Short abstract
Objective
To investigate the involvement of long non-coding RNA (lncRNA) Cancer Susceptibility 11 (CASC11) in patients with coronary artery disease (CAD).
Methods
This case–control study enrolled patients with CAD and age- and sex-matched healthy control subjects. The plasma levels of lncRNA CASC11 and transforming growth factor-beta 1 (TGF-β1) were measured. Diagnostic values of lncRNA CASC11 and TGF-β1 for CAD were determined using receiver operating characteristic curve analysis. Correlations between plasma levels of lncRNA CASC11 and TGF-β1 were analysed using linear regression.
Results
The study enrolled 82 patients with CAD and 82 healthy controls. Plasma levels of lncRNA CASC11 were downregulated in patients with CAD, while plasma TGF-β1 levels were upregulated in patients with CAD, compared with healthy controls. Plasma levels of lncRNA CASC11 and TGF-β1 distinguished patients with CAD from healthy controls and were inversely correlated in both groups. LncRNA CASC11 over-expression mediated the downregulation of TGF-β1 in human primary coronary artery endothelial cells, while TGF-β1 over-expression showed no significant effects on lncRNA CASC11 levels. An 8-year follow-up study showed that low lncRNA CASC11 levels were closely correlated with a higher mortality rate in patients with CAD.
Conclusion
LncRNA CASC11 is downregulated in CAD and inhibits TGF-β1.
Keywords: Coronary artery disease, lncRNA CASC11, transforming growth factor-β1, mortality
Introduction
Coronary artery disease (CAD), also known as ischaemic heart disease, is caused by damaged major blood vessels, which are responsible for the supplying oxygen, blood and nutrients to the heart.1 Despite efforts made regarding the treatment and prevention of CAD, this disease remains one of the most common causes of death all over the world including China.2 As a result, more than 40% of deaths in China are caused by CAD.3 In addition, the incidence of CAD is increasing as a consequence of the development of modern society and changes in people’s lifestyles.4 Several risk factors, such as smoking, diabetes mellitus, dietary intake, obesity and dyslipidaemia, have been identified for CAD.5 However, the pathogenesis of this disease remains unclear, leading to difficulties in the development of novel therapeutic approaches.
Genetic alterations were frequently observed in patients with CAD, indicating the critical roles of genetic factors in this disease.6,7 Research has demonstrated that long (>200 nucleotides) non-coding RNAs (lncRNAs) without any protein-coding ability can participate in physiological processes and disease progression by regulating the expression of genes.8,9 Expression of certain lncRNAs is altered during CAD, while functional investigations on lncRNAs in CAD are rare.10 LncRNA Cancer Susceptibility 11 (CASC11) plays a pivotal role in many types of cancer, such as gastric cancer,11 suggesting that it might also be of interest in CAD. A preliminary whole-genome transcriptome analysis undertaken by this current research team demonstrated the downregulation of lncRNA CASC11 in CAD (data not shown), so this present study investigated its potential involvement in patients with CAD.
Patients and methods
Study population
This case–control study recruited consecutive patients with CAD from the Outpatient Department of the Health and Medical Centre of the People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi City, Xinjiang Province, China between December 2009 and December 2011. A group of patients were selected from a larger group to participate in the study on the basis of the inclusion and exclusion criteria. All patients were confirmed to have CAD using coronary angiography. The inclusion criteria were as follows: (i) newly diagnosed; (ii) no therapies had been initiated. The exclusion criteria were as follows: (i) CAD patients transferred from other hospitals; (ii) any therapies for any clinical disorders were administered within 3 months prior to admission; (iii) any other obvious clinical disorders were observed. During the same time period, the current study enrolled healthy age- and sex-matched volunteers from the local population. All healthy participants from Urumqi City received systemic physiological examinations and the values of their physiological indicators were all within the normal range. All of the patients and healthy participants were informed of the experimental details and they provided written informed consent. The study was approved by the Ethics Committee of the Health and Medical Centre of the People’s Hospital of Xinjiang Uygur Autonomous Region.
Plasma samples
Before the initiation of any therapies, whole peripheral blood (5 ml) was collected from each patient with CAD and each healthy volunteer after an overnight fast. Whole peripheral blood was collected in tubes containing 1.5 mg/ml ethylenediaminetetra-acetic acid. Following centrifugation using an Allegra X-30 centrifuge (Beckman Coulter, Brea, CA, USA) at room temperature for 15 min at 1200 g, plasma was separated and stored in liquid nitrogen at –80°C before undertaking the following experiments.
Biochemical analyses
Biochemical data were retrieved from the medical records of the study participants.
Follow-up
Patients with CAD were followed up for 8 years after their initial diagnosis. Their survival conditions were monitored and recorded. This study did not include patients that were lost to follow-up or those that died of other causes.
Cells and transient transfections
LncRNA CASC11 and transforming growth factor (TGF)-β1 expressing pcDNA3 vectors were constructed by Sangon (Shanghai, China). Human coronary artery endothelial cells (HCAECs; ATCC, Gaithersburg, MD, USA) were transfected with pcDNA3 vectors expressing lncRNA CASC11 and TGF-β1, or empty pcDNA3 vector (negative control [NC]) using Lipofectamine® 2000 (Invitrogen, Shanghai, China). Cells were harvested at 24 h after transfection to perform the following experiments. Cells without any treatments were used as control (C) cells.
ELISA
The levels TGF-β1 in the plasma from patients with CAD and the healthy controls were measured using an enzyme-linked immunosorbent assay (ELISA) kits (ab100647; Abcam, Cambridge, UK). Levels of TGF-β1 in plasma were measured in ng/ml. The minimum detectable concentration was 80 pg/ml. Intra- and interassay coefficients of variation for all ELISAs were <5% and <10%, respectively.
Quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
TRIzol® reagent (Invitrogen) was used to extract total RNA from plasma (0.2 ml plasma per 1 ml TRIzol®) and HCAECs (105 cells per 1 ml TRIzol®). All RNA samples of satisfactory quality were subjected to DNase I digestion (Invitrogen). Following that, reverse transcriptase AMV (Sigma-Aldrich, St Louis, MO, USA) and SensiFAST™ SYBR® No-ROX One-Step Kit (Bioline, Boston, MA, USA) were used to carry out reverse transcriptions and prepare quantitative PCR mixtures, respectively. All experiments were repeated three times. Using 18S rRNA and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal housekeeping controls, the levels of lncRNA CASC11 and TGF-β1 RNA were analysed. The primer sequences were as follows: 5′-GCTGCAGAAGGTCCGAAGA-3′ (forward) and 5′-TTCACCACGTCCAGTTGCT-3′ (reverse) for CASC11; 5′-AAGAAGTCACCCGCGTGCT-3′ (forward) and 5′-TGTGTGATGTCTTTGGTTTTGT-3′ (reverse) for 18S rRNA; 5′-GGAGCGAGATCCCTCCAAAA-3′ (forward) and 5′-GGCTGTTGTCATACTTCTCATG-3′ (reverse) for TGF-β1; and 5′-CTACCACATCCAAGGAAGCA-3′ (forward) and 5′-TTTTTCGTCACTACCTCCCC-3′ for human GAPDH. The cycling programme involved preliminary denaturation at 95°C for 1 min, followed by 40 cycles of 95°C for 10 s and 58°C for 50 s. All PCR reactions were performed on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The relative amounts of lncRNA CASC11 and TGF-β1 mRNA to 18S rRNA and GAPDH were calculated using the 2–ΔΔCT method.
Western blot analysis
Total protein from HCAECs (105 cells per 1 ml RIPA buffer) was extracted using RIPA buffer (Sangon). After denaturing, the proteins were separated using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (10% gel). Following protein transfer to polyvinylidene fluoride membranes (Invitrogen) and blocking (2 h in 5% non-fat milk at room temperature), the membranes were incubated with rabbit anti-GAPDH primary polyclonal antibodies (1:800; ab9485; Abcam, Cambridge, UK) and rabbit anti-TGF-β1 primary polyclonal antibodies (1:800; ab92486; Abcam) overnight at 4°C. After washing the membranes in Tris-buffered saline Tween 20 (TBST; pH 7.4) three times (15 min per wash), the membranes were incubated with goat anti-rabbit immunoglobulin-horseradish peroxidase secondary antibodies (1:800; MBS435036; MyBioSource, Cambridge, UK) for 2 h at room temperature. Immunodetection was undertaken using an enhanced chemiluminescence method (Sigma-Aldrich). Image J version 1.46 software (National Institutes of Health, Bethesda, MA, USA) was used to analyse and normalize the signals.
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Data are presented as mean ± SD, which were calculated from three replicates. Differences between patient and control groups were determined using the unpaired t-test. Differences among different cell transfection groups were analysed using one-way analysis of variance and Tukey test. The diagnostic analysis was performed using receiver operating characteristic (ROC) curve. Correlations were analysed by performing linear regression. The patients with CAD were stratified in to high and low lncRNA CASC11 level groups. Based on follow-up data, the Kaplan–Meier method was used to plot survival curves. A log-rank test was performed to compare the survival curves. A P-value < 0.05 was considered statistically significant.
Results
The case–control study identified 228 patients with CAD and selected 82 (49 males and 33 females; mean ± SD age, 48.5 ± 9.1 years; age range, 33–63 years). A total of 82 age- and sex-matched healthy volunteers were also enrolled in the study (49 males and 33 females; mean ± SD age, 47.9 ± 8.6 years; age range, 33–64 years). The clinical and demographic characteristics of the patients and healthy controls are presented in Table 1. All 82 patients completed 8-year follow-up. The patients with CAD were stratified into high (n = 39) and low (n = 43) lncRNA CASC11 level groups.
Table 1.
Clinical and demographic characteristics of the patients (n = 82) and age- and sex-matched healthy controls (n = 82) that participated in a study to investigate the role of the long non-coding RNA Cancer Susceptibility 11 in coronary artery disease (CAD).
| Characteristic | CAD groupn = 82 | Control groupn = 82 |
|---|---|---|
| Sex | ||
| Male | 49 | 49 |
| Female | 33 | 33 |
| Age, years | 48.5 ± 9.1 | 47.9 ± 8.6 |
| Total cholesterol, mmol/l | 3.9 ± 1.1 | 3.9 ± 0/9 |
| Triglycerides, mmol/l | 1.0 ± 0.7 | 1.3 ± 0.5 |
| High-density lipoprotein cholesterol, mmol/l | 1.1 ± 0.1 | 1.1 ± 0.2 |
| Low-density lipoprotein cholesterol, mmol/l | 2.1 ± 0.8 | 2.1 ± 0.8 |
| Aspartate aminotransferase, U/l | 22.0 ± 4.4 | 21.7 ± 4.5 |
| Serum creatinine, µmol/l | 57.0 ± 7.3 | 64.4 ± 6.5 |
| Unbound thyroxine, pmol/l | 14.3 ± 2.3 | 15.1 ± 1.9 |
Data are presented as mean ± SD.
No significant between-group differences (P ≥ 0.05); unpaired t-test.
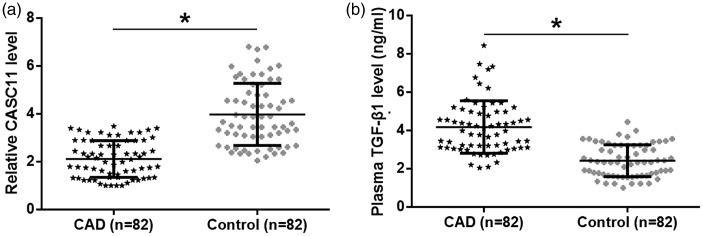
The plasma levels of lncRNA CASC11 and TGF-β1 were detected using qRT–PCR and ELISA, respectively, in the CAD and control groups. Compared with the control group, lncRNA CASC11 was significantly downregulated (Figure 1a, P < 0.05), while TGF-β1 was significantly upregulated in the plasma of patients in the CAD group (Figure 1b, P < 0.05).
Figure 1.
The plasma levels of long non-coding RNA Cancer Susceptibility 11 (CASC11) (a) and transforming growth factor (TGF)-β1 (b) in patients with coronary artery disease (CAD) and healthy control subjects as determined using quantitative reverse transcription–polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. Data are presented as mean (central black horizontal line) ± SD (error bars). Between-group comparisons undertaken with unpaired t-test; *P < 0.05.
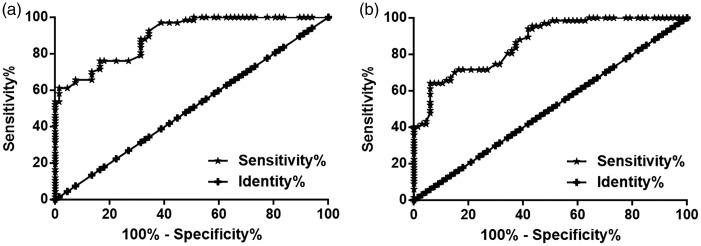
The diagnostic values of lncRNA CASC11 and TGF-β1 for CAD were determined by performing a ROC curve analysis. In this analysis, patients with CAD were the true positives and the healthy controls were the true negatives. For plasma lncRNA CASC11, the area under the curve (AUC) was 0.90 (AUC > 0.65 identified diagnostic potential) (Figure 2a). For plasma TGF-β1, the AUC was 0.87 (Figure 2b).
Figure 2.
The diagnostic potential of plasma levels of long non-coding RNA (lncRNA) Cancer Susceptibility 11 (CASC11) (a) and transforming growth factor (TGF)-β1 (b) for coronary artery disease (CAD) was determined using receiver operating characteristic (ROC) curve analyses. Plasma levels of lncRNA CASC11 and TGF-β1 distinguished CAD patients from healthy controls by ROC curve analysis.
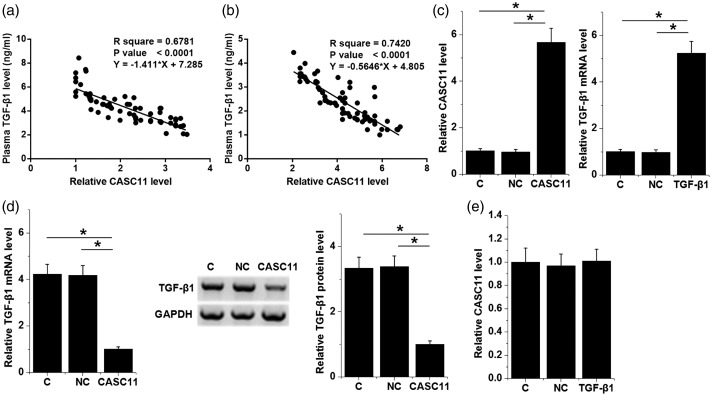
Correlations between plasma levels of lncRNA CASC11 and TGF-β1 were analysed by performing linear regression. Plasma levels of lncRNA CASC11 and TGF-β1 were inversely and significantly correlated in both the CAD group (Figure 3a) and control group (Figure 3b). The relationship between lncRNA CASC11 and TGF-β1 was further analysed by performing over-expression experiments in HCAECs. Compared with the control (C, untransfected cells) and the negative control (NC, empty vector transfection) groups, the levels of lncRNA CASC11 and TGF-β1 mRNA were significantly increased in HCAECs transfected with lncRNA CASC11 and TGF-β1 expression vectors at 24 h after transfection (Figure 3c; P < 0.05 for all comparisons). Moreover, HCAECs with lncRNA CASC11 over-expression showed significantly downregulated TGF-β1 at both the mRNA and protein levels (Figure 3d; P < 0.05 for all comparisons). However, TGF-β1 over-expression showed no significant effects on lncRNA CASC11 mRNA levels (Figure 3e).
Figure 3.
Studies of the effect of long non-coding RNA (lncRNA) Cancer Susceptibility 11 (CASC11) on transforming growth factor (TGF)-β1. Linear regression analyses demonstrated that plasma levels of lncRNA CASC11 and TGF-β1 were inversely and significantly correlated in both the CAD group (a) and the control group (b). The levels of lncRNA CASC11 and TGF-β1 mRNA were significantly increased in human coronary artery endothelial cells (HCAECs) transfected with lncRNA CASC11 and TGF-β1 expression vectors compared with the control (C) and negative control (NC) groups (c). The over-expression of lncRNA CASC11 mRNA mediated the downregulation of TGF-β1 mRNA and protein in HCAECs compared with the C and NC groups (d); The over-expression of TGF-β1 mRNA showed no significant effects on lncRNA CASC11 mRNA levels (e). Data are presented as mean ± SD. Differences among different cell transfection groups were analysed using one-way analysis of variance and Tukey test; *P < 0.05.
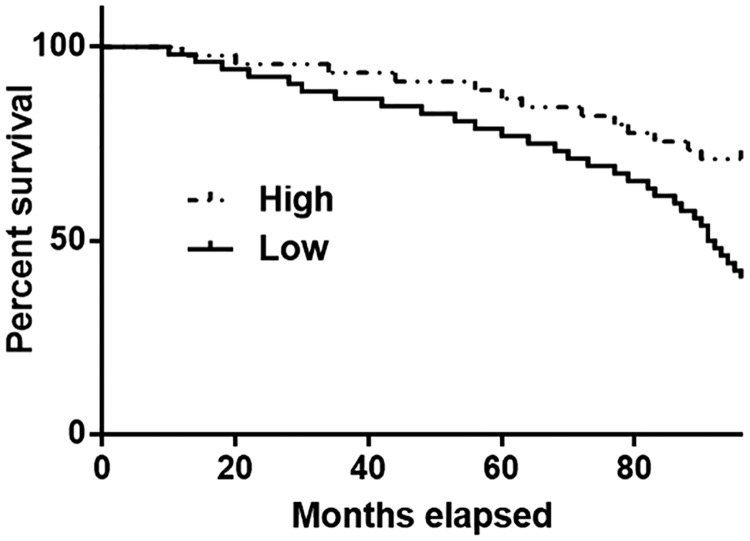
The cut-off value for CASC11 that was used to stratify patients into high and low lncRNA CASC11 level groups was 2.12. The prognostic value of lncRNA CASC11 for CAD was determined by performing a Kaplan–Meier survival curve analysis based on lncRNA CASC11 levels. During the 8-year follow-up, CAD patients in the low lncRNA CASC11 level group (n = 43) showed a significantly lower overall survival rate compared with patients in high lncRNA CASC11 level group (n = 39) (P < 0.05) (Figure 4).
Figure 4.
The prognostic value of long non-coding RNA (lncRNA) Cancer Susceptibility 11 (CASC11) for coronary artery disease (CAD) was determined by performing a Kaplan–Meier survival curve analysis based on lncRNA CASC11 levels. During the 8-year follow-up, CAD patients in the low CASC11 level group (n = 43) showed a significantly lower overall survival rate compared with patients in high CASC11 level group (n = 39) (P < 0.05).
Discussion
This current study investigated the expression pattern, clinical values and function of lncRNA CASC11 in patients with CAD. This current study demonstrated that lncRNA CASC11 levels were downregulated in patients with CAD and that changes in plasma lncRNA CASC11 levels may be of potential prognostic and diagnostic value as a biomarker for CAD. In addition, lncRNA CASC11 also downregulated TGF-β1 levels, which is involved in CAD.12,13
Coronary artery disease causes an unacceptably high mortality rate and accurate diagnosis and prognosis are extremely important.3With the advantage of their noninvasive nature, circulating biomarkers, such as blood lncRNAs, have shown promising potential in disease diagnosis.14 A novel lncRNA named CoroMarker has been identified as a promising biomarker for CAD.10 CoroMarker is upregulated in CAD and is independent of other CAD biomarkers.15 Another study showed that the polymorphisms of lncRNA MALAT1 could be used to predict CAD in a Chinese population.16 A preliminary whole-genome transcriptome analysis undertaken by this current research team demonstrated the downregulation of lncRNA CASC11 in CAD (data not shown). This present study further confirmed the downregulation of lncRNA CASC11 in patients with CAD. In addition, altered levels of plasma lncRNA CASC11 distinguished patients with CAD from healthy control subjects. Therefore, lncRNA CASC11 may be used to assist the diagnosis of CAD in clinical practice, while its sensitivity and specificity remain to be further investigated.
To the best of our knowledge, there are limited studies on the prognostic value of lncRNAs for CAD. This current 8-year follow-up study investigated the association between the levels of lncRNA CASC11 and the mortality rate of patients with CAD. These current data suggest that low levels of lncRNA CASC11 before treatment were closely correlated with poor survival of patients with CAD. Therefore, measuring the levels of lncRNA CASC11 before treatment may guide the development of individualized treatment strategies, thereby improving the survival of patients with CAD.
Transforming growth factor-β signalling controls cardiovascular function,17 such as maintenance of heart homeostasis.18 Upregulated levels of TGF-βs, such as TGF-β1, is closely correlated with the development of CAD.12,13 Over-expression of TGF-β1 promotes the development of CAD by regulating downstream genes, such as sphingosine kinase 1 and tissue inhibitor of metalloproteinase-1 to regulate heart cell behaviour, such as apoptosis.12,13 Similarly, this current study observed increased plasma levels of TGF-β1 in patients with CAD compared with healthy control subjects. This current study also demonstrated that lncRNA CASC11 over-expression downregulated TGF-β1 mRNA and protein levels. Therefore, lncRNA CASC11 may also be used as a therapeutic target for CAD.
This current study was limited by the small sample size. In addition, the mechanism that mediates the interaction between lncRNA CASC11 and TGF-β1 remains unclear. Future studies are needed to address these issues.
In conclusion, the levels of lncRNA CASC11 were downregulated in patients with CAD and had diagnostic and prognostic value. The over-expression of lncRNA CASC11 can downregulate TGF-β1. Therefore, regulation of lncRNA CASC11 may also prove beneficial for the treatment of this disease in the future.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Jianli Dang https://orcid.org/0000-0003-4602-6014
References
- 1.Hanson MA, Fareed MT, Argenio SL, et al. Coronary artery disease. Prim Care 2013; 40: 1–16. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation 2016; 133: 447–454. [DOI] [PubMed] [Google Scholar]
- 3.Hu SS, Kong LZ, Gao RL, et al. Outline of the report on cardiovascular disease in China, 2010. Biomed Environ Sci 2012; 25: 251–256. [DOI] [PubMed] [Google Scholar]
- 4.Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016; 4: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalen JE, Alpert JS, Goldberg RJ, et al. The epidemic of the 20(th) century: coronary heart disease. Am J Med 2014; 127: 807–812. [DOI] [PubMed] [Google Scholar]
- 6.Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet 2017; 18: 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res 2016; 118: 564–578. [DOI] [PubMed] [Google Scholar]
- 8.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 2014; 15: 7–21. [DOI] [PubMed] [Google Scholar]
- 9.Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res 2015; 116: 737–750. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Cai Y, Wu G, et al. Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin Sci (Lond) 2015; 129: 675–685. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Kang W, Lu X, et al. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle 2018; 17: 1886–1900. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Syrris P, Carter ND, Metcalfe JC, et al. Transforming growth factor-β1 gene polymorphisms and coronary artery disease. Clin Sci (Lond) 1998; 95: 659–667. [DOI] [PubMed] [Google Scholar]
- 13.Bogavac-Stanojevic N, Djurovic S, Jelic-Ivanovic Z, et al. Circulating transforming growth factor-β1, lipoprotein (a) and cellular adhesion molecules in angiographically assessed coronary artery disease. Clin Chem Lab Med 2003; 41: 893–898. [DOI] [PubMed] [Google Scholar]
- 14.Luo F, Wang T, Zeng L, et al. Diagnostic potential of circulating LncRNAs in human cardiovascular disease: a meta-analysis. Biosci Rep 2018; 38: BSR20181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Y, Yang Y, Chen X, et al. Circulating ‘lncRNA OTTHUMT00000387022’from monocytes as a novel biomarker for coronary artery disease. Cardiovasc Res 2016; 112: 714–724. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Ding H, Ouyang A, et al. LncRNA MALAT1 gene polymorphisms in coronary artery disease: a case–control study in a Chinese population. Biosci Rep 2019; 39: BSR20182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goumans MJ, Ten Dijke P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb Perspect Biol 2018; 10: a022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umbarkar P, Singh AP, Gupte M, et al. Cardiomyocyte SMAD4-dependent TGF-β signaling is essential to maintain adult heart homeostasis. JACC Basic Transl Sci 2019; 4: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]