Abstract
Background
Brain edema and neuronal apoptosis are closely associated with loss of neurological function and death in rats with subarachnoid hemorrhage (SAH). The present study investigated the effect of wogonoside on brain edema induced by SAH in rats and studied the mechanism involved.
Material/Methods
The rats were intra-gastrically administered 10, 20, 50, 100, 150 and 200 mg/kg doses of wogonoside 24 h prior to SAH induction. Western blotting was used to assess levels of pro-apoptotic protein, SIRT1, ZO-1, and p53 protein expression. Apoptotic nuclei were detected using immunofluorescence and TUNEL staining.
Results
Wogonoside treatment significantly suppressed edema formation in SAH-induced rats. Pre-treatment with wogonoside exhibited an inhibitory effect on SAH-induced extravascular Evans blue staining in rats. The expression of ZO-1, Occludin, and Claudin-5 proteins was increased by wogonoside in the SAH-induced rats. The inhibitory effect of SAH was completely reversed in the rats treated with the 200 mg/kg dose of wogonoside. The expression of SIRT1 protein was upregulated, and p53 and AC-p53 were downregulated by wogonoside in SAH rats. Wogonoside treatment significantly reduced SAH-mediated promotion of Bax, Puma, Noxa, Bid, and cleaved Caspase-3 expression. In the SAH-induced rats, pre-treatment with wogonoside reduced the TUNEL-positive cell count.
Conclusions
The present study demonstrated that wogonoside prevents brain edema development and apoptosis of neurons in rats by promoting SIRT1 expression and suppression of p53 activation. Therefore, wogonoside has therapeutic potential for the treatment of edema and needs to be investigated further to completely define the mechanism involved.
MeSH Keywords: Angioedema, Apoptosis, Occludin
Background
Subarachnoid hemorrhage (SAH) is a deadly disorder, accounting for about 30% of stroke patient deaths at the very initial stage and 10% at the advanced stage [1]. The average mortality rate of the patients with SAH is more than 50% [2]. The high mortality rate in early brain injury patients is associated with SAH [3]. The main factors involved in early brain injury are apoptosis of neurons and brain edema formation [4]. Therapeutic agents that can inhibit neuronal apoptosis and water content accumulation are believed to be of immense importance for the treatment of SAH. Induction of SAH has been found to activate various molecules that lead to increased permeability of the blood–brain barrier and apoptosis of neurons [5]. The molecules activated during SAH are p53, AKT, and mitogen-activated protein kinase [5]. The integrity and permeability of the blood-brain barrier is regulated by the expression of various tight-junction proteins [6]. The tight-junction protein zonao occludens (ZO) regulates the expression of occludins and claudins, which control blood–brain barrier permeability [6]. It is reported that during SAH, blood–brain barrier permeability is increased by the downregulation of tight-junction proteins [7]. Sirtuin 1 (SIRT1) is one of the deacetylase molecules associated with regulation of the cell cycle, apoptosis induction, and suppression of tumor growth by regulating the activation of p53 [8]. Acetylation of p53 leads to the increased expression of pro-apoptotic factors like Bax, Puma, and Bid [9]. Studies have shown that increased expression of SIRT1 protects against ischemic stroke in animal models [10].
Scutellaria is a member of the Labiatae family, which consists of around 400 species of annual and perennial herbs [11]. In traditional systems of medicine, Scutellaria has been used for the treatment of allergy, hepatitis, and inflammation, and as antioxidant [12]. Some of the compounds isolated from Scutellaria, which contains flavonoid nuclei, including baicalin, baicalein, and wogonin [13]. These flavonoid molecules are radical scavengers, anti-cancer agents, and antioxidants [14]. The heterocyclic molecules exhibit several biological activities, such as anti-cancer, anti-microbial, anti-Alzheimer’s effects [15–19]. It is reported that microglial cell inflammatory activation is inhibited by wogonin through suppression of NO and cytokines production [20]. In the present study, we assessed the effect of wogonoside (Figure 1) on brain edema induced by SAH in rats, and explored the mechanism involved. The results demonstrated that wogonoside suppressed SAH-induced edema and neuronal apoptosis in rats through downregulation of apoptotic protein expression and upregulation of junction protein expression.
Figure 1.
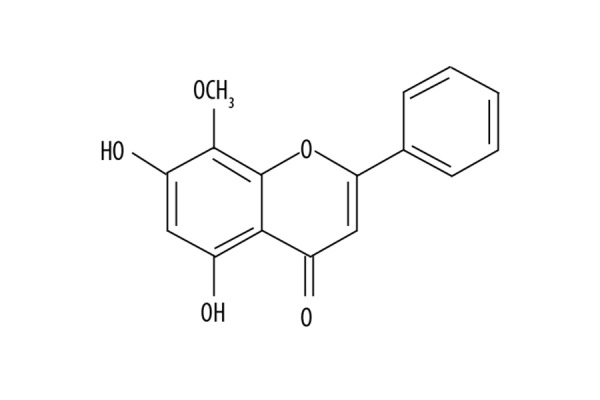
Chemical structure of wogonoside.
Material and Methods
Animals
A total of 40 male SpragueDawley rats (weight, 209–345 g) were supplied by the Animal Laboratory of Shandong University (Jinan, China). All the rats were caged singly with a 12/12h light/dark cycle in the animal house with a constant temperature of 24°C and humidity in the range of 55–60%. The rats were given free access to standard laboratory drinking water and rat chow. The experimental procedures on rats were conducted in compliance with the guidelines issued by the Animal Ethics Committee of Zhejiang University (Hangzhou, China). The study was approved by the Animal Ethics Committee, Medical University, Kunming, China.
Treatment strategy
We assigned the 40 rats to 8 groups of 5 rats each: a Sham group, an SAH group, and 10, 20, 50, 100, 150, and 200 mg/kg wogonoside treatment groups. The wogonoside treatment groups were intra-gastrically administered 10, 20, 50, 100, 150, and 200 mg/kg doses 24 h prior to SAH induction. The Sham and SAH groups were given normal saline alone in equal volumes.
Induction of SAH
We used a previously reported method for induction of SAH in the rats [21]. Briefly, the rats were intra-peritoneally injected with 50 mg/kg doses of 1% pentobarbital sodium anaesthesia. The common, internal, and external carotid arties were carefully exposed. After ligation of the external carotid arty, a nylon suture was pierced through it into the internal carotid artery. The suture was pushed through the internal carotid artery into the intracranial artery, which was indicated by resistance, and from that the point suture was pushed 5 mm more to cause perforation in the artery wall. Sham group rats underwent a similar procedure, but the suture was withdrawn as soon as resistance was felt.
Brain edema
At 24 h of SAH induction the rats were intra-peritoneally injected with 100 mg/kg doses of 1% pentobarbital sodium anaesthesia. The brains were excised to separate the cerebellum, brain stem, and left and right hemispheres. The parts were weighed to record wet weight and then dried in an oven at 105°C to measure the dry weight. Brain edema formation was assessed by measurement of the water content determined by the difference between dry and wet weights.
Evans blue extravasation
The integrity of the blood-brain barrier was determined using Evans blue dye. Two rats from each group were injected with 5 ml/kg doses of 2% Evans blue dye solution at 24 h of SAH induction. The rats were anaesthetised at 1 h of dye injection using 1% pentobarbital sodium anaesthesia to excise and subsequently separate the brain parts. After weighing, brain samples were homogenized and then centrifuged for 1 h at 6000 g. The supernatant collected was treated with an equal volume of TCA and ethanol mixture in the ratio of 1: 3. The mixture was incubated overnight at 4°C and then centrifuged for 45 min at 15 000 g. A spectro-fluorophotometer was used for determination of Evans blue (Cell Signaling Technology, Inc., Danvers, MA, USA) concentration in the supernatant.
Immunofluorescence and terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labeling (TUNEL) staining
The excised rat brains were fixed for 48 h in 4% paraformaldehyde at 4°C. After dehydration in a 30% solution of glucose fluid, the brain samples were frozen at −30°C followed by sectioning into 3-μm slices using a CM1850 cryomicrotome. The permeabilized sections were treated with 10% goat serum and 0.3% Triton X-100 at room temperature for 1.5 h. The apoptosis in neuronal cells of cerebral cortex was detected by TUNEL staining using a Cell Death Detection kit. Incubation of the slides was performed with TUNEL reagent in the dark for 3 h at 37°C. Counterstaining of the slides was carried out with DAPI for 1 min at room temperature. The slides were sealed with nail polish and examined under a Leica fluorescence microscope (Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Western blot analysis
A portion of cerebral cortex close to the optic chiasm was subjected to Western blotting. The tissue samples were homogenized in a PRO200 homogenizer (PRO Scientific, Inc., Oxford, CT, USA) with 460 μl of radioimmunoprecipitation lysis buffer, and the protein concentration was determined by bicinchoninic protein assay (Beyotime, Beijing). The protein extracts were separated using 12.5% SDS page by gel electrophoresis and then transferred onto the polyvinylidene difluoride membranes. The membranes were previously blocked by treatment with TBS and Tween-20 with 10% skimmed milk. The membranes were incubated at 4°C overnight with primary antibodies against SIRT1, p53, ZO-1, Caspase-3, Occluding, Claudin-5, and β-actin (all from Cell Signaling Technology, Inc.). After washing the membrane, incubation was performed for 2 h at room temperature with goat anti-rabbit IgG-conjugated horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). Detection of the complexes was carried out using the ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and Lab Software version 4.0 was used for quantification.
Statistical analysis
The data are expressed as mean±SD of 3 experiments carried out independently. The data analysis was made using SPSS 20.0 (IBM Corp., Armonk, NY, USA). The differences were determined by ANOVA and least significant difference test. Values were considered significant statistically at P<0.05.
Results
Wogonoside suppressed brain water accumulation and Evans blue extravasation
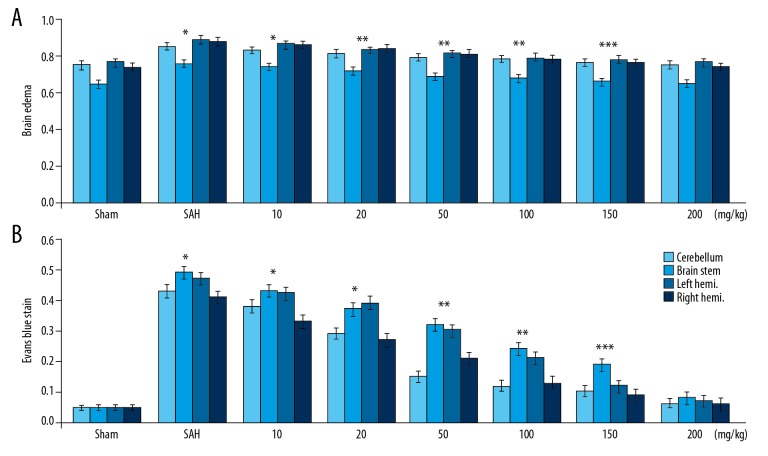
SAH induction caused a marked increase in the accumulation of water content in the rat brain compared to the Sham group (Figure 2). There was no significant increase in the water content accumulation in SAH-induced rats pre-treated with 200 mg/kg dose of wogonoside. The extravascular Evans blue was raised by ~12-fold in rats following SAH induction. Pre-treatment with wogonoside exhibited an inhibitory effect on SAH-induced extravascular Evans blue in rats.
Figure 2.
Effect of wogonoside on brain edema and Evans blue extravasation in SAH rats. (A) Brain water content in SAH-induced rats pre-treated with various doses of wogonoside. (B) Changes in Evans blue extravasation in SAH-induced rats with wogonoside pre-treatment. * P<0.05, ** P<0.02 and *** P<0.01 vs. Sham group.
Wogonoside upregulates ZO-1, Occludin, and Claudin-5 protein expression in rats with SAH
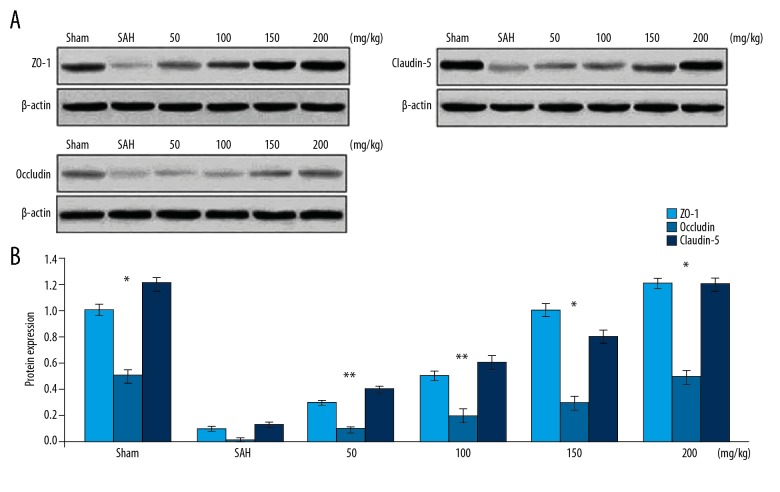
The expression of proteins corresponding to ZO-1, Occludin, and Claudin-5 in rats was reduced significantly following induction of SAH (Figure 3). Pre-treatment of the rats with wogonoside prevented SAH-induced reduction of ZO-1, Occludin, and Claudin-5 expression. Wogonoside pre-treatment lead to increased ZO-1, Occludin, and Claudin-5 expression in a concentration-dependent manner. The inhibitory effect of SAH was completely reversed in the rats treated with a 200 mg/kg dose of wogonoside.
Figure 3.
Effect of wogonoside on tight-junction protein expression. (A) In SAH-induced rats changes in expression of ZO-1, Occludin, and Claudin-5 proteins by wogonoside pre-treatment was assessed using Western blotting. (B) The semi-quantification by densitometric analysis. * P<0.05 and ** P<0.02 vs. Sham group.
Wogonoside regulates SIRT1 and p53 level in rats with SAH
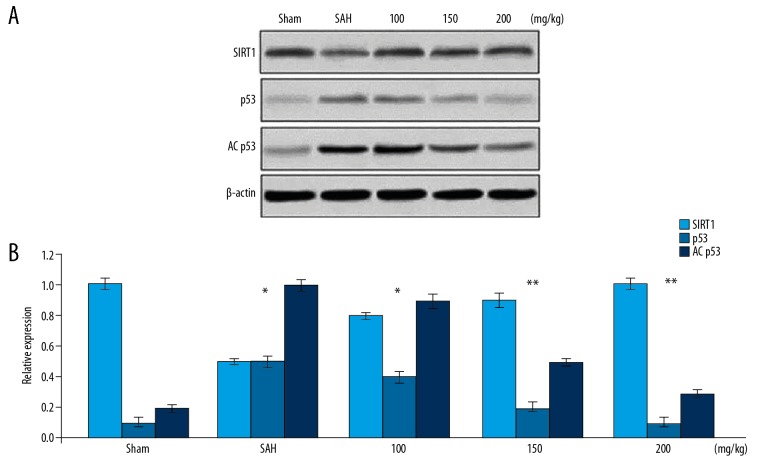
The expression of SIRT1 protein was markedly suppressed in rats following 24 h of SAH induction (Figure 4). Pre-treatment of the rats with wogonoside markedly prevented reduction of SIRT1 protein expression in comparison to the untreated group. The levels of p53 and AC-p53 were upregulated by SAH induction in the rats compared to the Sham group. Wogonoside pre-treatment of the rats with SAH caused a marked downregulation of p53 and AC-p53 levels.
Figure 4.
Effect of wogonoside on SIRT1, p53, and AC-p53 expression. (A) Western blotting was used for determination of changes in SIRT1, p53, and AC-p53 protein levels in SAH-induced rats pre-treated with wogonoside. (B) The values were quantified. * P<0.05 and ** P<0.02 vs. Sham group.
Wogonoside suppresses SAH-induced upregulation of pro-apoptotic molecules
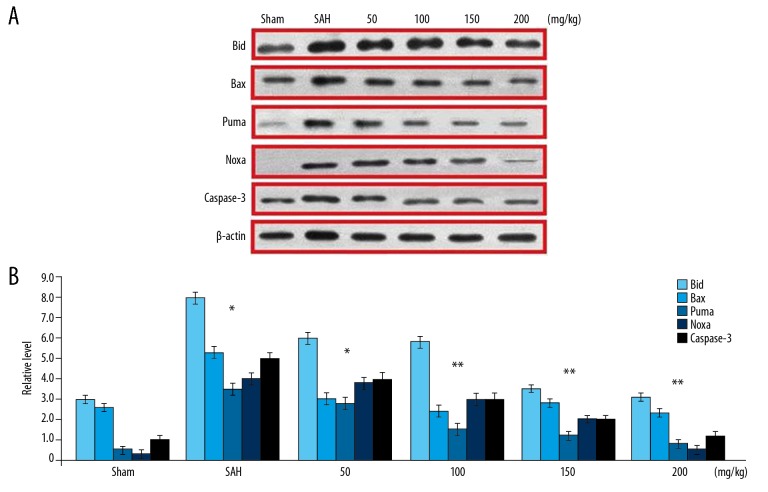
SAH induction in rats led to a marked increase in the levels of Bax, Puma, Noxa, and Bid mRNA (Figure 5). However, wogonoside pre-treatment of the rats significantly reduced SAH-mediated promotion of mRNA corresponding to Bax, Puma, Noxa, and Bid. Induction of SAH in the rats significantly increased the level of cleaved Caspase-3 (Figure 5). In wogonoside pre-treated rats, the expression of cleaved Caspase-3 was significantly lower in comparison to the SAH group. In group of rats treated with 200 mg/kg wogonoside, the expression of cleaved Caspase-3 was very similar to that in the Sham group rats.
Figure 5.
Effect of wogonoside pre-treatment on SAH-induced pro-apoptotic proteins. (A) The expression of Bid, Bax, Puma, Noxa, and cleaved Caspase-3 protein was analysed by Western blotting. (B) The values were quantified. * P<0.05 and ** P<0.02 vs. Sham group.
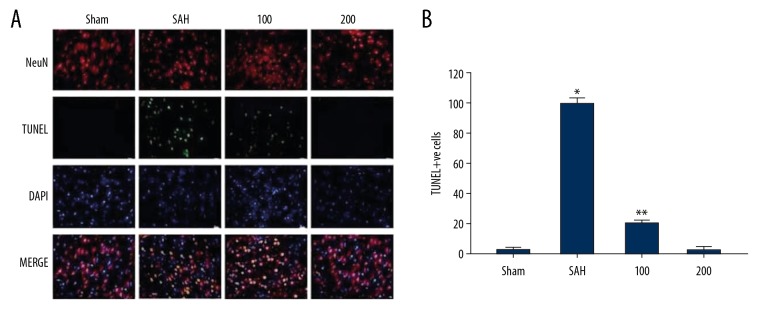
Wogonoside decreased the apoptotic neuron count in SAH rats
The immunofluorescence staining showed a markedly higher number of TUNEL-positive cells in SAH-induced rats (Figure 6). The number of TUNEL-positive cells increased to 38.5/100 cells in SAH-induced rats in comparison to 1.9/100 cells in the Sham group. In the SAH-induced rats, pre-treatment with wogonoside significantly inhibited the increase of TUNEL-positive cell count compared to the Sham group.
Figure 6.
Effect of wogonoside pre-treatment on SAH-induced increase of TUNEL-positive cell count. (A) TUNEL-positive cell count in the rat cerebral cortex was determined by immunofluorescence staining. (B) The quantification of TUNEL-positive cells in the rat cerebral cortex. * P<0.05 and * P<0.01 vs. Sham group.
Wogonoside reduced SAH-induced mortality in rats
Mortality was significantly higher in the untreated SAH rat group than in the Sham control group (Figure 7). Treatment of SAH-induced rats with wogonoside significantly improved mortality in dose-dependent manner. None of the rats died due to SAH in the 150 and 200 mg/kg wogonoside treatment groups.
Figure 7.
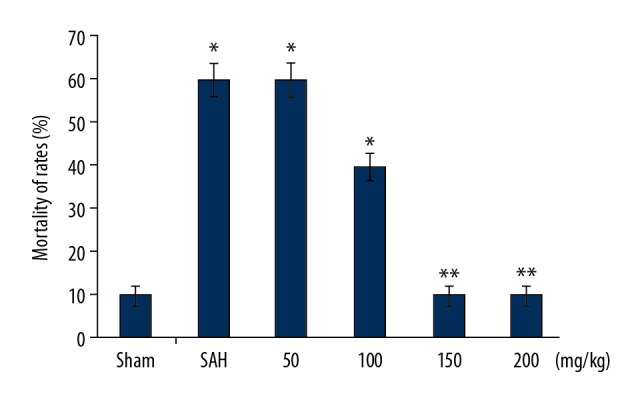
Effect of wogonoside pre-treatment on SAH-induced mortality. Mortality due to SAH was recorded in wogonoside-treated and Sham control rats during the study. * P<0.05 and * P<0.01 vs. Sham group.
Discussion
The present study investigated the effect of wogonoside on brain edema induced by SAH in rats and explored the mechanism involved. The results demonstrated that wogonoside suppressed SAH-induced edema and neuronal apoptosis in the rats through downregulation of apoptotic protein expression and upregulation of junction protein expression.
Early brain injury following SAH is associated with the development of disability and leads to patient mortality [1,21]. The characteristic features of EBI include brain edema, neuronal inflammation, apoptosis of neurons, and ischemia [1,21]. Brain edema is mainly caused by impairment of the blood-brain barrier and is the major factor responsible for the high mortality rate of patients after SAH [22]. There is excessive accumulation of water in the brain after impairment in the blood-brain barrier, leading to vasogenic edema [23]. Treatment of blood-brain barrier impairment by chemotherapeutic agents plays a vital role in the improvement of prognosis of SAH patients [24]. The main component maintaining the integrity of the blood-brain barrier are tight-junction proteins [25]. The tight-junction protein ZO-1 regulates the expression of Claudin-5 and Occludin, which in turn are linked with the maintenance of blood-brain barrier integrity [26]. It is well known that Occludin and Claudin-5 are the major components which regulate blood-brain barrier permeability [27]. In the present study, SAH markedly increased the accumulation of water in brains of rats, leading to the development of edema. Treatment of the SAH-induced rats with wogonoside markedly suppressed water content accumulation compared to the untreated group. These findings suggest that wogonoside treatment inhibits edema development in rats with induced SAH. Investigation of the effect of wogonoside on expression of tight-junction proteins revealed a significant increase in the levels of ZO-1, Occludin, and Claudin-5 in rat cerebral tissues. Therefore, wogonoside inhibited edema development in the SAH-induced rats through upregulation of ZO-1, Occludin, and Claudin-5 proteins.
Downregulation of SIRT1 in the cortical tissues following SAH indicates its association with blood-brain barrier impairment [28]. It has been shown that inhibition of SIRT1 expression causes impairment of the blood-brain barrier by activation of matrix metalloproteinases [29]. It is reported that blood-brain barrier permeability is promoted by metalloproteinases through downregulation of tight-junction proteins [30]. In the present study, SIRT1 expression was markedly decreased by SAH induction in rat cerebral tissues. Treatment of the SAH-induced rats with wogonoside caused a marked increase in the expression of SIRT1 in comparison to the untreated group. It is reported that the p53 pathway is directly associated with breakdown of the blood-brain barrier and edema development through activation of MMP-9 [5]. AC-p53 plays an important role in the Puma and Bax transcription activation process, thereby inducing apoptosis [31]. In the present study, SAH induction increased the level of p53 in the cerebral cortex of rats. Treatment of the SAH rats with wogonoside caused suppression of p53 expression in a dose-dependent manner. The expression of AC-p53 was also suppressed in the cerebral cortex of SAH-induced rats treated with wogonoside. These results suggest that wogonoside prevents neuronal apoptosis induced by SAH in rats through SIRT1 activation and p53 downregulation.
Conclusions
The present study demonstrates that wogonoside prevents brain edema development and apoptosis of neurons in rats by promoting SIRT1 expression and suppression of p53 activation. Therefore, wogonoside has therapeutic potential for the treatment of edema and needs to be investigated further to define the mechanism involved.
Footnotes
Conflict of interest
None.
Source of support: PhD Research Fund of Kunming Medical University, 2016BS006; Joint Special Fund for the Application Foundation Plan of Yunnan Province, 2018FE001 (-140)
References
- 1.Bederson JB, Connolly ES, Jr, Batjer HH, et al. American Heart Association: Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 2.King JT., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997;7:659–68. [PubMed] [Google Scholar]
- 3.Fujii M, Yan J, Rolland WB, et al. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–46. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nau R, Haase S, Bunkowski S, Brück W. Neuronal apoptosis in the dentate gyrus in humans with subarachnoid hemorrhage and cerebral hypoxia. Brain Pathol. 2002;12:329–36. doi: 10.1111/j.1750-3639.2002.tb00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan J, Chen C, Hu Q, et al. The role of p53 in brain edema after 24 h of experimental subarachnoid hemorrhage in a rat model. Exp Neurol. 2008;214:37–46. doi: 10.1016/j.expneurol.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Ghribi O, Geiger JD. Caffeine protects against disruptions of the blood brain barrier in animal models of Alzheimer’s and Parkinson’s diseases. J Alzheimer’s Dis. 2010;20(Suppl 1):S127–41. doi: 10.3233/JAD-2010-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugawara T, Jadhav V, Ayer R, et al. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 2009;40:1530–32. doi: 10.1161/STROKEAHA.108.531699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WJ, Rivera MN, Coffman EJ, Haber DA. The WTX tumor suppressor enhances p53 acetylation by CBP/p300. Mol Cell. 2012;45:587–97. doi: 10.1016/j.molcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill J, Calvert JW, Marcantonio S, Zhang JH. p53 may play an orchestrating role in apoptotic cell death after experimental subarachnoid hemorrhage. Neurosurgery. 2007;60:531–45. doi: 10.1227/01.NEU.0000249287.99878.9B. [DOI] [PubMed] [Google Scholar]
- 10.Becatti M, Taddei N, Cecchi C, et al. SIRT1 modulates MAPK pathways in ischemic reperfused cardiomyocytes. Cell Mol Life Sci. 2012;69:2245–60. doi: 10.1007/s00018-012-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis J. A dictionary of the flowering plant and ferns. Cambridge University Press; 1966. [Google Scholar]
- 12.Tiwari R, Trivedi M, Guang ZC, et al. Agrobacterium rhizogenes mediated transformation of Scutellaria baicalensis and production of flavonoids in hairy roots. Biol. Plantarum. 2008;52(1):26–35. [Google Scholar]
- 13.Kitamura K, Honda M, Yoshizaki H, et al. Baicalin, an inhibitor of HIV-1 production in vitro. Antivir Res. 1998;37(2):131–40. doi: 10.1016/s0166-3542(97)00069-7. [DOI] [PubMed] [Google Scholar]
- 14.Ikemoto S, Sugimura K, Yoshida N, et al. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology. 2000;55:951–55. doi: 10.1016/s0090-4295(00)00467-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HZ, Zhao ZL, Zhou CH. Recent advance in oxazole-based medicinal chemistry. Eur J Med Chem. 2018;144:444–92. doi: 10.1016/j.ejmech.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Shen YF, Wu XH, et al. Synthesis and biological evaluation of coumarin derivatives containing imidazole skeleton as potential antibacterial agents. Eur J Med Chem. 2018;143:958–69. doi: 10.1016/j.ejmech.2017.11.100. [DOI] [PubMed] [Google Scholar]
- 17.Bistrović A, Krstulović L, Harej A, et al. Design, synthesis and biological evaluation of novel benzimidazole amidines as potent multi-target inhibitors for the treatment of non-small cell lung cancer. Eur J Med Chem. 2018;143:1616–34. doi: 10.1016/j.ejmech.2017.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Xu YX, Wang H, Li XK, et al. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinylthiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2018;143:33–47. doi: 10.1016/j.ejmech.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar J, Khan AA, Ali Z, et al. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur J Med Chem. 2017;125:143–89. doi: 10.1016/j.ejmech.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Piao HZ, Jin SA, Chun HS, et al. Neuroprotective effect of wogonin: Potential roles of inflammatory cytokines. Arch Pharm Res. 2004;27:930–36. doi: 10.1007/BF02975846. [DOI] [PubMed] [Google Scholar]
- 21.Broderick JP, Brott TG, Duldner JE, et al. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–47. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 22.Claassen J, Carhuapoma JR, Kreiter KT, et al. Global cerebral edema after subarachnoid hemorrhage: Frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–32. doi: 10.1161/01.str.0000015624.29071.1f. [DOI] [PubMed] [Google Scholar]
- 23.Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–29. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin induced stabilization of blood brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–90. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kniesel U, Wolburg H. Tight junctions of the blood brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang S, Jensen JP, Ludwig RL, et al. Mdm2 is a RING finger dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–51. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 27.Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood brain barrier endothelial cells. Eur J Cell Biol. 2000;79:707–17. doi: 10.1078/0171-9335-00101. [DOI] [PubMed] [Google Scholar]
- 28.Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase 9 gene knock out on the proteolysis of blood brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L, Liu H, Yue L, et al. Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF-κB signaling pathway following subarachnoid hemorrhage in mice. Mol Neurobiol. 2017;54:1612–21. doi: 10.1007/s12035-016-9776-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XM, Zhang X, Zhang XS, et al. SIRT1 inhibition by sirtinol aggravates brain edema after experimental subarachnoid hemorrhage. J Neurosci Res. 2014;92:714–22. doi: 10.1002/jnr.23359. [DOI] [PubMed] [Google Scholar]
- 31.Sykes SM, Mellert HS, Holbert MA, et al. Acetylation of the p53 DNA binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–51. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]