression, the drug combination could reduce G alpha protein levels whereas the reduction in K-RAS was significantly reduced in cells expressing ATG16L1 A300 (Supplementary Figure 9).
As was observed in the pancreatic cancer cells expressing a mutant K-RAS, so in the uveal melanoma cells expressing a mutant G alpha protein (see Supplemental Figure S1), knock down of Rubicon enhanced the basal expression of YAP and the levels of YAP S397 phosphorylation, that again argues that a cell lacking LC3 associated phagocytosis / Rubicon has higher YAP levels and that it attempts to reduce this elevated expression by elevating S397 phosphorylation and ubiquitination (Supplemental Figure 10). Prior data had shown that neratinib could cause K-RAS V12-GFP and K-RAS V12-RFP to rapidly colocalize into intracellular vesicles of varying sizes [1, 20]. Knock down of Rubicon considerably reduced the ability of RAS-tagged proteins from localizing inside the cell as vesicles (Supplemental Figure 11). Of note, in cells lacking Beclin1 or Rubicon, where a modest induction of K-RAS V12-GFP vesicularization was observed, no vesicularization of K-RAS V12-RFP occurred.
Discussion
We had previously published in H1975 NSCLC cells that express a mutant active ERBB1 that the irreversible ERBB1/2/4 inhibitor neratinib had greater potential to down-regulate the expression of ERBB1 than another irreversible ERBB1/2/4 inhibitor, afatinib [1, 14]. Neratinib reduced the protein levels of ERBB1, ERBB2, K-RAS and N-RAS in other tumor cell types to a significantly greater extent than afatinib; though afatinib, presumably acting through ERBB1/2/4 could down-regulate the receptors. Unlike afatinib, neratinib not only inhibits the ERBB1/2/4 receptor tyrosine kinase family but also multiple members of the Ste20 family of serine/threonine kinases, including MST3 and MST4. In solid tumor cells and blood cancer cells neratinib prevented MST3/4 autophosphorylation and the phosphorylation of a downstream substrate Ezrin. In both types of tumor cell neratinib reduced the expression of K-RAS. Others have also recently shown that neratinib and afatinib in solid tumor cell lines can reduce ERBB1/2/3 expression [24, 25].
The kinase Sterile 20 (Ste20) is a component of the pheromone-response pathway in budding yeast. Several mammalian homologs to Ste20 have been identified including the PAKs, the GCN kinase family (MAP4Ks) and MST1/2/3/4 [26–28]. The Mammalian Sterile-Twenty-like kinases, MST1/2, are core signaling components of the Hippo Pathway [29, 30]. Hippo controls organ size and tissue homeostasis by regulating apoptosis and cell proliferation. Simplistically, MST1/2 phosphorylate and activate the Large Tumor Suppressor, LATS1/2, as well as the Nuclear Dbf2-related, NDR1/2, kinases [31]. Essential in this process are the scaffold proteins of the Mps one binder family, MOB1, MOB4 and SAV1 [32]. Other scaffold proteins such as RASSF1A and Salvador can also activate MST1/2 by forming SARAH domain dependent complexes [33]. The amount of MST1:MOB1 complex versus the MST4:MOB4 complex can influence, for example, whether pathway signaling promotes growth or cell death [34]. Neratinib can act to inhibit MST1/2 /3/4 but does not inhibit either LATS1/2 or NDR1/2; it in fact our data shows it activates LATS1/2. Activated LATS1/2 or NDR1/2 phosphorylate and cause cytoplasmic sequestration of the transcriptional co-activator Yes-associated protein (YAP) and its partner TAZ. Cytosolic YAP and TAZ can then be ubiquitinated and proteolytically degraded. e.g. requiring autophagy [35]. YAP is an oncogene that enhances transcription of genes involved in cell proliferation by partnering with TEAD family of transcription factors; thus, inactivation of YAP promotes cell death [36]. More recently, and in addition to MST1/2, other members of the Ste20-like MAP4K kinase family have been shown to perform activating phosphorylation of LATS1/2. MST1/2 are not required for the regulation of YAP/TAZ. Knock out of LATS1/2, but not of MST1/2, abolishes YAP/TAZ phosphorylation [37]. MAP4K1/2/3 and MAP4K4/6/7 are now known to be direct LATS1/2-activating kinases. Thus, combined deletion of the MAP4Ks and MST1/2, but neither subset alone, can suppress the phosphorylation of LATS1/2 and YAP/TAZ i.e. MAP4Ks as components of an expanded Hippo Pathway.
MAP4K4 family kinases function upstream of NDR1/2, and NDR1/2 as well as LATS1/2 kinases phosphorylate additional substrates besides YAP [38]. MST1/2/3 phosphorylate NDR1/2 on T444/T442, and the binding of the scaffold MOB1 to NDR1/2 is required to support the auto-phosphorylation of NDR1/2 on S281/S282. The activation of NDR1/2 can be mediated via different pathways: the inhibition of protein phosphatase 2A; the mutation of an autoinhibitory segment; and membrane targeting of NDR1/2 [39]. Hence, NDR1/2 can be regulated via different signaling mechanisms involving altered subcellular distribution and phosphorylation status. MAP4Ks can function as upstream kinases of NDR1/2 and LATS1/2. MAP4K4 can function as an NDR1/2 kinase as part of osmotic stress signaling and MAP4K4 has been identified as a LATS1/2 kinase in an unbiased kinome screen [40–42]. Further analyses have revealed that several members of the MAP4Ks family can function upstream of LATS1/2.
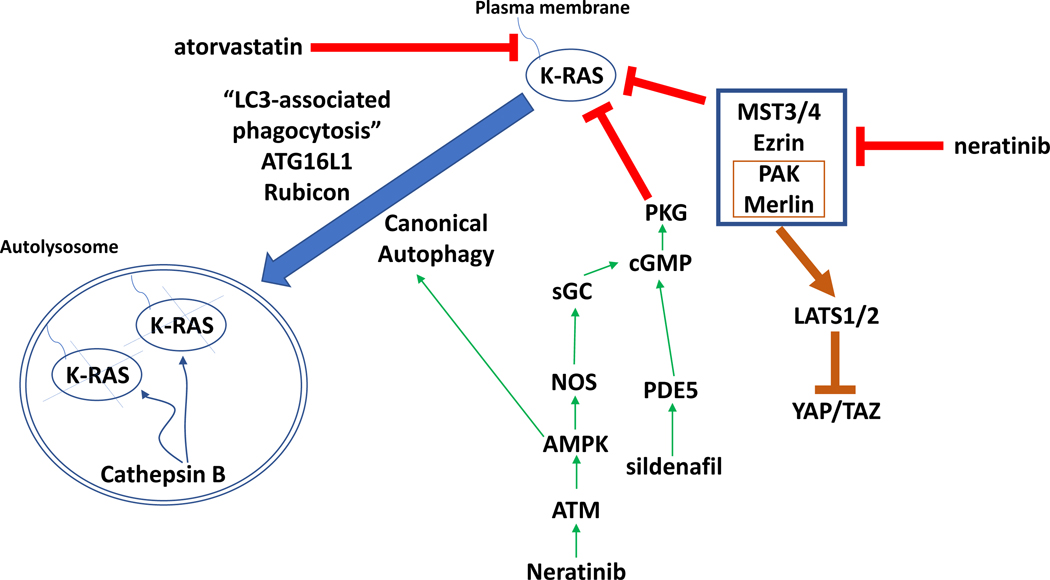
It has been shown that oncogenic RAS can alter signaling by the Hippo pathway. The transcription factor YAP is essential for neoplastic initiation and the growth of K-RAS-addicted pancreatic carcinomas (Figure 9) [43]. YAP activation can bypass the K-RAS dependency in pancreatic cancer [38]. And, YAP can also substitute for mutant K-RAS in a murine lung cancer model [45]. With respect to neratinib, YAP can mediate resistance to drugs such as the B-RAF inhibitor vemurafenib or the MEK1/2/5 inhibitor trametinib that target mutant K-RAS signaling [46]. In myoblasts expressing a mutant K-RAS, YAP cooperates to enhance proliferation and prevent death in vitro and in vivo [47].
Figure 9. Putative mechanisms by which neratinib down-regulates mutant K-RAS expression and inactivates Hippo Pathway function.
Neratinib via inhibition of MST3/4 causes Rubicon-dependent phagocytosis and in parallel, neratinib activates ATM-AMPK signaling that causes autophagosome formation. Collectively, this results in the cathepsin-dependent degradation of mutant K-RAS. Atorvastatin via reduced prenylation and sildenafil via increased PKG-dependent phosphorylation of K-RAS also independently act to lower mutant K-RAS levels. As part of this process at the plasma membrane, neratinib reduces PAK1 phosphorylation that leads to dephosphorylation of the PAK1 substrate Merlin/NF2. Dephosphorylated Merlin facilitates activation of LATS1/2, and active LATS1/2 phosphorylate YAP and TAZ. Phosphorylated YAP and TAZ leave the nucleus, preventing them from acting as transcriptional coactivators.
How non-canonical autophagic players, such as Rubicon, function in vesicular trafficking pathways, is of great interest [21]. Rubicon is essential for LAP and our data demonstrated that LAP was essential for K-RAS and G alpha Q / 11 down-regulation and for neratinib-based drug combination lethality. Muniz-Feliciano et al demonstrated in retinal pigment epithelial cells of the eye that Rubicon-dependent phagocytosis and LAP can activate ERBB1 signaling leading to increased mTOR phosphorylation and inhibition of classic autophagy [48]. Silencing of Rubicon prevents ERBB1 activation; in our systems, this would be caused by neratinib exposure. At least in our short-term assays, loss of Rubicon also enhanced YAP expression, arguing that the YAP/Rubicon axis could play an important role in tumor cells with altered phagocytosis. ATG16L1 isoform expression has a major impact on a person’s susceptibility to Crohn’s Disease [22, 23]. One facet of cells expressing ATG16L1 A300, more often found in European Americans, is that these cells are less capable of ingesting and digesting via autophagy, gut bacteria [49]. The T300A variant has been linked to an enhanced risk of gastric cancer [50]. It has been shown that selective autophagy is lowered in many cell types using T300A knock-in mice compared to T300 mice, which agrees with our findings showing a reduced autophagic down-regulation of K-RAS and ERBB1 [51] (Supplemental Figure 11). The ATG16L1 T300A isoform is associated with elevated production of IL-1β. The biology of neratinib regulating both autophagy, LAP and Ste20 kinase / stress signaling will require in the future multiple additional studies.
Materials and Methods
Materials.
Reagents, including siRNA catalogue numbers, and the performance of all experimental procedures were all as described in references [1–4]. Afatinib was purchased from Selleckchem (Houston, TX). Neratinib was supplied by Puma Biotechnology Inc. (Los Angeles, CA). Cell culture materials were purchased from GIBCOBRL (GIBCOBRL Life Technologies, Grand Island, NY). Established cell lines were purchased from the ATCC. The GFP and RFP tagged K-RAS V12 plasmids were provided by Dr. John Hancock (Department of Integrative Biology and Pharmacology, University of Texas Health Science Center, Houston TX, USA). PDX isolates of human head and neck cancer were kindly provided by Dr. John Lee (Chan Soon-Shiong Institute of Molecular Medicine, Culver City, CA, USA). Commercially available validated short hairpin RNA molecules to knock down RNA / protein levels were validated in house and purchased from Qiagen (Valencia, CA) (Supplemental Figure 12). Antibodies directed against RAS proteins: Thermo-Fisher (Waltham MA) N-RAS PA5–14833; K-RAS PA5–44339. Based on data from Waters et al, we used two validated siRNA tools to knock down K-RAS or N-RAS for antibody validation purposes; custom made NRAS-5 CCUGAGUACUGACCUAAGAdTdT and K-RAS Silencer s7940 [52]. Knock down of K-RAS or N- RAS reduced fluorescent staining by ~80%. Methods of approach were as described [1–4] (see supplemental Methods section).
RAS membrane localization:
Madin-Darby canine kidney (MDCK) cells stably co-expressing mGFP–K-RasG12V and mCherry-CAAX, a general endomembrane marker, were treated with drugs for 4 – 48 h and analyzed by confocal microscopy [5]. The extent of K-RasG12V mislocalization was calculated using Manders coefficients, which quantify the fraction of mCherry-CAAX colocalizing with mGFP–K-RasG12V. The greater the value of the Manders coefficient, the more extensive the displacement of mGFP–K-RasG12V from the PM. For the images taken at 24 and 48h all confocal imaging parameters were kept constant between samples to evaluate GFP-expression intensity.
MDCK epithelial cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)–high-glucose medium supplemented with sodium pyruvate, 2 mM l-glutamine, and 10% bovine calf serum. Cells grown on coverslips were fixed with 4% PFA, followed by 50 mM NH4Cl treatment to quench aldehyde groups. The coverslips were mounted in Mowiol and visualized by confocal microscopy (Nikon A1) using a 60× objective. For the images taken at 24 and 48h all confocal imaging parameters were kept constant between samples to evaluate GFP-expression intensity.
Data analysis.
Comparison of the effects of various treatments (in triplicate three times) was using one-way ANOVA and a two tailed Student’s t-test, with corrections for multiple comparisons. Differences with a p-value of < 0.05 were considered statistically significant. Experiments are the means of multiple individual points from multiple experiments (± SD).
Supplementary Material
Acknowledgements
Support for the present study was funded from philanthropic funding from Massey Cancer Center and the Universal Inc. Chair in Signal Transduction Research. PD acknowledges funding by the Commonwealth Health Research Board (CHRB) of Virginia. JFH acknowledges funding by CPRIT RP170233. NCI funding from neither the DT nor MCT2 study sections was forthcoming for studies presented in this manuscript.
Abbreviations:
- ERK
extracellular regulated kinase
- PI3K
phosphatidyl inositol 3 kinase
- ca
constitutively active
- dn
dominant negative
- ER
endoplasmic reticulum
- AIF
apoptosis inducing factor
- AMPK
AMP-dependent protein kinase
- mTOR
mammalian target of rapamycin
- JAK
Janus Kinase
- STAT
Signal Transducers and Activators of Transcription
- MAPK
mitogen activated protein kinase
- PTEN
phosphatase and tensin homologue on chromosome ten
- ROS
reactive oxygen species
- CMV
empty vector plasmid or virus
- si
small interfering
- SCR
scrambled
- IP
immunoprecipitation
- VEH
vehicle
- SIL
sildenafil
- VAL
sodium valproate
- ATOR
atorvastatin
Footnotes
There are no conflicts of interest to report for PD, LB, JLR, JL, AP, DT, JM and JFH. ASL is a shareholder and paid officer of Puma Biotechnology.
References.
- 1.Booth L, Roberts JL, Poklepovic A, Avogadri-Connors F, Cutler RE, Lalani AS, Dent P. HDAC inhibitors enhance neratinib activity and when combined enhance the actions of an anti-PD-1 immunomodulatory antibody in vivo. Oncotarget 2017; 8: 90262–90277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth L, Roberts JL, Rais R, Cutler RE Jr, Diala I, Lalani AS, Hancock JF, Poklepovic A, Dent P. Neratinib augments the lethality of [regorafenib + sildenafil]. J Cell Physiol. 2019;234:4874–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth L, Roberts JL, Rais R, Kirkwood J, Avogadri-Connors F, Cutler RE Jr, Lalani AS, Poklepovic A, Dent P. [Neratinib + Valproate] exposure permanently reduces ERBB1 and RAS expression in 4T1 mammary tumors and enhances M1 macrophage infiltration. Oncotarget. 2017; 9:6062–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth L, Roberts JL, Sander C, Lalani AS, Kirkwood J, Hancock JF, Poklepovic A, Dent P. Neratinib and Entinostat combine to rapidly reduce the expression of K-RAS, N-RAS, Gαq and Gα11 and kill uveal melanoma cells. Cancer Biol. Ther. 2018; DOI: 10.1080/15384047.2018.1551747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho KJ, Casteel DE, Prakash P, Tan L, van der Hoeven D, Salim AA, Kim C, Capon RJ, Lacey E, Cunha SR, Gorfe AA, Hancock JF. AMPK and Endothelial Nitric Oxide Synthase Signaling Regulates K-Ras Plasma Membrane Interactions via Cyclic GMP-Dependent Protein Kinase 2. Mol Cell Biol. 2016;36:3086–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Rahman O Statin treatment and outcomes of metastatic pancreatic cancer: a pooled analysis of two phase III studies. Clin Transl Oncol. 2018. November 21. doi: 10.1007/s12094-018-1992-3. [DOI] [PubMed] [Google Scholar]
- 7.Archibugi L, Arcidiacono PG, Capurso G. Statin use is associated to a reduced risk of pancreatic cancer: A meta-analysis. Dig Liver Dis. 2019;51:28–37. [DOI] [PubMed] [Google Scholar]
- 8.Hamada T, Khalaf N, Yuan C, Morales-Oyarvide V, Babic A, Nowak JA, Qian ZR, Ng K, Rubinson DA, Kraft P, Giovannucci EL, Stampfer MJ, Fuchs CS, Ogino S, Wolpin BM. Prediagnosis Use of Statins Associates With Increased Survival Times of Patients With Pancreatic Cancer. Clin Gastroenterol Hepatol. 2018;16:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jian-Yu E, Graber JM, Lu SE, Lin Y, Lu-Yao G, Tan XL. Effect of Metformin and Statin Use on Survival in Pancreatic Cancer Patients: a Systematic Literature Review and Meta-analysis. Curr Med Chem. 2018;25:2595–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Liang M, Sun C, Qu G, Shi T, Min M, Wu Y, Sun Y. Statin Use and Risk of Pancreatic Cancer: An Updated Meta-analysis of 26 Studies. Pancreas. 2019;48:142–150. doi: 10.1097/MPA.0000000000001226. [DOI] [PubMed] [Google Scholar]
- 11.Chandra A, Grecco HE, Pisupati V, Perera D, Cassidy L, Skoulidis F, Ismail SA, Hedberg C, Hanzal-Bayer M, Venkitaraman AR, Wittinghofer A, Bastiaens PI. The GDI-like solubilizing factor PDEδ sustains the spatial organization and signalling of Ras family proteins. Nat Cell Biol. 2011;14:148–58. [DOI] [PubMed] [Google Scholar]
- 12.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–51. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed AD, Shah N, Hettmer S, Vargesson N, Wackerhage H. Analysis of the relationship between the KRAS G12V oncogene and the Hippo effector YAP1 in embryonal rhabdomyosarcoma. Sci Rep. 2018; 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson BJ, Sahai E. MST kinases in development and disease. J Cell Biol. 2015;210:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Fang Y, Xu S, Reis C, Zhang J. Mammalian Sterile20-like Kinases: Signalings and Roles in Central Nervous System. Aging Dis. 2018;9:537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang OH, Azizian N, Guo M, Capello M, Deng D, Zang F, Fry J, Katz MH, Fleming JB, Lee JE, Wolff RA, Hanash S, Wang H, Maitra A. Prognostic and Functional Significance of MAP4K5 in Pancreatic Cancer. PLoS One. 2016;11:e0152300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, Flores F, Yu FX, Halder G, Guan KL. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu CL, Lee EX, Gordon KL, Paz EA, Shen WC, Ohnishi K, Meisenhelder J, Hunter T, La Spada AR. MAP4K3 mediates amino acid-dependent regulation of autophagy via phosphorylation of TFEB. Nat Commun. 2018;9:942. doi: 10.1038/s41467-018-03340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh H, Walker AJ, Amiri-Kordestani L, Cheng J, Tang S, Balcazar P, Barnett-Ringgold K, Palmby TR, Cao X, Zheng N, Liu Q, Yu J, Pierce WF, Daniels SR, Sridhara R, Ibrahim A, Kluetz PG, Blumenthal GM, Beaver JA, Pazdur R. U.S. Food and Drug Administration Approval: Neratinib for the Extended Adjuvant Treatment of Early-Stage HER2-Positive Breast Cancer. Clin Cancer Res. 2018;24:3486–3491. [DOI] [PubMed] [Google Scholar]
- 20.Booth L, Roberts JL, Tavallai M, Webb T, Leon D, Chen J, McGuire WP, Poklepovic A, Dent P. The afatinib resistance of in vivo generated H1975 lung cancer cell clones is mediated by SRC/ERBB3/c-KIT/c-MET compensatory survival signaling. Oncotarget. 2016; 7:19620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez J LAP it up, fuzz ball: a short history of LC3-associated phagocytosis. Curr Opin Immunol. 2018; 55:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavoie S, Conway KL, Lassen KG, Jijon HB, Pan H, Chun E, Michaud M, Lang JK, Gallini Comeau CA, Dreyfuss JM, Glickman JN, Vlamakis H, Ananthakrishnan A, Kostic A, Garrett WS, Xavier RJ. The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife. 2019;8. pii: e39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang MH, Okazaki T, Kugathasan S, Cho JH, Isaacs KL, Lewis JD, Smoot DT, Valentine JF, Kader HA, Ford JG, Harris ML, Oliva-Hemker M, Cuffari C, Torbenson MS, Duerr RH, Silverberg MS, Rioux JD, Taylor KD, Nguyen GC, Wu Y, Datta LW, Hooker S, Dassopoulos T, Kittles RA, Kao LW, Brant SR. Contribution of higher risk genes and European admixture to Crohn’s disease in African Americans. Inflamm Bowel Dis. 2012; 18:2277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moll HP, Pranz K, Musteanu M, Grabner B, Hruschka N, Mohrherr J, Aigner P, Stiedl P, Brcic L, Laszlo V, Schramek D, Moriggl R, Eferl R, Moldvay J, Dezso K, Lopez-Casas PP, Stoiber D, Hidalgo M, Penninger J, Sibilia M, Győrffy B, Barbacid M, Dome B, Popper H, Casanova E. Afatinib restrains K-RAS-driven lung tumorigenesis. Sci Transl Med. 2018;10. pii: eaao2301. doi: 10.1126/scitranslmed.aao2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruspig B, Monteverde T, Neidler S, Hock A, Kerr E, Nixon C, Clark W, Hedley A, Laing S, Coffelt SB, Le Quesne J, Dick C, Vousden KH, Martins CP, Murphy DJ. The ERBB network facilitates KRAS-driven lung tumorigenesis. Sci Transl Med. 2018;10. pii: eaao2565. doi: 10.1126/scitranslmed.aao2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang JW, Kim MK, Bae SC. Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway. Small GTPases. 2018; 20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawat SJ, Chernoff J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem Sci. 2015; 40:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae SJ, Luo X. Activation mechanisms of the Hippo kinase signaling cascade. Biosci Rep. 2018;38. pii: BSR20171469. doi: 10.1042/BSR20171469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong D, Zhao Y, Men T, Teng CB. Hippo signaling pathway in liver and pancreas: the potential drug target for tumor therapy. J Drug Target. 2015;23:125–33. [DOI] [PubMed] [Google Scholar]
- 30.Patel SH, Camargo FD, Yimlamai D. Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis. Gastroenterology. 2017;152:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hergovich A The Roles of NDR Protein Kinases in Hippo Signalling. Genes (Basel). 2016;7 pii: E21. doi: 10.3390/genes7050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avruch J, Zhou D, Fitamant J, Bardeesy N, Mou F, Barrufet LR. Protein kinases of the Hippo pathway: regulation and substrates. Semin Cell Dev Biol. 2012;23:770–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bitra A, Sistla S, Mariam J, Malvi H, Anand R. Rassf Proteins as Modulators of Mst1 Kinase Activity. Sci Rep. 2017;7:45020. doi: 10.1038/srep45020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen M, Zhang H, Shi Z, Li Y, Zhang X, Gao Z, Zhou L, Ma J, Xu Q, Guan J, Cheng Y, Jiao S, Zhou Z. The MST4-MOB4 complex disrupts the MST1-MOB1 complex in the Hippo-YAP pathway and plays a pro-oncogenic role in pancreatic cancer. J Biol Chem. 2018; 293:14455–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Deng J. Ubiquitination-deubiquitination in the Hippo signaling pathway.Oncol Rep. 2019;41:1455–1475. [DOI] [PubMed] [Google Scholar]
- 36.Crawford JJ, Bronner SM, Zbieg JR. Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: a patent review. Expert Opin Ther Pat. 2018. doi: 10.1080/13543776.2018.1549226. [DOI] [PubMed] [Google Scholar]
- 37.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, Flores F, Yu FX, Halder G, Guan KL. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regulation Hergovich A. and functions of mammalian LATS/NDR kinases: looking beyond canonical Hippo signalling. Cell Biosci. 2013;3:32. doi: 10.1186/2045-3701-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hergovich A The Roles of NDR Protein Kinases in Hippo Signalling. Genes (Basel). 2016;7 pii: E21. doi: 10.3390/genes7050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stegert MR1, Hergovich A, Tamaskovic R, Bichsel SJ, Hemmings BA. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol Cell Biol. 2005;25:11019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell. 2015;34:642–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S, Cho YS, Yue T, Ip YT, Jiang J. Overlapping functions of the MAP4K family kinases Hppy and Msn in Hippo signaling. Cell Discov. 2015;1:15038. doi: 10.1038/celldisc.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, Chetram M, Joshi M, Wang F, Kallakury B, Toretsky J, Wellstein A, Yi C. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, Chang Q, Chu GC, Al-Khalil R, Jiang S, Xia H, Fletcher-Sananikone E, Lim C, Horwitz GI, Viale A, Pettazzoni P, Sanchez N, Wang H, Protopopov A, Zhang J, Heffernan T, Johnson RL, Chin L, Wang YA, Draetta G, DePinho RA. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu PC, Miao J, Huang Z, Yang YL, Xu Z, You J, Dai Y, Yeh CC, Chan G, Liu S, Urisman A, Yang CT, Jablons DM, You L. Inhibition of yes-associated protein suppresses brain metastasis of human lung adenocarcinoma in a murine model. J Cell Mol Med. 2018;22:3073–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nussinov R, Tsai CJ, Jang H, Korcsmáros T, Csermely P. Oncogenic KRAS signaling and YAP1/β-catenin: Similar cell cycle control in tumor initiation. Semin Cell Dev Biol. 2016;58:79–85. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed AD, Shah N, Hettmer S, Vargesson N, Wackerhage H. Analysis of the relationship between the KRAS G12V oncogene and the Hippo effector YAP1 in embryonal rhabdomyosarcoma. Sci Rep. 2018;8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muniz-Feliciano L, Doggett TA, Zhou Z, Ferguson TA. RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye. Autophagy. 2017;13:2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadaghian Sadabad M, Regeling A, de Goffau MC, Blokzijl T, Weersma RK, Penders J, Faber KN, Harmsen HJ, Dijkstra G. The ATG16L1-T300A allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn’s disease patients. Gut. 2015;64:1546–52. [DOI] [PubMed] [Google Scholar]
- 50.Burada F, Ciurea ME, Nicoli R, Streata I, Vilcea ID, Rogoveanu I, Ioana M. ATG16L1 T300A Polymorphism is Correlated with Gastric Cancer Susceptibility. Pathol Oncol Res. 2016;22:317–22 [DOI] [PubMed] [Google Scholar]
- 51.Lassen KG, Kuballa P, Conway KL, Patel KK, Becker CE, Peloquin JM, Villablanca EJ, Norman JM, Liu TC, Heath RJ, Becker ML, Fagbami L, Horn H, Mercer J, Yilmaz OH, Jaffe JD, Shamji AF, Bhan AK, Carr SA, Daly MJ, Virgin HW, Schreiber SL, Stappenbeck TS, Xavier RJ. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A. 2014;111:7741–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waters AM, Ozkan-Dagliyan I, Vaseva AV, Fer N, Strathern LA, Hobbs GA, Tessier-Cloutier B, Gillette WK, Bagni R, Whiteley GR, Hartley JL, McCormick F, Cox AD, Houghton PJ, Huntsman DG, Philips MR, Der CJ. Evaluation of the selectivity and sensitivity of isoform-and mutation-specific RAS antibodies. Sci Signal. 2017;10. pii: eaao3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.