Short abstract
Objective
Huang-Lian-Jie-Du-Tang (HLJDT), a traditional Chinese medicine, improves cognitive ability in rat models of Alzheimer’s disease (AD). The objective of this study was to evaluate the protective effects of HLJDT on learning and memory impairment that are caused by Aβ25–35.
Methods
Rats were randomly assigned to the following groups: control (water), Aβ25–35, donepezil hydrochloride 1.05 mg/kg, HLJDT 6 g/kg, HLJDT 3 g/kg, and HLJDT 1.5 g/kg and the corresponding drugs were administered for 28 days by oral gavage. HLJDT for the prevention of Aβ25–35-induced injury in rats and the underlying mechanisms were assessed. Aβ25–35 and amyloid precursor protein (APP) levels were measured in the hippocampal specimens. Total superoxide dismutase (T-SOD), glutathione (GSH), and malondialdehyde (MDA) levels in the hippocampus were also measured. The ultrastructure of CA1 hippocampal region was observed using electron microscopy.
Results
HLJDT treatment ameliorated impaired learning and memory significantly, decreased Aβ25–35, and APP levels in the hippocampus, increased T-SOD and GSH activity and decreased the MDA concentration, and alleviated the nuclear and cytoplasmic abnormalities of the hippocampal CA 1 region that were induced by Aβ25–35 injection.
Conclusions
HLJDT might decrease hippocampal vulnerability to Aβ25–35, suggesting its potential neuroprotective effect in AD.
Keywords: Alzheimer’s disease, β-amyloid, Aβ25–35, Huang-Lian-Jie-Du Decoction, memory deficits, hippocampus
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that is characterized by progressive cognitive deterioration, which is accompanied by reduced daily activities and behavioral changes.1 β-amyloid (Aβ), the main component of the amyloid plaques and the crucial pathophysiological feature of AD, is derived from proteolytic cleavage of amyloid precursor protein (APP). Aβ accumulation is considered to be a principal cause of the neurodegeneration that is typically observed in AD and it has been associated with AD-like memory impairment.2
Oxidative stress (OS) is an abnormal redox state that is caused by an imbalance between the generation and detoxification of reactive oxygen species (ROS).3 ROS have been strongly implicated in AD pathophysiology.4 Several studies have indicated that Aβ-induced neuronal apoptosis and necrosis involves the participation of ROS. Aβ can lead to the production of intracellular ROS, which causes protein and lipid peroxidation in neurons.5
Despite many efforts, there is no efficient treatment for AD. Therapeutic strategies are currently limited to symptomatic treatment and do not slow disease progression.6 Drug research is based upon single-target-directed strategies such as acetylcholinesterase (AChE) inhibitors and neurotrophic factors,7 but these approaches do not seem to cure AD. Traditional Chinese medicines (TCMs) and their active components, which are known for their multi-target and multi-functional properties, have anti-depressive, anti-psychotic, anti-oxidant, anti-amyloid, and anti-inflammatory effects.7
Huang-Lian-Jie-Du-Tang (HLJDT), a TCM recipe, consists of Rhizoma coptidis, Radix scutellariae, Cortex phellodendri, and Fructus gardenia, and its main effects are believed to be “purging fire and removing toxins.” It is traditionally used to treat inflammation, Chinese toxic heat syndromes and infectious diseases.8 Preclinical pharmacological studies demonstrated that HLJDT possesses a broad range of functions9 and has potentially beneficial effects in diabetes,10 cerebrovascular diseases,11,12 and inflammation. HLJDT also influences the expression of numerous genes in a mouse model of dementia.7 Moreover, recent studies also revealed that HLJDT significantly improves cognitive ability in rats with AD, suggesting that HLJDT can alleviate age-related deterioration of learning and memory.13,14
Previous studies have suggested that the effects of HLJDT against cognitive deficits might involve prevention of decreased acetylcholine content15 or a reduction in OS. However, the mechanisms by which HLJDT enhances learning and memory are currently unknown. The present study aimed to evaluate the protective effects of HLJDT against learning and memory impairment that is induced by injection of Aβ25–35 in rats.
Materials and methods
Preparation of HLJDT
HLJDT was prepared based on a classic recipe including Rhizoma coptidis, Radix scutellariae, Cortex phellodendri, and Fructus gardenia (Table 1).13 The herbs were purchased from the Teaching Hospital of Chengdu University of TCM (Chengdu, China), and they were combined at a ratio of 3:2:2:3 (dry weight), mixed, and decocted twice with boiling water (1:10, w/v) for 1 hour.16 The resulting extract was filtered through five layers of gauze. The yield of HLJDT aqueous extraction was 20% (w/w) of the dry medicinal herbs.17 Coldspray ionization-mass spectrometry (CSI-MS) was used to detect the active components of the HLJDT extract.7
Table 1.
Huang-Lian-Jie-Du-Tang composition and herbal chemical constituents.
| Plant name | Chemical constituents |
|---|---|
| Coptis chinensis Franch | Berberine, coptisine, palmatine |
| Scutellaria baicalensis Georgi | Baicalin, baicalein, wogonin |
| Phellodendron amurense Ruprecht | Berberine, palmatine, phellodendrine |
| Gardenia jasminoides Ellis | Geniposide, genipin, gardenoside |
Animals
Male Sprague–Dawley rats were obtained from Chengdu Dossy Experimental Animals Co., Ltd. (China) and housed for 1 week for acclimation. The rats were maintained in cages at room temperature (22 ±1°C) under a standard 12-hour:12-hour light:dark cycle, with free access to food and water. Experiments were reviewed and approved by the Animal and Ethics Review Committee of Chengdu University of Traditional Chinese Medicine (No: 2013-03).
The rat model of AD was induced as previously described.18 Briefly, the rats were anaesthetized by intraperitoneal injection of 10% chloralhydrate and fixed in a stereotaxic device. Then, a midline incision was made on the scalp, and holes were drilled on both sides of the skull. The injection position was −3.0 mm posterior to the bregma, ± 2.0 mm lateral to the sagittal suture, and −4.0 mm below the dura. Aβ25–35 solution (Sigma-Aldrich, Inc., St. Louis, MO, USA; 5 µL, 2 μg/μL) or sterile saline was injected into the right and left hippocampal CA1 regions at a speed of 1 µL/minute using a microinjection device. After the injection was completed, the microinjection device was left at the site of injection for 5 minutes before slow withdrawal. After surgery, all rats were placed into cages, and 80,000 U/0.25 mL of penicillin was administered for 3 days by intramuscular injection.19 Rats were randomly assigned to control, Aβ25–35, donepezil hydrochloride (1.05 mg/kg), HLJDT 6 g/kg, HLJDT 3 g/kg, and HLJDT 1.5 g/kg groups. Rats in the control group were injected with sterile saline, while those in the other groups received Aβ25–35 by injection.
Drug administration
Animals in the control and Aβ25–35 groups were administered distilled water intragastrically for 28 days; those in the donepezil hydrochloride group were treated with donepezil hydrochloride (1.05 mg/kg) by oral gavage for 28 days; while animals in the HLJDT (1.5, 3, 6 g/kg) groups were administered the corresponding dosage of HLJDT by oral gavage for 28 days. Donepezil hydrochloride (a reversible AChE inhibitor) was selected as a positive control because two previous studies reported that berberine, an important active ingredient of Coptis chinensis, inhibits acetylcholine hydrolysis.
Morris water maze test
Learning and memory with regard to spatial orientation were assessed using the Morris water maze (MWM) test, performed as described previously.20,21 The MWM consisted of a 50-cm-high circular pool that was 120 cm in diameter and filled with water (24 ± 0.5°C) to a depth of 24 cm. A hidden, submerged platform was placed in one quadrant (quadrant IV) at a depth of 2 cm below the pool surface. The test was conducted in two parts: a spatial navigation test (conducted on days 1 to 4) to assess spatial learning and a spatial probe task (conducted on day 5) to assess spatial memory.
For the spatial learning task (days 1 to 4), the animals were placed in the tank in quadrant I, II, or III, facing the pool wall, and circumnavigated the pool to search for the escape platform in quadrant IV. Three trials (90 seconds per trial) were completed per day, and the escape latency (time to find the hidden platform) was recorded for each trial. If an animal failed to locate the platform during the 90-second trial, the animal was guided to the platform and an escape latency of 90 seconds was recorded. The mean escape latency was used as an indicator of the ability of the animal to learn the location of the hidden platform.
For the spatial probe test (day 5), which tests the ability of each animal to remember where it had been located during the spatial learning task, the hidden platform was removed. Each rat underwent three trials (90 seconds per trial, starting from quadrants I, II, or III). The number of crossings of the platform quadrant (NCP), swimming time in the platform quadrant (STP), and swimming distance in the platform quadrant (SDP) were recorded to assess spatial memory performance.22,23
Immunohistochemistry
After completing the MWM test, three rats were randomly selected per group, and each was anesthetized with 10% urethane (1 g/kg) and laid in the supine position to fully expose the thorax and heart. The rats were perfused through the ascending aorta with cold saline solution (4°C), followed by 4% paraformaldehyde in phosphate-buffered saline (PBS; 4°C). The whole brain was removed carefully, fixed in 4% paraformaldehyde for 24 hours, dehydrated, and embedded in paraffin. The hippocampal CA1 region was used for immunohistochemical analysis.24
Coronal sections (5-µm thickness) of the brain were taken from the region extending 5 mm anterior to the bilateral injection points (used for Aβ25–35 administration) to 5 mm posterior to the injection points. Following quenching with endogenous peroxidase and blocking with normal goat serum, the sections were incubated with anti-rat Aβ primary antibody (1:1000; Abcam, Cambridge, MA, USA) or anti-rat APP primary antibody (1: 500; Abcam) overnight at 4°C. PBS was used as a control. After washing in PBS three times (2 minutes per wash), the sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Zhongshan Company, Beijing, China; 1:2000) for 20 minutes at 37°C. Sections were stained with 3ʹ3-diaminobenzidine. The sections were counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and mounted using neutral gum. The density of staining for Aβ and APP was measured using Image Pro Plus 5.0 software (Media Cybernetics, Bethesda, MD, USA). Brown staining represented positive staining for Aβ or APP proteins, and the staining density was taken to indicate the protein expression level. Photographs were taken at a magnification of 400×.
Western blot
Eight rats were selected randomly from each group. The brains were removed carefully. The right hippocampal tissue was weighed and homogenized in lysis buffer (20 mM Tris-HCl, pH 7.4, 140 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3NO4, 10 mM NaF, 0.1% Nonidet-40, and 1 mM EDTA) at a proportion of 1:9 (homogenate:buffer) using a teflon/glass homogenizer. The homogenates were centrifuged at 10,000 × g for 10 minutes at 4°C. Each sample was placed in sample buffer (0.5 M Tris-HCl pH 6.8, 10% glycerol, 2% [w/v] sodium dodecyl sulfate, 5% [v/v] 2-β-mercaptoethanol, and 0.05% bromophenol blue) and denatured using boiling at 95 to 100°C for 5 minutes. Protein estimation was performed using the bicinchoninic acid (BCA) method (BCA-1, Sigma-Aldrich, Inc.), in accordance with the manufacturer’s instructions. The samples were separated by electrophoresis on 10% acrylamide gels. Proteins were transferred to polyvinylidene fluoride membranes (Immobilon™, Millipore Corp., Billerica, MA, USA) using a transblot apparatus (Bio-Rad, Hercules, CA, USA). Membranes were blocked for 2 hours at room temperature in TBST buffer (Tris 50 mM, NaCl 1.5%, and Tween 20 0.05%; pH 7.5). The blots were probed with anti-Aβ (ab68896; rabbit polyclonal; 1:1000; Abcam), anti-APP (ab17467; rabbit polyclonal; 1:1000; Abcam), and anti-β-actin antibodies (bs-0061R; rabbit polyclonal; Bioss Co., Beijing, China) at 4°C overnight. Blots were washed three times in TBST buffer and incubated for 1 hour with an HRP-labeled anti-mouse IgG secondary antibody (Bioss Co.). Quantity One Software (Bio-Rad) was used to analyze the results.
Enzyme-linked immunosorbent assay
The hippocampal specimens were removed from the brain and homogenized in 10% saline in a glass homogenizer (n = 6/group). Enzyme linked immunosorbent assay (ELISA) kits (Life Technologies Co., Grand Island, NY, USA) were used to quantify the Aβ25–35 and APP protein levels. The samples and standards (Aβ25–35 peptide and APP protein) were diluted, added to a 96-well plate, and incubated for 1 hour at 37°C. HRP-conjugated antibody was added to per well. The plate was incubated for 1 hour at 37°C and washed four times using PBS. Tetramethylbenzidine (100 μL) was added to each well, and then the plate was incubated for 15 minutes at 37°C in the dark. Next, 50 μL of 1 M H2SO4 was added to per well. The absorbance was read at 450 nm using a microplate reader (Varioskan Flash, Thermo Fisher, Rockford, IL, USA) within 15 minutes.
Assessment of SOD, GSH activity, and MDA concentration
Left hippocampal tissue specimens were snap-frozen in liquid nitrogen immediately after dissection and stored at −80°C until use. The SOD and GSH activity and the MDA concentration in the hippocampal tissue were evaluated using specific commercial assay kits (SOD: #A001; MDA: #A003-1; GSH: #A006; all from Nanjing Jiancheng Bioengineering Institute, Nanjing, China), in accordance with the manufacturer’s protocol. SOD activity was determined using the xanthine oxidase method (hydroxylamine method). The reaction system contained 20 μL of WST-1 enzyme reaction solution, 20 μL of sample, and 200 µL of enzyme substrate. The reaction mixtures were incubated at 37°C for 20 minutes. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the reduction of WST-1 formazan, as detected at 550 nm. Superoxide ions reacted with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazan dye featuring absorbance at 550 nm. SOD activity was expressed in U/mL in tissues. The spectrophotometric method was used for measuring GSH. Briefly, 100 µL of the sample, 100 µL of oxidized glutathione solution, and 25 µL of NADPH solution (6 mM) were mixed and the resulting reaction product was measured at 405 nm. The thiobarbituric acid (TBA) method was used to measure MDA. A volume of 100 μL of the sample and 100 μL of TBA reaction solution were mixed and heated to 95°C for 40 minutes. After cooling, the supernatant was collected by centrifugation at 2800 ×g for 10 minutes, and the MDA concentration was detected in the supernatant at 532 nm. For SOD, MDA, and GSH, the intra-batch coefficient of variation (CV) was 3.52%, 4.11%, and 3.86%, respectively; the inter-batch CV was 1.7%, 3.5%, and 1.2%, respectively; the recovery rate was 103%, 104%, and 102%, respectively; and the testing range was 5.0 to 122.1 U/mL, 0.5 to 113.0 nmol/mL, and 0.3 to 147.1 mg GSH/L, respectively. These kits use a linear standard curve. The positive control was provided in the kit and consisted of the purified end-product of the reaction. Distilled water was used as negative control.
Ultrastructural examination
The remaining rats in each group were anesthetized with 10% urethane (1 g/kg) and perfused with 100 mL of cold saline solution (4°C), followed by freshly prepared 4% paraformaldehyde in PBS (4°C). The CA1 hippocampal regions were quickly removed from brain tissue and incubated overnight with 2.5% glutaraldehyde at 4°C. After acetone dehydration, the tissue specimens were embedded to prepare ultrathin sections and then stained with uranyl acetate and lead citrate for electron microscopy (Hitachi, Ibaraki, Japan).24
Statistical analysis
Data are presented as the mean ± standard error of the mean. Levene’s test for equality of variances followed by a t-test for independent samples was performed. Multiple mean comparisons were analyzed using a one-way analysis of variance, followed by Tukey’s post hoc test for multiple comparisons. All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
Eighty-four male Sprague–Dawley rats (6–8 weeks old, 200 ± 20 g) were randomly divided into the following groups: control, Aβ25–35, donepezil hydrochloride 1.05 mg/kg, HLJDT 6 g/kg, HLJDT 3 g/kg, and HLJDT 1.5 g/kg. There were 14 rats in each group.
Effects of HLJDT on spatial learning and memory deficits induced by Aβ25–35 in male rats
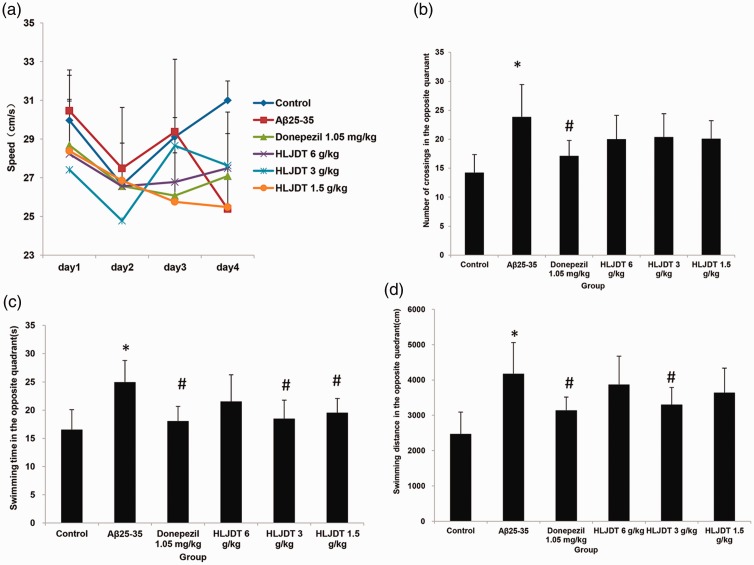
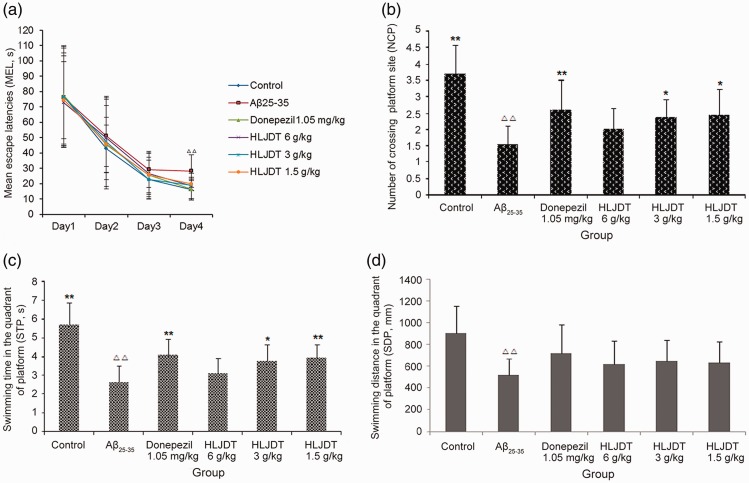
Learning and memory functions were examined using the MWM test (Figure 1). In the spatial navigation test (used to assess spatial learning performance), Aβ25–35 injection induced cognitive deficits that were alleviated by treatment with HLJDT or donepezil hydrochloride (Figure 1). The escape latency of the Aβ25–35 group on day 4 was significantly longer compared with control animals (P < 0.01). The escape latency on day 4 was significantly shorter in the donepezil hydrochloride group and in all three HLJDT groups compared with the Aβ25–35 group (P < 0.05 and P < 0.01, respectively), as shown in Figure 2a. There were no noticeable differences in swimming speed between the various groups on any of days 1 to 4 (data not shown), excluding the effects of physical energy on escape latency.
Figure 1.
Spatial learning and memory in rats assessed using the Morris water maze test. (a) Mean swimming speed. (b) Number of crossings to the opposite quadrant (spatial probe test). (c) Swimming duration in the opposite quadrant (spatial probe test). (d) Swimming distance in the opposite quadrant (spatial probe test). HLDJT, Huang-Lian-Jie-Du-Tang; *P<0.05 vs. the control group; #P<0.05 vs. the Aβ25–35 group (n = 14/group).
Figure 2.
Spatial learning and memory in rats assessed using the Morris water maze test. (a) Mean escape latency (spatial navigation test). (b) Swimming duration in the platform quadrant (spatial probe test). (c) Number of crossings to the platform quadrant (spatial probe test). (d) Swimming distance in the platform quadrant (spatial probe test). HLDJT, Huang-Lian-Jie-Du-Tang; △△P < 0.01, △P < 0.05 vs. the control group; *P < 0.05, **P < 0.01 vs. the Aβ25–35 group (n = 14/group).
In the spatial probe test (which was used to evaluate spatial memory performance), the NCP, STP, and SDP were significantly decreased in the Aβ25–35 group compared with the control group (P < 0.01; Figure 2b–d). Treatment with donepezil hydrochloride and HLJDT (at 3 and 1.5 g/kg) significantly increased NCP and STP compared with the Aβ25–35 group (P < 0.05 and P < 0.01, respectively), but no remarkable difference in NCP and STP was observed between the HLJDT 6 g/kg and Aβ25–35 groups. The donepezil hydrochloride group and all HLJDT groups showed a tendency towards an increase in SDP, but these tendencies were not statistically significant. In addition, the number of crossings in the opposite quadrant, swimming time in the opposite quadrant, and swimming distance in the opposite quadrant were all significantly increased in the Aβ25–35 group compared with the control group (P < 0.01 for all; Figure 2b–d). Treatment with donepezil hydrochloride and 3 g/kg HLJDT significantly decreased swimming time and swimming distance in the opposite quadrant compared with the Aβ25–35 group (P < 0.05 for both), but no significant effects of 6 g/kg HLJDT were observed (Figure 2c–d). Compared with the Aβ25–35 group, the donepezil hydrochloride group also showed a reduction in the number of crossings in the opposite quadrant (P < 0.05), although a similar trend was observed in all three HLJDT groups (Figure 2c).
Effects of HLJDT on Aβ protein expression in the rat hippocampus
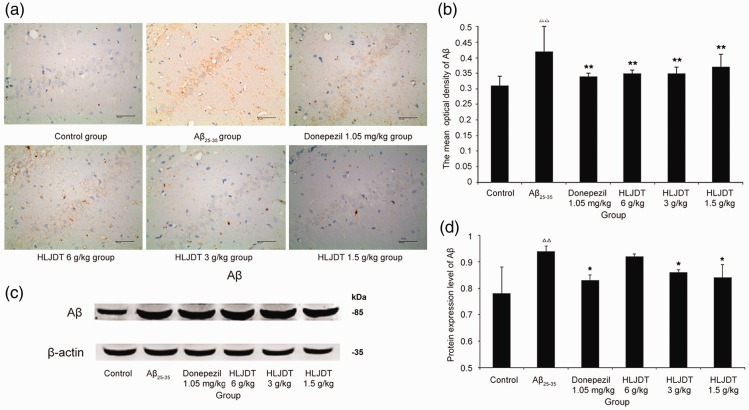
The hippocampal CA1 region was selected for immunohistochemical assessments. A distinct yellow staining was observed in the cytoplasm and plasma membrane of cone-shaped cells, representing Aβ expression. As shown in Figure 3a–b, the number of cells immunoreactive for Aβ significantly increased in the Aβ25–35 group compared with the control (P < 0.01). The Aβ expression levels in rats that received Aβ25–35 were significantly attenuated by HLJDT administration (P < 0.01 for all).
Figure 3.
Beta-amyloid (Aβ) expression in rat hippocampal tissue. (a) Light micrographs (400×) of the rat hippocampal CA1 region stained for Aβ using immunohistochemistry techniques. (b) Quantification of the data for Aβ expression obtained using immunohistochemistry techniques. (c) Representative western blots showing Aβ protein expression levels in the rat hippocampus. (d) Quantification of Aβ expression data that were obtained using western blot techniques. HLDJT, Huang-Lian-Jie-Du-Tang; △△P < 0.01, △P < 0.05 vs. the control group; *P < 0.05, **P < 0.01 vs. the Aβ25–35 group (n = 8/group).
As shown in Figure 3c–d (immunoblots), the amount of Aβ protein significantly increased in the Aβ25–35 group compared with the control group (P < 0.01), but decreased in the HLJDT 3 g/kg and 1.5 g/kg groups compared with the Aβ25–35 group (P < 0.05 for both). Taken together, these results indicate that administration with HLJDT reduced Aβ protein level in hippocampal tissue of Aβ25–35 induced experimental AD rats. Similar results were observed when the rats were treated with donepezil hydrochloride, but this was not the case for treatment with HLJDT 6 g/kg (Figure 3).
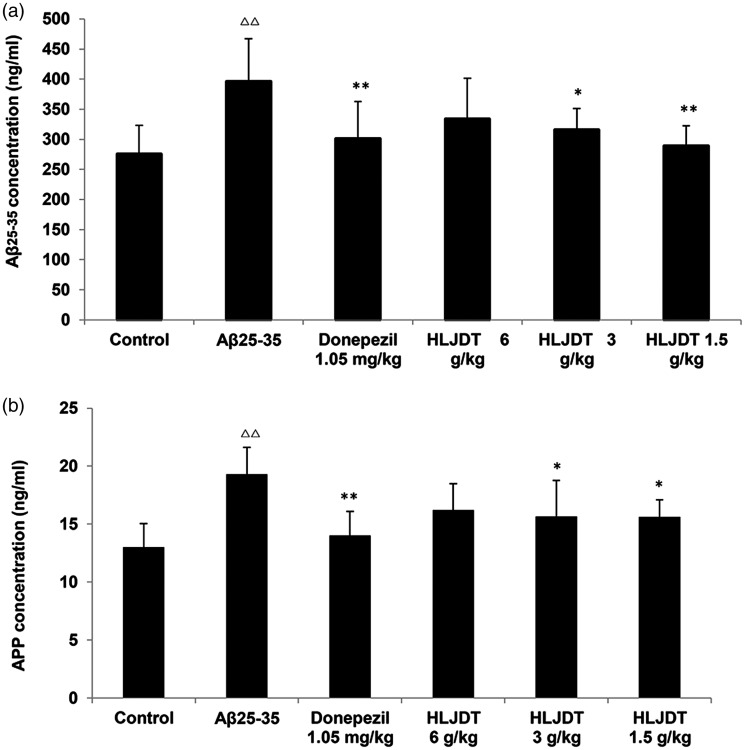
ELISA was used to quantify the Aβ25–35 protein levels in rat hippocampus. As shown in Figure 4a, the Aβ25–35 protein levels were significantly higher in the Aβ25–35 group (+44.0%, P < 0.01 vs. controls), but were lower in the HLJDT 3 and 1.5 g/kg groups (−20.2% and −27.0%, P < 0.05 and P < 0.01 vs. the Aβ25–35 group). These results show that HLJDT treatment decreased the Aβ25–35 protein levels in hippocampal tissues in rat models of AD. Treatment with donepezil hydrochloride also alleviated the Aβ25–35 protein levels (−24.0%, P < 0.01 vs. the Aβ25–35 group). No significant difference was observed in the HLJDT 6 g/kg group (Figure 4a). As shown in Figure 4b, the APP protein levels were noticeably higher in the Aβ25–35 group (+48.5%, P < 0.01 vs. the control group), but lower in the HLJDT 3 g/kg group (−18.7%, P < 0.05 vs. the Aβ25–35 group) and HLJDT 1.5 g/kg group (−19.2%, P < 0.05 vs. the Aβ25–35 group). This suggests that treatment with HLJDT decreased APP protein levels in the hippocampal tissues of rats with experimental AD (Figure 4b).
Figure 4.
Protein levels of beta-amyloid (Aβ) and amyloid precursor protein (APP) in the rat hippocampus. (a) Aβ25-35 levels; (b) APP levels; △P < 0.05, △△P < 0.01 vs. the control group; *P < 0.05, **P < 0.01 vs. the Aβ25-35 group (n = 6/group).
Effects of HLJDT on APP protein expression in the hippocampus
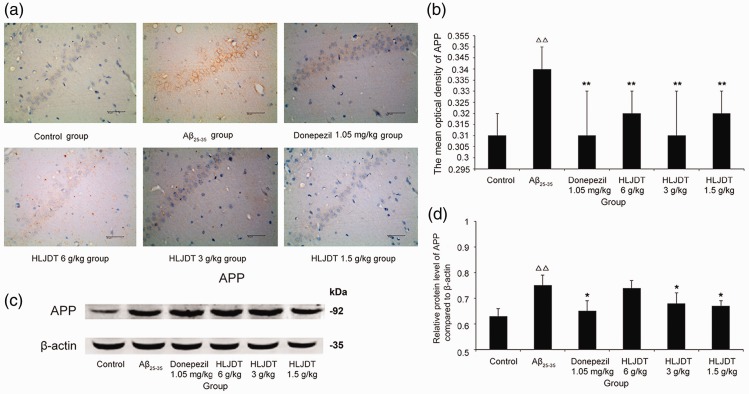
The hippocampal CA1 region was chosen for immunohistochemical analysis. A distinct yellow signal was observed in the cytoplasm and membrane of cone-shaped cells, representing APP expression. As shown in Figure 5a–b, the number of cells immunoreactive for APP increased remarkably in the Aβ25–35 group compared with controls (P < 0.01). Following treatment with HLJDT or donepezil hydrochloride, APP overexpression in Aβ25–35-treated rats was significantly attenuated (P < 0.01 for all).
Figure 5.
Amyloid precursor protein (APP) expression in the hippocampal CA1 region of rats. (a) Light micrographs (400×) of the rat hippocampal CA1 region stained for APP using immunohistochemistry techniques. (a) control group; (b) Aβ25-35 group; (c) donepezil hydrochloride 1.05 mg/kg group; (d) HLJDT 6 g/kg group; (e) HLJDT 3 g/kg group; (f) HLJDT 1.5 g/kg group. (b) Quantification of the data for APP expression obtained using immunohistochemistry techniques. (c) Representative western blots showing APP protein expression levels in the rat hippocampus. (d) Quantification of the data for APP expression obtained using western blot techniques. HLDJT, Huang-Lian-Jie-Du-Tang; △△P < 0.01, △P < 0.05 vs. the control group; *P < 0.05, **P < 0.01 vs. the Aβ25–35 group (n = 8/group).
APP protein levels, quantified by western blotting, significantly increased in the Aβ25–35 group compared with the control group (P < 0.01), but they were lower in the HLJDT 3 g/kg and 1.5 g/kg groups than in the Aβ25–35 group (P < 0.05 for both) (Figure 5c–d). These results show that administration of HLJDT decreased APP protein levels in hippocampal tissue in rats with experimental AD. As shown in Figure 5c–d, treatment with donepezil hydrochloride also significantly alleviated APP overexpression (P < 0.05).
Effects of HLJDT on total SOD (T-SOD), GSH activity, and MDA concentration in the hippocampus
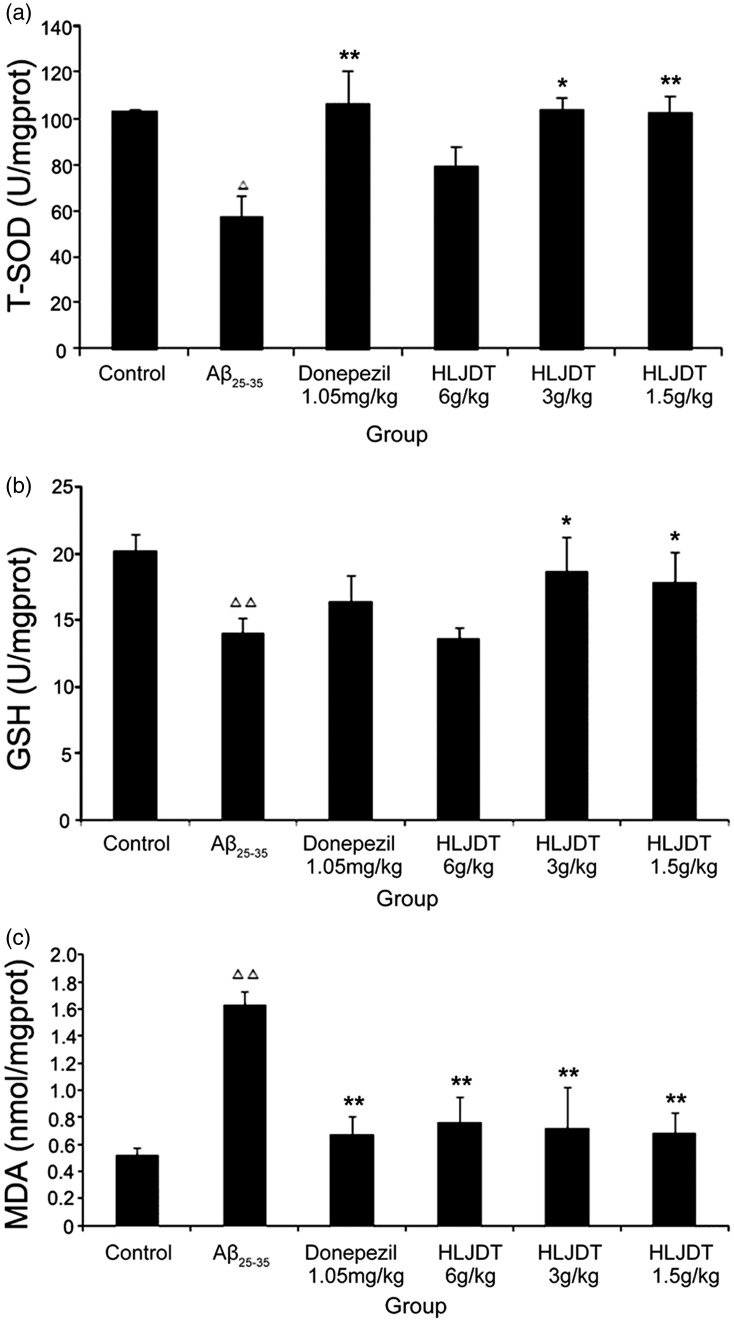
As shown in Figure 6a–b, Aβ25–35 injection suppressed T-SOD and GSH activity in the hippocampus. Treatment with donepezil hydrochloride and HLJDT (3 g/kg or 1.5 g/kg) significantly increased T-SOD (P < 0.01 for donepezil hydrochloride and HLJDT 1.5 g/kg; P < 0.05 for HLJDT 3 g/kg) and GSH activity (P < 0.01 for donepezil hydrochloride; P < 0.05 for HLJDT 3 g/kg and 1.5 g/kg). MDA content showed the opposite trend and was significantly increased in the Aβ25–35 group compared with the control group, HLJDT groups, and donepezil hydrochloride group (P < 0.01 for all) (Figure 6c).
Figure 6.
Total superoxide dismutase (T-SOD) activity, glutathione (GSH) activity, and malondialdehyde (MDA) level in the rat hippocampus. (a) T-SOD activity. (b) GSH activity. (c) MDA level. HLDJT, Huang-Lian-Jie-Du-Tang; △△P < 0.01, △P < 0.05 vs. the control group; *P < 0.05, **P < 0.01 vs. the Aβ25-35 group (n = 8/group).
Effects of HLJDT on ultrastructural abnormalities in the rat hippocampal CA1 region
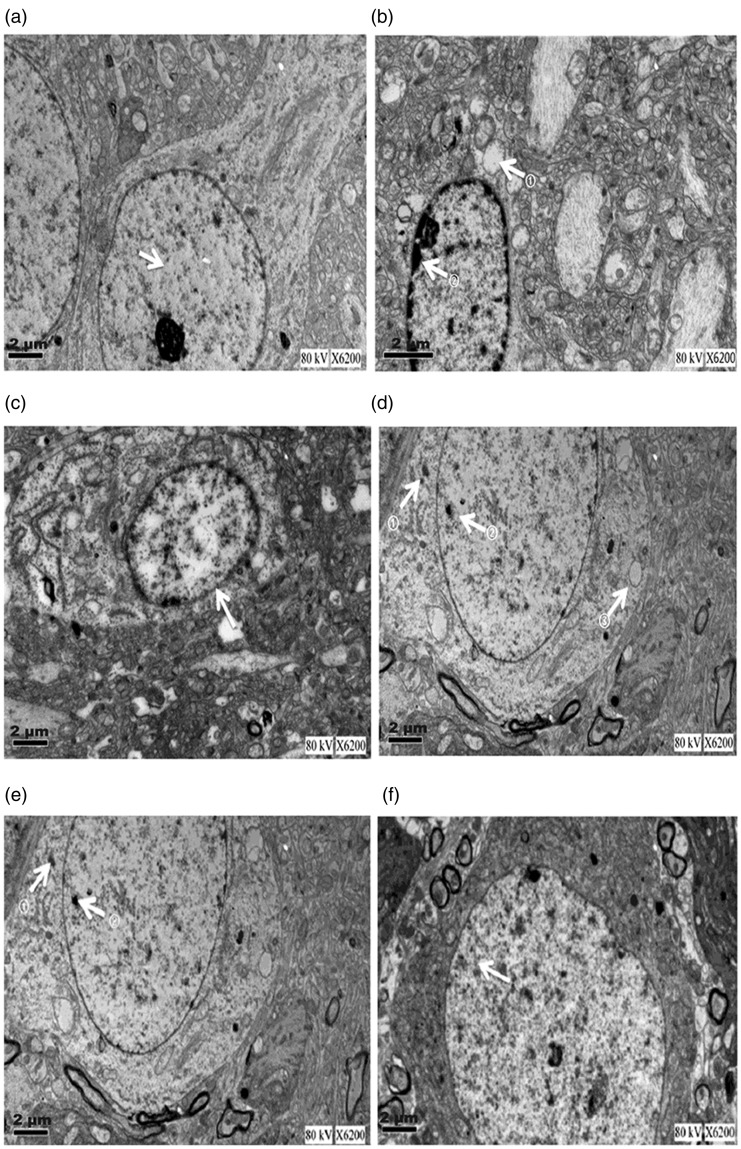
The ultrastructure of the hippocampal CA1 region was examined using electron microscopy. In the control group, the chromatin was well distributed, the nuclear membrane was clear, and ribosomes and mitochondria were abundant (Figure 7a–f). In the Aβ25–35 group, there were marked abnormalities in the ultrastructure of the hippocampal CA1 region, as follows: the chromatin was aggregated, the endoplasmic reticulum was expanded, the ribosome number was reduced, and the mitochondria showed swelling with disappearance of the ridges. Compared with the Aβ25–35 group, the donepezil hydrochloride and HLJDT groups tended to exhibit fewer abnormalities in the ultrastructure of the CA1 region (Figure 7a–f).
Figure 7.
Ultrastructural changes in the rat hippocampal CA1 region. Electron microscopy was used to assess the ultrastructural changes (1250×). (a) Control group. Arrow: chromatin homogeneity in the hippocampal cell. (b) Aβ25-35 group. Upper arrow: vacuolar region in the cytoplasm; lower arrow: chromatin agglutination on the nuclear membrane. (c) Donepezil hydrochloride group. Arrow: relatively complete nuclear membrane (although its integrity was not as high as that of the control group). (d) HLJDT 6 g/kg group. Arrows: small amounts of lipofuscin. (e) HLJDT 3 g/kg group. Arrows: small amounts of lipofuscin. (f) HLJDT 1.5 g/kg group. Arrow: small amount of lipofuscin. HLDJT, Huang-Lian-Jie-Du-Tang.
Discussion
In the present study, we investigated the protective effects of HLJDT on ameliorating the deficits in learning and memory induced by injection of Aβ25–35 in rats. The current study results reveal that animals with experimental AD (induced by hippocampal CA1 injection of Aβ25–35) showed increased deposition of Aβ in the hippocampal CA1 region and a noticeable decline in learning and memory compared with controls. However, experimental AD rats treated with HLJDT showed decreased Aβ and APP levels in the hippocampus and improvements in learning and memory, as shown by the MWM test.
Aβ is a major constituent in the histopathological anomalies of AD. Its aggregation in the brains of patients with AD can affect synaptic structure and plasticity leading to neurotoxicity and neuronal dysfunction.25 Our results showed that administration of HLJDT reduced Aβ and APP protein levels in hippocampal tissue from rats in which Aβ25–35 had experimentally induced AD. In the triple transgenic mouse model, modified HLJDT was shown to mediate the reduction of both the plaque load and memory impairment and HLJDT treatment increased APP and Aβ;26 HLJDT and baicalein treatment was shown to increase the amyloidogenic metabolism of APP in TgCRND8 mice expressing human APP with Swedish (K670N/M671L) and Indiana (V717F) mutations.27 However, our study likely differs from the previous reports because the model systems used are different, and thus, physiological differences could possibly give rise to the varying results.
Intracellular neurofibrillary tangles and extracellular formation of Aβ plaques in aggregated form along with metal ions such as copper, iron, or zinc are the hallmarks of AD. ROS production can be catalyzed by redox active metal ions such as copper that is bound to the Aβ, and these can cause oxidative damage to the Aβ peptide and to surrounding proteins and lipids.28 SOD can scavenge superoxide and H2O2, abrogating Aβ toxicity in vitro, which is considered to be the first-line tool of the antioxidant defense system.29 GSH, a critical intracellular antioxidant and scavenger of reactive intermediates, is thought to suppress toxic Aβ protein aggregation.30 MDA, another known protein oxidation indicator, is an end-product of lipid peroxidation and it generally reflects the lipid peroxidation level and the extent of injury.31 MDA was found to be increased in multiple brain regions and cerebrospinal fluid of patients with AD.32 In the present study, SOD and GSH activity was reduced significantly in the Aβ25–35 group, while MDA levels were significantly increased. Therapy with HLJDT significantly improved these parameters, with a higher activity of SOD and GSH and a lower level of MDA. These findings indicate that HLJDT could effectively attenuate OS induced by Aβ25–35 injection.
The hippocampus plays a prominent role in the process of memory and learning.33 Hippocampal neuronal apoptosis is one of the most important causes of amnesia in human neurodegenerative disorders.19 In addition, neuronal alterations in the hippocampus are strongly correlated with cognitive decline and AD progression.34 Therefore, we further investigated the ultrastructural changes that occurred because of Aβ25–35 injection in the hippocampal CA1 region and the effects of HLJDT treatment. The Aβ25–35 group showed ultrastructural defects and nuclear injury in cells of the hippocampal area CA1. These results indicate that the interaction between Aβ and OS can cause ultrastructural changes in CA1 cells. Treatment with HLJDT alleviated these abnormalities. A plausible mechanism by which HLJDT exerts a protective effect against insult by Aβ25–35 might involve interruption of the positive feedback loop between APP cleavage, Aβ deposition, and ROS generation. Further research is needed to explore the molecular mechanisms by which HLJDT reduces the aggregation of Aβ and generation of ROS in the hippocampus and thus alleviates deficits in learning and memory.
Based on our observations, the optimal dose of HLJDT in rats was 3 g/kg. It may be that the higher HLJDT dose (6 g/kg) exerted additional (currently unknown) effects that partially negated the beneficial actions on hippocampal Aβ, APP, and ROS levels and thereby also affected learning and memory performance. Consistent with this possibility are previous observations that low-dose berberine, an important active ingredient of Coptis chinensis, exerts a protective effect in cultured neuronal cells whereas high-dose berberine does not have this effect.35 Other studies have also shown concentration-dependent effects of berberine in cancer cells.36,37 The most effective concentration of HLJDT in rats (3 g/kg) was equivalent to the daily clinical dose of HLJDT that is used in patients. However, the dependence of the beneficial HLJDT effects on the dosage used warrants additional studies.
In conclusion, HLJDT improved symptoms that were induced by Aβ25–35 injection into the rat hippocampus by facilitating better learning and memory abilities, decreased hippocampal Aβ and APP levels, higher T-SOD and GSH activity, and lower MDA concentrations. These findings highlight the neuroprotective activity of HLJDT in AD.
Declaration of conflicting interest
The authors declare that there is no conflict of interest
Funding
This work was supported by the Science and Technology Agency Projects of Sichuan Province (No. 20132054).
ORCID iD
Wenbin Wu https://orcid.org/0000-0001-8784-6137
References
- 1.Tillement L, Lecanu L, Papadopoulos V. Alzheimer's disease: effects of beta-amyloid on mitochondria. Mitochondrion 2011; 11: 13–21. [DOI] [PubMed] [Google Scholar]
- 2.Song XY, Hu JF, Chu SF, et al. Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3beta/tau signaling pathway and the Abeta formation prevention in rats. Eur J Pharmacol 2013; 710: 29–38. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Wang W, Li L, et al. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim Biophys Acta 2014; 1842: 1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darvesh AS, Carroll RT, Bishayee A, et al. Oxidative stress and Alzheimer’s disease: Dietary polyphenols as potential therapeutic agents. Expert Rev Neurother 2010; 10: 729–745. [DOI] [PubMed] [Google Scholar]
- 5.Xu PX, Wang SW, Yu XL, et al. Rutin improves spatial memory in Alzheimer's disease transgenic mice by reducing Abeta oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res 2014; 264: 173–180. [DOI] [PubMed] [Google Scholar]
- 6.Bonda DJ, Wang X, Perry G, et al. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology 2010; 59: 290–294. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Cheng XR, Zhou WX, et al. Gene expression patterns of hippocampus and cerebral cortex of senescence-accelerated mouse treated with Huang-Lian-Jie-Du decoction. Neurosci Lett 2008; 439: 119–124. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Wang JS, Kong LY. Anti-inflammatory effects of Huang-Lian-Jie-Du decoction, its two fractions and four typical compounds. J Ethnopharmacol 2011; 134: 911–918. [DOI] [PubMed] [Google Scholar]
- 9.Xie SL, Peng LY, Yang K, et al. The advances in mechanism research of HLJDT to treat Alzheimer’s disease. Chin J Infor TCM 2014; 21: 121–123. [Google Scholar]
- 10.Zhang XJ, Deng YX, Shi QZ, et al. Hypolipidemic effect of the Chinese polyherbal Huanglian Jiedu decoction in type 2 diabetic rats and its possible mechanism. Phytomedicine 2014; 21: 615–623. [DOI] [PubMed] [Google Scholar]
- 11.Hwang YS, Shin CY, Huh Y, et al. Hwangryun-Hae-Dok-tang (Huanglian-Jie-Du-Tang) extract and its constituents reduce ischemia-reperfusion brain injury and neutrophil infiltration in rats. Life Sci 2002; 71: 2105–2117. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Bian H, Li Y, et al. Preconditioning with the traditional Chinese medicine Huang-Lian-Jie-Du-Tang initiates HIF-1alpha-dependent neuroprotection against cerebral ischemia in rats. J Ethnopharmacol 2014; 154: 443–452. [DOI] [PubMed] [Google Scholar]
- 13.Wu WB, Li B, Wang F, et al. Effects of Huanglian Jiedu decoction on learning and memorizing ability and expression of the related protein kinase in AD rats induced by Aβ25–35. Chin J TCM and Pharmacy 2014; 29: 1416–1420. [Google Scholar]
- 14.Fang Q, Zhan XP, Mo JL, et al. The effect of huanglian jiedu tang on Alzheimer’s disease and its influence on cytokines. Chin J Chin Materia Medica 2004; 29: 575–578. [PubMed] [Google Scholar]
- 15.Li B, Xie SL, Peng LY, et al. Effects of Huanglianjiedu decoction on learning-memory ability and cholinergic system of memory disorder model induced by scopolamine in mice. Pharmac Clin of Chin Materia Medica 2013; 29: 1–3. [Google Scholar]
- 16.Xu J, Murakami Y, Matsumoto K, et al. Protective effect of Oren-gedoku-to (Huang-Lian-Jie-Du-Tang) against impairment of learning and memory induced by transient cerebral ischemia in mice. J Ethnopharmacol 2000; 73: 405–413. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Hu Z, Wang S, et al. Protective effects of Huang-Lian-Jie-Du-Tang and its component group on collagen-induced arthritis in rats. J Ethnopharmacol 2013; 150: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 18.Deng J, Shen C, Wang YJ, et al. Nicotine exacerbates tau phosphorylation and cognitive impairment induced by amyloid-beta 25-35 in rats. Eur J Pharmacol 2010; 637: 83–88. [DOI] [PubMed] [Google Scholar]
- 19.Tian X, Zhang L, Wang J, et al. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Abeta25-35-induced oxidative stress and neuronal apoptosis in rats. Behav Brain Res 2013; 242: 1–8. [DOI] [PubMed] [Google Scholar]
- 20.Fan G, Feng C, Li Y, et al. Selection of nutrients for prevention or amelioration of lead-induced learning and memory impairment in rats. Ann Occup Hyg 2009; 53: 341–351. [DOI] [PubMed] [Google Scholar]
- 21.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984; 11: 47–60. [DOI] [PubMed] [Google Scholar]
- 22.Nie J, Luo Y, Huang XN, et al. Icariin inhibits beta-amyloid peptide segment 25-35 induced expression of beta-secretase in rat hippocampus. Eur J Pharmacol 2010; 626: 213–218. [DOI] [PubMed] [Google Scholar]
- 23.Li WZ, Wu WY, Huang DK, et al. Protective effects of astragalosides on dexamethasone and Abeta25-35 induced learning and memory impairments due to decrease amyloid precursor protein expression in 12-month male rats. Food Chem Toxicol 2012; 50: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 24.Lan Z, Chen L, Fu Q, et al. Paeoniflorin attenuates amyloid-beta peptide-induced neurotoxicity by ameliorating oxidative stress and regulating the NGF-mediated signaling in rats. Brain Res 2013; 1498: 9–19. [DOI] [PubMed] [Google Scholar]
- 25.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 2007; 8: 101–112. [DOI] [PubMed] [Google Scholar]
- 26.Durairajan SSK, Iyaswamy A, Shetty SG, et al. A modified formulation of Huanglian-Jie-Du-Tang reduces memory impairments and beta-amyloid plaques in a triple transgenic mouse model of Alzheimer’s disease. Sci Rep 2017; 7: 6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durairajan SS, Huang YY, Yuen PY, et al. Effects of Huanglian-Jie-Du-Tang and its modified formula on the modulation of amyloid-beta precursor protein processing in Alzheimer’s disease models. PLoS One 2014; 9: e92954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheignon C, Tomas M, Bonnefont-Rousselot D, et al. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 2018; 14: 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celsi F, Ferri A, Casciati A, et al. Overexpression of superoxide dismutase 1 protects against beta-amyloid peptide toxicity: Effect of estrogen and copper chelators. Neurochem Int 2004; 44: 25–33. [DOI] [PubMed] [Google Scholar]
- 30.Woltjer RL, Nghiem W, Maezawa I, et al. Role of glutathione in intracellular amyloid-alpha precursor protein/carboxy-terminal fragment aggregation and associated cytotoxicity. J Neurochem 2005; 93: 1047–1056. [DOI] [PubMed] [Google Scholar]
- 31.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med 2002; 33: 37–44. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev 2013; 2013: 316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaisler-Salomon I, Kravitz E, Feiler Y, et al. Hippocampus-specific deficiency in RNA editing of GluA2 in Alzheimer’s disease. Neurobiol Aging 2014; 35: 1785–1791. [DOI] [PubMed] [Google Scholar]
- 34.Stempler S, Waldman YY, Wolf L, et al. Hippocampus neuronal metabolic gene expression outperforms whole tissue data in accurately predicting Alzheimer’s disease progression. Neurobiol Aging 2012; 33: 2230.e13–2230.e21. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Li C, Chen S, et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol 2017; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serafim TL, Oliveira PJ, Sardao VA, et al. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother Pharmacol 2008; 61: 1007–1018. [DOI] [PubMed] [Google Scholar]
- 37.Tsang CM, Lau EP, Di K, et al. Berberine inhibits Rho GTPases and cell migration at low doses but induces G2 arrest and apoptosis at high doses in human cancer cells. Int J Mol Med 2009; 24: 131–138. [DOI] [PubMed] [Google Scholar]