Short abstract
Objective
This study aimed to investigate the effects of propofol and sevoflurane on cytotoxicity of natural killer (NK) cells in patients with gastric cancer.
Methods
Patients with gastric cancer were anesthetized by propofol or sevoflurane. Peripheral blood NK cells were isolated and co-cultured with BGC-823 gastric cancer cell culture supernatant, and the rate of apoptosis and effector molecules were analyzed by flow cytometry. Effects of propofol and sevoflurane on NK cell function and SMAD4 protein expression were investigated.
Results
Cytotoxicity of NK cells in patients with gastric cancer was inhibited before surgery, but it was enhanced in patients who were anesthetized by propofol compared with those who had sevoflurane. In vitro co-culture with BGC-823 cells significantly inhibited the cytotoxicity of NK cells, which was abolished by treatment of propofol or transforming growth factor (TGF)-β1. SMAD4 protein expression in the NK cell nucleus was significantly downregulated by TGF-β1 treatment and BGC-823 supernatant co-culture, and this expression could be restored by propofol.
Conclusions
Cytotoxicity of NK cells in patients with gastric cancer is low, but it can be promoted by propofol. Propofol regulates cytotoxicity in NK cells by promoting SMAD4, thereby affecting cellular function.
Keywords: Propofol, sevoflurane, tumor killing activity, peripheral blood natural killer cells, gastric cancer, cytotoxicity, SMAD4
Introduction
Gastric cancer is one of the most common malignant tumors of the digestive tract, with the highest incidence and mortality among tumors worldwide.1,2 Surgery combined with radiotherapy and chemotherapy represents the main clinical treatment for gastric cancer. Tumor recurrence and metastasis are the main reasons restricting the clinical treatment effects of gastric cancer.3,4 Regulation of various cells in the microenvironment of gastric cancer has an important effect on the development of tumor cells, and formation of the immunosuppressive microenvironment is closely related to progression of gastric cancer and a poor prognosis.5,6 There are a large number of infiltrating immune cells in gastric cancer tissue, including T cells, B cells, and natural killer (NK) cells. Elevated levels of these activated immune cells and related effector molecules in tumor tissues usually indicate a longer survival time.7,8 In T, B, and NK cell recombination activating gene 2 and interleukin 2 receptor double knockout (Rag2P-/-PIl2rgP-/-P) mice, tumors spontaneously form and sarcoma easily develops.9,10 These data indicate that immune cells in the tumor microenvironment are closely related to occurrence and development of tumors, and enhancing the function of immune cells is likely to be a new strategy for immunotherapy of gastric cancer.
Innate immunity is the first line of defense against infection and surveillance of tumors.11,12 NK cells are important in the body’s immune system.13 Unlike CD8+ cytotoxic T cells (CD8+CTL), which recognize tumor antigens in a major histocompatibility complex (MHC) class I molecule-dependent manner, there is no rearrangement of genes encoding antigen recognition-related proteins in development of NK cells. Therefore, recognition of NK cells to tumor antigens is not restricted by MHC.14,15 When cancer cells downregulate MHC class I molecules to escape recognition and death by CD8+CTLs, NK cells directly recognize malignant cancer cells through their surface-activated receptors, and secrete molecular targets (e.g., granzyme, perforin, and interferon-γ) to induce apoptosis in cancer cells.16 NK cells also express the immunoglobulin G low affinity receptor CD16, which binds to the Fc fragment of immunoglobulin G to kill cancer cells with antibody-dependent cell-mediated cytotoxicity.17 There is increasing evidence that activation and infiltration of NK cells are directly related to the prognosis of cancer.18,19 However, most NK cells in infiltrating tumor tissues are significantly inhibited, and the mechanism has not yet been clearly defined.
The perioperative period is an important time for tumor metastasis and recurrence in patients suffering from tumors. Tumor cells show different changes in immune activity after surgery, and during surgery, patients have different degrees of stress responses.20,21 Therefore, perioperative management is important for postoperative recovery of patients with cancer. Anesthesia is an important procedure in surgery, and it can reduce stimulation of the operation to patients and decrease the stress response.22 Recent studies have shown that, in addition to analgesic sedation, anesthetics can affect the biological functions of tumor cells and immune cells.23,24 Propofol and sevoflurane are two commonly used anesthetics in the clinical setting. Intravenous infusion of propofol is widely used in the clinic because of the fast onset, short duration of infusion, rapid and complete recovery, decreased adverse reactions, and no significant accumulation after continuous infusion.25 At present, the effects of these two anesthetic methods on postoperative immune function in patients with cancer are still unclear. Therefore, this study aimed to investigate the function and mechanism of these two anesthetics on CD3-CD56+ NK cells in peripheral blood of patients with gastric cancer.
Materials and methods
Patients
Patients with gastric cancer who were admitted to our hospital from September 2016 to December 2017 were included in this study. None of these patients had a previous history of malignant tumors, history of chemotherapy and radiotherapy, autoimmune diseases, or long-term medication history. These patients with gastric cancer were subdivided on the basis of the presence or absence of lymph node metastasis. Twenty patients had lymph node metastasis and 16 patients had no lymph node metastasis. According to the TNM staging criteria from the International Union Against Cancer in 2003, there were 11 cases of stage I, 11 cases of stage II, eight cases of stage III, and four cases of stage IV. Prior written and informed consent was obtained from every patient and the study was approved by the ethics review board of the First Affiliated Hospital of Jinzhou Medical University.
Anesthesia
Before anesthesia, the patients were subjected to monitoring of an electrocardiogram, invasive blood pressure, oxygen saturation, rectal temperature, and central venous pressure. In the propofol group, anesthesia was induced with 2.5 mg/kg propofol, 1 µg/kg remifentanil, and 0.15 mg/kg cisatracurium besilate by intravenous injection. This was followed by maintaining intravenous infusion of 4 mg/kg/hour propofol and 0.2 to 0.3 µg/kg/hour remifentanil. In the sevoflurane group, the patients were subjected to inhalation of 8% sevoflurane in fresh gas flow (5 L/min). Patients were induced with 1 to 2 µg/kg remifentanil and 0.15 mg/kg cisatracurium besilate by intravenous injection, followed by maintaining intravenous infusion of 0.2 to 0.3 µg/kg/min remifentanil and inhalation of 2% to 3% sevoflurane. The anesthesia depth index was monitored for all of the patients. The bispectral index was maintained at a level of 50 to 60 by adjusting the propofol infusion rate and the sevoflurane inhalation concentration.
Collection of tissue and peripheral blood samples
After the operation, freshly resected tumor tissues and corresponding adjacent tissues were collected. After washing with phosphate-buffered saline (PBS), the tissues were immersed in fresh complete medium. Tumor sand paracancerous tissues were ground into a single cell suspension using the MagicFilter® disposable filter on a clean bench. Mononuclear lymphocytes were isolated using the Ficoll lymphocyte separation solution (Haoyang Biotech, Tianjin, China) according to the manufacturer’s instructions.
Peripheral blood samples were collected from each patient (5 mL for each time point) at 1 hour before induction of anesthesia, at the end of surgery, and at 24 hours after surgery. Blood samples were added to EDTAk2 anti-coagulation tubes (BD, Franklin Lakes, NJ, USA). Mononuclear lymphocytes were isolated using the Ficoll lymphocyte separation solution according to the manufacturer’s instructions. Peripheral blood samples from 10 normal healthy people were used as the control group.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells were isolated from peripheral blood of patients with gastric cancer by the Ficoll lymphocyte separation method. A 5× volume of sterile PBS solution was then added to the monocytes, which were centrifuged at 200 × g for 6 minutes. The supernatant was discarded and the cells were re-suspended with sterile PBS.
Detection of apoptosis
For patients in the propofol and sevoflurane groups, NK cells were sorted by the magnetic bead sorting method. The cells were cultured with complete medium, which contained 200 IU interleukin-2, for 24 hours. Cells were then co-cultured with gastric cancer BGC-823 cells at a ratio of 3:1 in a 37°C, 5% CO2 incubator for 6 hours. The cells were collected and rinsed with pre-cooled PBS, and then stained with ANXN V FITC APOPTOSIS DTEC KIT I reagent (BD, Franklin Lakes, NJ, USA) according the manufacturer’s instructions. The rate of apoptosis was detected by flow cytometry. Cells that were positive for annexin V alone were regarded as early apoptotic cells, while cells that were positive for propidium iodide (PI) alone were regarded as necrotic cells.
Detection of NK cell killing effects
NK cell markers in isolated mononuclear lymphocytes were detected by flow cytometry. The mononuclear lymphocyte population was measured by forward/side scatter gating and the CD3-CD56+ cell population was selected for further analysis. Peripheral blood mononuclear cells were cultured in vitro for 72 hours and then centrifuged at 800 × g for 5 minutes. Expression levels of perforin and granzymes in CD3-CD56+ NK cells were detected. The experiment was performed in triplicate.
Quantitative real-time polymerase chain reaction
Total RNA was extracted with Trizol and cDNA was obtained from reverse transcription. Quantitative real-time polymerase chain reaction (PCR) was performed with the BeyoFast™ SYBR Green qPCR Mix kit (Beyotime, Beijing, China). The PCR system consisted of 10 µl of qRT-PCR Mix, 0.5 µl of each primer (upstream primer sequence: 5′-GATCATCGGGGGACATGAGG-3′; downstream primer sequence: 5′-GGTCGGCTCCTGTTCTTTGA-3′), 2 µl of cDNA, and 7 µl of ddH2O. Reaction conditions were as follows: 95°C for 10 minutes, 95°C for 1 minute, and 60°C for 30 seconds for a total of 40 cycles.
Western blot analysis
The isolated NK cells were lysed with radio-immunoprecipitation assay buffer. Nuclear protein was extracted with the Extraction Kit (P0027; Beyotime). Protein concentrations were determined with the bicinchoninic acid method (Beyotime). A total of 10 µL of protein sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and this was then electronically transferred onto a polyvinylidenefluoride membrane. After blocking with 50 g/L non-fat milk at room temperature for 1 hour, the membrane was incubated with appropriate primary antibodies (granzyme B [GZMB], 1:1000; SMAD4, 1:1000; and glyceraldehyde-3-phosphate dehydrogenase, 1:4000) (BD) at 4°C overnight. The membrane was then incubated with goat anti-mouse or goat anti-rabbit HRP-conjugated secondary antibody (1:4000) at room temperature for 1 hour. After washing with TBST, color development was performed with the electrochemiluminescence method.
Co-culture of gastric cancer cells and NK cells
The culture supernatant of the gastric cancer cell line was collected and mixed with the complete medium RPMI 1640 at a 1:1 ratio to prepare the conditional medium. NK cells were isolated from human peripheral blood with the magnetic bead method. Gastric cancer and NK cells were co-cultured with the CM medium containing 100 IU interleukin-2 for 48 hours. Expression and function of GZMB in NK cells were then detected by flow cytometry.
Propofol treatment of NK cells
NK cells were isolated from peripheral blood and then divided into the following three groups: (1) the normal culture group (control group); (2) the co-culture group in which NK cells were co-cultured with conditioned medium from gastric cancer cells; and (3) the co-culture plus treatment group in which co-cultured cells were treated with 25 µg/mL of propofol. Expression and function of GZMB in NK cells were detected by flow cytometry.
Cell transfection
For transfection, 5 × 106 NK cells were collected and rinsed twice with pre-cooled PBS. These NK cells were re-suspended in 500 µL electroporation buffer. A total of 100 nM small interfering RNA (siRNA) sequence (siRNA to SMAD4: 5′-AAC TAC AAA TGG AGG TCA TCC-3′) was added and the cell suspension was transferred to an electric rotor under the following conditions: 250 V, 5 ms, and a 0.4-mm cuvette.
Statistical analysis
Data are expressed as mean ± standard deviation. Graph Pad Prism 6.0 software (BD) was used for statistical analysis. One-way analysis of variance was performed for multiple group comparisons and the t-test was used for comparison between two groups. P < 0.05 was considered as statistically significant.
Results
Phenotype and function of CD3-CD56+ NK cells in peripheral blood of patients in the propofol and sevoflurane groups
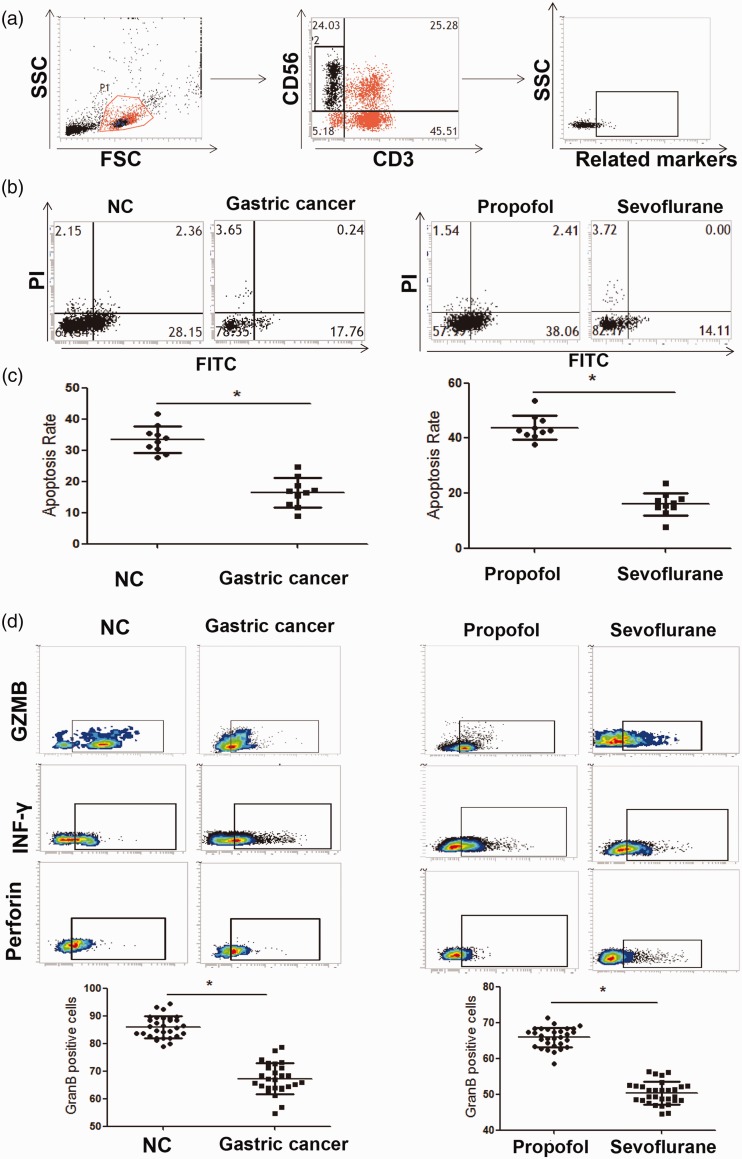
Thirty-six patients with gastric cancer, including 27 men and 9 women, with an average age of 47.6 years (ranging from 35–68 years), were included in this study. Flow cytometry was performed to investigate the killing effects of CD3-CD56+ NK cells on BGC-823 tumor cells. We found that before surgery, the mean rate of peripheral blood NK cell-induced tumor cell apoptosis was significantly lower in the gastric cancer group compared with the control group (16.4±0.34 vs. 32.7±0.76, P < 0.05). Moreover, after surgery, the ability of NK cells to induce apoptosis in BGC-823 tumor cells in the propofol group was significantly stronger than that in the sevoflurane group (44.1±0.68 vs. 16.5 ± 0.21, P < 0.05). This result suggested that propofol promoted the killing effects of NK cells on tumor cells. Further analysis showed that the mean GZMB expression level in peripheral blood NK cells in the control group was significantly higher than that in the gastric cancer group (P < 0.05). Moreover, after surgery, the mean GZMB expression level in peripheral blood NK cells in the propofol group (65.5± 0.83) was significantly higher than that in the sevoflurane group (50.5 ± 0.49, P < 0.05) (Figure 1). However, no significant differences were observed in expression of interferon-γ and perforin expression between the groups. These results indicated that, after surgery, the killing effects of NK cells in patients with gastric cancer who were anesthetized with propofol were stronger than those in patients who were anesthetized with sevoflurane.
Figure 1.
Tumor killing activity and GZMB expression in peripheral blood NK cells.
(a) Flow cytometry analysis of the NK cell phenotype in peripheral blood. (b) Flow cytometry analysis of the pro-apoptotic ability of NK cells on tumor cells. (c) Statistical analysis of apoptosis rates in tumor cells. (d) Flow cytometry analysis of molecular changes in NK cells. Experiments were performed in triplicate. *P<0.05, compared with the NC group. NK: natural killer; SSC: side scatter; FSC: forward scatter; NC: normal control; FITC: fluorescein isothiocyanate; GZMB: granzyme B.
Gastric cancer cells inhibit granulocyte expression and tumor killing ability of NK cells in vitro
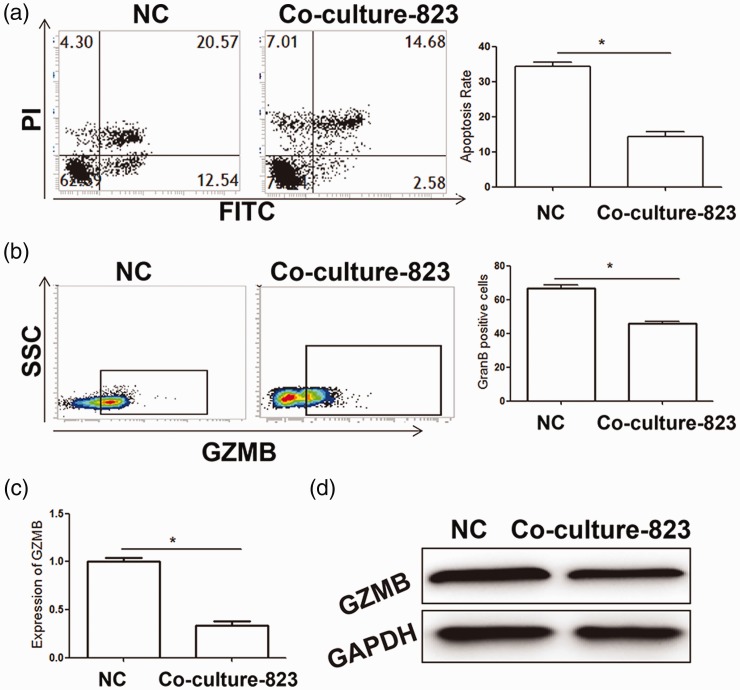
The perforin-GZMB pathway is the main mechanism by which NK cells kill tumor cells and GZMB expression levels are directly related to the killing ability of NK cells. Previous studies have reported that tumor cells secrete cytokines to inhibit immune cell activity.26 Therefore, in our study, isolated NK cells were treated with the culture supernatant of BGC-823 tumor cells (the main components of gastric cancer tissue) and cellular function was assessed. After co-culture, the mean apoptosis rate in the co-culture group was significantly lower than that in the control group (P < 0.05). This finding suggested that the culture supernatant of BGC-823 tumor cells inhibited the killing effects of NK cells on tumor cells. Moreover, GZMB expression levels in NK cells in the co-culture group were significantly downregulated compared with the control group (P < 0.05) (Figure 2). These results suggested that gastric cancer cells inhibited GZMB expression and the tumor killing ability of NK cells in vitro.
Figure 2.
Effects of culture supernatant of BGC-823 gastric cancer cells on NK cell killing activity.
(a) Flow cytometry analysis of pro-apoptotic effects of NK on tumor cells after co-culture. (b) Flow cytometry analysis of GZMB expression in NK cells after co-culture. (c, d) mRNA and protein expression levels of GZMB were detected by quantitative real-time polymerase chain reaction (c) and western blot analysis (d), respectively. Experiments were performed in triplicate. *P<0.05, compared with the NC group. NK: natural killer; NC: normal control; FITC: fluorescein isothiocyanate; GZMB: granzyme B; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Propofol inhibits negative regulation of gastric cancer cells on NK cell function
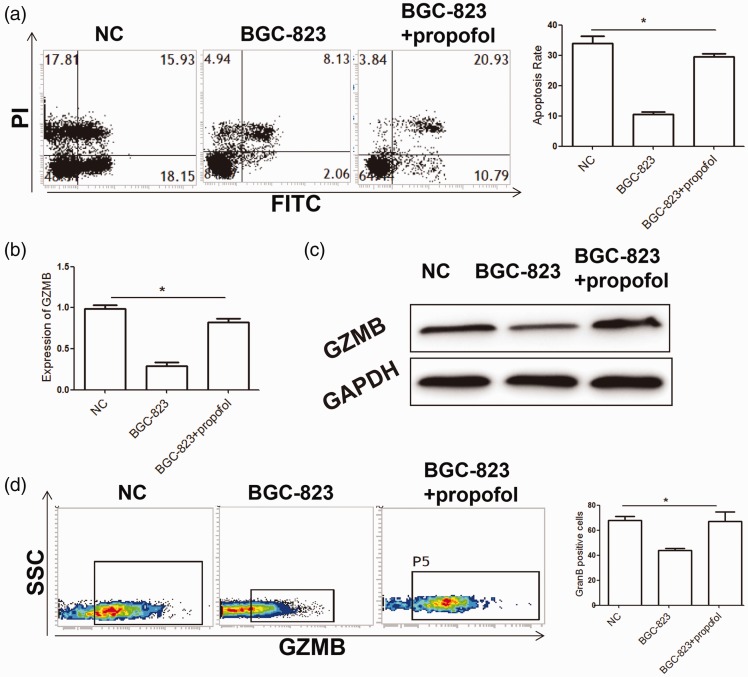
We further investigated whether propofol promotes the killing ability of NK cells. We found that, after treatment with propofol, the killing ability of NK cells on BGC-823 cells in the tumor cell co-culture group was significantly stronger than that in the propofol co-culture group (P < 0.05). This finding indicated that propofol inhibited negative regulation of tumor cells on NK cells, thereby promoting the killing ability of NK cells on tumor cells. Our results from flow cytometry also showed that the number of GZMB+ cells in NK cells in the propofol co-culture group was significantly higher than that in the BGC-823 co-culture group (P < 0.05). Additionally, quantitative real-time PCR and western blot analysis showed that propofol treatment promoted mRNA and protein expression levels of GZMB in NK cells (Figure 3). Taken together, these results suggested that propofol inhibited the negative regulation effects of gastric cancer cells on NK cell function.
Figure 3.
Propofol abolishes inhibition of gastric cancer cells on peripheral blood NK cells.
(a) Flow cytometry analysis of effects of propofol on the killing activity of NK cells after co-culture. (b, c) mRNA and protein expression levels of GZMB were detected by quantitative real-time polymerase chain reaction (b) and western blot analysis (c), respectively. (d) Flow cytometry analysis of the effect of propofol on GZMB expression in NK cells. Experiments were performed in triplicate. *P<0.05, compared with the NC group. NK: natural killer; PI: propidium iodide; NC: normal control; FITC: fluorescein isothiocyanate; GZMB: granzyme B; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; SSC: side scatter.
Propofol inhibits negative regulation of TGF-β1 on NK cell function
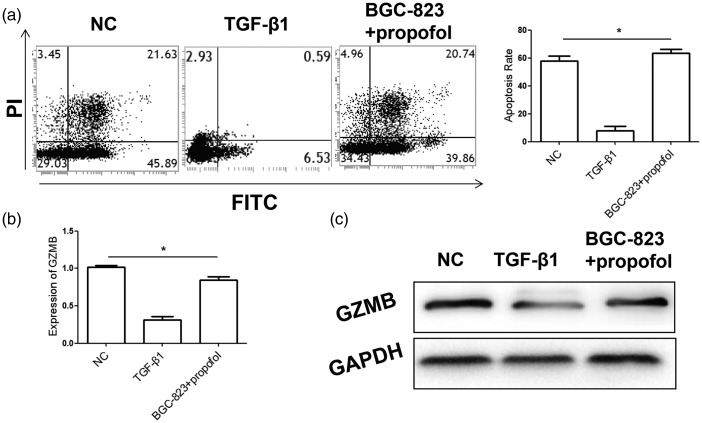
Because TGF-β1 is an important cytokine for negative regulation of NK cell function in tumor cells, the effects of propofol on TGF-β1-induced changes in NK cellular function were further analyzed. We found that the apoptosis rate of BGC-823 gastric cancer cells induced by TGF-β1-treated NK cells was significantly lower compared with that in the control group (P < 0.05). However, the apoptosis rate of gastric cancer cells was significantly higher after co-culture of BGC-823 tumor cells and NK cells in the propofol group than that in the TGF-β1 group (P < 0.05). Moreover, quantitative real-time PCR and western blot analysis showed that GZMB expression levels in NK cells in the propofol group were significantly higher than those in the TGF-β1 group (Figure 4). These results suggested that propofol inhibited the negative regulation effects of TGF-β1 on NK cell function, and thus promoted the killing effects of NK cells.
Figure 4.
Propofol abolishes TGF-β1-inducing inhibition of NK cell killing activity.
(a) Flow cytometry analysis for detecting the pro-apoptotic ability of NK cells on tumor cells after co-culture. (b, c) mRNA and protein expression levels of GZMB in NK cells were detected by quantitative real-time polymerase chain reaction (b) and western blot analysis (c), respectively. Experiments were performed in triplicate. *P<0.05, compared with the NC group. NK: natural killer; PI: propidium iodide; NC: normal control; TGF-β1: transforming growth factor-β1; GZMB: granzyme B; FITC: fluorescein isothiocyanate; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
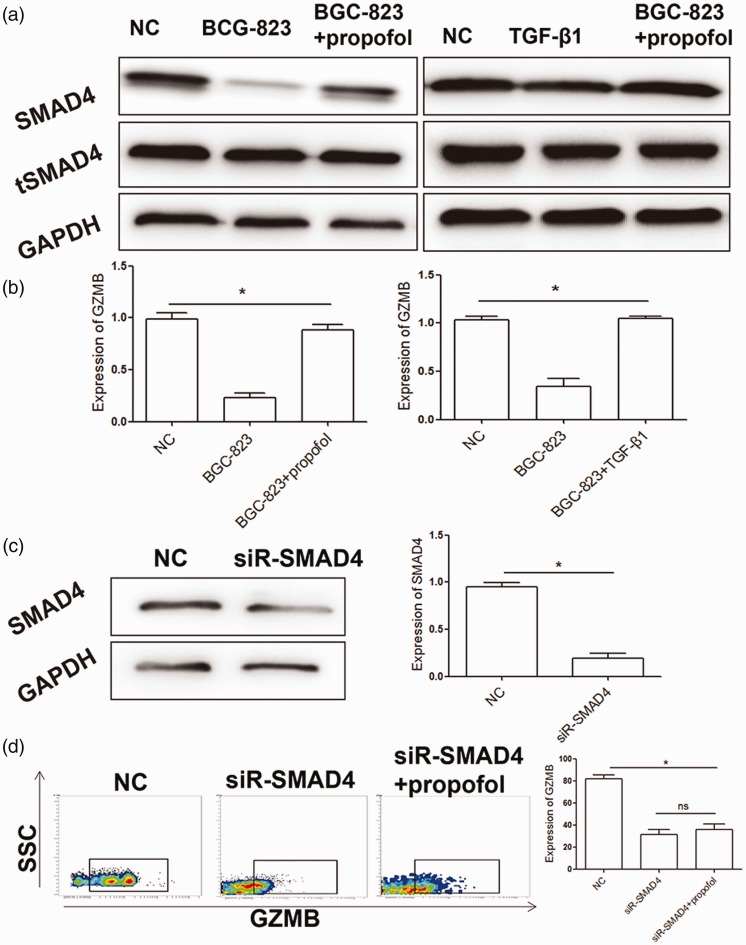
Propofol regulates tumor killing ability of NK cells through the SMAD4 pathway
SMAD4 is the only co-SMAD protein in the TGF-β1 signaling pathway, and it is able to promote transcription and translation of GZMB in NK cells. Therefore, SMAD4 protein expression levels in NK cells were detected. There were no significant changes in total SMAD4 protein expression levels between the control and propofol groups. After isolating protein in the nucleus, we found that SMAD4 protein expression levels in the propofol group were significantly higher than those in the BGC-823 co-culture group (P < 0.05). This finding suggested that the recovery effects of propofol on NK cell function might be related to nuclear importation of SMAD4. Moreover, SMAD4 protein expression levels after TGF-β1 antibody treatment were higher than those with BGC-823 co-culture (P < 0.05), which suggested that TGF-β1 promoted importation of SMAD4 into the nucleus. We also found that GZMB protein expression levels in NK cells was significantly decreased (P < 0.05), and protein expression of GZMB could not be restored by propofol treatment (Figure 5). Taken together, these results suggest that propofol regulates the tumor killing ability of NK cells through the SMAD4 pathway.
Figure 5.
Propofol regulates NK cell activity via the SMAD4 pathway.
(a, b) Western blot analysis of the effect of propofol on SMAD4 protein levels in NK cells under different culture conditions. (c) Western blot analysis for detecting the interference effects of SMAD4. (d) Restoration effect of propofol on GZMB expression in NK cells after SMAD4 interference. Experiments were performed in triplicate. *P<0.05, compared with the NC group. NK: natural killer; NC: normal control; TGF-β1: transforming growth factor-β1; GZMB: granzyme B; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; siR-SMAD4: small interfering RNA targeting Smad4; tSMAD4: total SMAD4.
Discussion
Previous studies have shown that patients with gastric cancer often have recurrence of tumors and distant metastasis after surgery, which might lead to death.27,28 However, surveillance of the postoperative immune system is an important method of reducing recurrence of tumors and metastasis. Because of the body’s stress responses, postoperative immune function is affected, and its functional status has an important effect on perioperative metastasis of tumor cells.29 A previous study also showed that anesthetics exerted direct or indirect effects on the body’s immune system, thereby regulating immune activity.30 Studying the effects of different anesthetics on immune cells might be useful for guidance of clinical anesthesia. In this study, we found that the main killing effector molecule GZMB in peripheral blood NK cells of patients with gastric cancer who were anesthetized with propofol showed significantly higher expression than that of patients who were anesthetized with sevoflurane anesthesia. In vitro experiments showed that propofol upregulated GZMB expression in NK cells through the SMAD4 signaling pathway, thus promoting tumor cell killing activity.
A large body of evidence has shown that NK cells can kill and inhibit proliferation and metastasis of various solid tumor cells.31,32 These cells function as the main effector cells in natural immunity, including in liver cancer and breast cancer. However, unfortunately, most NK cells infiltrating into solid tumor tissues are in a low-activity state. Additionally, in some patients with tumors, peripheral blood NK cell activity is lower than that in healthy people, limiting the tumor killing effects of NK cells.33 In recent years, anesthetic drugs have been reported to affect tumor cells and the immune system as follows. Liu et al.34 showed that etomidate affected the immune system of patients with lung adenocarcinoma, thereby affecting development of tumors.34 Moreover, high concentrations of ropivacaine or bupivacaine have shown inhibitory effects on proliferation of colon cancer cells in vitro.35 Gong et al.36 showed that sufentanil anesthesia affected the ratio of peripheral blood Treg cells. These results suggest that anesthetics can affect the body’s immune cells or tumor cells, thus affecting the patient’s prognosis. In this study, the effects of two commonly used anesthetics (i.e., propofol and sevoflurane) on the activity of peripheral NK cells in patients with gastric cancer were analyzed. We found that NK cells in the propofol group had stronger killing effects on gastric cancer cells compared with those in the sevoflurane group. Further analysis showed that GZMB expression levels in NK cells in the propofol group were significantly higher than those in the sevoflurane group. There is evidence that decreased NK cell activity in patients with tumors is closely related to regulation of the tumor microenvironment s follows. Peng et al. showed that tumor-associated macrophage cells secreted TGF-β1 to inhibit the activity of NK cells. In our study, BGC-823 gastric cancer cells downregulated GZMB expression in NK cells, which inhibited their killing activity. Therefore, the recovery effect of propofol on GZMB expression was also investigated. After co-culture of propofol, the apoptosis-promoting effect of NK cells on BGC-823 gastric cancer cells was upregulated and GZMB levels were also restored. These results indicate that propofol can upregulate the tumor killing activity of NK cells in vitro. In fact, propofol can directly regulate the function of immune cells and tumor cells. Zhou et al.37 found that propofol promoted the killing activity of peripheral blood NK cells in esophageal squamous cell carcinoma in vitro. Moreover, Liu et al.38 found that propofol inhibited proliferation, metastasis, and anti-apoptotic activity of hepatoma cells by downregulating miR-374a. Furthermore, Liu et al.39 showed that propofol improved the killing activity of peripheral blood NK cells in colon cancer cells. Based on these findings, we believe that propofol anesthesia is beneficial for enhancing postoperative tumor killing activity of NK cells.
TGF-β1 can inhibit various immune cells, such as NK and T cells. A previous study showed that TGF-β1 levels were significantly increased in gastric cancer tissues and cell lines.40 In our study, an in vitro model of TGF-β1-inhibiting NK cells was established. We found that propofol restored tumor killing ability and GZMB expression in NK cells. These results suggest that propofol can restore the TGF-β1-mediated inhibition of NK cell function. SMAD4 is an important intracytoplasmic signaling cascade molecule in the TGF-β signaling pathway, and it is responsible for transducing signals into the nucleus, functioning as a transcription factor. A recent study showed that SMAD4 plays an important role in development and maturation of NK cells.41 Cortez et al.42 found that Smad4 inhibited transformation of NK cells into a phenotype of group 1 innate lymphoid cells by inhibiting non-canonical TGF-β signaling. Additionally, Wang et al.43 found that SMAD4 promoted TGF-β1-independent NK cell homeostasis and maturation, which directly regulated GZMB. Therefore, SMAD4 protein expression levels in NK cells were examined in our study. We found that SMAD4 protein expression levels in the NK cell nucleus were significantly downregulated with TGF-β1 treatment and BGC-823 supernatant co-culture, and they could be restored by propofol treatment. These results suggest that TGF-β1 promotes nuclear translocation of SMAD4 protein. Additionally, after interfering with SMAD4 expression in NK cells, the killing ability of NK cells on gastric cancer cells was significantly weakened, while treatment of propofol could not restore GZMB expression. These results suggest that propofol regulates GZMB expression through the SMAD4 pathway, thereby affecting the tumor cell killing effects of NK cells.
In conclusion, our study shows that the cytotoxicity of NK cells in peripheral blood of patients with gastric cancer who are anesthetized by propofol is stronger than that in the patients who are anesthetized by sevoflurane. Moreover, propofol promotes nuclear importation of SMAD4 in NK cells and upregulates GZMB expression, thereby enhancing the tumor cell killing effects of NK cells.
Acknowledgements
We thank the staff from the Department of Anesthesiology, the First Affiliated Hospital of Jinzhou Medical University for their advice and guidance, and the staff from the Laboratory, Jinzhou Medical University for their support in technology and equipment.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Bar-Zeev M, Kelmansky D, Assaraf YG, et al. β-Casein micelles for oral delivery of SN-38 and elacridar to overcome BCRP-mediated multidrug resistance in gastric cancer. Eur J Pharm Biopharm 2018; 133: 240–249. DOI: 10.1016/j.ejpb.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Fan Y, Che X, Hou K, et al. MiR-940 promotes the proliferation and migration of gastric cancer cells through up-regulation of programmed death ligand-1 expression. Exp Cell Res 2018; 373: 180–187. DOI: 10.1016/j.yexcr.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Karaszewska B, Kang YK, et al. A multicenter phase II study of AMG 337 in patients with MET-amplified gastric/gastroesophageal junction/esophageal adenocarcinoma and other MET-amplified solid tumors. Clin Cancer Res 2018; 25: 2414–2423. DOI: 10.1158/1078-0432.ccr-18-1337. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Qiu R, Yu S, et al. Paclitaxel-resistant gastric cancer MGC803 cells promote epithelial-to-mesenchymal transition and chemoresistance in paclitaxel-sensitive cells via exosomal delivery of miR1555p. Int J Oncol 2019; 54: 326–338. DOI: 10.3892/ijo.2018.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leiting JL, Grotz TE. Optimizing outcomes for patients with gastric cancer peritoneal carcinomatosis. World J Gastrointest Oncol 2018; 10: 282–289. DOI: 10.4251/wjgo.v10.i10.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong D, Zhang X, Li R, et al. Deletion of TMEM268 inhibits growth of gastric cancer cells by downregulating the ITGB4 signaling pathway. Cell Death Differ 2018; 26: 1453–1466. DOI: 10.1038/s41418-018-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W, Zhang H, Han F, et al. CD155T/TIGIT signaling regulates CD8(+) T-cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res 2017; 77: 6375–6388. DOI: 10.1158/0008-5472.can-17-0381. [DOI] [PubMed] [Google Scholar]
- 8.Lu B, Teng X, Fu G, et al. Analysis of PD-L1 expression in trophoblastic tissues and tumors. Hum Pathol 2019; 84: 202–212. DOI: 10.1016/j.humpath.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Mehta R, Shah A, Almhanna K. Pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction cancer: an evidence-based review of place in therapy. Onco Targets Ther 2018; 11: 6525–6537. DOI: 10.2147/ott.s152513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tada Y, Togashi Y, Kotani D, et al. Targeting VEGFR2 with Ramucirumab strongly impacts effector/activated regulatory T cells and CD8(+) T cells in the tumor microenvironment. J Immunother Cancer 2018; 6: 106. DOI: 10.1186/s40425-018-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park R, Williamson S, Kasi A, et al. Immune therapeutics in the treatment of advanced gastric and esophageal cancer. Anticancer Res 2018; 38: 5569–5580. DOI: 10.21873/anticanres.12891. [DOI] [PubMed] [Google Scholar]
- 12.Yang R, Chang Q, Meng X, et al. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer 2018; 9: 3295–3302. DOI: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castriconi R, Carrega P, Dondero A, et al. Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Front Immunol 2018; 9: 2324. DOI: 10.3389/fimmu.2018.02324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin X, Liu T, Wang Z, et al. Expression of the inhibitory receptor TIGIT is up-regulated specifically on NK cells with CD226 activating receptor from HIV-infected individuals. Front Immunol 2018; 9: 2341. DOI: 10.3389/fimmu.2018.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saetersmoen ML, Hammer Q, Valamehr B, et al. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin Immunopathol 2019; 41: 59–68. DOI: 10.1007/s00281-018-0721-x. [DOI] [PubMed] [Google Scholar]
- 16.Dou B, Zhou W, Li S, et al. Buyang Huanwu Decoction attenuates infiltration of natural killer cells and protects against ischemic brain injury. Cell Physiol Biochem 2018; 50: 1286–1300. DOI: 10.1159/000494587. [DOI] [PubMed] [Google Scholar]
- 17.Hjorton K, Hagberg N, Israelsson E, et al. Cytokine production by activated plasmacytoid dendritic cells and natural killer cells is suppressed by an IRAK4 inhibitor. Arthritis Res Ther 2018; 20: 238. DOI: 10.1186/s13075-018-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuch A, Zecher BF, Muller PA, et al. NK-cell responses are biased towards CD16-mediated effector functions in chronic hepatitis B virus infection. J Hepatol 2019; 70: 351–360. DOI: 10.1016/j.jhep.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Ricon I, Hanalis-Miller T, Haldar R, et al. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of beta-adrenergic and cyclooxygenase 2 signaling. Cancer 2019; 125: 45–56. DOI: 10.1002/cncr.31594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogenur M, Hillig T, Gogenur I. CytoTrack analysis reveals low presence of circulating tumor cells in the perioperative period in patients with non-metastatic colorectal cancer. Anticancer Res 2017; 37: 3099–3103. DOI: 10.21873/anticanres.11666. [DOI] [PubMed] [Google Scholar]
- 21.Piegeler T, Beck-Schimmer B. Anesthesia and colorectal cancer - the perioperative period as a window of opportunity? Eur J Surg Oncol 2016; 42: 1286–1295. DOI: 10.1016/j.ejso.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Sekandarzad MW, van Zundert AAJ, Doornebal CW, et al. Regional anesthesia and analgesia in cancer care: is it time to break the bad news? Curr Opin Anaesthesiol 2017; 30: 606–612. DOI: 10.1097/aco.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 23.Desmond F, McCormack J, Mulligan N, et al. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res 2015; 35: 1311–1319. [PubMed] [Google Scholar]
- 24.Yan T, Zhang GH, Wang BN, et al. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-beta and prognosis after breast cancer surgery: a prospective, randomized and controlled study. BMC Anesthesiol 2018; 18: 131. DOI: 10.1186/s12871-018-0588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown MB, Dugat DR, Lyon SD, et al. Comparison of methohexital and propofol as induction agents for evaluation of laryngeal function in healthy dogs. Vet Surg 2019; 48: 70–78. DOI: 10.1111/vsu.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeevan-Raj B, Gehrig J, Charmoy M, et al. The transcription factor Tcf1 contributes to normal NK cell development and function by limiting the expression of granzymes. Cell Rep 2017; 20: 613–626. DOI: 10.1016/j.celrep.2017.06.071. [DOI] [PubMed] [Google Scholar]
- 27.Aragon-Sanabria V, Kim GB, Dong C. From cancer immunoediting to new strategies in cancer immunotherapy: the roles of immune cells and mechanics in oncology. Adv Exp Med Biol 2018; 1092: 113–138. DOI: 10.1007/978-3-319-95294-9_7. [DOI] [PubMed] [Google Scholar]
- 28.Wijn DH, Groeneveld GH, Vollaard AM, et al. Influenza vaccination in patients with lung cancer receiving anti-programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur J Cancer 2018; 104: 182–187. DOI: 10.1016/j.ejca.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Hinchcliff E, Hong D, Le H, et al. Characteristics and outcomes of patients with recurrent ovarian cancer undergoing early phase immune checkpoint inhibitor clinical trials. Gynecol Oncol 2018; 151: 407–413. DOI: 10.1016/j.ygyno.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuzier R, Izard P, Daboussi A, et al. A case report of sustained resolution of cancer pain by continuous perineural infusion of local anaesthetic. Eur J Pain 2019; 23: 31–34. DOI: 10.1002/ejp.1295. [DOI] [PubMed] [Google Scholar]
- 31.Liu P, Chen L, Zhang H. Natural killer cells in liver disease and hepatocellular carcinoma and the NK cell-based immunotherapy. J Immunol Res 2018; 2018: 1206737. DOI: 10.1155/2018/1206737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun H, Xu J, Huang Q, et al. Reduced CD160 expression contributes to impaired NK-cell function and poor clinical outcomes in patients with HCC. Cancer Res 2018; 78: 6581–6593. DOI: 10.1158/0008-5472.can-18-1049. [DOI] [PubMed] [Google Scholar]
- 33.Jewett A, Kos J, Fong Y, et al. NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis. Semin Cancer Biol 2018; 53: 178–188. DOI: 10.1016/j.semcancer.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Dong W, Wang T, et al. Effects of etomidate and propofol on immune function in patients with lung adenocarcinoma. Am J Transl Res 2016; 8: 5748–5755. [PMC free article] [PubMed] [Google Scholar]
- 35.Bundscherer A, Malsy M, Gebhardt K, et al. Effects of ropivacaine, bupivacaine and sufentanil in colon and pancreatic cancer cells in vitro. Pharmacol Res 2015; 95–96: 126–131. DOI: 10.1016/j.phrs.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Gong L, Qin Q, Zhou L, et al. Effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T cells frequencies. Int J Clin Exp Pathol 2014; 7: 7708–7716. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou M, Dai J, Zhou Y, et al. Propofol improves the function of natural killer cells from the peripheral blood of patients with esophageal squamous cell carcinoma. Exp Ther Med 2018; 16: 83–92. DOI: 10.3892/etm.2018.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu SQ, Zhang JL, Li ZW, et al. Propofol inhibits proliferation, migration, invasion and promotes apoptosis through down-regulating miR-374a in hepatocarcinoma cell lines. Cell Physiol Biochem 2018; 49: 2099–2110. DOI: 10.1159/000493814. [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Sun X, Du Y, et al. Propofol promotes activity and tumor-killing ability of natural killer cells in peripheral blood of patients with colon cancer. Med Sci Monit 2018; 24: 6119–6128. DOI: 10.12659/msm.911218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishimoto T, Miyake K, Nandi T, et al. Activation of transforming growth factor beta 1 signaling in gastric cancer-associated fibroblasts increases their motility, via expression of rhomboid 5 homolog 2, and ability to induce invasiveness of gastric cancer cells. Gastroenterology 2017; 153: 191–204.e116. DOI: 10.1053/j.gastro.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 41.Ozawa H, Ranaweera RS, Izumchenko E, et al. SMAD4 loss is associated with cetuximab resistance and induction of MAPK/JNK activation in head and neck cancer cells. Clin Cancer Res 2017; 23: 5162–5175. DOI: 10.1158/1078-0432.ccr-16-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortez VS, Ulland TK, Cervantes-Barragan L, et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat Immunol 2017; 18: 995–1003. DOI: 10.1038/ni.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Chu J, Yi P, et al. SMAD4 promotes TGF-beta-independent NK cell homeostasis and maturation and antitumor immunity. J Clin Invest 2018; 128: 5123–5136. DOI: 10.1172/jci121227. [DOI] [PMC free article] [PubMed] [Google Scholar]