Short abstract
Background
Chemotherapy is the standard treatment for non-small cell lung cancer (NSCLC). However, chemoresistance frequently occurs, making the treatment of NSCLC more difficult.
Method
We combined clinical and experimental studies to establish the role of microRNA (miR)-34c in NSCLC metastasis and chemoresistance.
Results
MiR-34c expression was significantly decreased in patients with NSCLC who showed a poor chemoresponse and metastasis. Overexpression of miR-34c sensitized NSCLC cells to paclitaxel and cisplatin both in vitro and in vivo. Furthermore, we found that NOTCH1 was target of miR-34c in NSCLC cells and played a key role in the effects of miR-34c on NSCLC.
Conclusion
NSCLC metastasis and chemoresistance are suppressed though the miR-34c/NOTCH1 axis. MiR-34c has important implications in the development of therapeutic strategies for metastasis and chemoresistance in NSCLC.
Keywords: Non-small cell lung cancer, miR-34c, metastasis, NOTCH1, chemoresistance, gene expression
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide.1,2 Chemotherapy is the standard treatment for advanced non-small cell lung cancer (NSCLC); however, chemoresistance and metastasis are huge clinical problems. The potential mechanisms underlying chemoresistance of NSCLC are of great interest in clinical oncology.
MicroRNAs (miRNAs) are gene regulators that play important roles in numerous biological processes.3 They are small non-coding RNA molecules of 18 to 22 nucleotides in length that regulate gene expression through interactions with complementary sequences in the 3′-untranslated regions (3′-UTRs) of target messenger RNAs (mRNAs).4 Growing numbers of studies are suggesting that miRNAs may play a key role in the modulation of drug resistance-related pathways in cancer cells.5–7 Recent studies have shown that miRNA 34c (miR-34c) is involved in the metastasis and chemoresponse of cancers8,9 and that the ectopic expression of miR-34c can induce cell cycle arrest and inhibit metastasis and chemoresistance of osteosarcoma cells.8 MiR-34c is one of the dysregulated miRNAs identified in NSCLC tissue. However, whether miR-34c-3p regulates the chemoresistance of NSCLC remains unknown.
In the present study, miR-34c was significantly decreased in patients with NSCLC. Overexpression of miR-34c enhanced NSCLC cell chemosensitivity. We combined clinical and experimental studies to establish the role of miR-34c in NSCLC metastasis and chemoresistance. Restoration of miR-34c dramatically enhanced the sensitivity of NSCLC cells to chemotherapy and inhibited NSCLC cell invasion. Therefore, our findings may be helpful for developing potential therapeutics against NSCLC metastasis and chemoresistance.
Materials and methods
Cell culture, oligonucleotide transfection, and human specimens
A549, H1299, and 293T cells were cultured in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum in a 5% carbon dioxide atmosphere at 37°C. Cells were transfected with 50 pmol of miRNA.
This study was approved by the Ethics Committee of The First Affiliated Hospital of Kunming Medical University. The specimens and adjacent tissues of 30 patients with NSCLC were used in this study. Verbal informed consent was obtained from each patient who participated in this study.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA was extracted from cells or tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subjected to qRT-PCR. The miR-34c and RNU6 expression levels were detected using the primer set from RiboBio Co. (Guangzhou, China). For miRNA-34c mimic (sequence: AGGCAGUGUAGUUAGCUGAUUGC), inhibitor (sequence: GCAAUCAGCUAACUACACUGCCU), or negative control molecules (Ruibo Bio-Technology, Guangzhou, China), RT-PCR was performed as described by Xu et al.8 All reagents for cell culture and transfection were purchased from Life Technologies (Carlsbad, CA, USA).
Cell viability
Twenty-four hours after transfection with the indicated nucleotide and/or a plasmid (Ruibo Bio-Technology), the cells were seeded into 96-well culture plates at a density of 5000 cells/well and incubated with paclitaxel or cisplatin (Sigma-Aldrich, St. Louis, MO, USA) for 24 hours. The Cell Counting Kit-8 (CCK-8) assay (Promega Corporation, Madison, WI, USA) was performed as described by Xu et al.8
Transwell invasion assay
Using 8.0-µm Transwell permeable supports (Corning Inc., Corning, NY, USA), a Transwell invasion assay was performed as described by Xu et al.8 Twenty-four hours after transfection, 5 × 104 cells in growth medium without serum were seeded into the upper wells of a BD BioCoat Chamber (BD Biosciences, Franklin Lakes, NJ, USA). The lower wells contained the same medium with 10% serum. After 48 hours of incubation, the cells that had migrated to the lower side of the chamber were fixed with 4% glutaraldehyde, stained with 0.1% crystal violet, and counted under microscopy.
Western blot
The indicated cells were transfected with the indicated oligonucleotides. At 72 hours after transfection, the cells were subjected to western blot. Western blot analysis was performed as described by Xu et al.8 All antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Stained cells were analyzed with a FACSCalibur Flow Cytometer (BD Biosciences).
Apoptosis assays
Apoptotic cells were detected using flow cytometry. At 48 hours after transfection, the cells were treated with the indicated drugs for 24 hours. The degree of cell apoptosis was assessed by an Annexin V–Propidium Iodide Apoptosis Detection Kit (BD Pharmingen; BD Biosciences) by flow cytometric analysis according to the manufacturer’s protocol.
Luciferase reporter assay
The NOTCH1 3′-UTR containing target sequences complementary to the miR-34c seed sequence was cloned downstream of the Firefly luciferase gene in the pMIR-REPORT luciferase vector (Life Technologies). Mutated NOTCH1 3′-UTR sequences were also cloned in the same vector. The indicated reporter constructs and 100-nM miR-34c mimics were cotransfected with the phRG-TK Renilla luciferase internal control plasmid (Promega Corporation) into 293T cells. Cell extracts were prepared 24 hours after transfection, the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega Corporation), and the ratio of Firefly/Renilla values was reported. The Dual-Luciferase Reporter Assay was performed as described by Xu et al.8
In vivo lung tumor xenograft model
Six-week-old female athymic (nu/nu) mice were purchased from the animal center of Kunming Medical University. A549 cells (1 × 107 cells) stably transfected with the control vector or miR-34c were constructed in our laboratory. The mice were divided into four groups using the random number method. After 3 weeks, all seven animals in each group had tumors of >5 mm in diameter, and either paclitaxel or cisplatin was intraperitoneally injected once a week for 6 weeks after the tumor volume reached 1500 mm3.
Statistical analysis
Statistical significance was analyzed by the unpaired Student’s t tests or one-way analysis of variance and Duncan’s multiple range test using the SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Decreased miR-34c was associated with chemoresistance and metastasis of NSCLC
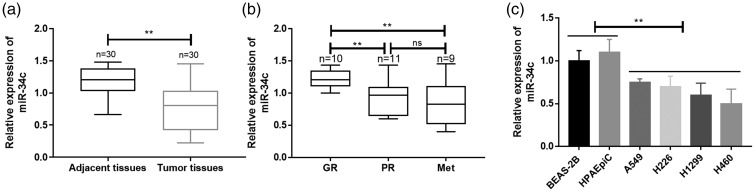
MiR-34c expression was significantly lower in tumor specimens than in adjacent lung tissues (p < 0.01) (Figure 1(a), Table 1). Additionally, miR-34c expression was significantly lower in NSCLC specimens that showed a poor than good chemoresponse (p < 0.01), but there was no distinction between a poor chemoresponse and metastasis (Figure 1(b)). Our data also showed that the expression of miR-34c was significantly lower in NSCLC cell lines than in human pulmonary alveolar epithelial cells and human bronchial epithelial cells (p < 0.01) (Figure 1(c)).
Figure 1.
Decreased miR-34c expression was associated with the development of chemoresistance and metastasis of NSCLC. (a) The expression of miR-34c in the primary tumors of patients with NSCLC was measured by qRT-PCR. (b) The expression of miR-34c in the GR, PR, and Met of patients with NSCLC was measured by qRT-PCR. (c) The expression of miR-34c was clarified in different NSCLC cell lines by qRT-PCR. miR, microRNA; NSCLC, non-small cell lung cancer; qRT-PCR, quantitative real-time polymerase chain reaction; GR, good chemoresponse; PR, poor chemoresponse; Met, lung metastasis; HPAEpiC, human pulmonary alveolar epithelial cells; BEAS-2B, human bronchial epithelial cells. **p < 0.01.
Table 1.
Characteristics of patients with non-small cell lung cancer (n = 30).
| Variable | Patients, n (%) |
|---|---|
| Age, years | |
| ≥60 | 11 (36.7) |
| <60 | 19 (63.3) |
| Sex | |
| Male | 13 (43.3) |
| Female | 17 (56.7) |
| Smoking habit | |
| No | 20 (66.7) |
| Yes | 10 (33.3) |
| Pathology | |
| Adenocarcinoma | 23 (76.7) |
| Non-adenocarcinoma | 7 (23.3) |
MiR-34c overexpression significantly enhanced NSCLC chemosensitivity
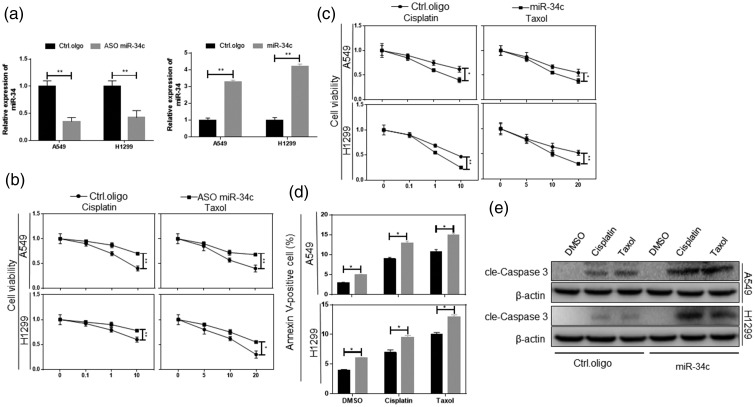
An association of miR-34c with metastasis and chemoresistance has been reported.10,11 Cisplatin and paclitaxel are commonly used anticancer drugs in the treatment of NSCLC, and patients with resistance to these drugs have a poor prognosis.12,13 We transfected antisense oligonucleotides (ASO) and mimics specific for miR-34c in A549 and H1299 cells and detected the miR-34c expression level 72 hours after transfection (Figure 2(a)). The CCK-8 assay showed that the inhibition of miR-34c caused the NSCLC cells to be resistant to all anticancer treatments (p < 0.05) (Figure 2(b)). Corresponding to this result, overexpression of miR-34c made the cells sensitive to cisplatin and paclitaxel, made them more sensitive to chemotherapy drugs, and increased the rate of drug-induced apoptosis (p < 0.05) (Figure 2(c)–(e)).
Figure 2.
Restoration of miR-34c significantly enhanced NSCLC cell chemosensitivity. (a) The indicated cells were transfected with miR-34c mimics or miR-34c ASO. At 72 hours after transfection, the expression of miR-34c was measured by qRT-PCR. (b) The indicated cells were treated with the indicated drugs and miR-34c ASO for 24 hours, and the cell viability was then analyzed by the CCK-8 assay. (c) The indicated cells were treated with the indicated drugs and miR-34c mimics for 24 hours, and the cell viability was then analyzed by the CCK-8 assay. (d) The indicated cells were treated with doxorubicin, cisplatin, or paclitaxel for 24 hours, and apoptosis was then analyzed by measuring the percentage of annexin V-positive cells using flow cytometry. (e) Cleaved caspase 3 was analyzed by western blot. miR, microRNA; NSCLC, non-small cell lung cancer; ASO, antisense oligonucleotides; qRT-PCR, quantitative real-time polymerase chain reaction, CCK-8, Cell Counting Kit-8. *p < 0.05, **p < 0.01.
Mir-34c inhibited NSCLC cell migration
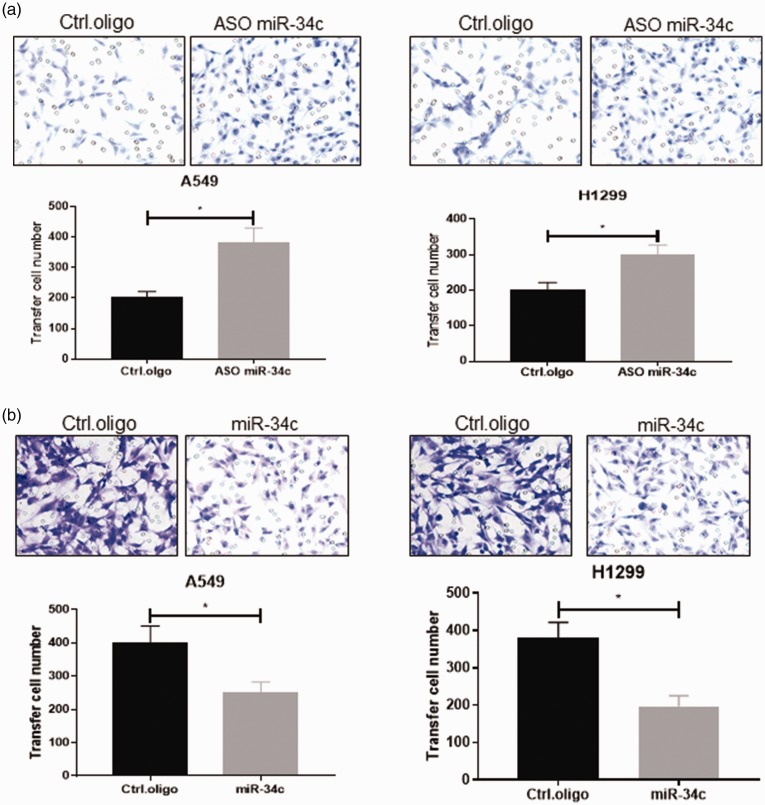
Next, we used the Transwell assay to detect the effect of miR-34c on NSCLC cell migration. As shown in Figure 3(a), a significantly higher migration rate was detected when miR-34c was knocked down (p < 0.05). However, a significantly lower migration rate was observed when miR-34c was overexpressed (p < 0.05) (Figure 3(b)).
Figure 3.
MiR-34c inhibited NSCLC cell migration. We used the Transwell assay to detect the effect of miR-34c on NSCLC cell migration. (a) When miR-34c was knocked down, a higher migration rate was detected in A549 and H1299 cell lines. (b) When miR-34c was overexpressed, NSCLC cell migration was suppressed. miR, microRNA; NSCLC, non-small cell lung cancer. *p < 0.05.
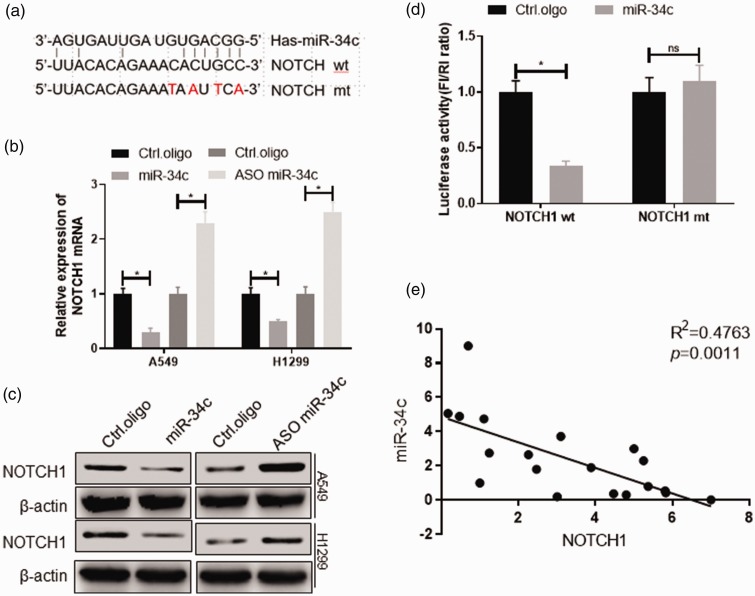
MiR-34c target NOTCH1 in NSCLC
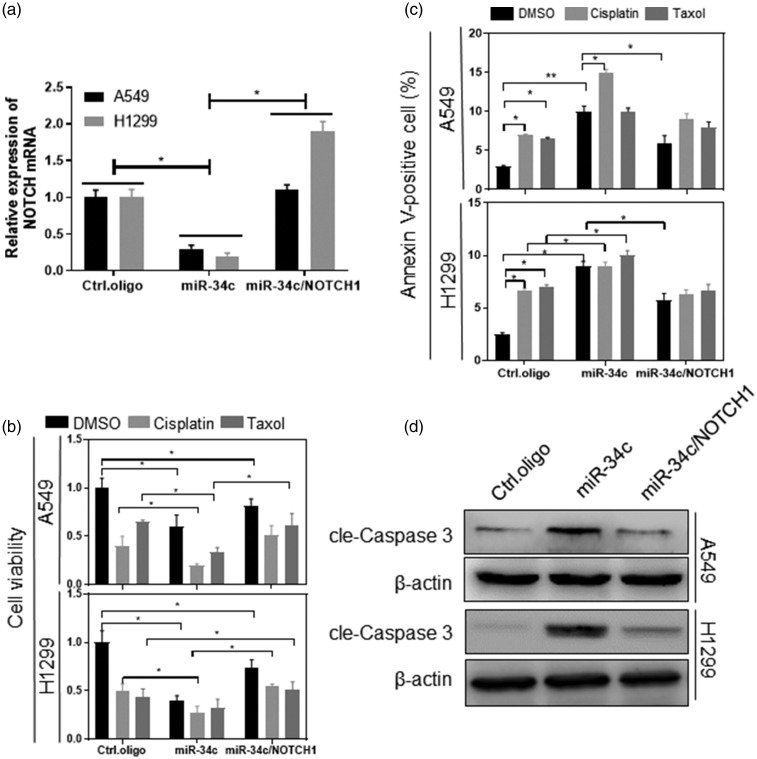
MiRNA acts mainly on the 3′-UTR region of the gene; therefore, we used an miRNA-target prediction program to identify putative target genes of miR-34c, of which NOTCH1 is a target (Figure 4(a)). As shown in Figure 4(b) and (c), overexpression of miR-34c inhibited NOTCH1 mRNA and protein expression, whereas interference with miR-34c promoted NOTCH1 mRNA and protein expression (p < 0.05). We then inserted four base mutations into the 3′-UTR of NOTCH1 and determined whether miR-34c regulates NOTCH1 by binding to the 3′-UTR region of NOTCH1 using the Dual-Luciferase Reporter Assay. The results showed that overexpression of miR-34c significantly inhibited the luciferase activity of wild-type NOTCH1 (p < 0.05) and that miR-34c had no significant effect on mutated NOTCH1 in 293T cells (Figure 4(d)). In addition, there was a significant negative correlation between miR-34c and NOTCH1 expression levels in patients with NSCLC (p = 0.0011) (Figure 4(e)).
Figure 4.
NOTCH1 is a target of miR-34c in NSCLC cells. (a) The miR-34c seed sequence was complementary to the 3′-UTR of human NOTCH1. 293T cells were transfected with the indicated nucleotides. At 48 hours after transfection, the expression of the indicated genes was measured using (b) qRT-PCR and (c) western blot. (d) 293T cells were cotransfected with a Firefly luciferase reporter plasmid encoding the wild-type or mutated 3′-UTR of human NOTCH1, with miR-34c mimics and a Renilla luciferase reporter for normalization. At 24 hours after transfection, luciferase activity was measured. (e) qPCR analysis in human NSCLC samples (n = 18) showed that the expression of miR-34c and NOTCH1 was negatively correlated. miR, microRNA; NSCLC, non-small cell lung cancer; qRT-PCR, quantitative real-time polymerase chain reaction. *p < 0.05, **p < 0.01.
MiR-34c regulates NSCLC cell chemosensitivity by NOTCH1 in vitro
We overexpressed miR-34c and NOTCH1 simultaneously to determine whether the function of miR-34c is related to NOTCH1 (Figure 5(a)). We then analyzed the chemosensitivity and apoptosis rate by flow cytometry and CCK-8, respectively, as shown in Figure 5(b). Overexpression of NOTCH1 significantly inhibited the high chemosensitivity induced by miR-34c (p < 0.05). In addition, NOTCH1 significantly inhibited apoptosis induced by miR-34c overexpression (p < 0.05) (Figure 5(c) and (d)).
Figure 5.
MiR-34c regulates NSCLC cell chemosensitivity through NOTCH1 in vitro. The indicated cells were transfected with the indicated nucleotides and plasmid. At 48 hours after transfection, the indicated (a) NOTCH1 mRNA was measured by qRT-PCR, (b) cell viability was analyzed by the CCK-8 assay, (c) apoptosis was analyzed by measuring the percentage of annexin V-positive cells using flow cytometry, and (d) protein expression was measured by western blot. miR, microRNA; NSCLC, non-small cell lung cancer; qRT-PCR, quantitative real-time polymerase chain reaction; CCK-8, Cell Counting Kit 8. *p < 0.05, **p < 0.01.
MiR-34c regulates NSCLC chemosensitivity in vivo
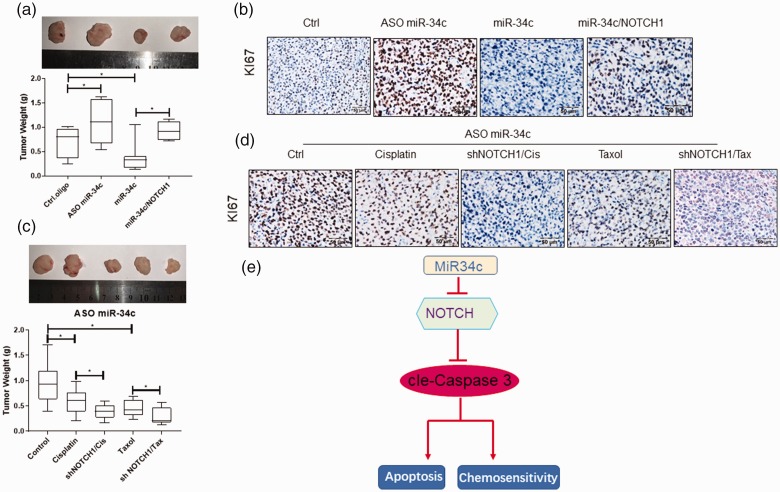
We used a xenograft model generated by the lentiviral transfected A549 to observe whether this theory is correct in vivo. When the average volume of the xenograft tumor reached 100 mm3, the mice underwent 4 weeks of cisplatin treatment. The results showed that in the A549 xenograft model, miR-34c mimics significantly suppressed the tumor weight (p < 0.05). However, miR-34c ASO increased the tumor weight. Short hairpin RNAs specific to NOTCH1 (shNOTCH1) significantly reversed the suppression of tumor growth by miR-34c mimics (p < 0.05) (Figure 6(a)). The immunohistochemistry assay verified that miR-34c suppressed the expression of Ki67 but that NOTCH1 reversed it (Figure 6(b)). Finally, we demonstrated the correlation between miR-34c ASO and NOTCH1 in regulating NSCLC chemotherapy resistance in vivo (Figure 6(c)). For this purpose, we investigated the effects of miR-34c on tumor growth in nude mice treated with paclitaxel and cisplatin. The combination of miR-34c inhibitor and paclitaxel or cisplatin treatment resulted in a significant decrease in tumor growth (p < 0.05). shNOTCH1 enhanced the inhibition of paclitaxel or cisplatin in the NSCLC xenograft model. As expected, the expression levels of Ki67 were significantly lower in the shNOTCH1 combined with cisplatin or paclitaxel treatment group than in the control group (p < 0.05) (Figure 6(d)).
Figure 6.
MiR-34c regulates NSCLC chemosensitivity in vivo. (a) The mice were sacrificed and the tumor weights were measured. miR-34c mimics significantly suppressed the tumor weight. However, miR-34c ASO increased the tumor weight. Upregulated NOTCH1 expression significantly reversed the suppression of tumor growth by miR-34c mimics. (b) The IHC assay verified that miR-34c suppressed the expression of Ki67 but that NOTCH1 reversed it. (c) The correlation between miR-34c ASO and siNOTCH1 in regulating NSCLC chemotherapy resistance was analyzed in vivo. The combination of miR-34c inhibitor and paclitaxel or cisplatin treatment resulted in a significant decrease in tumor growth. Short hairpin RNAs specific to NOTCH1 (shNOTCH1) enhanced the inhibition of paclitaxel or cisplatin in the NSCLC xenograft model. (d) The IHC assay verified the expression of Ki67. When miR-34c was suppressed, the expression level of Ki67 was significantly lower in the shNOTCH1 combined with cisplatin or paclitaxel treatment group than in the control. (e) A schematic model showing that miR-34c-3p target inhibiting NOTCH1 suppresses the chemosensitivity and metastasis of NSCLC. miR, microRNA; NSCLC, non-small cell lung cancer; ASO, antisense oligonucleotides; IHC, immunohistochemistry. *p < 0.05.
Discussion
As chemotherapeutic drugs have been increasingly used to treat patients with cancer, tumor cell chemoresistance has become a major problem in the clinical setting.14 In recent years, growing numbers of studies have indicated that chemoresistance-related genes are regulated by aberrant miRNA expression.15,16 In our study, miR-34c directly targeted and inhibited expression of NOTCH1 and played a role in regulating chemoresistance and migration of NSCLC cells. NOTCH1 and miR-3c have been previously investigated in some other types of cancer.8,17–21 Before the present study, however, the mechanisms of upregulated NOTCH1 expression in NSCLC had not been investigated.
Our data showed that miR-34c expression was significantly lower in NSCLC cells and tissue samples than in normal lung cells and paired adjacent tissue, respectively. Inhibition of miR-34c expression increased NSCLC cell chemoresistance and migration and reduced apoptosis; in contrast, overexpression of miR-34c expression significantly reduced NSCLC cell chemoresistance and migration and increased apoptosis. The Dual-Luciferase Reporter Assay showed that miR-34c was directly bound to the 3′-UTR of NOTCH1 mRNA, directly regulating the expression of NOTCH1. These results were also verified in the xenograft. Our data show that miR-34c functions as a tumor suppressor and that restoring expression of miR-34c may be useful for treatment of NSCLC.
Members of the miR-34c family act as tumor suppressors in some types of cancer.22–24 In our study, miR-34c was significantly decreased in chemotherapy-resistant tissue and acted as a chemosensitizer, which is consistent with its roles in osteosarcoma and lymphocytic leukemia.8,25,26 To verify this hypothesis, we examined whether restoration of miR-34c expression could dramatically suppress cell migration, prevent chemotherapy resistance, and promote apoptosis in vitro and in vivo. However, many factors involved in deregulation of miR-34c may differ among different types of cancer. MiR-34c-3p expression is reportedly increased in chronic lymphocytic leukemia and breast cancer.27
In summary, our data suggest that miR-34c plays a critical role in regulating chemotherapy resistance and migration and represses NOTCH1 to decrease chemotherapy resistance and suppress cell migration. Our study clarifies that together, miR-34c and NOTCH1 play a critical role in chemotherapy resistance. These findings suggest that miR-34c may be a novel therapeutic target in NSCLC.
Acknowledgements
We thank all the people and patients who participated in this study.
Authors’ contributions
Linzhu Yang and Xi Zhan conceived and designed this study. Linzhu Yang, Changcheng Lei, and Yunping Zhao performed the main experiments. Hong-wen Sun constructed the tumor xenograft model. Qinghe Yu, En-Ji Yang, and Xi Zhan were jointly involved in extracting the data and writing the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Natural Science Foundation of China (81060010).
ORCID iD
References
- 1.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008; 100: 1672–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol 2014; 9: 287–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lujambio A, Lowe SW. The microcosmos of cancer. Nature 2012; 482: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du L, Subauste MC, DeSevo C, et al. miR-337-3p and its targets STAT3 and RAP1A modulate taxane sensitivity in non-small cell lung cancers. PLoS One 2012; 7: e39167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng B, Wang R, Song HZ, et al. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer 2012; 118: 3365–3376. [DOI] [PubMed] [Google Scholar]
- 7.Tan S, Wu Y, Zhang CY, et al. Potential microRNA targets for cancer chemotherapy. Curr Med Chem 2013; 20: 3574–3581. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, Jin H, Xu CX, et al. MiR-34c inhibits osteosarcoma metastasis and chemoresistance. Med Oncol 2014; 31: 972. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Rao Q, Zhang H, et al. miR-34C disrupts the stemness of purified CD133(+) prostatic cancer stem cells. Urology 2016; 96: 177.e1–177.e9. [DOI] [PubMed] [Google Scholar]
- 10.Zhou YL, Xu YJ, Qiao CW. MiR-34c-3p suppresses the proliferation and invasion of non-small cell lung cancer (NSCLC) by inhibiting PAC1/MAPK pathway. Int J Clin Exp Pathol 2015; 8: 6312–6322. [PMC free article] [PubMed] [Google Scholar]
- 11.Catuogno S, Cerchia L, Romano G, et al. miR-34c may protect lung cancer cells from paclitaxel-induced apoptosis. Oncogene 2013; 32: 341–351. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Wang J, Liu L, et al. Curcumin increases the sensitivity of Paclitaxel-resistant NSCLC cells to Paclitaxel through microRNA-30c-mediated MTA1 reduction. Tumour Biol 2017; 39: 1010428317698353. [DOI] [PubMed] [Google Scholar]
- 13.Zang H, Peng J, Wang W, et al. Roles of microRNAs in the resistance to platinum based chemotherapy in the non-small cell lung cancer. J Cancer 2017; 8: 3856–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Periti P, Mini E. Drug resistance in cancer: an overview of the clinical aspects. J Chemother 1989; 1: 5–9. [DOI] [PubMed] [Google Scholar]
- 15.Fan Z, Cui H, Yu H, et al. MiR-125a promotes paclitaxel sensitivity in cervical cancer through altering STAT3 expression. Oncogenesis 2016; 5: e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Y, Wang P, Li Y, et al. miR-375 is upregulated in acquired paclitaxel resistance in cervical cancer. Br J Cancer 2013; 109: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae Y, Yang T, Zeng HC, et al. miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet 2012; 21: 2991–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Cao S, Xu P, et al. Changes in the expression of miR-34a and its target genes following spinal cord injury in rats. Med Sci Monit 2016; 22: 3981–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou L, Xu J, Li H, et al. MiR-34c represses muscle development by forming a regulatory loop with Notch1. Sci Rep 2017; 7: 9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu XD, Zhang LY, Zhu TC, et al. Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int J Clin Exp Pathol 2015; 8: 4525–4534. [PMC free article] [PubMed] [Google Scholar]
- 21.Sun N, Lei L, Wang Y, et al. Preliminary comparison of plasma notch-associated microRNA-34b and -34c levels in drug naive, first episode depressed patients and healthy controls. J Affect Disord 2016; 194: 109–114. [DOI] [PubMed] [Google Scholar]
- 22.Misso G, Di Martino MT, De Rosa G, et al. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids 2014; 3: e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ 2010; 17: 193–199. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Paranjape T, Müller RU, et al. The mir-34 microRNA is required for the DNA damage response in vivo in C. elegans and in vitro in human breast cancer cells. Oncogene 2009; 28: 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng D, Wang H, Li L, et al. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia 2018; 32: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Yang Q, Wang L, et al. Knockdown of the oncogene lncRNA NEAT1 restores the availability of miR-34c and improves the sensitivity to cisplatin in osteosarcoma. Biosci Rep 2018; 38: BSR20180375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achari C, Winslow S, Ceder Y, et al. Expression of miR-34c induces G2/M cell cycle arrest in breast cancer cells. BMC Cancer 2014; 14: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]